Study of Activity and Super-Capacitance Exhibited by Bifunctional Raney 2.0 Catalyst for Alkaline Water-Splitting Electrolysis
Abstract
1. Introduction
the best catalysts should bind atoms and molecules with an intermediate strength: not too weakly in order to be able to activate the reactants, and not too strongly to be able to desorb the products [7]
2. Materials and Methods
3. Results
3.1. Raney1
3.2. Raney2
4. Comparisons
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 316SS | 316-grade Stainless Steel |
| CE | Counter Electrode |
| CPE | Constant Phase Element |
| CV | Cyclic Voltammetry |
| EIS | Electrochemical Impedance Spectroscopy |
| HER | Hydrogen Evolution Reaction |
| ICT | Information and Communications Technology |
| OCP | Open-Circuit Potential |
| OER | Oxygen Evolution Reaction |
| PEM | Proton Exchange Membrane |
| RC | a Resistor–Capacitor network |
| RCR | a Resistor–Capacitor–Resistor network |
| RE | Reference Electrode |
| RQ | a Resistor–CPE(Q) network |
| SI | Supplementary Information |
| WE | Working Electrode |
References
- Jones, N. The Information Factories. Nat. Mag. 2018, 561, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Andrae, A.; Edler, T. On Global Electricity Usage of Communication Technology: Trends to 2030. Challenges 2015, 6, 117–157. [Google Scholar] [CrossRef]
- Gannon, W.J.; Jones, D.R.; Dunnill, C.W. Enhanced Lifetime Cathode for Alkaline Electrolysis Using Standard Commercial Titanium Nitride Coatings. Processes 2019, 7, 112. [Google Scholar] [CrossRef]
- Jones, D.; Phillips, R.; Gannon, W.J.; Rome, B.; Warwick, M.; Dunnill, C. Photocapacitive CdS/WOx nanostructures for solar energy storage. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Gannon, W.J.; Warwick, M.E.A.; Dunnill, C.W. Woven Stainless-Steel Mesh as a Gas Separation Membrane for Alkaline Water-Splitting Electrolysis. Membranes 2020, 10, 109. [Google Scholar] [CrossRef]
- Yu, F.; Yu, L.; Mishra, I.K.; Yu, Y.; Ren, Z.F.; Zhou, H.Q. Recent developments in earth-abundant and non-noble electrocatalysts for water electrolysis. Mater. Today Phys. 2018, 7, 121–138. [Google Scholar] [CrossRef]
- Medford, A.J.; Vojvodic, A.; Hummelshøj, J.S.; Voss, J.; Abild-Pedersen, F.; Studt, F.; Bligaard, T.; Nilsson, A.; Nørskov, J.K. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis. J. Catal. 2015, 328, 36–42. [Google Scholar] [CrossRef]
- Eftekhari, A. Electrocatalysts for hydrogen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 11053–11077. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the Exchange Current for Hydrogen Evolution. J. Electrochem. Soc. 2005, 152, J23. [Google Scholar] [CrossRef]
- Smith, A.J.; Trimm, D.L. The Preparation of Skeletal Catalysts. Annu. Rev. Mater. Res. 2005, 35, 127–142. [Google Scholar] [CrossRef]
- Schiller, G.; Henne, R.; Mohr, P.; Peinecke, V. High Performance Electrodes for an Advanced Intermittently Operated 10-kW Alkaline Water Electrolyzer. Int. J. Hydrogen Energy 1998, 23, 761–765. [Google Scholar] [CrossRef]
- Endoh, E.; Otouma, H.; Morimoto, T.; Oda, Y. New Raney nickel composite-coated electrode for hydrogen evolution. Int. J. Hydrogen Energy 1987, 12, 473–479. [Google Scholar] [CrossRef]
- Balej, J.; Divisek, J.; Schmitz, H.; Mergel, J. Preparation and properties of raney nickel electrodes on Ni-Zn base for H2 and O2 evolution from alkaline solutions Part I: Electrodeposition of Ni-Zn alloys from chloride solutions. J. Appl. Electrochem. 1992, 22, 705–710. [Google Scholar] [CrossRef]
