3.1. Thermodynamic Background
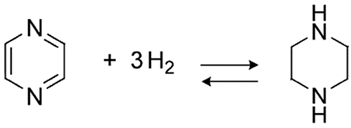
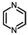
One of the typical for the hydrogen storage reactions is the hydrogenation/dehydrogenation of 2,5-dimethylpyrazine as it shown in Equation (1) below:
A chemical reaction basically takes place under thermodynamic and (or) kinetic control. The thermodynamic feasibility analysis of chemical processes enables the determination of the driving forces and could reduce the experimental efforts. Thermodynamic feasibility of a chemical process is determined by the sign of the Gibbs reaction energy,
, according to the Gibbs-Helmholtz equation:
where
is the change in the free energy of a chemical reaction;
is the reaction enthalpy, and
is the change in the entropy of a chemical reaction. The negative sign (
< 0) provides a qualitative indication of the general feasibility. Moreover, the magnitude of the gas-phase thermodynamic equilibrium constant
(at any temperature of interest
T) provides quantitative insight into the possible yield of the desired product (e.g., for
> 10 the yield is practically close to 99%). As a rule, the thermodynamic calculations are initially carried out at the reference temperature
T = 298 K. Using the standard molar isobaric heat capacities
of reaction participants, the feasibility of the reaction at any desired temperature can be derived with the aid of Kirchhoff’s law.
According to Equation (2), knowledge of standard molar enthalpies of formation and standard entropies of reaction participants is indispensable for the feasibility analysis. In the past decade, the high-level composite quantum-chemical methods become a valuable tool for obtaining theoretical
(g, 298)-values with so-called “chemical accuracy” of 4–5 kJ·mol
−1 [
21]. These QC methods differ considerably in terms of time and computing power. The QC calculations are currently being carried out successfully to obtain
(g)-values for small- and medium-sized molecules.
Predicting the entropies (required for Equation (2)) and heat capacities (required for the Kirchhoff’s Law) of organic molecules is undoubtedly a challenging task. In principle, they can be reliably calculated with help of quantum chemistry or using statistical mechanics if their structures, moments of inertia and vibration frequencies are known. The uncertainties of such calculations depend strongly on the size and flexibility of the molecules of interest. However, it is obvious from Equation (2) that the possible inaccuracy of the entropy predictions (calculated in usual units J.K−1.mol−1) compared to the enthalpic term (calculated in usual units kJ.mol−1) can be regarded as less pronounced. At least the inaccuracy of the entropy estimates is not crucial to get a correct trend of the –value.
The gas-phase thermodynamic functions
and
required for the thermodynamic analysis and optimization of the hydrogenation/dehydrogenation reactions can also be obtained with the high-level QC methods. For the practical applications the equilibrium constant,
, derived with the help of the quantum-chemistry have to be re-calculated to the liquid-phase thermodynamic equilibrium constant
. For example, for the hydrogenation reaction (1) the thermodynamic equilibrium constant
in the liquid phase can be calculated by Equation (3):
where
are the absolute vapor pressures of the pure reaction (1) participants (
i), which are either available in the literature or measured by any suitable method [
22]. In addition, the QC calculations can be performed at any desired temperature and the desired
-values in the liquid phase can be evaluated in the temperature range of the practical application.
The heat management of the hydrogenation/dehydrogenation reactions is based on the standard molar enthalpies of formation of reaction participants. The latter values can be obtained with the help of modern high-level QC methods for the ideal gas state. However, the hydrogenation/dehydrogenation reactions are usually carried out in the liquid phase. Therefore, the theoretical enthalpies of formation
(g) of potential hydrogen carriers, which are calculated by using the QC method have to be re-calculated into the practically relevant liquid phase values,
(liq), as follows:
where
is the standard molar vaporization enthalpy of the compound of interest. It is important that all thermochemical properties involved in Equation (4) have to be referenced to the same temperature (e.g., to the reference temperature
T = 298 K). The
(liq, 298 K)-values derived by Equation (4) can be further used for estimation of reaction enthalpies
(liq, 298 K) (according to the Hess’s Law):
In our experiences, however, there is a more elegant and reliable access to the desired
(liq, 298 K)-value over the theoretical enthalpy of a gas phase reaction,
(g)
QC. Indeed, the latter value can be calculated according to the Hess’s Law directly from the
H298 values available in the output of the QC method. This gas-phase
(g)
QC-value is related to the liquid phase reaction enthalpy,
(liq), by equation:
where
are the molar enthalpies of vaporization of the pure reaction participants
i at the reference temperature 298 K, and
is the stoichiometric coefficient. The required vaporization enthalpies of compounds involved in the hydrogenation reactions are measurable [
22] or they can be estimated with a reasonable accuracy, e.g., by the group-additivity [
6,
7,
8,
9]. It is also important to note, that only in this case the
(g)
QC-values for hydrogenation/dehydrogenation reactions are calculated from enthalpies
H298 of the reaction participants, which are directly available from the Gaussian output. This option is valuable to overcome common ambiguities [
23,
24] associated with the quantum-chemical methods by using conventional isodesmic, homodesmic, etc. reactions [
25]. Thus, the reasonable combination of experimental and theoretical methods opens up the possibility of a quick but reliable assessment of the energetics of reactions that are relevant for the hydrogen storage process.
