Experimental and Simulation Study on Coproduction of Hydrogen and Carbon Nanomaterials by Catalytic Decomposition of Methane-Hydrogen Mixtures
Abstract
1. Introduction
2. Materials and Methods
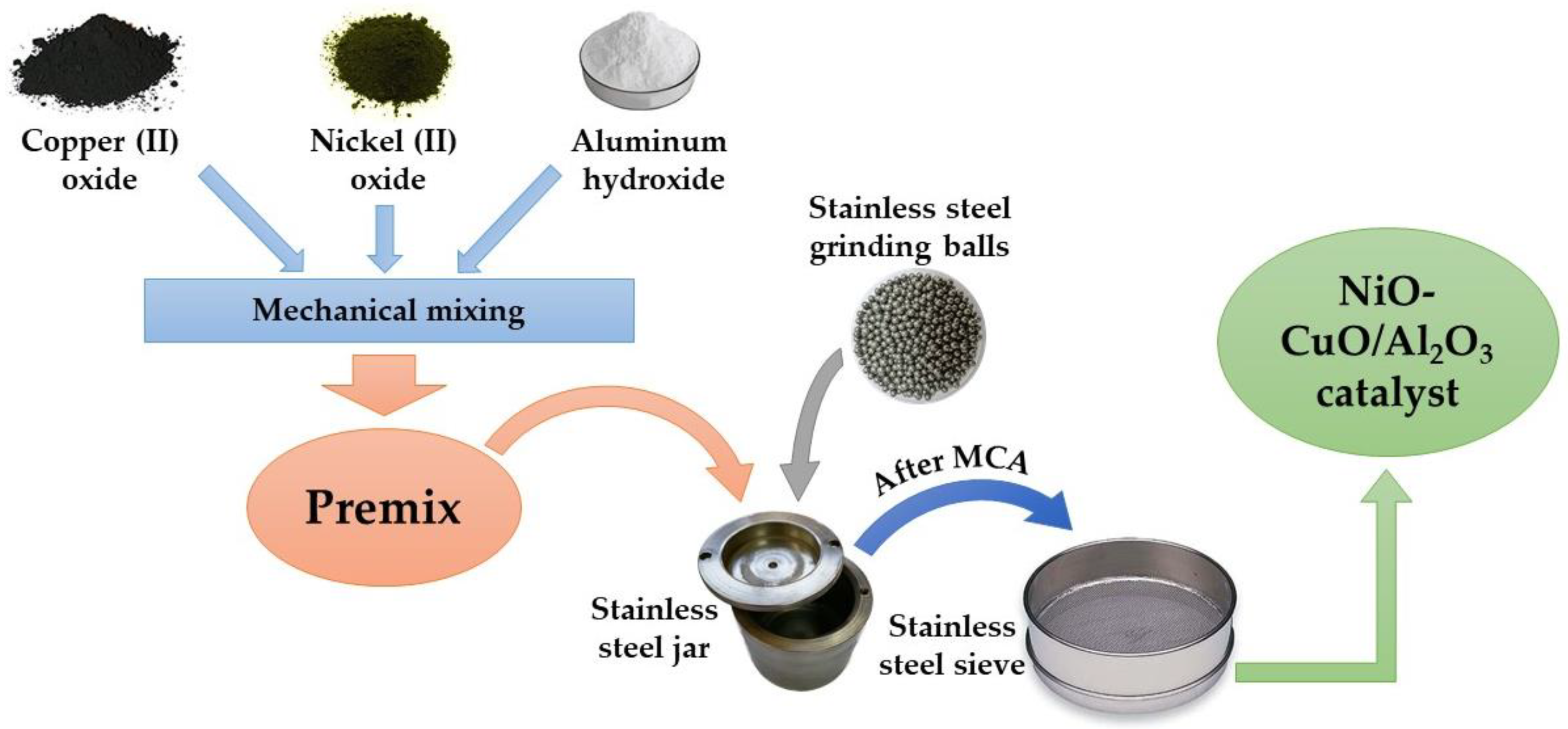
2.1. Synthesis of the Catalyst
2.2. Kinetic Studies on Decomposition of Methane–Hydrogen Mixture
2.3. Characterization of the Samples
3. Mathematical Modelling of the Process
3.1. Mathematical Model of the Reactor
- The volume of the reaction mixture is constant;
- Any changes in the volume of the reaction mixture caused by the reaction are negligible;
- An ideal mixing mode is realized in the reaction zone.