- De Giz, M.J.; Machado, S.A.S.; Avaca, L.A.; Gonzalez, E.R. High area Ni-Zn and Ni-Co-Zn codeposits as hydrogen electrodes in alkaline solutions. J. Appl. Electrochem. 1992, 22, 973–977. [Google Scholar] [CrossRef]
- Marozzi, C.A.; Chialvo, A.C. Development of electrode morphologies of interest in electrocatalysis. Part 1: Electrodeposited porous nickel electrodes. Electrochim. Acta 2000, 45, 2111–2120. [Google Scholar] [CrossRef]
- Birry, L.; Lasia, A. Studies of the hydrogen evolution reaction on Raney nickel–molybdenum electrodes. J. Appl. Electrochem. 2004, 34, 735–749. [Google Scholar] [CrossRef]
- Chade, D.; Berlouis, L.; Infield, D.; Cruden, A.; Nielsen, P.T.; Mathiesen, T. Evaluation of Raney nickel electrodes prepared by atmospheric plasma spraying for alkaline water electrolysers. Int. J. Hydrogen Energy 2013, 38, 14380–14390. [Google Scholar] [CrossRef]
- Divisek, J.; Malinowski, P.; Mergel, J.; Schmitz, H. Improved components for advanced alkaline water electrolysis. Int. J. Hydrogen Energy 1988, 13, 141–150. [Google Scholar] [CrossRef]
- Sheela, G.; Pushpavanam, M.; Pushpavanam, S. Zinc-nickel alloy electrodeposits for water electrolysis. Int. J. Hydrogen Energy 2002, 27, 627–633. [Google Scholar] [CrossRef]
- Herraiz-Cardona, I.; Ortega, E.; Vázquez-Gómez, L.; Pérez-Herranz, V. Electrochemical characterization of a NiCo/Zn cathode for hydrogen generation. Int. J. Hydrogen Energy 2011, 36, 11578–11587. [Google Scholar] [CrossRef]
- Herraiz-Cardona, I.; Ortega, E.; Pérez-Herranz, V. Impedance study of hydrogen evolution on Ni/Zn and Ni-Co/Zn stainless steel based electrodeposits. Electrochim. Acta 2011, 56, 1308–1315. [Google Scholar] [CrossRef]
- Herraiz-Cardona, I.; González-Buch, C.; Valero-Vidal, C.; Ortega, E.; Pérez-Herranz, V. Co-modification of Ni-based type Raney electrodeposits for hydrogen evolution reaction in alkaline media. J. Power Sources 2013, 240, 698–704. [Google Scholar] [CrossRef]
- González-Buch, C.; Herraiz-Cardona, I.; Ortega, E.; García-Antón, J.; Pérez-Herranz, V. Synthesis and characterization of macroporous Ni, Co and Ni-Co electrocatalytic deposits for hydrogen evolution reaction in alkaline media. Int. J. Hydrogen Energy 2013, 38, 10157–10169. [Google Scholar] [CrossRef]
- Solmaz, R.; Döner, A.; Kardaş, G. Preparation, characterization and application of alkaline leached CuNiZn ternary coatings for long-term electrolysis in alkaline solution. Int. J. Hydrogen Energy 2010, 35, 10045–10049. [Google Scholar] [CrossRef]
- Solmaz, R. Electrochemical preparation, characterization, and application of a novel cathode material, mild Steel/Ni/NiZn-Pt, for alkaline water electrolysis. Energy Sources Part A Recover. Util. Environ. Eff. 2014, 36, 1212–1218. [Google Scholar] [CrossRef]
- Solmaz, R.; Döner, A.; Doğrubaş, M.; Erdoğan, I.Y.; Kardaş, G. Enhancement of electrochemical activity of Raney-type NiZn coatings by modifying with PtRu binary deposits: Application for alkaline water electrolysis. Int. J. Hydrogen Energy 2016, 41, 1432–1440. [Google Scholar] [CrossRef]
- Solmaz, R.; Salcı, A.; Yüksel, H.; Doğrubaş, M.; Kardaş, G. Preparation and characterization of Pd-modified Raney-type NiZn coatings and their application for alkaline water electrolysis. Int. J. Hydrogen Energy 2017, 42, 2464–2475. [Google Scholar] [CrossRef]