In this work we used the data derived from traditional thermochemical methods (combustion calorimetry, vapor pressure measurements) together with the modern composite quantum-chemical methods G4 and G3MP2 methods to obtain Gibbs energies, reaction enthalpies, equilibrium constants, , and . These results, together with the thermodynamic functions of vaporization, derived from vapor pressure measurements, are used for the thermodynamic analysis of various nitrogen-containing LOHC hydrogenation reactions as follows.
3.2. Thermodynamics of Liquid-Gas, Crystal-Gas, and Crystal-Liquid Phase Transitions
Vapor pressures and vaporization enthalpies are an essential part of the thermodynamic analysis and they are involved in Equations (5) and (6). Results on the vapor pressure temperature dependences measured in this work by the transpiration method (see
Table S2) have been used to derive the standard molar enthalpies of vaporization,
, and standard molar sublimation enthalpies,
, of pyrazine derivatives at different temperatures. Vapor pressures of 2-methylquinoxaline have been measured for the first time. Vapor pressures of pyrazine derivatives available in the original literature have been evaluated in the same way as our own results (see details in ESI). Results of the data evaluation are compiled in
Table 1.
The single experimental vaporization enthalpy for quinoxaline was reported from the Correlation-Gas-Chromatography method [
27]. The enthalpies of sublimation for 2,3-dimethylquinoxaline compiled in
Table 1 also agree within the combined experimental uncertainties. Vapor pressures for this compound measured in this work by the transpiration method are in agreement (see
Figure S1) with those measured by the Knudsen mass-loss effusion technique [
29]. Thus, the weighted average value
(298 K) = (85.4 ± 0.7) kJ·mol
−1 was derived and applied for further thermochemical calculations.
We have carefully collected available
(298 K) and
(298 K) data on alkyl-pyrazine derivatives (see
Table S4) and together with our new complementary results on quinoxalines these data can be used for optimization of the hydrogenation/dehydrogenation reactions of LOHC.
Data on the thermodynamics of the solid-liquid phase transition for pyrazine derivatives are required for the mutual re-calculations of sublimation and vaporization enthalpies collected in
Table 1 according to the general equation:
Thermal behavior of 2,3-dimethylquinoxaline has been studied in this work for the first time. Available experimental data on enthalpies of fusion for methyl-substituted pyrazines and quinoxalines are compiled in
Table 2. For thermochemical calculations, the experimental enthalpies of fusion measured at
Tfus were adjusted to the reference temperature
T = 298 K (for details see ESI).
The energetics of the solid-liquid phase transition for pyrazine derivatives are also required for the re-calculation of the standard molar enthalpies of formation in the crystal state,
(cr), into practically more relevant standard molar enthalpies of formation in the liquid state,
(liq), according to a general equation:
provided that all values are adjusted to the reference temperature
T = 298 K. The liquid phase enthalpies of formation of pyrazine derivatives are given in
Table 2, column 7.
3.3. Consistency of Vaporization Enthalpies
Since the amount of experimental data on the vaporization/sublimation thermodynamics of quinoxalines is very limited, internal consistency needs to be proven using any kind of structure-property correlations. A valuable method to establish consistency of experimental data is the correlation gas-chromatography (CGC) [
38]. In this method experimental enthalpies of vaporization
(298 K) are correlated with the chromatographic Kovats indices (
Jx) on DB-1701 [
39] (see
Table 3). Earlier we demonstrated that vaporization enthalpy was linearly correlated with the
Jx in different homologous series of nitriles [
40], aliphatic ethers [
41], and alkylbenzenes [
42].