3.2. Kinetic Model of the Process
4. Results and Discussions
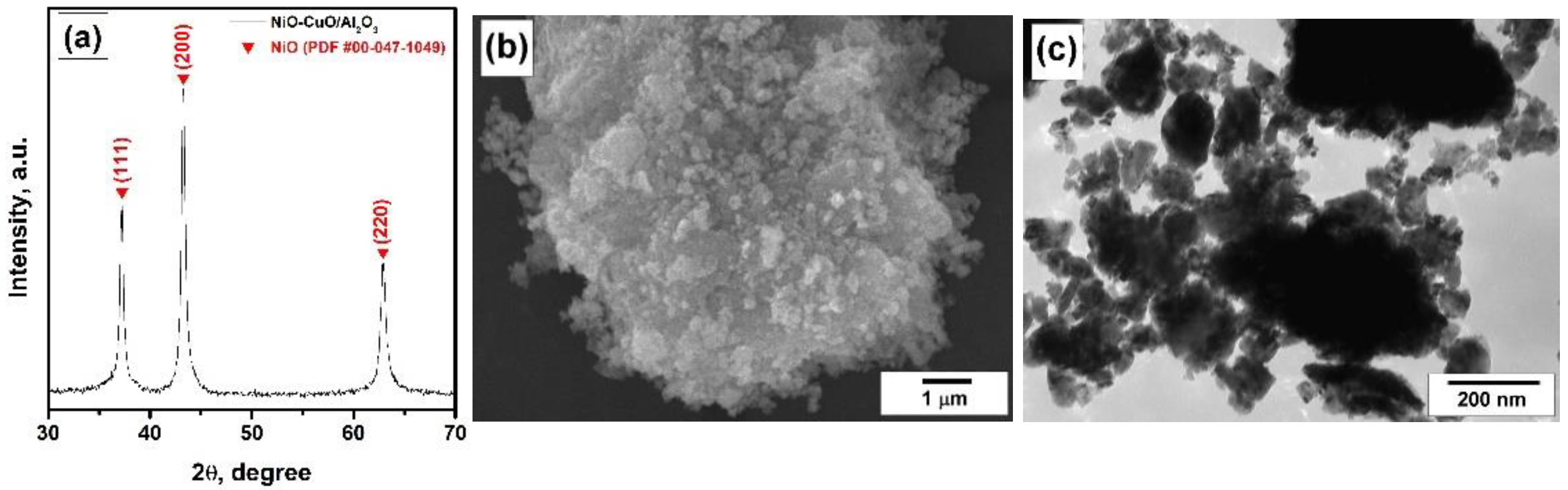
4.1. Characterization of the Catalyst
4.2. Kinetic Features of the Decomposition of Methane–Hydrogen Mixture
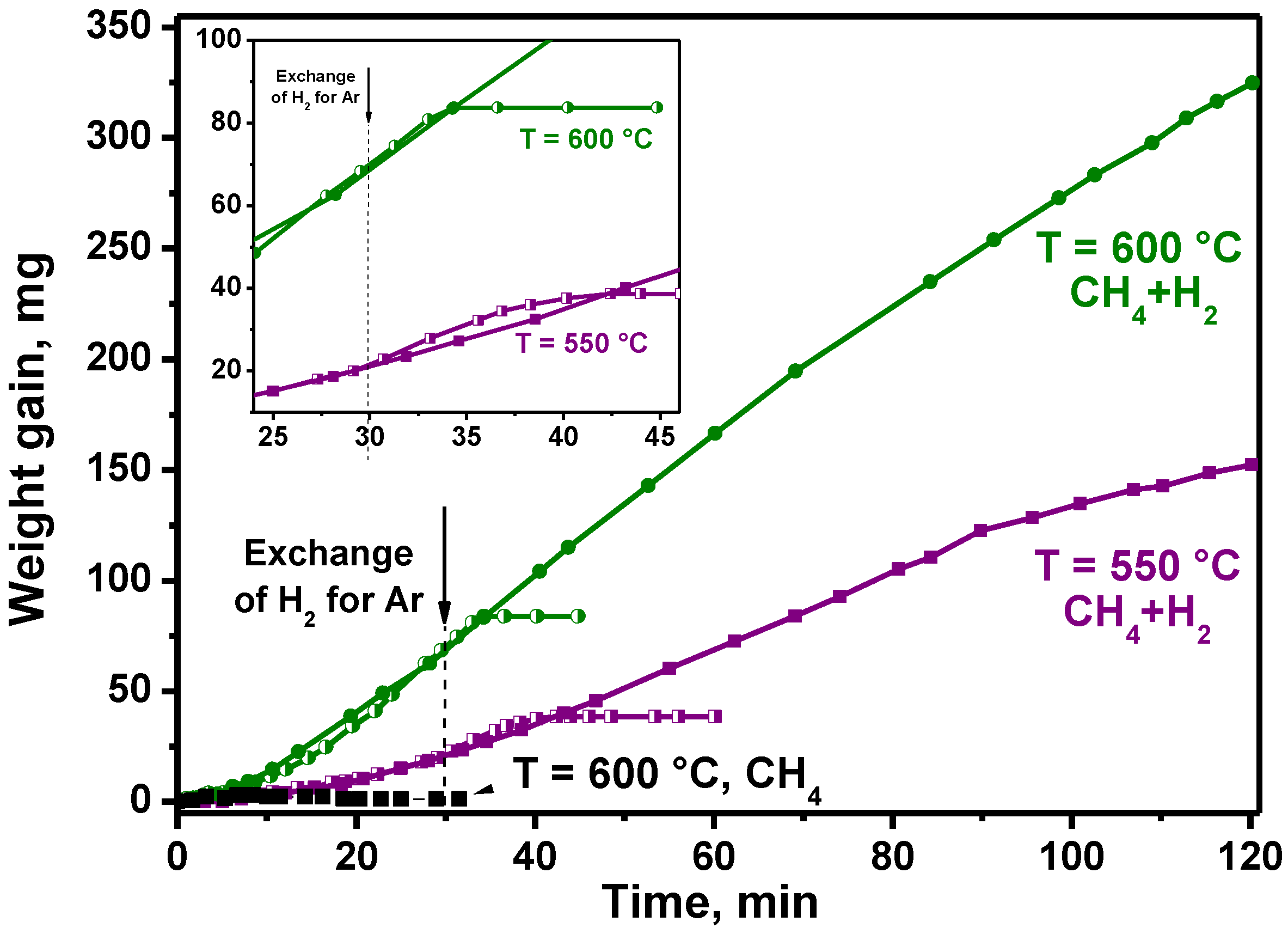
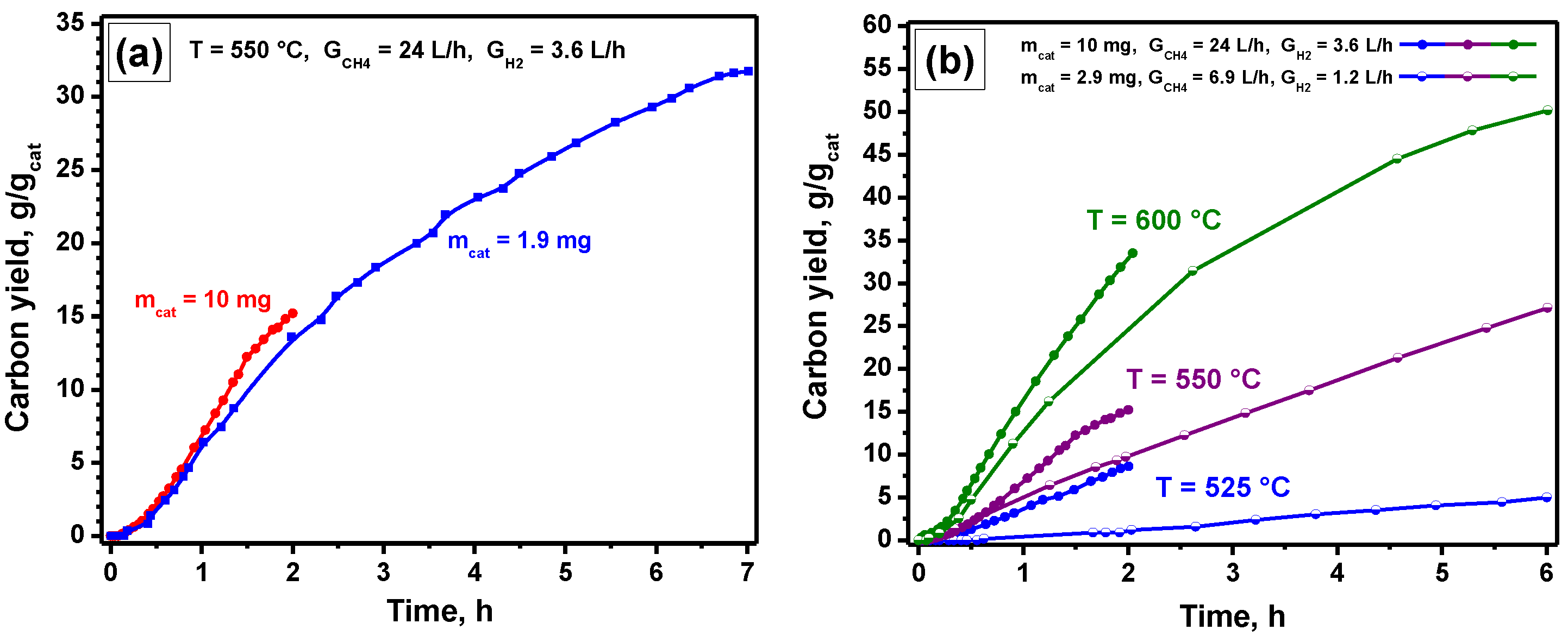
4.2.1. Effect of the Hydrogen Presence
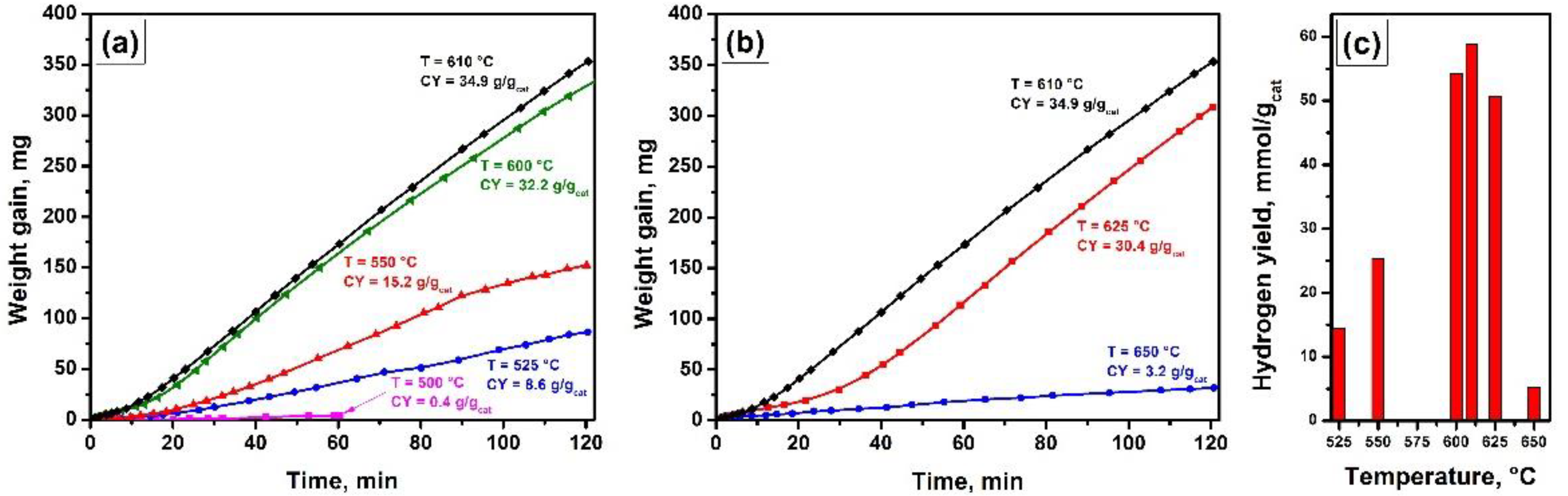
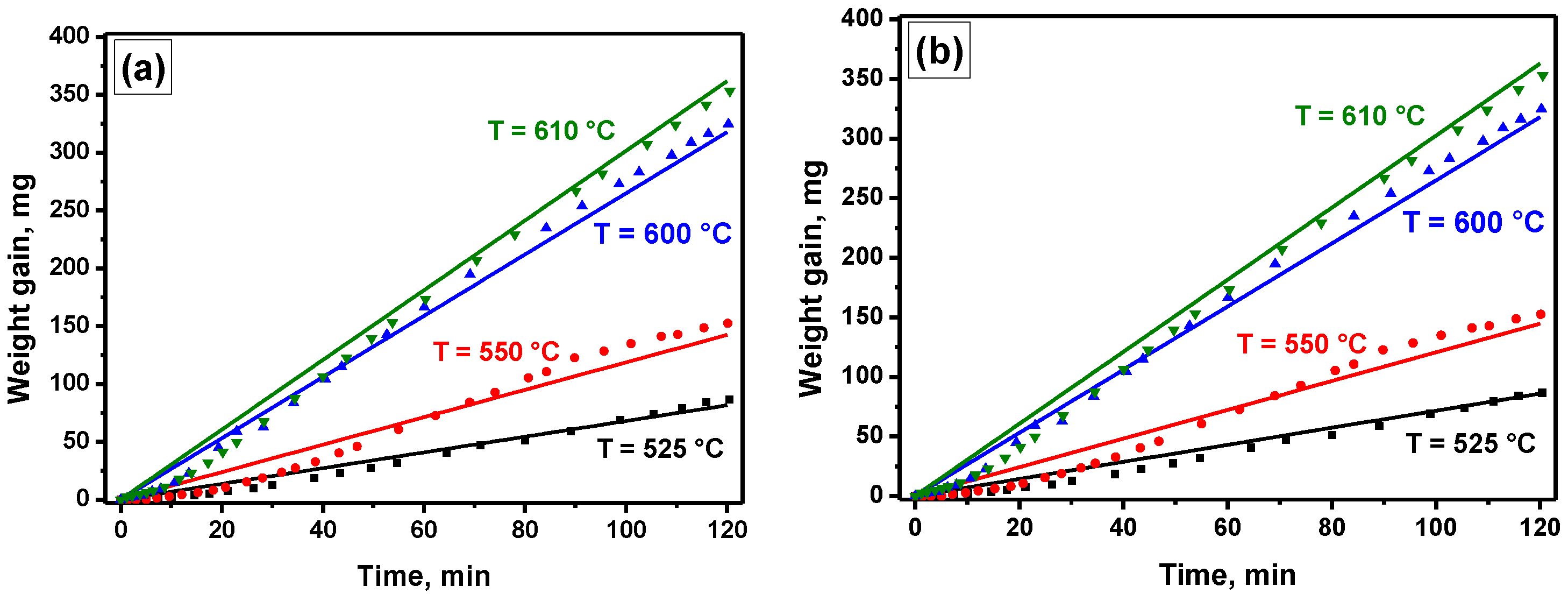
4.2.2. Effect of the Temperature
4.2.3. Studying the Stability of the Catalyst
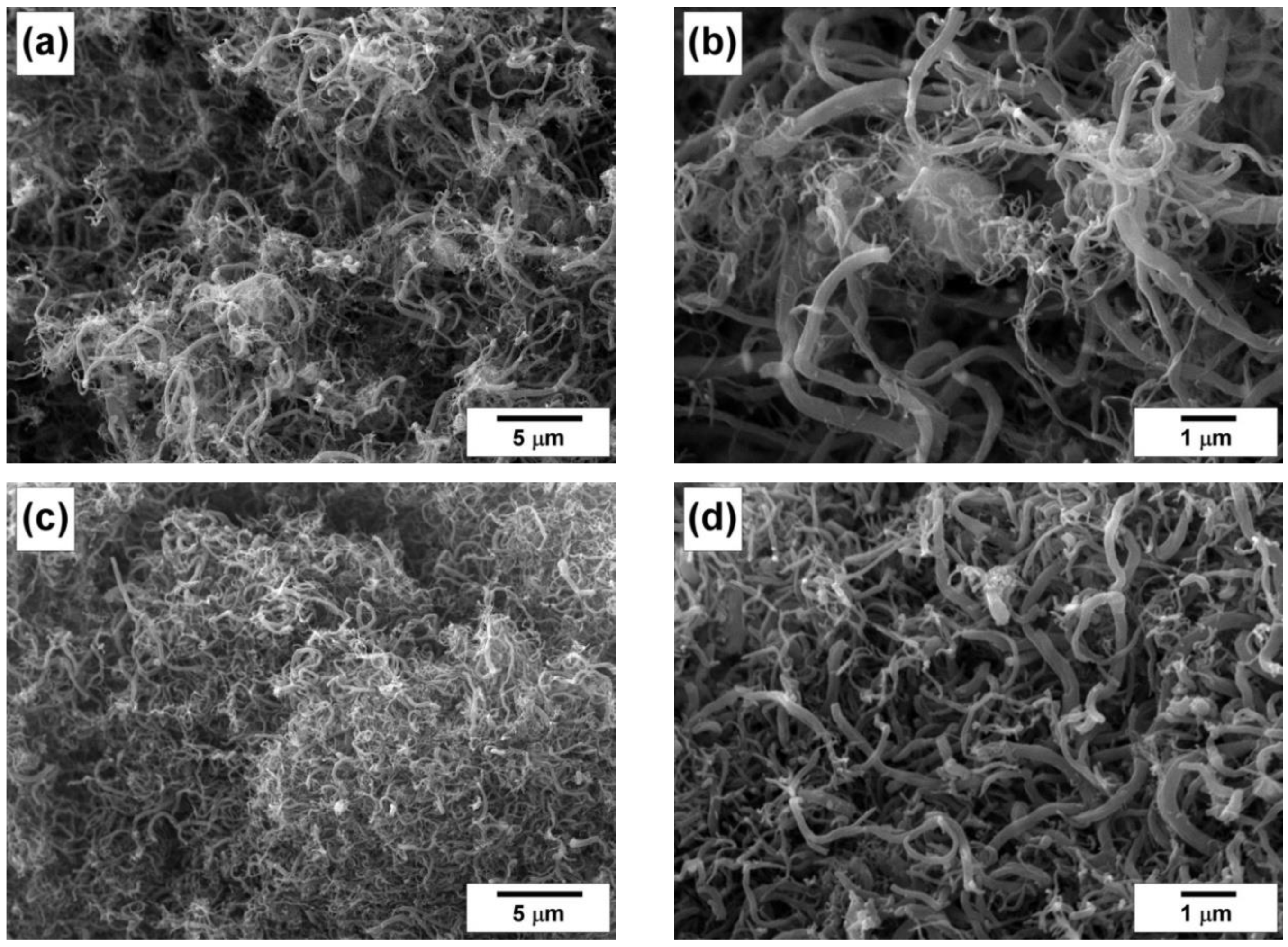
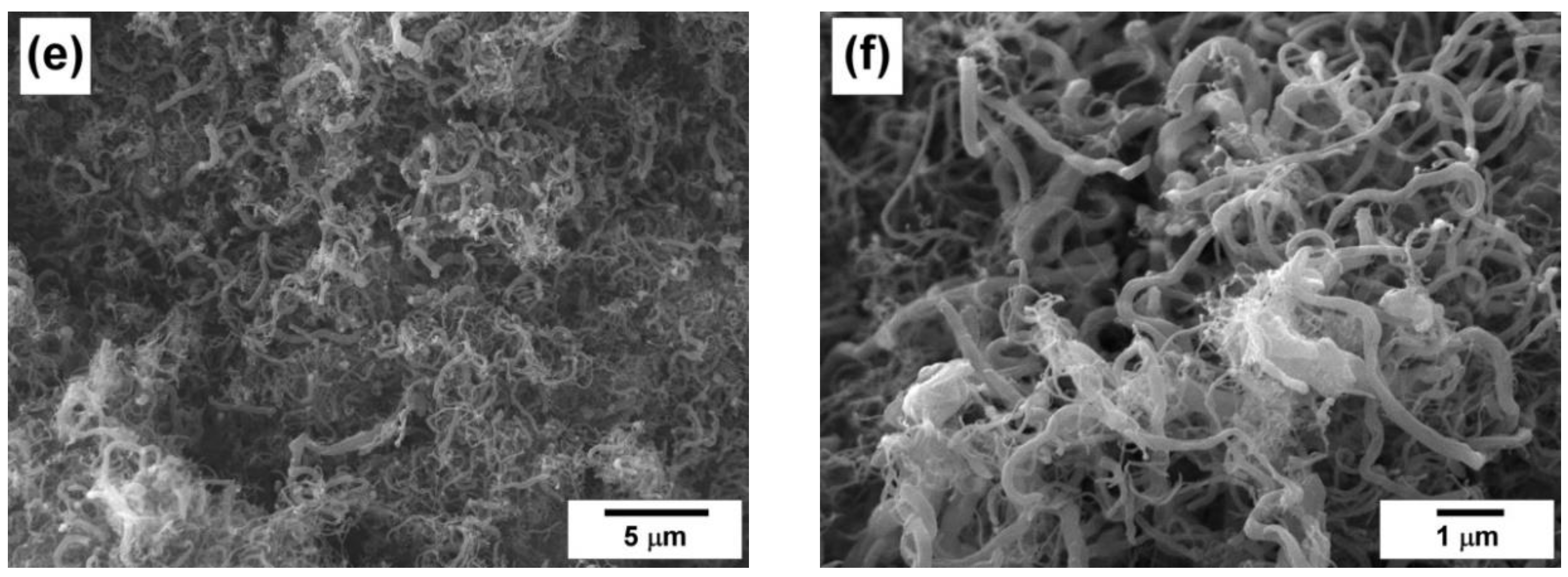
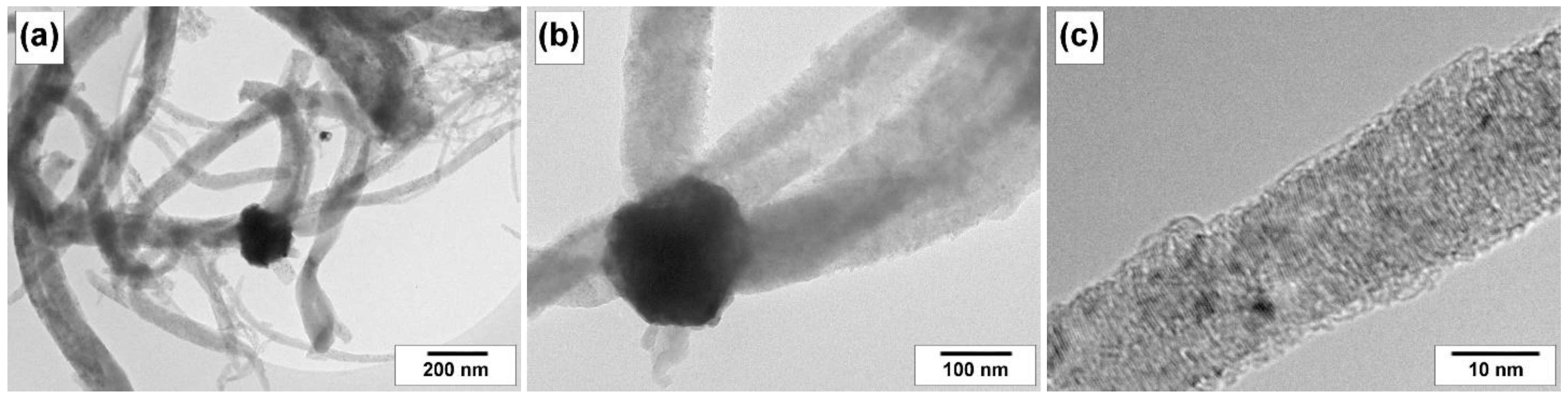
4.3. Characterization of the Carbon Product
4.4. Estimation of the Kinetic Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Ci | concentration of the i-th component, mol/m3 |
| G | flow rate of the gas mixture, L/h |
| mcat | weight of the catalyst, mg |
| r | rate of the reaction, mol/mcat3·s |
| t | time, h |
| Vcat | volume of the catalyst, m3 |
| Vmix | volume of the gas mixture inside the reactor, m3 |
| ν | stoichiometric coefficient |
| Pi | partial pressure of the i-th component, atm |
| k+ | rate constant of the direct reaction, mol/(mcat3·s·atm) |
| k− | rate constant of the reverse reaction, mol/(mcat3·s·atm1/2) |
References
- Da Rosa, A.V.; Ordóñez, J.C. Hydrogen production. In Fundamentals of Renewable Energy Processes; Academic Press: Cambridge, MA, USA, 2022; pp. 419–470. [Google Scholar] [CrossRef]
- Falcone, P.M.; Hiete, M.; Sapio, A. Hydrogen economy and sustainable development goals: Review and policy insights. Curr. Opin. Green Sustain. Chem. 2021, 31, 100506. [Google Scholar] [CrossRef]