- Solmaz, R. Gold-supported activated NiZn coatings: Hydrogen evolution and corrosion studies. Int. J. Energy Res. 2017, 41, 1452–1459. [Google Scholar] [CrossRef]
- Balej, J. Electrocatalysts for oxygen evolution in advanced water electrolysis. Int. J. Hydrogen Energy 1985, 10, 89–99. [Google Scholar] [CrossRef]
- Bates, M.K.; Jia, Q.; Doan, H.; Liang, W.; Mukerjee, S. Charge-Transfer Effects in Ni-Fe and Ni-Fe-Co Mixed-Metal Oxides for the Alkaline Oxygen Evolution Reaction. ACS Catal. 2016, 6, 155–161. [Google Scholar] [CrossRef]
- Tsay, P.; Hu, C.C. Non-Anomalous Codeposition of Iron-Nickel Alloys Using Pulse-Reverse Electroplating Through Means of Experimental Strategies. J. Electrochem. Soc. 2002, 149, C492. [Google Scholar] [CrossRef]
- Tozar, A.; Karahan, I.H. Structural and corrosion protection properties of electrochemically deposited nano-sized Zn-Ni alloy coatings. Appl. Surf. Sci. 2014, 318, 15–23. [Google Scholar] [CrossRef]
- Soares, D.M. Hydride Effect on the Kinetics of the Hydrogen Evolution Reaction on Nickel Cathodes in Alkaline Media. J. Electrochem. Soc. 1992, 139, 98. [Google Scholar] [CrossRef]
- Olivares-Ramírez, J.M.; Campos-Cornelio, M.L.; Godínez, J.U.; Borja-Arco, E.; Castellanos, R.H. Studies on the hydrogen evolution reaction on different stainless steels. Int. J. Hydrogen Energy 2007, 32, 3170–3173. [Google Scholar] [CrossRef]
- Sapountzi, F.M.; Gracia, J.M.; Weststrate, C.K.J.; Fredriksson, H.O.; Niemantsverdriet, J.H. Electrocatalysts for the generation of hydrogen, oxygen and synthesis gas. Prog. Energy Combust. Sci. 2017, 58, 1–35. [Google Scholar] [CrossRef]
- Divisek, J.; Schmitz, H.; Steffen, B. Electrocatalyst Materials Evolution for Hydrogen. Electrochim. Acta 1994, 39, 1723–1731. [Google Scholar] [CrossRef]
- Gannon, W.J.; Dunnill, C.W. Raney Nickel 2.0: Development of a high-performance bifunctional electrocatalyst. Electrochim. Acta 2019, 322, 134687. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Jovic, V. Determination of the Correct Value of Cdl From the Impedance Results Fitted by the Commercially Available Software. Technical Report; University of Belgrade: Belgrade, Serbia, 2003. [Google Scholar]
- McCrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking Hydrogen Evolving Reaction and Oxygen Evolving Reaction Electrocatalysts for Solar Water Splitting Devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef]
- Athanasiou, V.; Konkoli, Z. On the efficient simulation of electrical circuits with constant phase elements: The Warburg element as a test case. Int. J. Circuit Theory Appl. 2018, 46, 1072–1090. [Google Scholar] [CrossRef]
- Gannon, W.J.; Dunnill, C.W. Apparent disagreement between cyclic voltammetry and electrochemical impedance spectroscopy explained by time-domain simulation of constant phase elements. Int. J. Hydrogen Energy 2020, 45, 22383–22393. [Google Scholar] [CrossRef]
- Colli, A.N.; Girault, H.H.; Battistel, A. Non-precious electrodes for practical alkaline water electrolysis. Materials 2019, 12, 1336. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef] [PubMed]
- Bagotzky, V.S.; Khrushcheva, E.I.; Tarasevich, M.R.; Shumilova, N.A. Corrosion of platinum catalyst in alkaline solutions. J. Power Sources 1982, 8, 301–309. [Google Scholar] [CrossRef]
- Ganesh, V.; Lakshminarayanan, V. Preparation of high surface area nickel electrodeposit using a liquid crystal template technique. Electrochim. Acta 2004, 49, 3561–3572. [Google Scholar] [CrossRef]
