Geochemical Effects on Storage Gases and Reservoir Rock during Underground Hydrogen Storage: A Depleted North Sea Oil Reservoir Case Study
Abstract
1. Background
2. Methodology
2.1. Base Scenario
2.2. Sensitivity Analysis
3. Results and Discussion
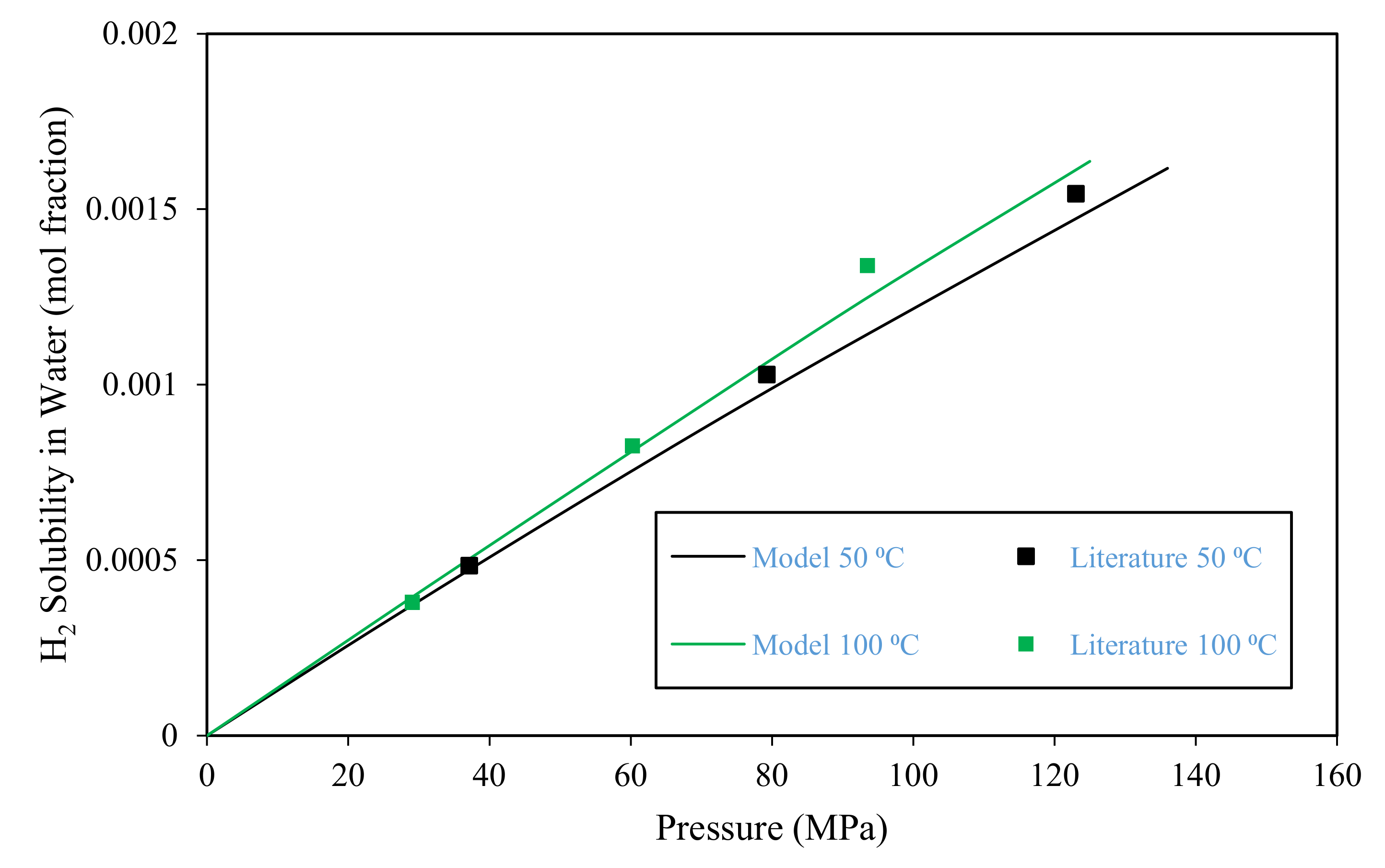