- Espegren, K.; Damman, S.; Pisciella, P.; Graabak, I.; Tomasgard, A. The role of hydrogen in the transition from a petroleum economy to a low-carbon society. Int. J. Hydrogen Energ. 2021, 46, 23125–23138. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Beswick, R.R.; Yan, Y. A green hydrogen economy for a renewable energy society. Curr. Opin. Chem. Eng. 2021, 33, 100701. [Google Scholar] [CrossRef]
- Franchi, G.; Capocelli, M.; De Falco, M.; Piemonte, V.; Barba, D. Hydrogen production via steam reforming: A critical analysis of MR and RMM technologies. Membranes 2020, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, H.; Liu, X.; Jiang, Q.; Hong, H. A strategy of mid-temperature natural gas based chemical looping reforming for hydrogen production. Int. J. Hydrogen Energ. 2022, 47, 12052–12066. [Google Scholar] [CrossRef]
- Pashchenko, D. Natural gas reforming in thermochemical waste-heat recuperation systems: A review. Energy 2022, 251, 123854. [Google Scholar] [CrossRef]
- Amin, A.M.; Croiset, E.; Epling, W. Review of methane catalytic cracking for hydrogen production. Int. J. Hydrogen Energ. 2011, 36, 2904–2935. [Google Scholar] [CrossRef]
- Abbas, H.F.; Wan Daud, W.M.A. Hydrogen production by methane decomposition: A review. Int. J. Hydrogen Energ. 2010, 35, 1160–1190. [Google Scholar] [CrossRef]
- Tezel, E.; Figen, H.E.; Baykara, S.Z. Hydrogen production by methane decomposition using bimetallic Ni–Fe catalysts. Int. J. Hydrogen Energ. 2019, 44, 9930–9940. [Google Scholar] [CrossRef]
