Enhancing the Hydrogen Storage Properties of AxBy Intermetallic Compounds by Partial Substitution: A Short Review
Abstract
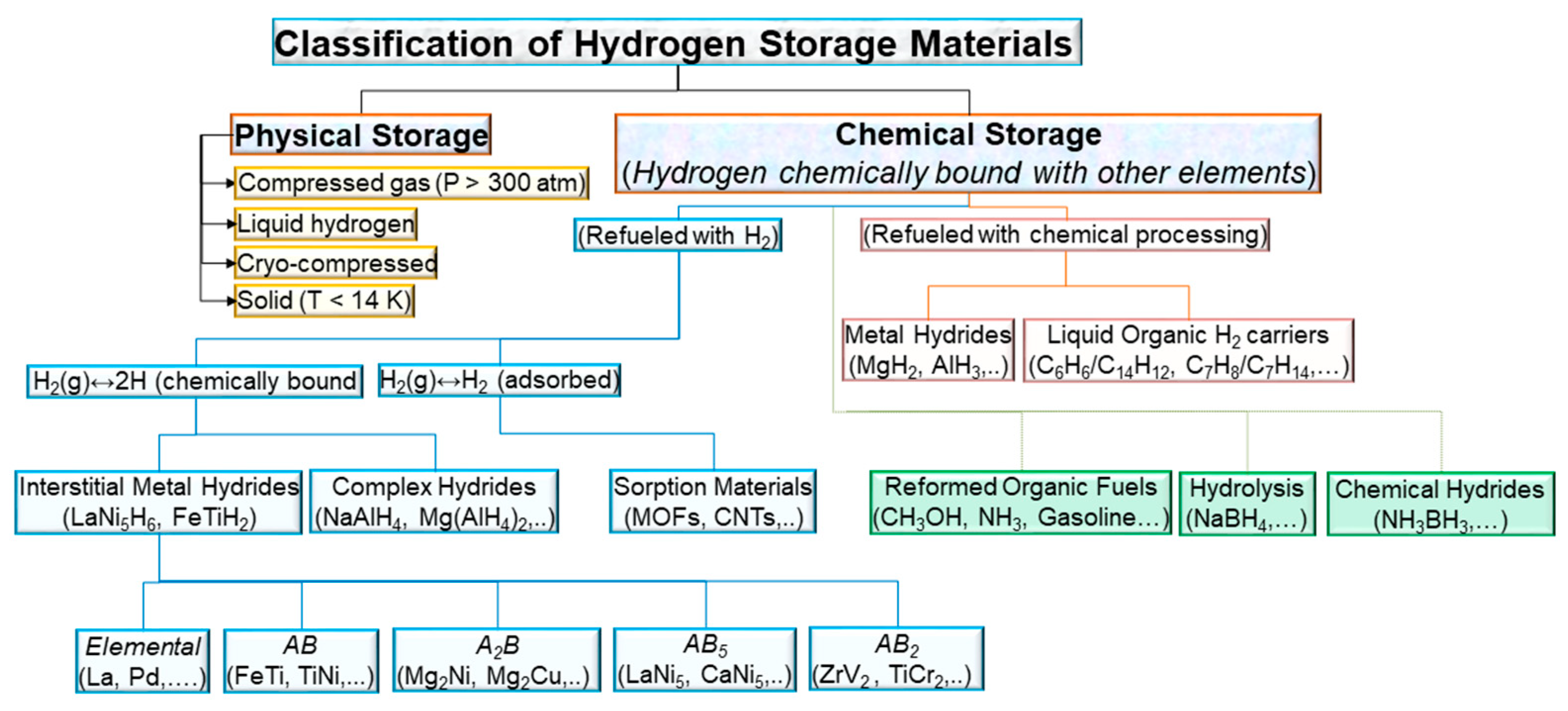
:1. Introduction
2. AB5-Type Alloys
3. AB2-Type Alloys
4. AB-Type Alloys
5. AB3-Type Alloys
6. Solid Solutions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schlapbach, L.; Züttel, A. Hydrogen-Storage Materials for Mobile Applications. In Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group; World Scientific: Singpore, 2011; pp. 265–270. [Google Scholar]
- Orimo, S.; Matsushima, T.; Fujii, H.; Fukunaga, T.; Majer, G.; Züttel, A.; Schlapbach, L. Nanostructured graphite-hydrogen systems prepared by mechanical milling method. Mol. Cryst. Liq. Cryst. 2002, 386, 173–178. [Google Scholar] [CrossRef]
- Von Colbe, J.B.; Ares, J.-R.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.M.; Guzik, M.N.; Jacob, I.; et al. Application of hydrides in hydrogen storage and compression: Achievements, outlook and perspectives. Int. J. Hydrog. Energy 2019, 44, 7780–7808. [Google Scholar] [CrossRef]
- Liu, T.; Wang, C.; Wu, Y. Mg-based nanocomposites with improved hydrogen storage performances. Int. J. Hydrog. Energy 2014, 39, 14262–14274. [Google Scholar] [CrossRef]
- Trudeau, M.L. Advanced Materials for Energy Storage. MRS Bull. 1999, 24, 23–26. [Google Scholar] [CrossRef]
- Wang, P.; Kang, X.-D. ChemInform Abstract: Hydrogen-Rich Boron-Containing Materials for Hydrogen Storage. ChemInform 2009, 40, 5400–5413. [Google Scholar] [CrossRef]
- Hong, S.-H.; Song, M.Y. Hydrogen desorption and absorption properties of Pd and MgO or nano-sized Ni-added MgH2 + LiBH4 composites. Mater. Res. Bull. 2013, 48, 3453–3458. [Google Scholar] [CrossRef]
- Rusman, N.; Dahari, M. A review on the current progress of metal hydrides material for solid-state hydrogen storage applications. Int. J. Hydrog. Energy 2016, 41, 12108–12126. [Google Scholar] [CrossRef]
- Jain, I.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrog. Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Dolotko, O.; Gupta, S.; Kobayashi, T.; McDonald, E.; Hlova, I.; Majzoub, E.; Balema, V.P.; Pruski, M.; Pecharsky, V.K. Mechanochemical reactions and hydrogen storage capacities in MBH4–SiS2 systems (MLi or Na). Int. J. Hydrog. Energy 2019, 44, 7381–7391. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Yuan, J.; Han, H.; Wu, Y. Effect of carbon nanotubes on the microstructural evolution and hydrogen storage properties of Mg(BH4). J. Alloy. Compd. 2018, 743, 11–16. [Google Scholar] [CrossRef]
- Afonso, G.; Bonakdarpour, A.; Wilkinson, D.P. Hydrogen Storage Properties of the Destabilized 4NaBH4/5Mg2NiH4 Composite System. J. Phys. Chem. C 2013, 117, 21105–21111. [Google Scholar] [CrossRef]
- Javadian, P.; Zlotea, C.; Ghimbeu, C.M.; Latroche, M.; Jensen, T.R. Hydrogen Storage Properties of Nanoconfined LiBH4–Mg2NiH4 Reactive Hydride Composites. J. Phys. Chem. C 2015, 119, 5819–5826. [Google Scholar] [CrossRef]
- Severa, G.; Ronnebro, E.; Jensen, C.M. Direct hydrogenation of magnesium boride to magnesium borohydride: Demonstration of >11 weight percent reversible hydrogen storage. Chem. Commun. 2010, 46, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Cao, Y.; Hu, L.; Fu, D.; Ma, J.; Youngblood, J. An efficient mechanochemical synthesis of alpha-aluminum hydride: Synergistic effect of TiF3 on the crystallization rate and selective formation of alpha-aluminum hydride polymorph. J. Hazard. Mater. 2019, 373, 141–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogdanović, B.; Hartwig, T.; Spliethoff, B. The development, testing and optimization of energy storage materials based on the MgH2 Mg system. Int. J. Hydrog. Energy 1993, 18, 575–589. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Kinetic investigation of the effect of milling time on the hydrogen sorption reaction of magnesium catalyzed with different Nb2O5 contents. J. Alloy. Compd. 2006, 407, 249–255. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Fast hydrogen sorption kinetics of nanocrystalline Mg using Nb2O5 as catalyst. Scr. Mater. 2003, 49, 213–217. [Google Scholar] [CrossRef]