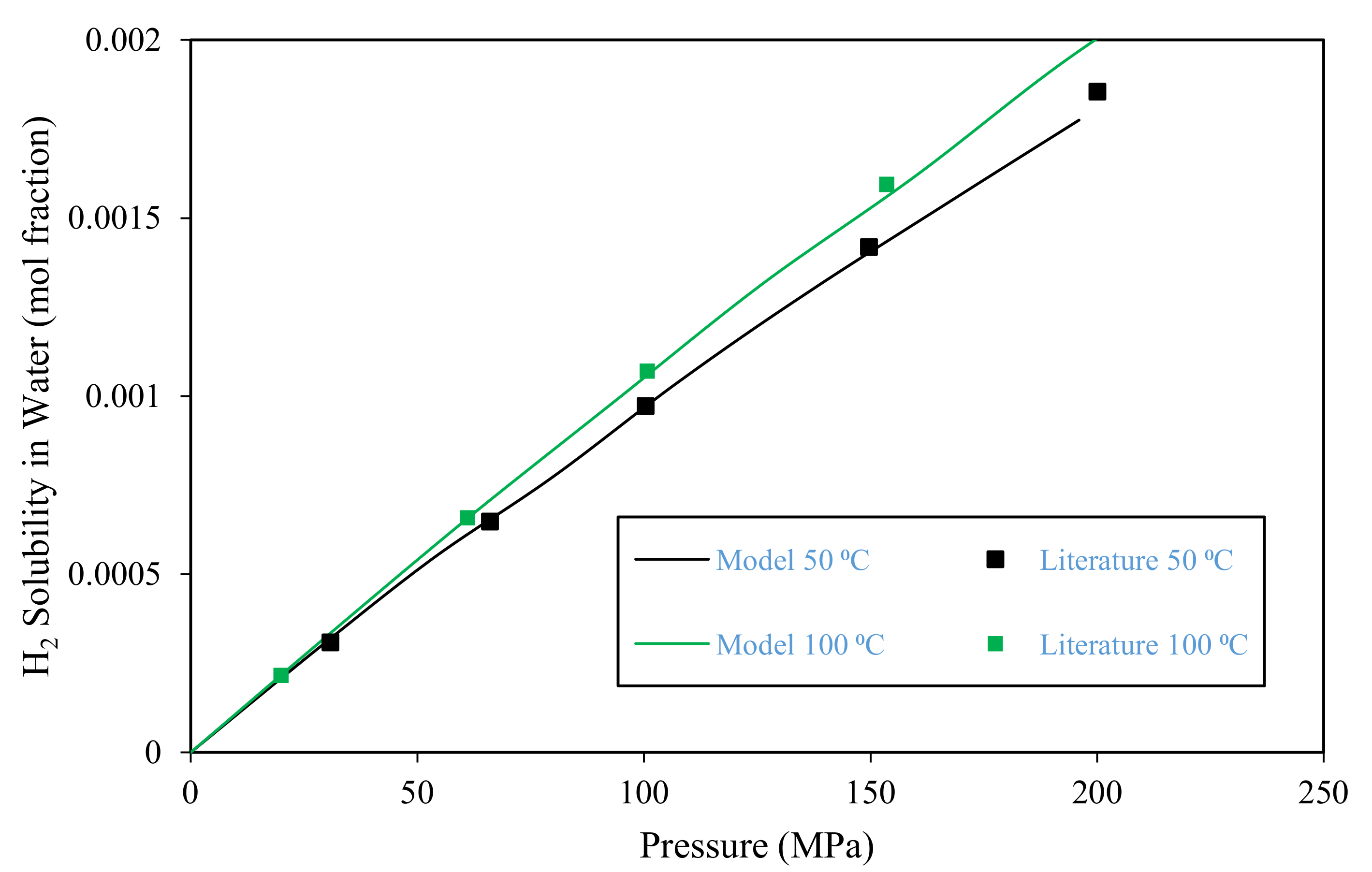
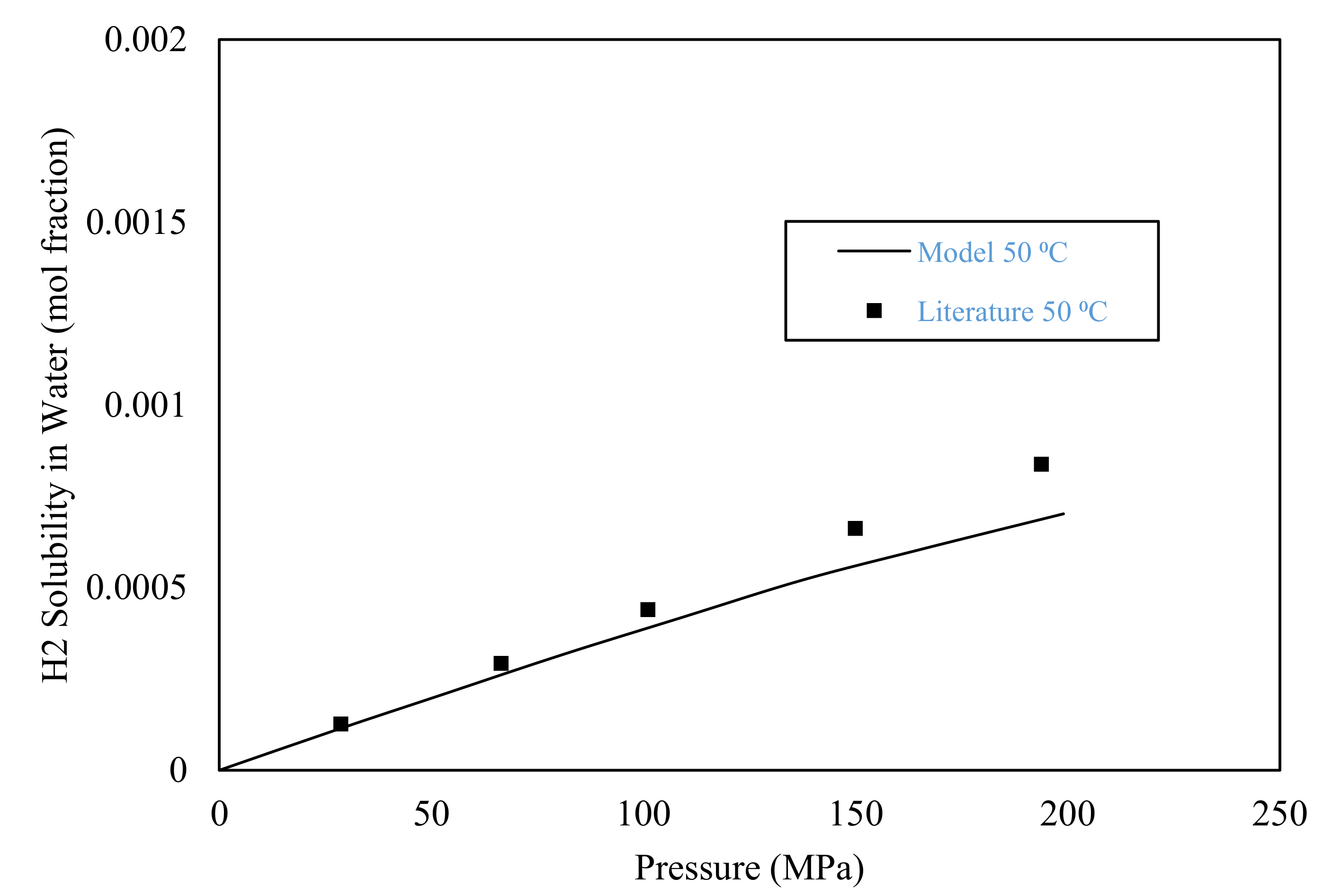
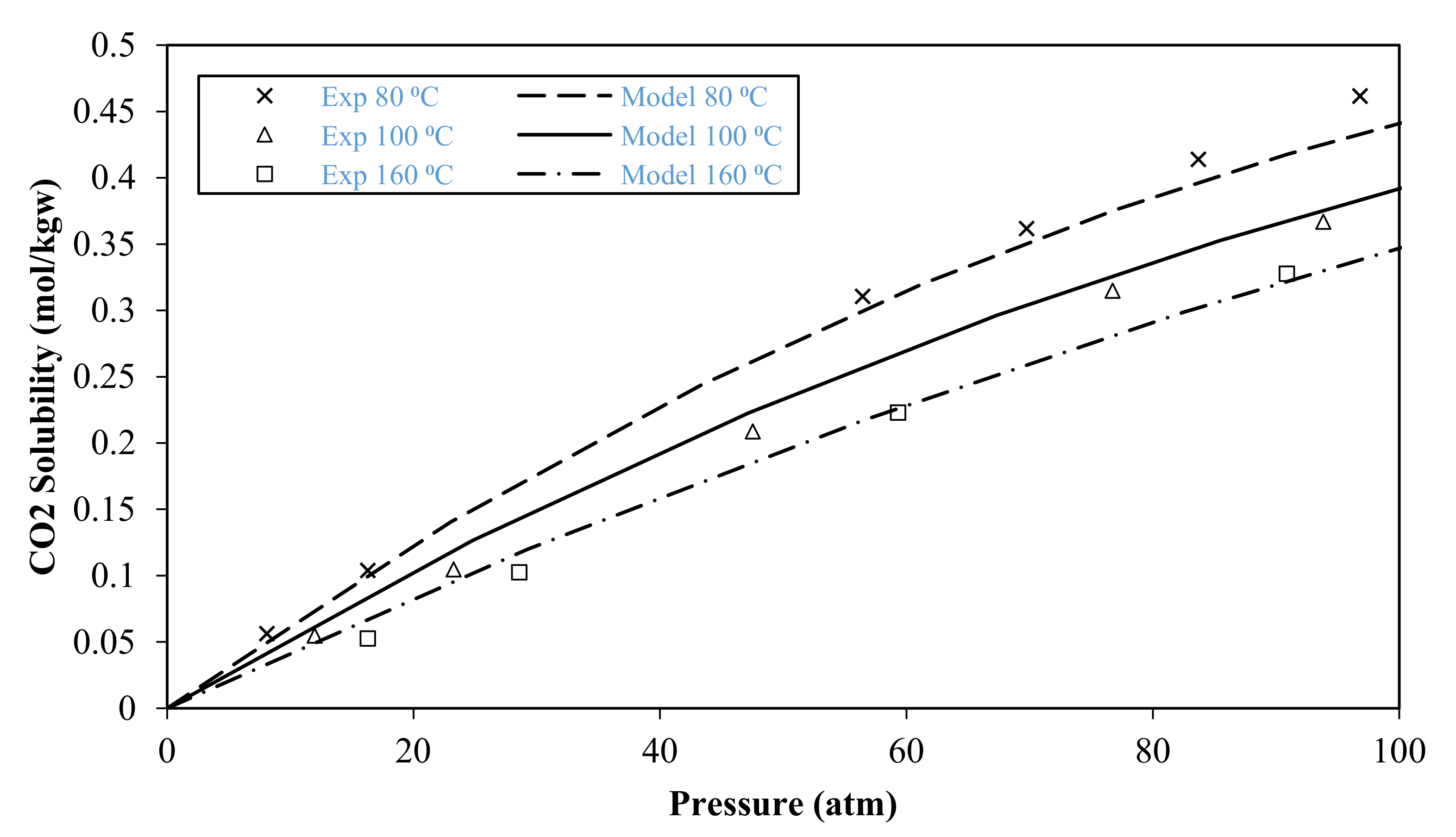
3.1. Model Validation
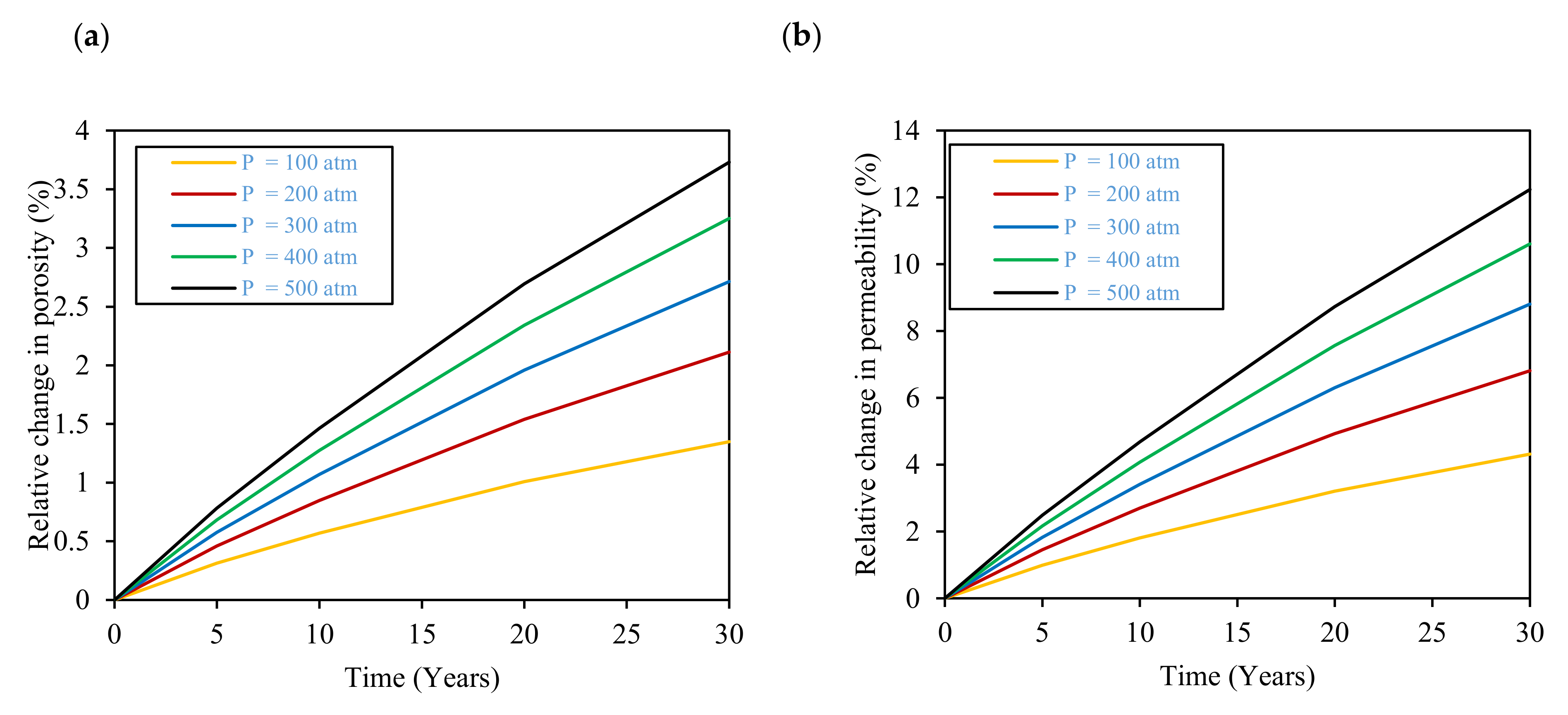
3.2. Effect of Pressure
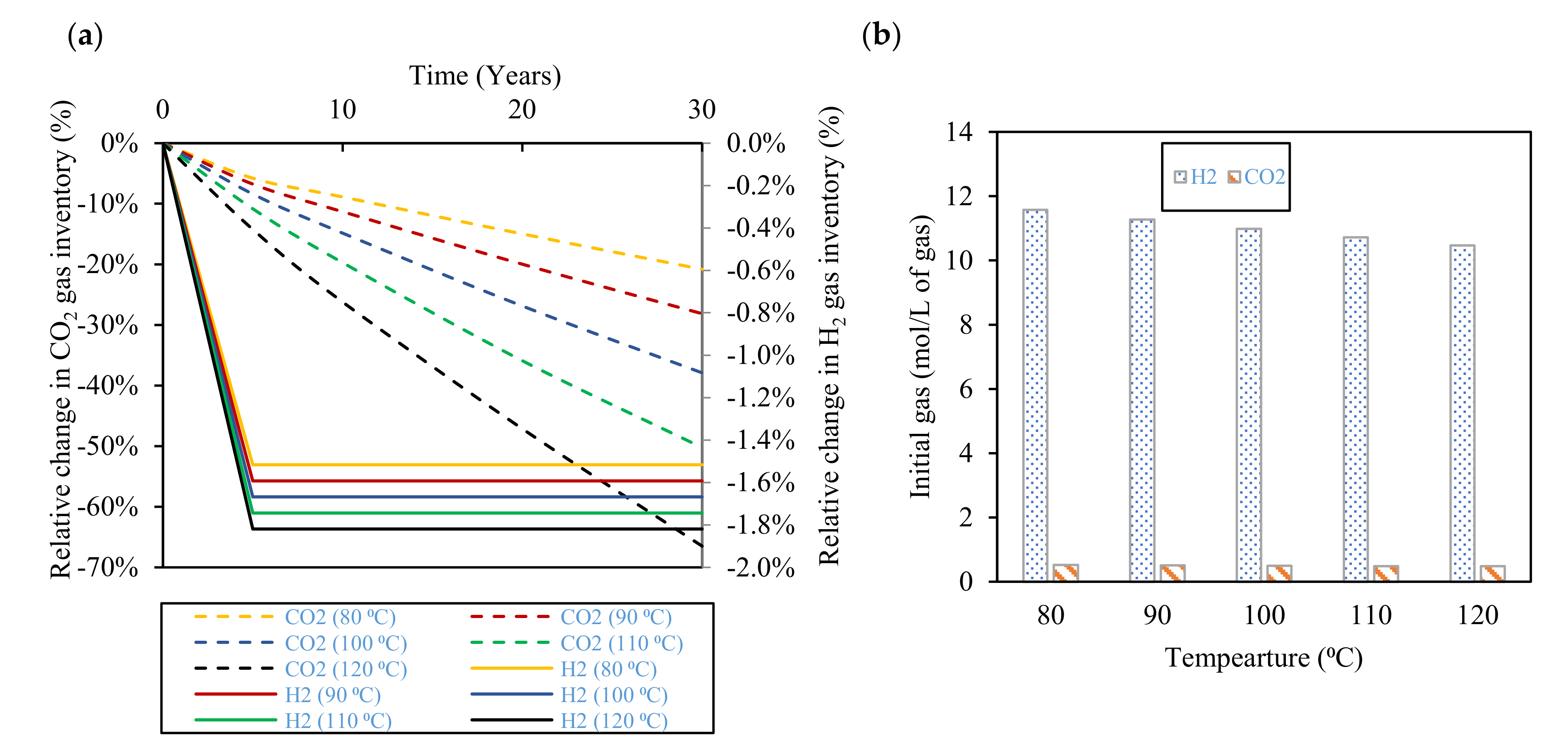
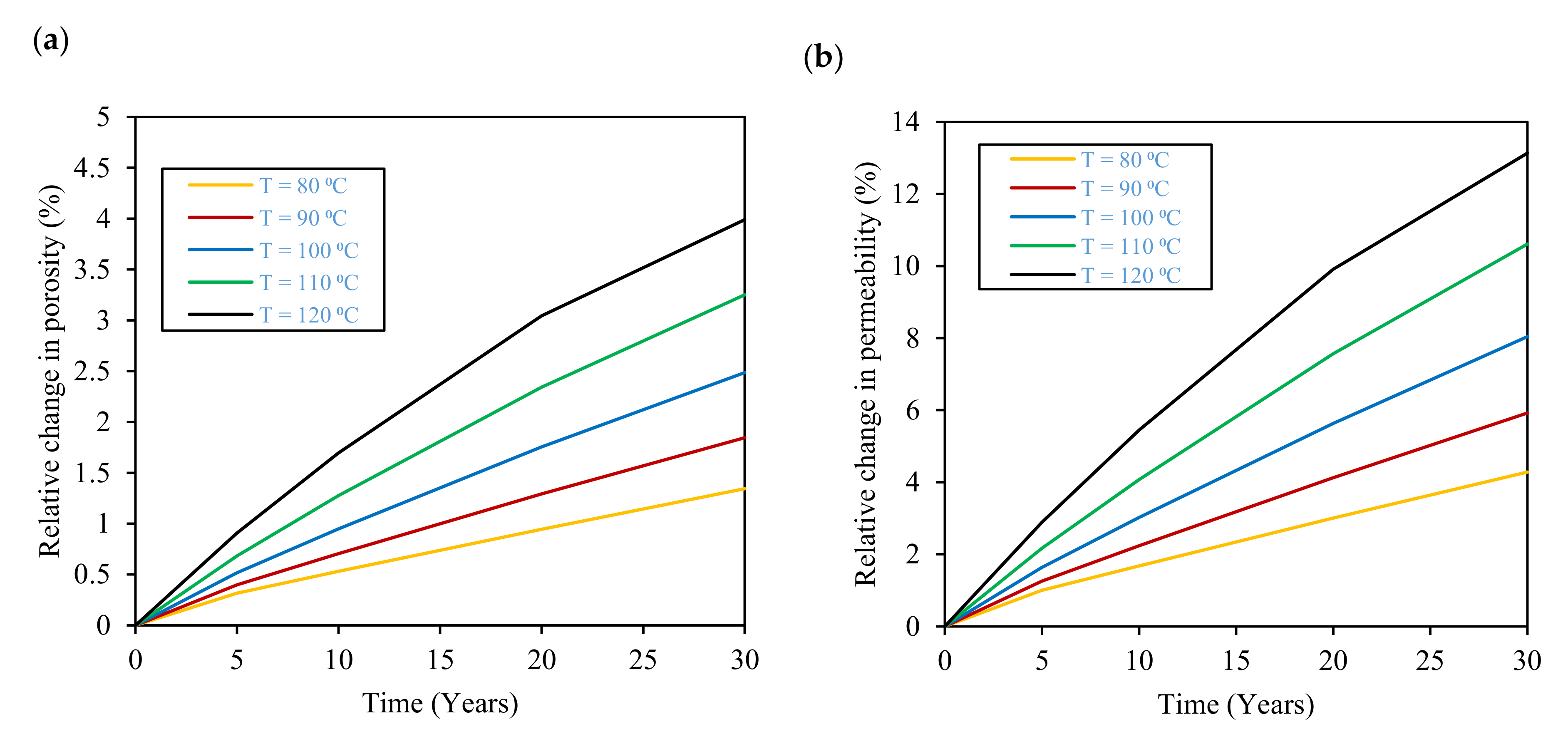