- Suffredini, H.B.; Cerne, J.L.; Crnkovic, F.C.; MacHado, S.A.S.; Avaca, L.A. Recent developments in electrode materials for water electrolysis. Int. J. Hydrogen Energy 2000, 25, 415–423. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Yu, X.; Guo, Z. Facile one-step electrodeposition preparation of porous NiMo film as electrocatalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2015, 40, 2173–2181. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; Xu, W.; Wan, P.; Lu, Z.; Li, Y.; Sun, X. A 3D Nanoporous Ni-Mo Electrocatalyst with Negligible Overpotential for Alkaline Hydrogen Evolution. ChemElectroChem 2014, 1, 1138–1144. [Google Scholar] [CrossRef]
- Song, F.; Li, W.; Yang, J.; Han, G.; Liao, P.; Sun, Y. Interfacing nickel nitride and nickel boosts both electrocatalytic hydrogen evolution and oxidation reactions. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef]
- Panda, C.; Menezes, P.W.; Yao, S.; Schmidt, J.; Walter, C.; Hausmann, J.N.; Driess, M. Boosting Electrocatalytic Hydrogen Evolution Activity with a NiPt3@NiS Heteronanostructure Evolved from a Molecular Nickel-Platinum Precursor. J. Am. Chem. Soc. 2019, 141, 13306–13310. [Google Scholar] [CrossRef]
- Yu, F.; Zhou, H.; Huang, Y.; Sun, J.; Qin, F.; Bao, J.; Goddard, W.A.; Chen, S.; Ren, Z. High-performance bifunctional porous non-noble metal phosphide catalyst for overall water splitting. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Liu, P.; Liao, Z.; Liu, S.; Zhuang, X.; Chen, M.; Zschech, E.; Feng, X. Efficient hydrogen production on MoNi 4 electrocatalysts with fast water dissociation kinetics. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, K.; Wang, C.; Li, S.; Zhang, Q.; Chang, X.; Li, J.; Li, S.; Jia, S.; Wang, J.; et al. Nanometric Ni5P4 Clusters Nested on NiCo2O4 for Efficient Hydrogen Production via Alkaline Water Electrolysis. Adv. Energy Mater. 2018, 8, 2–7. [Google Scholar] [CrossRef]
- Men, Y.; Li, P.; Zhou, J.; Cheng, G.; Chen, S.; Luo, W. Tailoring the Electronic Structure of Co2P by N Doping for Boosting Hydrogen Evolution Reaction at All pH Values. ACS Catal. 2019, 9, 3744–3752. [Google Scholar] [CrossRef]
- Chen, W.; Mishra, I.K.; Qin, Z.; Yu, L.; Zhou, H.; Sun, J.; Zhang, F.; Chen, S.; Wenya, G.E.; Yu, Y.; et al. Nickel phosphide based hydrogen producing catalyst with low overpotential and stability at high current density. Electrochim. Acta 2019, 299, 756–761. [Google Scholar] [CrossRef]
- Herraiz-Cardona, I.; Ortega, E.; Vázquez-Gómez, L.; Pérez-Herranz, V. Double-template fabrication of three-dimensional porous nickel electrodes for hydrogen evolution reaction. Int. J. Hydrogen Energy 2012, 37, 2147–2156. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, Y.; Zheng, X.; Aoki, T.; Pattengale, B.; Huang, J.; He, X.; Bian, W.; Younan, S.; Williams, N.; et al. Atomically engineering activation sites onto metallic 1T-MoS 2 catalysts for enhanced electrochemical hydrogen evolution. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Xiang, R.; Duan, Y.; Peng, L.; Wang, Y.; Tong, C.; Zhang, L.; Wei, Z. Three-dimensional Core@Shell Co@CoMoO4 nanowire arrays as efficient alkaline hydrogen evolution electro-catalysts. Appl. Catal. B: Environ. 2019, 246, 41–49. [Google Scholar] [CrossRef]