- Oelerich, W.; Klassen, T.; Bormann, R. Comparison of the catalytic effects of V, V2O5, VN, and VC on the hydrogen sorption of nanocrystalline Mg. J. Alloy. Compd. 2001, 322, L5–L9. [Google Scholar] [CrossRef]
- Sargent, A.-L.; Sheppard, D.A.; Paskevicius, M.; Pistidda, C.; Dornheim, M.; Buckley, C.E. Reaction kinetic behaviour with relation to crystallite/grain size dependency in the Mg–Si–H system. Acta Mater. 2015, 95, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Vajo, J.J.; Mertens, F.; Ahn, C.C.; Bowman, J.R.C.; Fultz, B. Altering Hydrogen Storage Properties by Hydride Destabilization through Alloy Formation: LiH and MgH2 Destabilized with Si. J. Phys. Chem. B 2004, 108, 13977–13983. [Google Scholar] [CrossRef]
- Su, W.; Zhu, Y.; Zhang, J.; Liu, Y.; Yang, Y.; Mao, Q.; Li, L. Effect of multi-wall carbon nanotubes supported nano-nickel and TiF3 addition on hydrogen storage properties of magnesium hydride. J. Alloy. Compd. 2016, 669, 8–18. [Google Scholar] [CrossRef]
- Asselli, A.A.C.; Botta, W.J.; Huot, J. Formation reaction of Mg2FeH6: Effect of hydrogen absorption/desorption kinetics. Mater. Res. 2013, 16, 1373–1378. [Google Scholar] [CrossRef] [Green Version]
- Huot, J.; Hayakawa, H.; Akiba, E. Preparation of the hydrides Mg2FeH6 and Mg2CoH5 by mechanical alloying followed by sintering. J. Alloy. Compd. 1997, 248, 164–167. [Google Scholar] [CrossRef]
- Bogdanovic, B.; Hofmann, H.; Neuy, A.; Reiser, A.; Schlichte, K.; Spliethoff, B.; Wessel, S. Ni-doped versus undoped Mg-MgH2 materials for high temperature heat or hydrogen storage. J. Alloy. Compd. 1999, 292, 57–71. [Google Scholar] [CrossRef]
- Felderhoff, M.; Bogdanović, B. High Temperature Metal Hydrides as Heat Storage Materials for Solar and Related Applications. Int. J. Mol. Sci. 2009, 10, 325–344. [Google Scholar] [CrossRef] [PubMed]
- Puszkiel, J.; Riglos, M.V.C.; Ramallo-López, J.M.; Mizrahi, M.; Gemming, T.; Pistidda, C.; Larochette, P.A.; Von Colbe, J.B.; Klassen, T.; Dornheim, M.; et al. New Insight on the Hydrogen Absorption Evolution of the Mg–Fe–H System under Equilibrium Conditions. Metals 2018, 8, 967. [Google Scholar] [CrossRef] [Green Version]
- Witek, K.; Karczewski, K.; Karpowicz, M.; Polański, M. Mg2FeH6 Synthesis Efficiency Map. Crystals 2018, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- Polański, M.; Nawra, D.; Zasada, D. Mg2FeH6 synthesized from plain steel and magnesium hydride. J. Alloy. Compd. 2019, 776, 1029–1040. [Google Scholar] [CrossRef]
- Fadonougbo, J.O.; Jung, J.-Y.; Suh, J.-Y.; Lee, Y.-S.; Shim, J.-H.; Cho, Y.W. Low temperature formation of Mg2FeH6 by hydrogenation of ball-milled nano-crystalline powder mixture of Mg and Fe. Mater. Des. 2017, 135, 239–245. [Google Scholar] [CrossRef]
- Jung, J.Y.; Fadonougbo, J.O.; Suh, J.-Y.; Lee, Y.-S.; Huh, J.-Y.; Cho, Y.W. Synthesis of Mg2FeH6 by hydrogenation of Mg/Fe powder mixture prepared by cold roll milling in air: Effects of microstructure and oxygen distribution. Int. J. Hydrog. Energy 2018, 43, 16758–16765. [Google Scholar] [CrossRef]
- Fadonougbo, J.O.; Jung, J.-Y.; Suh, J.-Y.; Lee, Y.-S.; Shim, J.-H.; Fleury, E.; Cho, Y.W. The role of Fe particle size and oxide distribution on the hydrogenation properties of ball-milled nano-crystalline powder mixtures of Fe and Mg. J. Alloy. Compd. 2019, 806, 1039–1046. [Google Scholar] [CrossRef]
- Bobet, J. Study of Mg–M (M = Co, Ni and Fe) mixture elaborated by reactive mechanical alloying: Hydrogen sorption properties. Int. J. Hydrog. Energy 2001, 26, 493–501. [Google Scholar] [CrossRef]
- Rzeszotarska, M.; Czujko, T.; Polański, M. Mg2(Fe, Cr, Ni)HX complex hydride synthesis from austenitic stainless steel and magnesium hydride. Int. J. Hydrog. Energy 2020, 45, 19440–19454. [Google Scholar] [CrossRef]
- Fadonougbo, J.O.; Kim, H.-J.; Suh, B.-C.; Suh, J.-Y.; Lee, Y.-S.; Shim, J.-H.; Yim, C.D.; Cho, Y.W. Kinetics and thermodynamics of near eutectic Mg–Mg2Ni composites produced by casting process. Int. J. Hydrog. Energy 2020, 45, 29009–29022. [Google Scholar] [CrossRef]