For correlation with
(298 K)-values listed in
Table 1 and
Table S4, we have collected Kovats indices for low polarity gas-chromatographic column DB-1701 (see
Table 3). It has turned out that the
(298 K)-values of pyrazine derivatives and quinoxalines exhibit very good linear correlations with the
Jx-values:
The high correlation coefficient
R2 = 0.985 of the
(298 K) =
f(
Jx) dependence proves the reliability and internal consistency of the enthalpies of vaporization evaluated in the
Table 1. The uncertainties of the correlated results derived in
Table 3 were rated with ±1 kJ·mol
−1.
It is also important to show the internal consistency of the datasets of the quinoline and its methyl derivatives as well as the pyrazine and its methyl derivatives, as shown in
Figure S2. The difference between pairs of molecules is not expected to be the same, but the general trends appear to be consonant within a range of pyrazines, quinoxalines, and benzenes (see
Figure S2).
Vapor pressures and thermodynamics of solid-vapor, liquid-vapor, and solid-liquid phase transitions of pyrazine and quinoxaline derivatives, which were evaluated in this work (see
Table 1,
Table 2 and
Table 3) can be recommended for the thermodynamic analysis of reactions hydrogenation/dehydrogenation reactions and for the calculations of the liquid-phase thermodynamic equilibrium constants
Ka of these reactions in combination with the quantum-chemical methods as follows in
Section 3.7 and
Section 3.8.
3.4. Standard Molar Enthalpies of Formation of Pyrazine Derivatives
Standard molar enthalpies of formation of pyrazines and quinoxalines are required for the thermodynamic analysis of the hydrogenation/dehydrogenation reactions according to the Hess’s Law (Equation (5)) or according to Equation (6). The thermochemistry of pyrazine and quinoxaline derivatives has been studied intensively in established laboratories in Porto [
26,
28,
29,
43,
44]. The available experimental results together with our new measurements are compiled in
Table 4.
The results for
(liq or cr) from the combustion calorimetry, which are collected in
Table 4, and the vaporization/sublimation enthalpies of pyrazine derivatives, which were evaluated and averaged in this work (see
Table 1), were used together for the further calculation of the experimental gas phase standard molar enthalpies of formation,
(g)
exp at 298 K (see
Table 4, column 4). Since the available for each compound data on
(liq or cr) compiled in
Table 4, column 2 are originated from single sources, they were validated with help of the QC calculations. An agreement or disagreement between the experimental (
Table 4, column 4) and theoretical (
Table 4, column 5)
(g)
G4-values could provide a criterion for the mutual validation for both results.
In order to convert the
H298 values into theoretical standard enthalpies of formation
(g) we used the atomization procedure. It is known that enthalpies of formation resulting from the atomization process have to be corrected for a systematic shift [
23,
24]. In order to “correct” the theoretical
(g)
G4 values calculated by the atomization procedure, we used the linear correlation:
developed in our previous work [
19] with a set of 18 small heterocyclic compounds similar in shape to the diazines. The “corrected” in this way theoretical
(g)
G4 values (
Table 4, column 5) are in a good agreement with the experiment (within the boundaries of the experimental uncertainties). This agreement can be considered as an indicator for the mutual consistency of the experimental and theoretical results evaluated in this work.
3.5. Standard Molar Enthalpies of Formation of Hydrogenated Pyrazines as LOHC
Standard molar enthalpies of formation of hydrogenated pyrazines (e.g., piperazine as fully hydrogenated pyrazine) are also required for the thermodynamic analysis of the hydrogenation/dehydrogenation reactions according to the Hess’s Law (Equation (5)) or according to Equation (6). For the sake of clarity, the structures of hydrogenated pyrazine derivatives are given in
Table 5, column 2. It has turned out that the thermochemical data on piperazine derivatives are only available for the piperazine itself [
45,
46] (see
Table 5). However, in our recent work we have shown that the group-additivity method and quantum-chemical calculations can provide reliable enthalpies of vaporization and gas-phase enthalpies of formation of various aliphatic cyclic amines (cyclohexanamine,
N-methyl-cyclohexanamine, N,N-dimethyl-cyclohexanamine, and
N-cyclohexyl-cyclohexanamine) [
47]. The latter structures are related to the hydrogenated pyrazines investigated in this work. In the present work we propagate this combination of empirical and quantum chemical methods in order to derive the enthalpies of liquid phase formation of the LOHC candidates.