3.3. Effect of Temperature
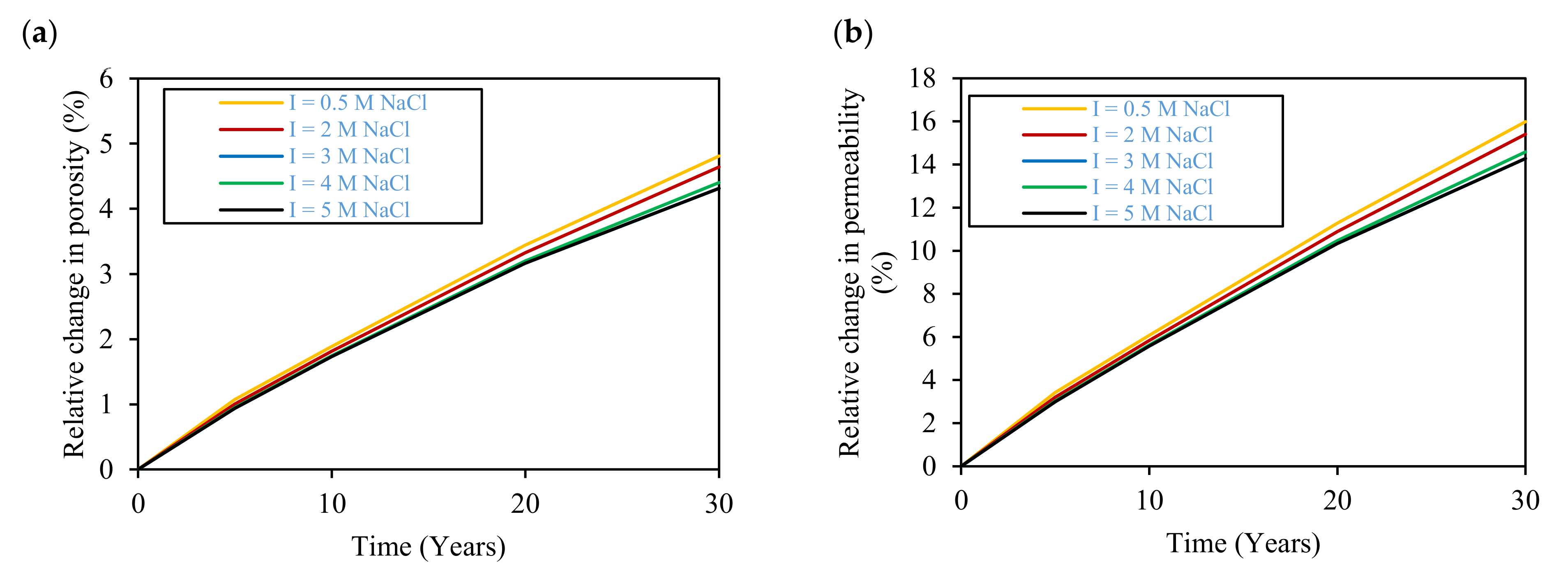
3.4. Effect of Salinity
4. Conclusions
- -
- The increase in reservoir pressure from 100 atm to 500 atm results in an increase in the dissolved hydrogen and carbon dioxide. Moreover, as a result of mineral dissolution reactions, the higher pressures result in an increase in the reservoir rock’s porosity and permeability. These porosity and permeability increases suggest that the reservoir’s storage capacity and ease of storage and withdrawal will be improved with time during storage. Nonetheless, due to the expected pressure changes during the injection and production stage of underground hydrogen storage, it would be important to understand the effects of pressure combined with the expected geochemical and geomechanical impacts on the reservoir rock’s strength during the underground hydrogen storage process.