- Shao, H.; He, L.; Lin, H.; Li, H.-W. Progress and Trends in Magnesium-Based Materials for Energy-Storage Research: A Review. Energy Technol. 2018, 6, 445–458. [Google Scholar] [CrossRef] [Green Version]
- Iba, H.; Akiba, E. Hydrogen absorption and modulated structure in Ti–V–Mn alloys. J. Alloy. Compd. 1997, 253, 21–24. [Google Scholar] [CrossRef]
- Principi, G.; Agresti, F.; Maddalena, A.; Russo, S.L. The problem of solid state hydrogen storage. Energy 2009, 34, 2087–2091. [Google Scholar] [CrossRef]
- US Department of Energy. DOE Technical Targets for Onboard Hydrogen Storage for Light-Duty Vehicles; Office of Energy Efficiency and Renewable Energy: Washington, DC, USA, 2017.
- Hirscher, M.; Yartys, V.; Baricco, M.; Von Colbe, J.B.; Blanchard, D.; Bowman, R.C.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for hydrogen-based energy storage—past, recent progress and future outlook. J. Alloy. Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrog. Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Ren, J.; Musyoka, N.M.; Langmi, H.W.; Mathe, M.; Liao, S. Current research trends and perspectives on materials-based hydrogen storage solutions: A critical review. Int. J. Hydrog. Energy 2017, 42, 289–311. [Google Scholar] [CrossRef]
- Abe, J.; Popoola, A.; Ajenifuja, E.; Popoola, O. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrog. Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Bannenberg, L.; Heere, M.; Benzidi, H.; Montero, J.; Dematteis, E.; Suwarno, S.; Jaroń, T.; Winny, M.; Orłowski, P.; Wegner, W. Metal (boro-) hydrides for high energy density storage and relevant emerging technologies. Int. J. Hydrog. Energy 2020, 45, 33687–33730. [Google Scholar] [CrossRef]
- Yartys, V.; Lototskyy, M.; Akiba, E.; Albert, R.; Antonov, V.; Ares, J.; Baricco, M.; Bourgeois, N.; Buckley, C.; Von Colbe, J.B.; et al. Magnesium based materials for hydrogen based energy storage: Past, present and future. Int. J. Hydrog. Energy 2019, 44, 7809–7859. [Google Scholar] [CrossRef]
- Crivello, J.-C.; Denys, R.V.; Dornheim, M.; Felderhoff, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; De Jongh, P.E.; Latroche, M.; Walker, G.; et al. Mg-based compounds for hydrogen and energy storage. Appl. Phys. A 2016, 122, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Baran, A.; Polański, M. Magnesium-Based Materials for Hydrogen Storage—A Scope Review. Materials 2020, 13, 3993. [Google Scholar] [CrossRef]
- Liu, W.; Webb, C.; Gray, E. Review of hydrogen storage in AB3 alloys targeting stationary fuel cell applications. Int. J. Hydrog. Energy 2016, 41, 3485–3507. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, A.; Ichikawa, T.; Kojima, Y.; Dey, G. Development of vanadium based hydrogen storage material: A review. Renew. Sustain. Energy Rev. 2017, 72, 791–800. [Google Scholar] [CrossRef]
- Klebanoff, L. Hydrogen storage technology: Materials and applications. Choice Rev. Online 2013, 50, 50. [Google Scholar] [CrossRef] [Green Version]
- Borzone, E.; Baruj, A.; Blanco, M.; Meyer, G. Dynamic measurements of hydrogen reaction with LaNi5−xSnx alloys. Int. J. Hydrog. Energy 2013, 38, 7335–7343. [Google Scholar] [CrossRef] [Green Version]
- Modibane, K.; Lototskyy, M.V.; Davids, M.; Williams, M.; Hato, M.; Molapo, K. Influence of co-milling with palladium black on hydrogen sorption performance and poisoning tolerance of surface modified AB5-type hydrogen storage alloy. J. Alloy. Compd. 2018, 750, 523–529. [Google Scholar] [CrossRef]
- Pan, H.; Liu, Y.; Gao, M.; Zhu, Y.; Lei, Y. The structural and electrochemical properties of La0.7Mg0.3 (Ni0.85Co0.15)x (x = 3.0–5.0) hydrogen storage alloys. Int. J. Hydrog. Energy 2003, 28, 1219–1228. [Google Scholar] [CrossRef]
- Sharma, V.K.; Kumar, E.A. Effect of measurement parameters on thermodynamic properties of La-based metal hydrides. Int. J. Hydrog. Energy 2014, 39, 5888–5898. [Google Scholar] [CrossRef]
- Prigent, J.; Joubert, J.-M.; Gupta, M. Modification of the hydrogenation properties of LaNi5 upon Ni substitution by Rh, Ir, Pt or Au. J. Alloy. Compd. 2012, 511, 95–100. [Google Scholar] [CrossRef]