The available experimental gas-phase enthalpy of formation of piperazine have been validated with help of the G4 calculations. A very good agreement between the experimental
(g)
exp = 31.6 ± 2.1 kJ·mol
−1 and theoretical
(g)
G4 = 32.0 ± 3.5 kJ·mol
−1 (see
Table 5) clearly demonstrated the ability of the high-level composite method G4 to provide reliable energetics of the hydrogenated pyrazine derivatives in the gas phase. However, in order to get the liquid phase
(liq, 298 K)-values according to Equation (4), vaporization enthalpies of hydrogenated pyrazine derivatives are required. In a series of our recent papers [
48,
49,
50,
51,
52] we have developed group-additivity parameters for prediction of
(298 K)-values of aliphatic nitrogen-containing compounds. Compilation of the group contributions required for estimation of vaporization enthalpies of different cyclic amines is given in
Table S6. These increments have been used to obtain
(298 K)-values for alkyl-piperazines listed in
Table 5, column 4. The validation of the increments is shown in
Table S7. Finally, in order to derive the liquid phase enthalpies of formation of hydrogenated pyrazines, required for thermodynamic analysis of the LOHC practical applications, we have combined the theoretical
(g, 298 K)-values from QC with vaporization enthalpies estimated by the group additivity procedure. These
(liq, 298 K)-values are given in
Table 5, column 3.
3.6. Energetics of the Hydrogenation Process: Structure-Energetics Relationships
When using LOHC technology for on-board energy supply in road vehicles, the energetics of the dehydrogenation/hydrogenation reaction is essential for optimizing the temperature range and the power density of the conversion units. Unfortunately, the body of thermochemical data on various LOHC compounds is very limited. For this reason, two methods (using Equations (5) and (6)) given in
Section 3.1 for evaluating the reaction enthalpies
(liq) have to be thoroughly validated with reliable data before using for a general thermodynamic analysis.
As an attestation, we used well-established experimental thermochemical data for piperazine
(liq, 298 K) = (−18.8 ± 0.8) kJ.mol
−1 [
46] and pyrazine
(liq, 298 K) = (161.2 ± 1.7) kJ.mol
−1 [
19] in order to calculate the experimental reaction enthalpy,
(298 K) = (−180.0 ± 1.9) kJ.mol
−1 according to Equation (5) for the following reaction:
For comparison, the practically indistinguishable reaction enthalpy
(liq) = −178.7 kJ·mol
−1 (see
Table 6) was calculated for this reaction according to Equation (6) by using the G4 calculated gas-phase enthalpy of reaction
(g)
G4 combined with the experimental vaporization enthalpies of reaction participants.
Very good agreement between the “pure” experimental
(liq)-value, which was derived using the Hess’s Law, and the theoretical value, which was estimated by Equation (6), was demonstrated for the typical reaction of the pyrazine hydrogenation/dehydrogenation (see Equation (11) and
Table 6). This fact can serve as a successful validity test for applying the combination of QC and group additivity methods to reliably predict the enthalpies of the hydrogenation/dehydrogenation reaction.
An important remark should be made about the quantum chemical calculations. The theoretical values,
(g)
G4, of the gas phase hydrogenation reaction derived in
Table 6 and discussed above were calculated according to the Hess’s Law from the
H298 values calculated by the G4 method which are directly available from the Gaussian 09 output. This way to get the
(g)
G4-values is preferable in comparison (see details in ESI) to other options to calculate the reaction enthalpy based on the Hess’s Law applied for the theoretical enthalpies of formation,
(g, 298 K), calculated by any common procedures (atomization, isodesmic reactions, homodesmic reactions, etc.) [
25]. This way is advantageous in order to overcome ambiguities associated with the quantum-chemical methods for calculations of
(g, 298 K), by using conventional reactions [
23,
24,
25].
The hydrogenation of aromatic compounds is a highly exothermic process and careful heat management is essential for the safety of the practical applications. In order to reveal the structure-energetics relationships for the hydrogenation reactions, the
(liq)
G4-values for series of pyrazine derivatives (
Table 6), quinoxaline derivatives (
Table 7), pyridine derivatives (
Table 8), and aniline derivatives (
Table 9).