- Silva, R.R.C.M.; Oliveira, H.A.; Guarino, A.C.P.F.; Toledo, B.B.; Moura, M.B.T.; Oliveira, B.T.M.; Passos, F.B. Effect of support on methane decomposition for hydrogen production over cobalt catalysts. Int. J. Hydrogen Energy 2016, 41, 6763–6772. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Chichkan, A.S. Production of hydrogen by methane catalytic decomposition over Ni–Cu–Fe/Al2O3 catalyst. Int. J. Hydrogen Energ. 2009, 34, 2979–2985. [Google Scholar] [CrossRef]
- Ping, D.; Wang, C.; Dong, X.; Dong, Y. Co-production of hydrogen and carbon nanotubes on nickel foam via methane catalytic decomposition. Appl. Surf. Sci. 2016, 369, 299–307. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Ibrahim, A.A.; Khan, W.U.; Seshan, K.; Al Otaibi, R.L.; Al-Fatesh, A.S. Hydrogen production via catalytic methane decomposition over alumina supported iron catalyst. Arab. J. Chem. 2018, 11, 405–414. [Google Scholar] [CrossRef]
- Villacampa, J.I.; Royo, C.; Romeo, E.; Montoya, J.A.; Del Angel, P.; Monzón, A. Catalytic decomposition of methane over Ni-Al2O3 coprecipitated catalysts: Reaction and regeneration studies. Appl. Catal. A-Gen. 2003, 252, 363–383. [Google Scholar] [CrossRef]
- Cazaña, F.; Latorre, N.; Tarifa, P.; Royo, C.J.; Sebastián, V.; Romeo, E.; Centeno, M.A.; Monzón, A. Performance of AISI 316L-stainless steel foams towards the formation of graphene related nanomaterials by catalytic decomposition of methane at high temperature. Catal. Today 2022, 383, 236–246. [Google Scholar] [CrossRef]
- Dussault, L.; Dupin, J.C.; Guimon, C.; Monthioux, M.; Latorre, N.; Ubieto, T.; Romeo, E.; Royo, C.; Monzón, A. Development of Ni–Cu–Mg–Al catalysts for the synthesis of carbon nanofibers by catalytic decomposition of methane. J. Catal. 2007, 251, 223–232. [Google Scholar] [CrossRef]