- Georgiadis, M.C.; Kikkinides, E.; Makridis, S.S.; Kouramas, K.; Pistikopoulos, E.N. Design and optimization of advanced materials and processes for efficient hydrogen storage. Comput. Chem. Eng. 2009, 33, 1077–1090. [Google Scholar] [CrossRef]
- Liu, J.J.; Li, K.; Cheng, H.H.; Yan, K.; Wang, Y.; Liu, Y.; Jin, H.M.; Zheng, Z. New insights into the hydrogen storage performance degradation and Al functioning mechanism of LaNi5−xAlx alloys. Int. J. Hydrog. Energy 2017, 42, 24904–24914. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, C.; Zhu, J.; Li, L. Structural and electrochemical hydrogen storage properties of Mg2Ni-based alloys. J. Alloy. Compd. 2011, 509, 5309–5314. [Google Scholar] [CrossRef]
- Pęska, M.; Dworecka-Wójcik, J.; Płociński, T.; Polański, M. The Influence of Cerium on the Hydrogen Storage Properties of La1−xCexNi5 Alloys. Energies 2020, 13, 1437. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Dou, S.; Liu, H.K. Effect of partial substitution of La with Ce, Pr and Nd on the properties of LaNi5-based alloy electrodes. J. Power Sources 1996, 63, 267–270. [Google Scholar] [CrossRef]
- Balasubramaniam, R.; Mungole, M.; Rai, K. Hydriding properties of MmNi5 system with aluminium, manganese and tin substitutions. J. Alloy. Compd. 1993, 196, 63–70. [Google Scholar] [CrossRef]
- Mungole, M. Hysteresis in MmNi5 systems with aluminium, manganese and tin substitutions. Int. J. Hydrog. Energy 1995, 20, 151–157. [Google Scholar] [CrossRef]
- Molinas, B.; Pontarollo, A.; Scapin, M.; Peretti, H.; Melnichuk, M.; Corso, H.; Aurora, A.; Gattia, D.M.; Montone, A. The optimization of MmNi5−xAlx hydrogen storage alloy for sea or lagoon navigation and transportation. Int. J. Hydrog. Energy 2016, 41, 14484–14490. [Google Scholar] [CrossRef]
- Iosub, V.; Latroche, M.; Joubert, J.-M.; Percheron-Guégan, A. Optimisation of MmNi5−xSnx (Mm = La, Ce, Nd and Pr, 0.27 < x < 0.5) compositions as hydrogen storage materials. Int. J. Hydrog. Energy 2006, 31, 101–108. [Google Scholar]
- Zhou, W.; Tang, Z.; Zhu, D.; Ma, Z.; Wu, C.; Huang, L.; Chen, Y. Low-temperature and instantaneous high-rate output performance of AB5-type hydrogen storage alloy with duplex surface hot-alkali treatment. J. Alloy. Compd. 2017, 692, 364–374. [Google Scholar] [CrossRef]
- Srivastava, S.; Upadhyaya, R. Investigations on synthesis, characterization and hydrogenation behavior of hydrogen storage alloys, Mm1−xCaxNi5−y−zAlyFez (x = 0, 0.05, 0.1, 0.2, 0.3; y = 0, 0.1; z = 0, 0.1) Mm1−xCaxNi5−y−zAlyFez (x = 0, 0.05, 0.1, 0.2, 0.3; y = 0, 0.1; z = 0, 0.1). Int. J. Hydrog. Energy 2007, 32, 4195–4201. [Google Scholar] [CrossRef]
- Srivastava, S.; Upadhyaya, R. Investigations of AB5-type hydrogen storage materials with enhanced hydrogen storage capacity. Int. J. Hydrog. Energy 2011, 36, 7114–7121. [Google Scholar] [CrossRef]
- Sandrock, G. A New Family of Hydrogen Storage Alloys Based on the System Nickel-Mischmetal-Calcium. In Proceedings of the 12th Intersociety Energy Conversion Engineering Conference, Washington, DC, USA, 28 August–2 September 1977; pp. 951–958. [Google Scholar]
- Shinar, J.; Shaltiel, D.; Davidov, D.; Grayevsky, A. Hydrogen sorption properties of the La1−xCaxNi5 and La (Ni1−xCux)5 systems. J. Less Comm. Metals 1978, 60, 209–219. [Google Scholar] [CrossRef]
- Wang, X.; Chen, C.; Wang, C.; Wang, Q. Hydrogen storage properties of Ml1−xCaxNi5 pseudobinary intermetallic compounds. J. Alloy. Compd. 1996, 232, 192–196. [Google Scholar] [CrossRef]
- Kumar, E.A.; Sharma, V. Simulation of Pressure Concentration Isotherms of MmNi5−xAlx Hydrides. In Proceedings of the 13th International Conference on Fuel Cell & Hydrogen Technologies, Kuala Lumpur, Malaysia, 22–23 November 2011. [Google Scholar]
- Sarma, V. On the mechanically pulverized MmNi4.6Fe0.4 as a viable hydrogen storage material. Int. J. Hydrog. Energy 2001, 26, 231–236. [Google Scholar] [CrossRef]
- Apostolov, A.; Stanev, N.; Tcholakov, P. Hydrogen desorption characteristics of MmNi5−xFex compounds. J. Less Common Metals 1985, 110, 127–129. [Google Scholar] [CrossRef]
- An, X.; Gu, Q.; Zhang, J.; Chen, S.; Yu, X.; Li, Q. Experimental investigation and thermodynamic reassessment of La–Ni and LaNi5–H systems. Calphad 2013, 40, 48–55. [Google Scholar] [CrossRef]