It has turned out, that a consequent methylation of the pyrazine ring (methyl, dimethyl, trimethyl, and tetramethyl) significantly reduce (see
Table 6) the liquid phase reaction enthalpy (from −178.7 kJ·mol
−1 observed for unsubstituted pyrazine to −145.4 kJ·mol
−1 for tetramethylpyrazine). Surprisingly, the sterically hindered tert-butylpyrazine has hydrogenation enthalpy (−175.8 kJ·mol
−1) comparable to the unsubstituted pyrazine, moreover the considerably more strained 2,6-di-tert-butylpyrazine also exhibit (see
Table 6) substantial energetic level (−156.1 kJ·mol
−1). This peculiarity of the sterically strained substituted pyrazines could be interesting for practical implementation in the LOHC technology.
Compared to the pyrazine ring, the quinoxaline ring has a hydrogen storage capacity (5 mole H
2) about 1.7 times greater, but this benefit is energetically covered by the 1.7 times larger (see
Table 7) hydrogenation enthalpy of quinoxaline (−299.7 kJ·mol
−1). The consequent methylation of the quinoxaline ring (methyl and dimethyl shown in
Table 7) is similar to the effect on the pyrazine ring and causes a gradual decrease in the liquid phase reaction enthalpy (from −299.7 kJ·mol
−1 observed for unsubstituted quinoxaline to −279.3 kJ·mol
−1 for 2,3-dimethylquinoxaline). Displacement of methyl-substituents and nitrogen atoms on the quinoxaline aromatic rings (e.g., results for 2,3-dimethylquinoxaline and 2,6-dimethyl-1,5-naphthyridine are given in
Table 7) is also not thermoneutral. Accordingly, the storage capacity (7 mole H
2) and hydrogenation enthalpy (−434.3 kJ·mol
−1) of phenazine are significantly larger comparable to the unsubstituted pyrazine and quinoxaline.
Hydrogenation of pyridine and its derivatives (see
Table 8) seems to be energetically equal (except for crowded 2-tert-butyl- and 2,6-di-tert-butyl-pyridine,) to analogous alkyl-pyrazines (see
Table 6). The sterically hindered tert-butyl-pyridines exhibit not only the highest for the pyridine series hydrogenation enthalpies, but also 2,6-di-tert-butyl-pyridines with
(liq)
G4 = −188.6 kJ·mol
−1 is apparently a leader in the series of monoheterocyclic compounds shown in
Table 6 and
Table 8.
Aniline derivatives (see
Table 9) can be considered as unbeatable low-cost nitrogen containing LOHC. The liquid phase hydrogenation enthalpies of aniline (−173.1 kJ·mol
−1), N-methyl-aniline (−174.4 kJ·mol
−1), and N,N-dimethyl-aniline (−180.2 kJ·mol
−1) listed in
Table 9 are apparently on the same level as those for pyrazine and methylpyrazine (see
Table 6). For diphenylamine the hydrogen storage capacity of 6 mole H
2 amounts the hydrogenation enthalpy −371.7 kJ·mol
−1. The increasing of hydrogen storage capacity up to 9 mole H
2 in tri-phenyl-amine has required the energetic penalty in the hydrogenation enthalpy of −571.2 kJ·mol
−1.
Chemical engineering of hydrogen storage frequently operates with a specific unity “reaction enthalpy per mole of hydrogen, kJ·mol
−1/H
2” for reaction enthalpy of the hydrogenation/dehydrogenation process. Following this line, we have calculated (see
Table 6,
Table 7,
Table 8 and
Table 9, last column) hydrogenation enthalpies of pyrazine, quinoxaline, pyridine, and aniline derivatives. Values of reaction enthalpy −59.7 kJ·mol
−1/H
2 calculated for unsubstituted pyrazine, −59.9 kJ·mol
−1/H
2 for unsubstituted quinoxaline, and 59.2 kJ·mol
−1/H
2 for hydrogenation of unsubstituted pyridine. Analysis of the reaction enthalpies per mole of hydrogen for miscellaneous LOHC candidates presented in
Table 6,
Table 7,
Table 8 and
Table 9 has revealed that the values scatter in very narrow range between 52 and 63 kJ·mol
−1/H
2. These values are not significantly higher in comparison to N-ethyl-carbazole with
(liq)
G4 = −50.5 kJ·mol
−1/H
2 [
2] (considered as the most acknowledged candidate from the N-containing aromatics), as well as comparable with pure hydrocarbon LOHC such as commercially available thermofluids biphenyl (−65.4 kJ·mol
−1/H
2) [
56], benzyltoluenes (Marlothem LH
®) and dibenzyltoluenes (Marlotherm SH
®) (−63.5 and −65.4 kJ·mol
−1/H
2, respectively) [
57].