- Gao, M.Y.; Yang, C.; Zhang, Q.B.; Zeng, J.R.; Li, X.T.; Hua, Y.X.; Xu, C.Y.; Dong, P. Facile electrochemical preparation of self-supported porous Ni-Mo alloy microsphere films as efficient bifunctional electrocatalysts for water splitting. J. Mater. Chem. A 2017, 5, 5797–5805. [Google Scholar] [CrossRef]
- Liu, C.; Gong, T.; Zhang, J.; Zheng, X.; Mao, J.; Liu, H.; Li, Y.; Hao, Q. Engineering Ni2P-NiSe2 heterostructure interface for highly efficient alkaline hydrogen evolution. Appl. Catal. B Environ. 2020, 262. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, Y.; Lin, H.; Zhang, H.; Shen, M.; Xie, S.; Zhang, Y.; Gao, Q.; Tang, Y. Porous nanoMoC@graphite shell derived from a MOFs-directed strategy: An efficient electrocatalyst for the hydrogen evolution reaction. J. Mater. Chem. A 2016, 4, 6006–6013. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, H.C.; Hsu, C.S.; Lin, T.S.; Chang, C.J.; Chang, S.C.; Tsai, L.D.; Chen, H.M. Operando unraveling of the structural and chemical stability of P-substituted CoSe 2 electrocatalysts toward hydrogen and oxygen evolution reactions in alkaline electrolyte. ACS Energy Lett. 2019, 4, 987–994. [Google Scholar] [CrossRef]
- Xing, Z.; Li, Q.; Wang, D.; Yang, X.; Sun, X. Self-supported nickel nitride as an efficient high-performance three-dimensional cathode for the alkaline hydrogen evolution reaction. Electrochim. Acta 2016, 191, 841–845. [Google Scholar] [CrossRef]
- Lai, F.; Feng, J.; Ye, X.; Zong, W.; He, G.; Miao, Y.E.; Han, X.; Ling, X.Y.; Parkin, I.P.; Pan, B.; et al. Energy level engineering in transition-metal doped spinel-structured nanosheets for efficient overall water splitting. J. Mater. Chem. A 2019, 7, 827–833. [Google Scholar] [CrossRef]
- Zhu, W.; Yue, X.; Zhang, W.; Yu, S.; Zhang, Y.; Wang, J.; Wang, J. Nickel sulfide microsphere film on Ni foam as an efficient bifunctional electrocatalyst for overall water splitting. Chem. Commun. 2016, 52, 1486–1489. [Google Scholar] [CrossRef] [PubMed]
- Haque, F.; Zavabeti, A.; Zhang, B.Y.; Datta, R.S.; Yin, Y.; Yi, Z.; Wang, Y.; Mahmood, N.; Pillai, N.; Syed, N.; et al. Ordered intracrystalline pores in planar molybdenum oxide for enhanced alkaline hydrogen evolution. J. Mater. Chem. A 2019, 7, 257–268. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, X.Y.; Paik, U. Nickel cobalt phosphides quasi-hollow nanocubes as an efficient electrocatalyst for hydrogen evolution in alkaline solution. Chem. Commun. 2016, 52, 1633–1636. [Google Scholar] [CrossRef]
- Liang, H.W.; Brüller, S.; Dong, R.; Zhang, J.; Feng, X.; Müllen, K. Molecular metal-Nx centres in porous carbon for electrocatalytic hydrogen evolution. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, Y.; Chen, H.M.; Hu, Z.; Hung, S.F.; Ma, N.; Dai, J.; Lin, H.J.; Chen, C.T.; Zhou, W.; et al. An Amorphous Nickel–Iron-Based Electrocatalyst with Unusual Local Structures for Ultrafast Oxygen Evolution Reaction. Adv. Mater. 2019, 31, 1–7. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, X.; Voznyy, O.; Comin, R.; Bajdich, M.; Arquer, F.P.G.D.; Dinh, C.T.; Fan, F.; Yuan, M.; Janmohamed, A.; et al. Homogeneously dispersed multimetal oxygen-evolving catalysts. Science 2016, 352, 333–337. [Google Scholar] [CrossRef]
- Xu, X.; Song, F.; Hu, X. A nickel iron diselenide-derived efficient oxygen-evolution catalyst. Nat. Commun. 2016, 7, 12324. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhao, C. Electrodeposition of hierarchically structured three-dimensional nickel–iron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 2015, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Yu, H.; Jiang, G.; Jia, J.; Qin, B.; Yi, B.; Shao, Z. Construction of orderly hierarchical FeOOH/NiFe layered double hydroxides supported on cobaltous carbonate hydroxide nanowire arrays for a highly efficient oxygen evolution reaction. J. Mater. Chem. A 2018, 6, 3397–3401. [Google Scholar] [CrossRef]