- Verbetsky, V.; Sirotina, R.; Umerenko, E. Absorption of hydrogen by MmNi5 alloys. Int. J. Hydrog. Energy 1996, 21, 935–938. [Google Scholar] [CrossRef]
- Rozdzynska-Kielbik, B.; Iwasieczko, W.; Drulis, H.; Pavlyuk, V.; Bala, H. Hydrogenation equilibria characteristics of LaNi5−xZnx intermetallics. J. Alloy. Compd. 2000, 298, 237–243. [Google Scholar] [CrossRef]
- Abu Dakka, M.I.; Jain, I. Comparative study of hydrogen in La(28.9)Ni(67.55)Si(3.55) and LaNi. Int. J. Hydrog. Energy 2000, 25, 773–777. [Google Scholar] [CrossRef]
- Bowman, R., Jr.; Luo, C.; Ahn, C.; Witham, C.; Fultz, B. The effect of tin on the degradation of LaNi5−ySny metal hydrides during thermal cycling. J. Alloy. Compd. 1995, 217, 185–192. [Google Scholar] [CrossRef]
- Briki, C.; Belkhiria, S.; Dhaou, M.H.; De Rango, P.; Jemni, A. Experimental study of the influences substitution from Ni by Co, Al and Mn on the hydrogen storage properties of LaNi3.6Mn0.3Al0.4Co0.7 alloy. Int. J. Hydrog. Energy 2017, 42, 10081–10088. [Google Scholar] [CrossRef]
- Yilmaz, F.; Ergen, S.; Hong, S.-J.; Uzun, O. Effect of Bismuth on hydrogen storage properties of melt-spun LaNi4.7−xAl0.3Bix (x = 0.0, 0.1, 0.2, 0.3) ribbons. Int. J. Hydrog. Energy 2018, 43, 20243–20251. [Google Scholar] [CrossRef]
- Bououdina, M.; Grant, D.M.; Walker, G. Review on hydrogen absorbing materials—structure, microstructure, and thermodynamic properties. Int. J. Hydrog. Energy 2006, 31, 177–182. [Google Scholar] [CrossRef]
- Kim, M.-H.; Debnath, M.R.; Wang, Y.-I.; Suh, J.-Y.; Fleury, E.; Kim, D. Effect of Co on the degradation of the hydrogen permeability of Ni–Nb–Zr amorphous membranes. Metals Mater. Int. 2014, 20, 215–219. [Google Scholar] [CrossRef]
- Zhao, Y.; Choi, I.-C.; Seok, M.-Y.; Kim, M.-H.; Kim, D.-H.; Ramamurty, U.; Suh, J.-Y.; Jang, J.-I. Effect of hydrogen on the yielding behavior and shear transformation zone volume in metallic glass ribbons. Acta Mater. 2014, 78, 213–221. [Google Scholar] [CrossRef]
- Zhao, Y.; Choi, I.-C.; Seok, M.-Y.; Ramamurty, U.; Suh, J.-Y.; Jang, J.-I. Hydrogen-induced hardening and softening of Ni–Nb–Zr amorphous alloys: Dependence on the Zr content. Scr. Mater. 2014, 93, 56–59. [Google Scholar] [CrossRef]
- Fadonougbo, J.O.; Suh, J.-Y.; Han, S.; Shim, C.-H.; Kim, G.-H.; Kim, M.-H.; Fleury, E.; Cho, Y.W. Hydrogen-induced decomposition of Cu–Zr binary amorphous metallic alloys. J. Alloy. Compd. 2016, 660, 456–460. [Google Scholar] [CrossRef]
- Fadonougbo, J.O.; Suh, J.-Y.; Shim, C.-H.; Kim, G.-H.; Fleury, E.; Cho, Y.W. Nanometer-scale phase separation and formation of delta ZrH2 in Cu–Zr binary amorphous alloys. J. Alloy. Compd. 2017, 721, 646–652. [Google Scholar] [CrossRef]
- Park, J.-G.; Jang, H.-Y.; Han, S.-C.; Lee, P.S.; Lee, J.-Y. The thermodynamic properties of Ti–Zr–Cr–Mn laves phase alloys. J. Alloy. Compd 2001, 325, 293–298. [Google Scholar] [CrossRef]
- Liu, B.-H.; Kim, N.-M.; Lee, K.-Y.; Lee, J.-Y. Hydrogen storage properties of TiMn2-based alloys. J. Alloy. Compd. 1996, 240, 214–218. [Google Scholar] [CrossRef]
- Kojima, Y.; Kawai, Y.; Towata, S.-I.; Matsunaga, T.; Shinozawa, T.; Kimbara, M. Development of metal hydride with high dissociation pressure. J. Alloy. Compd. 2006, 419, 256–261. [Google Scholar] [CrossRef]
- Kandavel, M.; Bhat, V.; Rougier, A.; Aymard, L.; Nazri, G.-A.; Tarascon, J.-M. Improvement of hydrogen storage properties of the AB2 Laves phase alloys for automotive application. Int. J. Hydrog. Energy 2008, 33, 3754–3761. [Google Scholar] [CrossRef]
- Morii, K.; Shimizu, T. Hydriding characteristics in (Ti,Zr)(Ni,Mn,X)2 alloys. J. Alloy. Compd. 1995, 231, 524–527. [Google Scholar] [CrossRef]
- Manickam, K.; Grant, D.M.; Sardari, P.T. Optimization of AB2 type alloy composition with superior hydrogen storage properties for stationary applications. Int. J. Hydrog. Energy 2015, 40, 16288–16296. [Google Scholar] [CrossRef]