- Cazaña, F.; Latorre, N.; Tarifa, P.; Labarta, J.; Romeo, E.; Monzón, A. Synthesis of graphenic nanomaterials by decomposition of methane on a Ni-Cu/biomorphic carbon catalyst. Kinetic and characterization results. Catal. Today 2018, 299, 67–79. [Google Scholar] [CrossRef]
- Shen, Y.; Lua, A.C. Synthesis of Ni and Ni–Cu supported on carbon nanotubes for hydrogen and carbon production by catalytic decomposition of methane. Appl. Catal. B-Environ. 2015, 164, 61–69. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Mishakov, I.V.; Korneev, D.V.; Bauman, Y.I.; Nalivaiko, A.Y.; Gromov, A.A. Selected aspects of hydrogen production via catalytic decomposition of hydrocarbons. Hydrogen 2021, 2, 122–133. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Bauman, Y.I.; Streltsov, I.A.; Korneev, D.V.; Vinokurova, O.B.; Vedyagin, A.A. The regularities of the formation of carbon nanostructures from hydrocarbons based on the composition of the reaction mixture. Resour. Effic. Technol. 2016, 2, 61–67. [Google Scholar] [CrossRef]
- Wang, S.; Tan, K.H. Flexural performance of reinforced carbon nanofibers enhanced lightweight cementitious composite (CNF-LCC) beams. Eng. Struct. 2021, 238, 112221. [Google Scholar] [CrossRef]
- Wang, L.; Aslani, F. Development of self-sensing cementitious composites incorporating CNF and hybrid CNF/CF. Construct. Build. Mater. 2021, 273, 121659. [Google Scholar] [CrossRef]
- Petukhova, E.S.; Fedorov, A.L.; Bauman, Y.I.; Zdanovich, A.A.; Mishakov, I.V.; Matsko, M.A. Reinforcing of polyethylene with carbon nanofibers: An approach to improve CNF distribution via pre-coating of CNF surface by PE. J. Phys. Conf. Series 2021, 1889, 022089. [Google Scholar] [CrossRef]
- Nanni, F.; Travaglia, P.; Valentini, M. Effect of carbon nanofibres dispersion on the microwave absorbing properties of CNF/epoxy composites. Compos. Sci. Technol. 2009, 69, 485–490. [Google Scholar] [CrossRef]