- Yan, F.; Wang, Y.; Li, K.; Zhu, C.; Gao, P.; Li, C.; Zhang, X.; Chen, Y. Highly Stable Three-Dimensional Porous Nickel-Iron Nitride Nanosheets for Full Water Splitting at High Current Densities. Chem. Eur. J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, Y.; Liu, D.; Zou, Y.; Wang, S. Water-Plasma-Enabled Exfoliation of Ultrathin Layered Double Hydroxide Nanosheets with Multivacancies for Water Oxidation. Adv. Mater. 2017, 29, 1–7. [Google Scholar] [CrossRef]
- Nai, J.; Lu, Y.; Yu, L.; Wang, X.; Lou, X.W.D. Formation of Ni–Fe Mixed Diselenide Nanocages as a Superior Oxygen Evolution Electrocatalyst. Adv. Mater. 2017, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.F.; Gu, L.F.; Wang, J.W.; Wu, J.X.; Liao, P.Q.; Li, G.R. Bimetal-Organic Framework Derived CoFe2O4/C Porous Hybrid Nanorod Arrays as High-Performance Electrocatalysts for Oxygen Evolution Reaction. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Tian, X.; Wang, X.; Yu, Y.; Owusu, K.A.; He, L.; Mai, L. Porous Nickel-Iron Selenide Nanosheets as Highly Efficient Electrocatalysts for Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2016, 8, 19386–19392. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.; Wang, H.; Liang, Y.; Wu, J.Z.; Zhou, J.; Wang, J.; Regier, T.; Wei, F.; Dai, H. An Advanced Ni - Fe Layered Double Hydroxide Electrocatalyst for Water Oxidation. J. Am. Chem. Soc. 2013, 135, 8452–8455. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, W.; Zhu, W.; Yang, Q.; Lei, X.; Liu, J.; Li, Y.; Sun, X.; Duan, X. Three-dimensional NiFe layered double hydroxide film for high-efficiency oxygen evolution reaction. Chem. Commun. 2014, 50, 6479. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, W.; Dong, J.; Lu, X.F.; Lou, X.W.D. Intramolecular electronic coupling in porous iron cobalt (oxy)phosphide nanoboxes enhances the electrocatalytic activity for oxygen evolution. Energy Environ. Sci. 2019, 12, 3348–3355. [Google Scholar] [CrossRef]
- Smith, R.D.L.; Prévot, M.S.; Fagan, R.D.; Trudel, S.; Berlinguette, C.P. Water Oxidation Catalysis: Electrocatalytic Response to Metal Stoichiometry in Amorphous Metal Oxide Films Containing Iron, Cobalt, and Nickel. J. Am. Chem. Soc. 2013, 135, 11580–11586. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tu, W.; Zhang, B.; Yin, S.; Huang, Y.; Kraft, M.; Xu, R. Nickel Nanoparticles Encapsulated in Few-Layer Nitrogen-Doped Graphene Derived from Metal–Organic Frameworks as Efficient Bifunctional Electrocatalysts for Overall Water Splitting. Adv. Mater. 2017, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Anantharaj, S.; Reddy, P.N.; Kundu, S. Core-Oxidized Amorphous Cobalt Phosphide Nanostructures: An Advanced and Highly Efficient Oxygen Evolution Catalyst. Inorg. Chem. 2017, 56, 1742–1756. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.P.; Liu, Y.P.; Ren, T.Z.; Yuan, Z.Y. Self-supported cobalt phosphide mesoporous nanorod arrays: A flexible and bifunctional electrode for highly active electrocatalytic water reduction and oxidation. Adv. Funct. Mater. 2015, 25, 7337–7347. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Pi, Y.; Shao, Q.; Tan, Y.; Huang, X. Double Perovskite LaFex Ni1-x O 3 Nanorods Enable Efficient Oxygen Evolution Electrocatalysis. Angew. Chem. Int. Ed. 2019, 58, 2316–2320. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Chen, T.; Zhang, J.; Zhang, J.; Lou, X.W. Unveiling the Activity Origin of Electrocatalytic Oxygen Evolution over Isolated Ni Atoms Supported on a N-Doped Carbon Matrix. Adv. Mater. 2019, 31, 1–7. [Google Scholar] [CrossRef]