- Li, J.; Jiang, X.; Li, G.; Li, X. Development of Ti1.02Cr2−x−yFexMny (0.6 ≤ x ≤ 0.75, y = 0.25, 0.3) alloys for high hydrogen pressure metal hydride system. Int. J. Hydrog. Energy 2019, 44, 15087–15099. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lee, K.-Y.; Lee, H.-H.; Kim, D.-M.; Yu, J.-S.; Jung, J.-H.; Lee, S.-G. Hydrogen-Storage Material Employing Ti-Mn Alloy System. U.S. Patent 5,888,317A, 30 March 1999. [Google Scholar]
- Li, J.; Xu, L.; Jiang, X.; Li, X. Study on the hydrogen storage property of (TiZr0.1)xCr1.7−y FeyMn0.3 (1.05 < x < 1.2, 0.2 < y < 0.6) alloys. Prog. Nat. Sci. Mater. Int. 2018, 28, 470–477. [Google Scholar]
- Corgnale, C.; Sulic, M. Techno-Economic Analysis of High-Pressure Metal Hydride Compression Systems. Metals 2018, 8, 469. [Google Scholar] [CrossRef] [Green Version]
- Pickering, L.; Lototskyy, M.V.; Davids, M.W.; Sita, C.; Linkov, V. Induction melted AB2-type metal hydrides for hydrogen storage and compression applications. Mater. Today Proc. 2018, 5, 10470–10478. [Google Scholar] [CrossRef]
- Ulmer, U.; Dieterich, M.; Pohl, A.; Dittmeyer, R.; Linder, M.P.; Fichtner, M. Study of the structural, thermodynamic and cyclic effects of vanadium and titanium substitution in laves-phase AB2 hydrogen storage alloys. Int. J. Hydrog. Energy 2017, 42, 20103–20110. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; Liu, L.; Xiao, X.; Wang, C.; Jiang, L.; Chen, L. Effect of rare earth doping on the hydrogen storage performance of Ti1.02Cr1.1Mn0.3Fe0.6 alloy for hybrid hydrogen storage application. J. Alloy. Compd. 2018, 731, 524–530. [Google Scholar] [CrossRef]
- Puszkiel, J.; Bellosta von Colbe, J.M.; Jepsen, J.; Mitrokhin, S.V.; Movlaev, E.; Verbetsky, V.; Klassen, T. Designing an AB2-Type Alloy (TiZr-CrMnMo) for the Hybrid Hydrogen Storage Concept. Energies 2020, 13, 2751. [Google Scholar] [CrossRef]
- Wu, T.; Xue, X.; Zhang, T.; Hu, R.; Kou, H.; Li, J. Role of Ni addition on hydrogen storage characteristics of ZrV2 Laves phase compounds. Int. J. Hydrog. Energy 2016, 41, 10391–10404. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, C.; Ouyang, L.; Liu, J.; Zhu, M.; Sun, T.; Wang, H. High-pressure hydrogen storage performances of ZrFe2 based alloys with Mn, Ti, and V addition. Int. J. Hydrog. Energy 2020, 45, 9836–9844. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, H.; Ouyang, L.; Liu, J.; Zhu, M. Achieving high equilibrium pressure and low hysteresis of Zr–Fe based hydrogen storage alloy by Cr/V substitution. J. Alloy. Compd. 2019, 806, 1436–1444. [Google Scholar] [CrossRef]
- Blasius, A.; Gonster, U. Mössbauer surface studies on Tife hydrogen storage material. Appl. Phys. A 1980, 22, 331–332. [Google Scholar] [CrossRef]
- Reilly, J.J.; Wiswall, R.H. Formation and properties of iron titanium hydride. Inorg. Chem. 1974, 13, 218–222. [Google Scholar] [CrossRef]
- Sandrock, G.D.; Reilly, J.J.; Johnson, J.R. Metallurgical Considerations in the Production and use of FeTi Alloys for Hydrogen Storage. In Proceedings of the Intersociety Energy Conversion Engineering Conference, State Line, NV, USA, 12 September 1976. [Google Scholar]
- Edalati, K.; Matsuda, J.; Iwaoka, H.; Toh, S.; Akiba, E.; Horita, Z. High-pressure torsion of TiFe intermetallics for activation of hydrogen storage at room temperature with heterogeneous nanostructure. Int. J. Hydrog. Energy 2013, 38, 4622–4627. [Google Scholar] [CrossRef]
- Davids, M.W.; Lototskyy, M.V.; Nechaev, A.; Naidoo, Q.; Williams, M.; Klochko, Y. Surface modification of TiFe hydrogen storage alloy by metal-organic chemical vapour deposition of palladium. Int. J. Hydrog. Energy 2011, 36, 9743–9750. [Google Scholar] [CrossRef]
- Qu, H.; Du, J.; Pu, C.; Niu, Y.; Huang, T.; Li, Z.; Lou, Y.; Wu, Z. Effects of Co introduction on hydrogen storage properties of Ti–Fe–Mn alloys. Int. J. Hydrog. Energy 2015, 40, 2729–2735. [Google Scholar] [CrossRef]
- Yamashita, I.; Tanaka, H.; Takeshita, H.; Kuriyama, N.; Sakai, T.; Uehara, I. Hydrogenation characteristics of TiFe1−xPdx (0.05 ≤ x ≤ 0.30) alloys. J. Alloy. Compd. 1997, 253, 238–240. [Google Scholar] [CrossRef]
- Jang, T.; Han, J.; Jai-Young, L. Effect of substitution of titanium by zirconium in TiFe on hydrogenation properties. J. Less Common Metals 1986, 119, 237–246. [Google Scholar] [CrossRef]