- -
- Temperature increase enhances hydrogen gas and carbon dioxide gas dissolution and, consequently, gas losses, where losses of 2% and 67% in the stored hydrogen and carbon dioxide gases are expected at 120 °C after 30 years of storage, respectively. Moreover, higher temperatures and salinities limit microbial activity inside the reservoir and restrain the rate of biotic reactions that consume stored hydrogen. Therefore, the evaluation of the temperature and salinity effects on any UHS application should consider both biotic and abiotic reactions. However, there is a lack of experimental data on biotic reactions that must be properly addressed in future research.
- -
- In addition to the changes in porosity and permeability, this study shows that the losses of carbon dioxide gas inside the reservoir during underground hydrogen storage as a result of aqueous and mineral reactions are significant and may require remedial carbon dioxide gas to be injected to maintain cushion gas requirements.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Carden, P.O.; Paterson, L. Physical, chemical and energy aspects of underground hydrogen storage. Int. J. Hydrogen Energy 1979, 4, 559–569. [Google Scholar] [CrossRef]
- Gallo, A.B.; Simões-Moreira, J.R.; Costa, H.K.M.; Santos, M.M.; Moutinho dos Santos, E. Energy storage in the energy transition context: A technology review. Renew. Sustain. Energy Rev. 2016, 65, 800–822. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- McCay, M.H.; Shafiee, S. Hydrogen: An energy carrier. In Future Energy: Improved, Sustainable and Clean Options for Our Planet; Elsevier: Amsterdam, The Netherlands, 2020; pp. 475–493. [Google Scholar] [CrossRef]
- Das, D.; Veziroǧlu, T.N. Hydrogen production by biological processes: A survey of literature. Int. J. Hydrogen Energy 2001, 26, 13–28. [Google Scholar] [CrossRef]
- Davison, J.; Arienti, S.; Cotone, P.; Mancuso, L. Co-production of hydrogen and electricity with CO2 capture. Int. J. Greenh. Gas Control 2010, 4, 125–130. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Flesch, S.; Pudlo, D.; Albrecht, D.; Jacob, A.; Enzmann, F. Hydrogen underground storage—Petrographic and petrophysical variations in reservoir sandstones from laboratory experiments under simulated reservoir conditions. Int. J. Hydrogen Energy 2018, 43, 20822–20835. [Google Scholar] [CrossRef]
- Tarkowski, R. Underground hydrogen storage: Characteristics and prospects. Renew. Sustain. Energy Rev. 2019, 105, 86–94. [Google Scholar] [CrossRef]
- Zivar, D.; Kumar, S.; Foroozesh, J. Underground hydrogen storage: A comprehensive review. Int. J. Hydrogen Energy 2021, 46, 23436–23462. [Google Scholar] [CrossRef]
- Tarkowski, R. Perspectives of using the geological subsurface for hydrogen storage in Poland. Int. J. Hydrogen Energy 2017, 42, 347–355. [Google Scholar] [CrossRef]
- Tarkowski, R.; Uliasz-Misiak, B.; Tarkowski, P. Storage of hydrogen, natural gas, and carbon dioxide—Geological and legal conditions. Int. J. Hydrogen Energy 2021, 46, 20010–20022. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Haq, B.; Al Shehri, D.; Al-Ahmed, A.; Rahman, M.M.; Zaman, E. A review on underground hydrogen storage: Insight into geological sites, influencing factors and future outlook. Energy Rep. 2022, 8, 461–499. [Google Scholar] [CrossRef]
- Hematpur, H.; Abdollahi, R.; Rostami, S.; Haghighi, M.; Blunt, M.J. Review of underground hydrogen storage: Concepts and challenges. Adv. Geo-Energy Res. 2022, 7, 111–131. [Google Scholar] [CrossRef]
- Pan, B.; Yin, X.; Ju, Y.; Iglauer, S. Underground hydrogen storage: Influencing parameters and future outlook. Adv. Colloid Interface Sci. 2021, 294, 102473. [Google Scholar] [CrossRef]
- Raza, A.; Arif, M.; Glatz, G.; Mahmoud, M.; Al Kobaisi, M.; Alafnan, S.; Iglauer, S. A holistic overview of underground hydrogen storage: Influencing factors, current understanding, and outlook. Fuel 2022, 330, 125636. [Google Scholar] [CrossRef]
- Delshad, M.; Umurzakov, Y.; Sepehrnoori, K.; Eichhubl, P.; Batista Fernandes, B.R. Hydrogen Storage Assessment in Depleted Oil Reservoir and Saline Aquifer. Energies 2022, 15, 8132. [Google Scholar] [CrossRef]
- Green, D.W.; Willhite, G.P. Enhanced Oil Recovery. Henry, L. Doherty Memorial Fund of AIME, Society of Petroleum Engineers: Richardson, TX, USA, 1998; Volume 6. [Google Scholar]