- He, S.; Yang, E.-H. Strategic strengthening of the interfacial transition zone (ITZ) between microfiber and cement paste matrix with carbon nanofibers (CNFs). Cement Concrete Compos. 2021, 119, 104019. [Google Scholar] [CrossRef]
- Shelepova, E.V.; Vedyagin, A.A.; Mishakov, I.V.; Noskov, A.S. Simulation of hydrogen and propylene coproduction in catalytic membrane reactor. Int. J. Hydrogen Energ. 2015, 40, 3592–3598. [Google Scholar] [CrossRef]
- Shelepova, E.V.; Vedyagin, A.A. Theoretical prediction of the efficiency of hydrogen production via alkane dehydrogenation in catalytic membrane reactor. Hydrogen 2021, 2, 362–376. [Google Scholar] [CrossRef]
- Zavarukhin, S.G.; Kuvshinov, G.G. Mathematic modeling of the process of production of nanofibrous carbon from methane in an isothermal reactor with a fixed bed of the Ni–Al2O3 catalyst. Chem. Eng. J. 2006, 120, 139–147. [Google Scholar] [CrossRef]
- Zavarukhin, S.G.; Kuvshinov, G.G. Mathematical modeling of the continuous process for synthesis of nanofibrous carbon in a moving catalyst bed reactor with recirculating gas flow. Chem. Eng. J. 2008, 137, 681–685. [Google Scholar] [CrossRef]
- Chen, Q.; Lua, A.C. Kinetic reaction and deactivation studies on thermocatalytic decomposition of methane by electroless nickel plating catalyst. Chem. Eng. J. 2020, 389, 124366. [Google Scholar] [CrossRef]
- Zhang, Y.; Smith, K.J. A kinetic model of CH4 decomposition and filamentous carbon formation on supported Co catalysts. J. Catal. 2005, 231, 354–364. [Google Scholar] [CrossRef]