- Bronca, V.; Bergman, P.; Ghaemmaghami, V.; Khatamian, D.; Manchester, F. Hydrogen absorption characteristics of an FeTi + misch metal alloy. J. Less Common Metals 1985, 108, 313–325. [Google Scholar] [CrossRef]
- Guéguen, A.; Latroche, M. Influence of the addition of vanadium on the hydrogenation properties of the compounds TiFe0.9Vx and TiFe0.8Mn0.1Vx (x = 0, 0.05 and 0.1). J. Alloy. Compd. 2011, 509, 5562–5566. [Google Scholar] [CrossRef]
- Lee, S.M.; Perng, T.P. Microstructural Correlations with the Hydrogenation Kinetics of FeTi1+ε Alloys. J. Alloy. Compd. 1991, 177, 107–118. [Google Scholar] [CrossRef]
- Emami, H.; Edalati, K.; Matsuda, J.; Akiba, E.; Horita, Z. Hydrogen storage performance of TiFe after processing by ball milling. Acta Mater. 2015, 88, 190–195. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Chin, Z.-H.; Perng, T.-P. Hydrogenation of TiFe by high-energy ball milling. J. Alloy. Compd. 2000, 307, 259–265. [Google Scholar] [CrossRef]
- Edalati, K.; Matsuda, J.; Yanagida, A.; Akiba, E.; Horita, Z. Activation of TiFe for hydrogen storage by plastic deformation using groove rolling and high-pressure torsion: Similarities and differences. Int. J. Hydrog. Energy 2014, 39, 15589–15594. [Google Scholar] [CrossRef]
- Johnson, J.; Reilly, J. The Use of Manganese Substituted Ferrotitanium Alloys for Energy Storage. In Proceedings of the The Use of Manganese Substituted Ferrotitanium Alloys for Energy Storage, Miami Beach, FL, USA, 5–7 December 1977. [Google Scholar]
- Shang, H.; Zhang, M.; Li, Y.; Qi, Y.; Guo, S.; Zhao, D. Effects of adding over-stoichiometrical Ti and substituting Fe with Mn partly on structure and hydrogen storage performances of TiFe alloy. Renew. Energy 2019, 135, 1481–1498. [Google Scholar] [CrossRef]
- Wu, C.Y.; Li, J.C. Phase-Structure of the Ti1cu1-Xfex System. Metall. Trans. A Phys. Metall. Mater. Sci. 1989, 20, 981–985. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Cuevas, F.; Latroche, M. Hydrogen storage properties of Mn and Cu for Fe substitution in TiFe0.9 intermetallic compound. J. Alloy. Compd. 2021, 851, 156075. [Google Scholar] [CrossRef]
- Nagai, H.; Kitagaki, K.; Shoji, K.-I. Hydrogen Storage Characteristics of FeTi Containing Zirconium. Trans. Jpn. Inst. Metals 1988, 29, 494–501. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-M.; Perng, T.-P. Correlation of substitutional solid solution with hydrogenation properties of TiFe1−x Mx (M= Ni, Co, Al) alloys. J. Alloy. Compd. 1999, 291, 254–261. [Google Scholar] [CrossRef]
- Kuziora, P.; Kunce, I.; McCain, S.; Adkins, N.J.E.; Polański, M. The influence of refractory metals on the hydrogen storage characteristics of FeTi-based alloys prepared by suspended droplet alloying. Int. J. Hydrog. Energy 2020, 45, 21635–21645. [Google Scholar] [CrossRef]
- Jain, P.; Gosselin, C.; Huot, J. Effect of Zr, Ni and Zr 7 Ni 10 alloy on hydrogen storage characteristics of TiFe alloy. Int. J. Hydrog. Energy 2015, 40, 16921–16927. [Google Scholar] [CrossRef]
- Yang, T.; Wang, P.; Xia, C.Q.; Liu, N.; Liang, C.Y.; Yin, F.X.; Li, Q. Effect of chromium, manganese and yttrium on microstructure and hydrogen storage properties of TiFe-based alloy. Int. J. Hydrog. Energy 2020, 45, 12071–12081. [Google Scholar] [CrossRef]
- Ha, T.; Lee, S.-I.; Hong, J.; Lee, Y.-S.; Kim, D.-I.; Suh, J.-Y.; Cho, Y.W.; Hwang, B.; Lee, J.; Shim, J.-H. Hydrogen storage behavior and microstructural feature of a TiFe–ZrCr2 alloy. J. Alloy. Compd. 2021, 853, 157099. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, Y.-S.; Suh, J.-Y.; Huh, J.-Y.; Cho, Y.W. Tailoring the equilibrium hydrogen pressure of TiFe via vanadium substitution. J. Alloy. Compd. 2021, 854, 157263. [Google Scholar] [CrossRef]
- Ouyang, L.; Huang, J.; Wang, H.; Liu, J.; Zhu, M. Progress of hydrogen storage alloys for Ni–MH rechargeable power batteries in electric vehicles: A review. Mater. Chem. Phys. 2017, 200, 164–178. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, H.; Yue, Y.; Wu, X.; Chen, N.; Lei, Y. Cycling durability and degradation behavior of La–Mg–Ni–Co-type metal hydride electrodes. J. Alloy. Compd. 2005, 395, 291–299. [Google Scholar] [CrossRef]