- Jadhawar, P.; Yang, J.; Chapoy, A.; Tohidi, B. Subsurface Carbon Dioxide Sequestration and Storage in Methane Hydrate Reservoirs Combined with Clean Methane Energy Recovery. Energy Fuels 2021, 35, 1567–1579. [Google Scholar] [CrossRef]
- Oldenburg, C.; Stevens, S.; Benson, S. Carbon sequestration in natural gas reservoirs: Enhanced gas recovery and natural gas storage. In Proceedings of the Greenhouse Gas Control Technologies—6th International Conference, Kyoto, Japan, 1–4 October 2002; pp. 691–696. [Google Scholar] [CrossRef]
- Yekta, A.E.; Pichavant, M.; Audigane, P. Evaluation of geochemical reactivity of hydrogen in sandstone: Application to geological storage. Appl. Geochem. 2018, 95, 182–194. [Google Scholar] [CrossRef]
- Bo, Z.; Zeng, L.; Chen, Y.; Xie, Q. Geochemical reactions-induced hydrogen loss during underground hydrogen storage in sandstone reservoirs. Int. J. Hydrogen Energy 2021, 46, 19998–20009. [Google Scholar] [CrossRef]
- Hassannayebi, N.; Azizmohammadi, S.; De Lucia, M.; Ott, H. Underground hydrogen storage: Application of geochemical modelling in a case study in the Molasse Basin, Upper Austria. Environ. Earth Sci. 2019, 78, 177. [Google Scholar] [CrossRef]
- Hemme, C.; van Berk, W. Hydrogeochemical Modeling to Identify Potential Risks of Underground Hydrogen Storage in Depleted Gas Fields. Appl. Sci. 2018, 8, 2282. [Google Scholar] [CrossRef]
- Hagemann, B.; Rasoulzadeh, M.; Panfilov, M.; Ganzer, L.; Reitenbach, V. Hydrogenization of underground storage of natural gas: Impact of hydrogen on the hydrodynamic and bio-chemical behavior. Comput. Geosci. 2016, 20, 595–606. [Google Scholar] [CrossRef]
- Gregory, S.P.; Barnett, M.J.; Field, L.P.; Milodowski, A.E. Subsurface microbial hydrogen cycling: Natural occurrence and implications for industry. Microorganisms 2019, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Bernardez, L.A.; De Lima, L.R.P.A.; De Jesus, E.B.; Ramos, C.L.S.; Almeida, P.F. A kinetic study on bacterial sulfate reduction. Bioprocess Biosyst. Eng. 2013, 36, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Machel, H.G. Bacterial and thermochemical sulfate reduction in diagenetic settings—Old and new insights. Sediment. Geol. 2001, 140, 143–175. [Google Scholar] [CrossRef]
- Jørgensen, B.B.; Isaksen, M.F.; Jannasch, H.W. Bacterial sulfate reduction above 100°C in deep-sea hydrothermal vent sediments. Science 1992, 258, 1756–1757. [Google Scholar] [CrossRef]
- Thaysen, E.M.; McMahon, S.; Strobel, G.J.; Butler, I.B.; Ngwenya, B.T.; Heinemann, N.; Wilkinson, M.; Hassanpouryouzband, A.; McDermott, C.I.; Edlmann, K. Estimating microbial growth and hydrogen consumption in hydrogen storage in porous media. Renew. Sustain. Energy Rev. 2021, 151, 111481. [Google Scholar] [CrossRef]
- Amid, A.; Mignard, D.; Wilkinson, M. Seasonal storage of hydrogen in a depleted natural gas reservoir. Int. J. Hydrogen Energy 2016, 41, 5549–5558. [Google Scholar] [CrossRef]
- Laban, M. Hydrogen Storage in Salt Caverns: Chemical Modelling and Analysis of Large-Scale Hydrogen Storage in Underground Salt Caverns. Master’s Thesis, Delft University of Technology, Delft, The Netherlands, 2020. [Google Scholar]
- Zeng, L.; Hosseini, M.; Keshavarz, A.; Iglauer, S.; Lu, Y.; Xie, Q. Hydrogen wettability in carbonate reservoirs: Implication for underground hydrogen storage from geochemical perspective. Int. J. Hydrogen Energy 2022, 47, 25357–25366. [Google Scholar] [CrossRef]
- Zeng, L.; Keshavarz, A.; Xie, Q.; Iglauer, S. Hydrogen storage in Majiagou carbonate reservoir in China: Geochemical modelling on carbonate dissolution and hydrogen loss. Int. J. Hydrogen Energy 2022, 47, 24861–24870. [Google Scholar] [CrossRef]
- Parkhurst, D.; Appelo, C. Description of Input and Examples for PHREEQC Version 3: A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey: Denver, CO, USA, 2013. [Google Scholar]
- Saeed, M.; Jadhawar, P.; Ayirala, S.C.; Abhishek, R.; Zhou, Y. Modelling the effects of reservoir parameters and rock mineralogy on wettability during low salinity waterflooding in sandstone reservoirs. J. Pet. Sci. Eng. 2022, 215, 110676. [Google Scholar] [CrossRef]
- Saeed, M.; Jadhawar, P.; Zhou, Y.; Abhishek, R. Triple-layer surface complexation modelling: Characterization of oil-brine interfacial zeta potential under varying conditions of temperature, pH, oil properties and potential determining ions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127903. [Google Scholar] [CrossRef]