- Borghei, M.; Karimzadeh, R.; Rashidi, A.; Izadi, N. Kinetics of methane decomposition to COx-free hydrogen and carbon nanofiber over Ni–Cu/MgO catalyst. Int. J. Hydrogen Energ. 2010, 35, 9479–9488. [Google Scholar] [CrossRef]
- Zavarukhin, S.G.; Kuvshinov, G.G. The kinetic model of formation of nanofibrous carbon from CH4–H2 mixture over a high-loaded nickel catalyst with consideration for the catalyst deactivation. Appl. Catal. A-Gen. 2004, 272, 219–227. [Google Scholar] [CrossRef]
- Wang, H.Y.; Lua, A.C. Deactivation and kinetic studies of unsupported Ni and Ni–Co–Cu alloy catalysts used for hydrogen production by methane decomposition. Chem. Eng. J. 2014, 243, 79–91. [Google Scholar] [CrossRef]
- Demicheli, M.C.; Ponzi, E.N.; Ferretti, O.A.; Yeramian, A.A. Kinetics of carbon formation from CH4-H2 mixtures on nickel-alumina catalyst. Chem. Eng. J. 1991, 46, 129–136. [Google Scholar] [CrossRef]
- Alstrup, I.; Tavares, M.T. Kinetics of carbon formation from CH4 + H2 on silica-supported nickel and Ni-Cu catalysts. J. Catal. 1993, 139, 513–524. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2006; 928p. [Google Scholar]
- Salmi, T.O.; Mikkola, J.-P.; Warna, J.P. Chemical Reaction Engineering and Reactor Technology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2010; 644p. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Buyanov, R.A. The formation of carbon filaments upon decomposition of hydrocarbons catalysed by iron subgroup metals and their alloys. Russ. Chem. Rev. 2000, 69, 623–638. [Google Scholar] [CrossRef]


| # | Temperature, °C | CY, g/gcat | SSA, m2/g | Vpore, cm3/g | CRC, wt% |
|---|---|---|---|---|---|
| 1 | 550 | 15.2 | 174 | 0.16 | 6.74 |
| 2 | 600 | 32.2 | 147 | 0.19 | 3.33 |