- Song, F.; Hu, X. Ultrathin Cobalt - Manganese Layered Double Hydroxide Is an Efficient Oxygen Evolution Catalyst. J. Am. Chem. Soc. 2014, 136, 16481–16484. [Google Scholar] [CrossRef]
- Liu, G.; Wang, K.; Gao, X.; He, D.; Li, J. Fabrication of mesoporous NiFe2O4 nanorods as efficient oxygen evolution catalyst for water splitting. Electrochim. Acta 2016, 211, 871–878. [Google Scholar] [CrossRef]
- Bikkarolla, S.K.; Papakonstantinou, P. CuCo2O4 nanoparticles on nitrogenated graphene as highly efficient oxygen evolution catalyst. J. Power Sources 2015, 281, 243–251. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, A.; Li, L.; Ai, L. Nickel-cobalt layered double hydroxide nanosheets as high-performance electrocatalyst for oxygen evolution reaction. J. Power Sources 2015, 278, 445–451. [Google Scholar] [CrossRef]

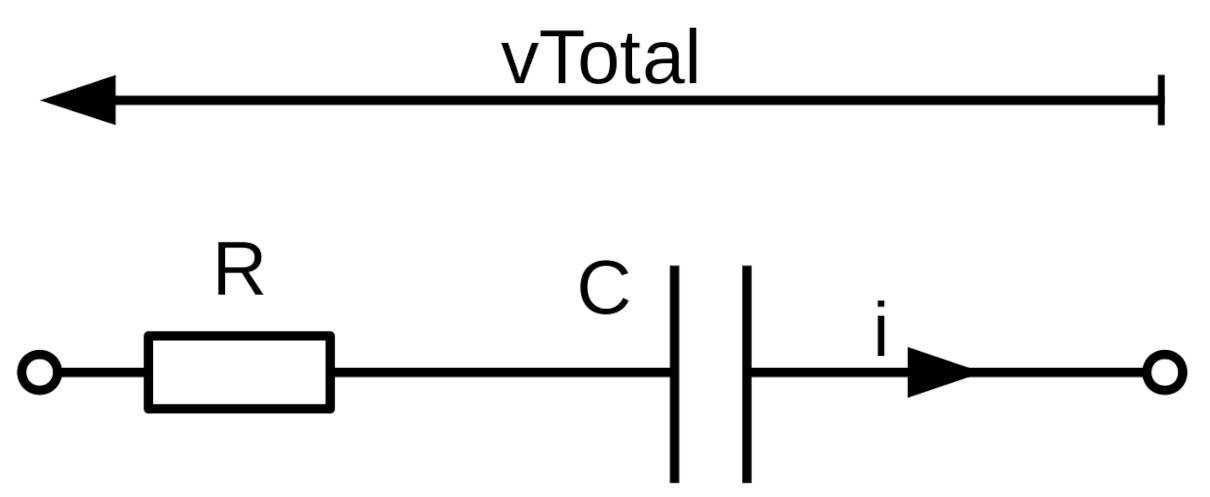




| Trace | n | RF | |||
|---|---|---|---|---|---|
| Ω cm2 | sn Ω−1 cm−2 | mF cm−2 | |||
| EIS at 10 | 8.57 (0.8%) | 0.148 (2.5%) | 0.68 (1.9%) | 166 | 4150 |
| EIS at 31 | 8.52 (0.7%) | 0.149 (2.2%) | 0.68 (1.6%) | 167 | 4180 |
| EIS at 100 | 8.47 (0.7%) | 0.188 (2.3%) | 0.60 (1.9%) | 255 | 6370 |
| CV at 10 −1 | 9.45 | 0.206 | 0.72 | 266 | 6650 |
| Coating | Reaction | Current | Tafel | Over- | Voltage |
|---|---|---|---|---|---|
| Density | Slope | Potential | |||
| mA cm−2 | mV dec−1 | mV | V | ||
| Raney2 | HER | 10 | 50 | 28 | |
| Raney2 | HER | 100 | 111 | 91 | |
| Raney2 | OER | 10 | 34 | 291 | |
| Raney2 | OER | 100 | 62 | 328 | |
| Raney2 | Both | 10 | 84 | 320 | 1.55 |
| Raney2 | Both | 100 | 173 | 419 | 1.65 |
| Ramp Rate | n | RF | |||
|---|---|---|---|---|---|
| mV s−1 | Ω cm2 | sn Ω−1 cm−2 | F cm−2 | ||
| 10 | 9.41 | 0.811 | 0.67 | 2.21 | 55,170 |
| 6.7 | 9.61 | 0.784 | 0.71 | 1.80 | 45,000 |
| 3.3 | 9.84 | 0.762 | 0.72 | 1.70 | 42,490 |
| Average | 9.62 | 0.786 | 0.70 | 1.89 | 47,150 |
| Lead Author(s) | Year | Catalyst | Substrate | Electrolyte | Tafel Slope mV dec | Overpotential mV |
|---|---|---|---|---|---|---|
| Wang Mingyong [48] | 2015 | NiMo | Cu | 10 wt% NaOH | 137 | 7 |
| Wang Yuhang [49] | 2014 | 3D NiMo | Cu foam | 1 NaOH | 11 | |
| Song Fuzhang [50] | 2018 | Ni3N/Ni | NF | 1 KOH | 12 | |
| C Panda [51] | 2019 | NiPt3@NiS | NF | 1 KOH | 24 | 12 |
| Yu Fang [52] | 2018 | FeP/Ni2P | NF | 1 KOH | 24.2 | 14 |
| Zhang Jian [53] | 2017 | MoNi4 / MoO2 | NF | 1 KOH | 30 | 15 |
| Zhang Tao [54] | 2018 | Ni5P4@NiCo2O4 | NF | 1 KOH | 27 | 27 |
| W. Gannon [37] | 2019 | Raney2 | 316SS | 1 KOH | 50 | 28 |
| R. Solmaz [28] | 2017 | NiZn-Au | Cu/Ni | 1 KOH | 66 | 31 |
| Men Yana [55] | 2019 | Ni−Co2P | CC | 1 KOH | 51 | 34 |
| Chen Weiwu [56] | 2019 | S-NiP | NF | 1 KOH | 44 | 35 |
| Herraiz-Cardona [57] | 2012 | Ni | Cu foam | 30 wt% KOH | 103 | 41 |
| Huang Yichao [58] | 2019 | 1T-MoS2 | CFP | 1 KOH | 52 | 43 |
| Xiang Rui [59] | 2019 | PtC | NF | 1 KOH | 53 | 46 |
| Xiang Rui [59] | 2019 | Co@CoMoO4 | NF | 1 KOH | 85 | 46 |