3.7. Thermodynamic Analysis of the Hydrogenation Process in the Gas Phase
Quantification of energetics of the hydrogenation/dehydrogenation reactions is important part of the LOHC-technology optimization. However, the general thermodynamic analysis based on standard molar Gibbs energies,
, provide direct access to the yield and the degree of conversion of the hydrogenation/dehydrogenation reactions. As it has been shown in previous section, the high-level composite QC methods are reliable for energetics of chemical reactions. Admittedly, the quantum chemical calculations for the
, and especially for
are very demanding. In order to test the ability of the G4 method to provide reliable
—values, we have calculated standard molar Gibbs energy of the well-known ethylbenzene dehydrogenation reaction with reliable experimental data [
58]. As can be seen from result given in
Table S12, the excellent agreement between theoretical and experimental
and
values achieved at 298 K and 400 K provide confidence in results with G4 method calculations. Thus, in this work we used the G4 method for calculation of thermodynamic functions involved in Equation (2): the standard molar Gibbs energies, the standard molar enthalpies of formation, and the standard molar entropies of hydrogenation/dehydrogenation reactions of miscellaneous LOHC (see
Table 10 and
Table 11).
For practical purpose, it has been reasonable to perform thermodynamic analysis of hydrogenation/dehydrogenation reactions at least at two significantly different temperatures. We selected the acknowledged reference temperature T = 298 K and practically relevant T = 400 K. The latter temperature represents an acceptable level for the on-board energy delivery on vehicles. Calculations at the reference temperature T = 298 K are practically irrelevant, but they help to assess the trend of the equilibrium constants temperature dependences.
The theoretical gas-phase thermodynamic data for hydrogenation of pyrazine and 2,5-dimethylpyrazine, including chemical equilibrium constants
at both temperatures are collected in
Table 10 and
Table S13. It is obvious, that the equilibrium of the hydrogenation/dehydrogenation reactions at room temperatures is completely shifted to the left side (i.e., to the starting molecule), but the position of equilibrium is strongly temperature dependent and, already at 400 K, the dehydrogenation reactions become more thermodynamically favorable.
Thermodynamic analysis of the hydrogenation reactions of miscellaneous LOHC candidates in the gas phase calculated using G4 method is summarized in
Table 11. Taking into account reversibility of the hydrogenation/dehydrogenation processes, the thermodynamic functions
(g),
(g), and
(g) given in
Table 11 for the hydrogenation reaction will get the opposite sign for the dehydrogenation reaction. Results collected in
Table 11 help to reveal that hydrogenation reactions are definitely ″driven by enthalpy″. Indeed, let us compare thermodynamic data obtained for reactions which involve methylpyrazine and tetramethylpyrazine. Both reactions exhibit quite similar reaction entropies
(g): −380.5 J·mol
−1·K
−1 for methylpyrazine and −383.6 J·mol
−1·K
−1 for tetramethylpyrazine, although the
(g)-values are different by only 16.9 kJ·mol
−1 (−166.3 kJ·mol
−1 for methylpyrazine and −149. 4 kJ·mol
−1 for tetramethylpyrazine). Nevertheless, this difference is quite decisive for the level of the gas-phase equilibrium constant
: 1.8 × 10
9 for methylpyrazine and 1.3 × 10
6 for tetramethylpyrazine. The similar trend was observed within all three series of the LOHC candidates—pyrazine, pyridine, and aniline derivatives. This finding has reinforced importance of precise studies of energetics of the hydrogenation/dehydrogenation process in order to select most suitable LOHC candidate and optimize conditions for application.