- Tavassoli, S.; Korrani, A.K.N.; Pope, G.A.; Sepehrnoori, K. Low-salinity surfactant flooding—A multimechanistic enhanced-oil-recovery method. SPE J. 2016, 21, 744–760. [Google Scholar] [CrossRef]
- Saeed, M.; Jadhawar, P. Surface Complexation Modeling of HPAM Polymer–Brine–Sandstone Interfaces for Application in Low-Salinity Polymer Flooding. Energy Fuels 2023, 37, 6585–6600. [Google Scholar] [CrossRef]
- Jordan, M.; Sorbie, K.; Jiang, P.; Yuan, M.; Todd, A.; Taylor, K. Mineralogical Controls on Inhibitor Adsorption/Desorption in Brent Group Sandstone and Their Importance in Predicting and Extending Field Squeeze Lifetimes. In Proceedings of the European Production Operations Conference and Exhibition, Aberdeen, UK, 15–17 March 1994. [Google Scholar] [CrossRef]
- Chabab, S.; Théveneau, P.; Coquelet, C.; Corvisier, J.; Paricaud, P. Measurements and predictive models of high-pressure H2 solubility in brine (H2O+NaCl) for underground hydrogen storage application. Int. J. Hydrogen Energy 2020, 45, 32206–32220. [Google Scholar] [CrossRef]
- Alvarez, J.; Crovetto, R.; Fernandez-Prini, R. The Dissolution of N2 and of H2 in water from room temperature to 640 K. Ber. Bunsenges. Phys. Chem. 1988, 92, 935–940. [Google Scholar] [CrossRef]
- Wiebe, R.; Gaddy, V.L. The Solubility of Hydrogen in Water at 0, 50, 75 and 100° from 25 to 1000 Atmospheres. J. Am. Chem. Soc. 1934, 56, 76–79. [Google Scholar] [CrossRef]
- Tödheide, K.; Franck, E.U. Das zweiphasengebiet und die kritische kurve im system kohlendioxid-wasser bis zu drucken von 3500 bar. Z. Phys. Chem. 1963, 37, 387–401. [Google Scholar] [CrossRef]
- Ahmadi, P.; Chapoy, A. CO2 solubility in formation water under sequestration conditions. Fluid Phase Equilibria 2018, 463, 80–90. [Google Scholar] [CrossRef]
- Rumpf, B.; Nicolaisen, H.; Öcal, C.; Maurer, G. Solubility of carbon dioxide in aqueous solutions of sodium chloride: Experimental results and correlation. J. Solut. Chem. 1994, 23, 431–448. [Google Scholar] [CrossRef]
- Saeed, M.; Sayani, J.K.S.; Jadhawar, P. Evaluating the Performance of Various Cushion Gas Types for Underground Hydrogen Storage in an Aquifer. In Proceedings of the 1st International Conference on Green Hydrogen for Global Decarbonisation, Gandhinagar, India, 17–18 March 2023. [Google Scholar]
- Giammar, D.E.; Bruant, R.G.; Peters, C.A. Forsterite dissolution and magnesite precipitation at conditions relevant for deep saline aquifer storage and sequestration of carbon dioxide. Chem. Geol. 2005, 217, 257–276. [Google Scholar] [CrossRef]
- Stillings, L.L.; Brantley, S.L. Feldspar dissolution at 25°C and pH 3: Reaction stoichiometry and the effect of cations. Geochim. Cosmochim. Acta 1995, 59, 1483–1496. [Google Scholar] [CrossRef]



| Mineral Phases Reaction | Log K @ 25 °C |
|---|---|
| −20.573 | |
| −18 | |
| 7.435 | |
| 3.95 | |
| 8.48 | |
| −18.479 | |
| −40.267 | |
| −17.09 |
| Mineral | Weight% |
|---|---|
| Quartz | 78 |
| Feldspar | 11 |
| Muscovite | 2 |
| Carbonate | 1 |
| Pyrite | 2 |
| Kaolinite + Illite | 5 |
| Ion | Concentration (mg/l) |
|---|---|
| Sr2+ | 46 |
| Ba2+ | 67 |
| Cl− | 14,740 |
| SO42- | 18 |
| Mg2+ | 62 |
| Ca2+ | 290 |
| K+ | 190 |
| Na+ | 9210 |
| Fe3+ | <0.10 |
| Parameter | Base Case | Minimum | Maximum |
|---|---|---|---|
| Pressure (atm) | 400 | 100 | 500 |
| Temperature (°C) | 100 | 80 | 120 |
| Salinity (M NaCl) | 0.5 | 0.5 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, M.; Jadhawar, P.; Bagala, S. Geochemical Effects on Storage Gases and Reservoir Rock during Underground Hydrogen Storage: A Depleted North Sea Oil Reservoir Case Study. Hydrogen 2023, 4, 323-337. https://doi.org/10.3390/hydrogen4020023
Saeed M, Jadhawar P, Bagala S. Geochemical Effects on Storage Gases and Reservoir Rock during Underground Hydrogen Storage: A Depleted North Sea Oil Reservoir Case Study. Hydrogen. 2023; 4(2):323-337. https://doi.org/10.3390/hydrogen4020023
Chicago/Turabian StyleSaeed, Motaz, Prashant Jadhawar, and Stefano Bagala. 2023. "Geochemical Effects on Storage Gases and Reservoir Rock during Underground Hydrogen Storage: A Depleted North Sea Oil Reservoir Case Study" Hydrogen 4, no. 2: 323-337. https://doi.org/10.3390/hydrogen4020023
APA StyleSaeed, M., Jadhawar, P., & Bagala, S. (2023). Geochemical Effects on Storage Gases and Reservoir Rock during Underground Hydrogen Storage: A Depleted North Sea Oil Reservoir Case Study. Hydrogen, 4(2), 323-337. https://doi.org/10.3390/hydrogen4020023