| Gao M. [60] | 2017 | Ni-Mo MS | Cu | 1 KOH | 49 | 49 |
| Liu Caichi [61] | 2020 | Ni2P−NiSe2 | CC | 1 KOH | 72.6 | 66 |
| Shi Zhangping [62] | 2016 | nano MoC | GCE | 1 KOH | 50 | 77 |
| Zhu Yanping [63] | 2019 | CoSe1.26P1.42 | CC | 1 KOH | 90 | 92 |
| Xing Zhicai [64] | 2016 | Ni3N | NF | 1 KOH | 109 | 121 |
| Lai Feili [65] | 2019 | Fe−NiCo2O4@HNCP | GCE | 1 KOH | 47 | 124 |
| Zhu Wenxin [66] | 2016 | NiS−MS | Ni foam | 1 KOH | 83 | 134 |
| Farjana Haque [67] | 2019 | 2D Crys-AMO | NF | KOH | 50 | 138 |
| Feng Yi [68] | 2016 | Ni-Co-P-300 | not known | 1 KOH | 61 | 150 |
| Liang Hai-Wei [69] | 2015 | CoNx | C | 1 KOH | 75 | 170 |
| Lead Author(s) | Year | Catalyst | Substrate | Electrolyte | Tafel Slope mV dec | Overpotential mV |
|---|---|---|---|---|---|---|
| Yu Fang [52] | 2018 | FeP/Ni2P | NF | 1 KOH | 22.7 | 154 |
| Gao Chen [70] | 2019 | Amorphous LaNiFe | NF | 1 KOH | 36 | 189 |
| Bo Zhang [71] | 2016 | Gelled FeCoW | Au-plated NF | 1 KOH | 191 | |
| Xiang Xu [72] | 2016 | NixFe1-xSe2−DO | NF | 1 KOH | 28 | 195 |
| Lu Xunyu, Zhao Chuan [73] | 2015 | NiFe NSh | NF | 1 KOH | 28 | 215 |
| Chi Jun [74] | 2018 | FeOOH/NiFe | CCH NA | 1 KOH | 220 | |
| Feng Yan [75] | 2017 | NiFe-N NSh | CC | 1 KOH | 26 | 224 |
| Liu Rong [76] | 2017 | CoFe LDH NSh | NF | 1 KOH | 36 | 232 |
| Nai Jianwei [77] | 2017 | Ni-Fe-Se disks | GCE | 1 KOH | 26 | 240 |
| Lu Xue Feng [78] | 2017 | CoFe2O4/C NRA | NF | 1 KOH | 45 | 240 |
| Wang Zhaoyang [79] | 2016 | NiFeSe NSh | CC | 1 KOH | 47 | 229 |
| Gong Ming [80] | 2013 | NiFe-LDH | CNT | 1 KOH | 31 | 247 |
| Lu Zhiyi [81] | 2014 | NiFe-LDH NP | Nickel | 1 KOH | 43 | 250 |
| Zhang Huabin [82] | 2019 | FeCoP nanoboxes | CFP | 1 KOH | 31 | 269 |
| Rodney Smith [83] | 2013 | Fe40%Ni60% | FTO | KOH | 34 | 284 |
| Xu You [84] | 2017 | Ni@NC-800 | NF | 1 KOH | 45 | 285 |
| Sengeni Anantharaj [85] | 2017 | CoP NSt | GC | 1 KOH | 70 | 287 |
| Yun-Pei Zhu [86] | 2015 | CoP-MNA | NF | 1 KOH | 65 | 290 |
| W. Gannon [37] | 2019 | Raney2 | 316SS | 1 KOH | 38 | 293 |
| Wang Huaping [87] | 2019 | LaFexNi1-xO3 NR | GCE | 1 KOH | 50 | 302 |
| Zhang Huabin [88] | 2019 | HCM@Ni-N | Carbon | 1 KOH | 76 | 304 |
| Song Fang, Xile Hu [89] | 2014 | CoMn LDH | GC | 1 KOH | 43 | 324 |
| Guang Liu [90] | 2016 | NiFe2O4 NR | GC | 1 KOH | 44 | 342 |
| Santosh Bikkarolla [91] | 2015 | CuCo2O4 | NrGO | 1 KOH | 64 | 360 |
| Jing Jiang [92] | 2014 | NiCo-LDH NSh | NF | KOH | 113 | 420 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gannon, W.J.F.; Dunnill, C.W. Study of Activity and Super-Capacitance Exhibited by Bifunctional Raney 2.0 Catalyst for Alkaline Water-Splitting Electrolysis. Hydrogen 2021, 2, 1-17. https://doi.org/10.3390/hydrogen2010001
Gannon WJF, Dunnill CW. Study of Activity and Super-Capacitance Exhibited by Bifunctional Raney 2.0 Catalyst for Alkaline Water-Splitting Electrolysis. Hydrogen. 2021; 2(1):1-17. https://doi.org/10.3390/hydrogen2010001
Chicago/Turabian StyleGannon, William J. F., and Charles W. Dunnill. 2021. "Study of Activity and Super-Capacitance Exhibited by Bifunctional Raney 2.0 Catalyst for Alkaline Water-Splitting Electrolysis" Hydrogen 2, no. 1: 1-17. https://doi.org/10.3390/hydrogen2010001
APA StyleGannon, W. J. F., & Dunnill, C. W. (2021). Study of Activity and Super-Capacitance Exhibited by Bifunctional Raney 2.0 Catalyst for Alkaline Water-Splitting Electrolysis. Hydrogen, 2(1), 1-17. https://doi.org/10.3390/hydrogen2010001