| 3 | 625 | 30.1 | 120 | 0.18 | 3.56 |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Weight of the catalyst (mcat), mg | 10 | CH4 input concentration (CCH4,in), mol/m3 | 38.7 |
| Time (t), h | 2 | H2 input concentration (CH2,in), mol/m3 | 5.79 |
| Methane flow rate (GCH4), L/h | 24 | Volume of the gas mixture (Vmix), m3 | 0.245 × 10−3 |
| Hydrogen flow rate (GH2), L/h | 3.6 | Volume of the catalyst (Vcat), m3 | 7.3 × 10−9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shelepova, E.V.; Maksimova, T.A.; Bauman, Y.I.; Mishakov, I.V.; Vedyagin, A.A. Experimental and Simulation Study on Coproduction of Hydrogen and Carbon Nanomaterials by Catalytic Decomposition of Methane-Hydrogen Mixtures. Hydrogen 2022, 3, 450-462. https://doi.org/10.3390/hydrogen3040028
Shelepova EV, Maksimova TA, Bauman YI, Mishakov IV, Vedyagin AA. Experimental and Simulation Study on Coproduction of Hydrogen and Carbon Nanomaterials by Catalytic Decomposition of Methane-Hydrogen Mixtures. Hydrogen. 2022; 3(4):450-462. https://doi.org/10.3390/hydrogen3040028
Chicago/Turabian StyleShelepova, Ekaterina V., Tatyana A. Maksimova, Yury I. Bauman, Ilya V. Mishakov, and Aleksey A. Vedyagin. 2022. "Experimental and Simulation Study on Coproduction of Hydrogen and Carbon Nanomaterials by Catalytic Decomposition of Methane-Hydrogen Mixtures" Hydrogen 3, no. 4: 450-462. https://doi.org/10.3390/hydrogen3040028
APA StyleShelepova, E. V., Maksimova, T. A., Bauman, Y. I., Mishakov, I. V., & Vedyagin, A. A. (2022). Experimental and Simulation Study on Coproduction of Hydrogen and Carbon Nanomaterials by Catalytic Decomposition of Methane-Hydrogen Mixtures. Hydrogen, 3(4), 450-462. https://doi.org/10.3390/hydrogen3040028