Equilibrium of hydrogenation/dehydrogenation processes exhibit many similarities with polymerization/depolymerization processes, because the enthalpy also drives polymerizations [
59]. According to the Gibbs free energy equation (Equation (2)), the entropy term is negative. Thus, at low temperatures, the enthalpy term dominates over the entropy term, which allows hydrogenation or polymerization to occur. In polymer chemistry, ceiling temperature
is a practical measure of the trend of a polymer to revert to the starting monomers. At the ceiling temperature, the enthalpy term
and the entropy term
are equal. Above the ceiling temperature, the depolymerization begins to be thermodynamically favorable. At equilibrium
= 0 and we can re-arrange Equation (2) to get expression
=
leading to the definition of the ceiling temperature as the ratio (
/
). Using this analogy with the polymerization/depolymerization processes it is practically important to define an equilibrium temperature
, which is able to imply the reverse of thermodynamic feasibility from hydrogenation to dehydrogenation trend:
This temperature could be valuable indicator for design and selection of effective LOHC. This equilibrium temperature is desired to be not too low for the hydrogenation step in order to keep acceptable reaction rates. At the same time, for the dehydrogenation step the
is expected to be not too high, but sufficient for a good level of conversion and selectivity. Results for
calculations according to Equation (12) for pyrazine, pyridine, and aniline derivatives are given in
Table 11, last column. It is apparent from this table that alkyl-pyrazines exhibit the lowest equilibrium temperatures (except for unsubstituted pyrazine and methylpyrazine) at the level of 400 K. The pyridine derivatives show the
-values around 450 K and for aniline derivatives the highest
-values around 500 K are required. Thus, the pyrazine derivatives appear to be significantly more suitable LOHC candidates compared to the aniline derivatives. A short, but important, comment on calculations according to Equation (12) have to be done. All
calculations in
Table 11 have been performed by using thermodynamic functions referenced to 298 K. However, the choice of the reference temperature is generally irrelevant for this type of calculations. To set an example, for hydrogenation/dehydrogenation of 2,5-dimethylpyrazine the value
= 408 K was calculated in
Table 11. However, by using thermodynamic functions for this compound given in
Table 10 and referenced to 400 K, the value
= 406 K was estimated. The difference is marginal for theoretical and practical applications and it means that quantum-chemical calculations can be successfully applied for characterization and selection of efficient LOHC candidates.
3.8. Thermodynamic Equilibrium Constants of the Hydrogenation/Dehydrogenation Reactions from Quantum-Chemical Calculations
From a practical point of view, the most interesting thermodynamic result is the liquid phase equilibrium constant
. This constant provides preliminary information not only about the feasibility of the chemical reaction according to the Gibbs energy equation:
However, it is also important to assess the expected theoretical concentrations of the reaction participants. The liquid phase equilibrium constant
of the hydrogenation/dehydrogenation is related to the gas phase thermodynamic equilibrium constant,
, by Equation (3) (related exemplary to reactions 1 and 11, for the sake of brevity). Auxiliary data on vapor pressures at 298 K and 400 K are given in
Table S14. Saturated vapor pressures for the 2,5-dimethylpyrazine and trans-2,5-dimethyl-piperazine are available in the literature (see
Table S14). Using the theoretical
calculated with help of the G4-method and presented in
Table 10, the liquid phase thermodynamic equilibrium constant
was calculated. Values of the liquid phase equilibrium constants for reaction hydrogenation of 2,5-dimethylpyrazine to trans-2,5-dimethyl-piperazine: very large
= 8.3 × 10
7 at 298 K and very small
= 2.5 at 400 K indicate sufficient thermodynamic feasibility for the hydrogenation/dehydrogenation reversible process. This theoretical conclusion is in agreement with the practical observation for this reaction. Indeed, a new system for hydrogen storage based on the reversible interconversion between 2,5-dimethylpyrazine and 2,5-dimethylpiperazine by hydrogenation at 383 K and dehydrogenation at 411 K catalyzed by an iridium complex has been reported just recently [
60]. Reversible and repetitive transformations between 2,5-dimethylpyrazine and 2,5-dimethylpiperazine, accompanied by the uptake and release of three equivalents of hydrogen was repeated almost quantitatively at least four times without any loss of efficiency [
60].
As can be seen in
Table 10, the contribution from multiplication of pressures (
) in Equation (3) is altering from 1.2 to 5.9 depending on the structure of reaction participants and reaction temperature. Nevertheless, this
p-factor is hardly decisive for the general level of the
even at 400 K as compared to the level of
(see
Table 10) derived by the G4 method. This observation is of utmost importance for the successful application of quantum chemical methods to the advanced screening of suitable LOHC candidates. Indeed, even taking into account the use of time-consuming and resource-intensive high-level G* methods, the in silico assessment of reliable
-values for any hydrogenation/dehydrogenation reaction at any desired temperature opens the window of the possibility of reducing the experimental thermochemical effort and performing the general computational search for promising LOHC structures suitable for various transportation, household, etc. niche applications.