- Liao, B.; Lei, Y.; Chen, L.; Lü, G.; Pan, H.; Wang, Q. The effect of Al substitution for Ni on the structure and electrochemical properties of AB3-type La2Mg(Ni1−xAlx)9 (x = 0–0.05) alloys. J. Alloy. Compd. 2005, 404, 665–668. [Google Scholar] [CrossRef]
- Takeshita, T.; Wallace, W.E.; Craig, R.S. Solubility of hydrogen in rare earth-tricobalt compounds. Inorg. Chem. 1974, 13, 2283–2284. [Google Scholar] [CrossRef]
- Oesterreicher, H.; Clinton, J.; Bittner, H. Hydrides of La–Ni Compounds. Mater. Res. Bull. 1976, 11, 1241–1247. [Google Scholar] [CrossRef]
- Kadir, K.; Sakai, T.; Uehara, I. Synthesis and structure determination of a new series of hydrogen storage alloys; RMg2Ni9 (R = La, Ce, Pr, Nd, Sm and Gd) built from MgNi2 Laves-type layers alternating with AB5 layers. J. Alloy. Compd. 1997, 257, 115–121. [Google Scholar] [CrossRef]
- Kadir, K.; Sakai, T.; Uehara, I. Structural investigation and hydrogen capacity of YMg2Ni9 and (Y0.5Ca0.5)(MgCa)Ni9: New phases in the AB2C9 system isostructural with LaMg2Ni9. J. Alloy. Compd. 1999, 287, 264–270. [Google Scholar] [CrossRef]
- Kadir, K.; Sakai, T.; Uehara, I. Structural investigation and hydrogen storage capacity of LaMg2Ni9 and (La0.65Ca0.35)(Mg1.32Ca0.68)Ni9 of the AB2C9 type structure. J. Alloy. Compd. 2000, 302, 112–117. [Google Scholar] [CrossRef]
- Chen, J.; Kuriyama, N.; Takeshita, H.; Tanaka, H.; Sakai, T.; Haruta, M. Hydrogen Storage Alloys with PuNi3-Type Structure as Metal Hydride Electrodes. Electrochem. Solid State Lett. 2000, 3, 249–252. [Google Scholar] [CrossRef]
- Lim, K.L.; Liu, Y.; Zhang, Q.-A.; Chan, S.L.I. Effects of partial substitutions of cerium and aluminum on the hydrogenation properties of La (0.65−x) CexCa1.03Mg1.32Ni (9−y) Aly alloy. Int. J. Hydrog. Energy 2014, 39, 10537–10545. [Google Scholar] [CrossRef]
- Xin, G.; Wang, S.; Yuan, H.; Yang, K.; Jiang, L.; Liu, X. Promising hydrogen storage properties of cost-competitive La(Y)–Mg–Ca–Ni AB3-type alloys for stationary applications. RSC Adv. 2016, 6, 21742–21748. [Google Scholar] [CrossRef]
- Zang, J.; Zhang, Q.; Sun, D. Hydrogen storage performances of RCaMgNi9 (R = Nd, Gd and Er) compounds. J. Alloy. Compd. 2019, 794, 45–52. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, H.; Gao, M.; Li, R.; Lei, Y. Effect of Co content on the structural and electrochemical properties of the La0.7Mg0.3Ni3.4−xMn0.1Cox hydride alloys: II. Electrochemical properties. J. Alloy. Compd. 2004, 376, 304–313. [Google Scholar] [CrossRef]
- Liao, B.; Lei, Y.; Chen, L.; Lü, G.; Pan, H.; Wang, Q. Effect of Co substitution for Ni on the structural and electrochemical properties of La2Mg(Ni1−xCox)9 (x = 0.1–0.5) hydrogen storage electrode alloys. Electrochim. Acta 2004, 50, 1057–1063. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, D.; Yin, W.; Chai, Y.; Zhao, M. Crystallographic and electrochemical characteristics of La0.7Mg0.3Ni3.5−x (Al0.5Mo0.5)x (x = 0–0.8) hydrogen storage alloys. J. Power Sources 2006, 154, 290–297. [Google Scholar] [CrossRef]
- Yan, H.; Xiong, W.; Wang, L.; Li, B.; Li, J.; Zhao, X. Investigations on AB3-, A2B7-and A5B19-type LaYNi system hydrogen storage alloys. Int. J. Hydrog. Energy 2017, 42, 2257–2264. [Google Scholar] [CrossRef]
- Sandrock, G. A panoramic overview of hydrogen storage alloys from a gas reaction point of view. J. Alloy. Compd. 1999, 293, 877–888. [Google Scholar] [CrossRef]
- Yukawa, H.; Takagi, M.; Teshima, A.; Morinaga, M. Alloying effects on the stability of vanadium hydrides. J. Alloy. Compd. 2002, 330, 105–109. [Google Scholar] [CrossRef]
- Seo, C.-Y.; Kim, J.-H.; Lee, P.S.; Lee, J.-Y. Hydrogen storage properties of vanadium-based b.c.c. solid solution metal hydrides. J. Alloy. Compd. 2003, 348, 252–257. [Google Scholar] [CrossRef]
- Kumar, S.; Taxak, M.; Krishnamurthy, N. Hydrogen absorption kinetics of V–Al alloy. J. Therm. Anal. Calorim. 2013, 112, 5–10. [Google Scholar] [CrossRef]
- Kumar, S.; Taxak, M.; Krishnamurthy, N. Synthesis and hydrogen absorption kinetics of V4Cr4Ti alloy. J. Therm. Anal. Calorim. 2012, 112, 51–57. [Google Scholar] [CrossRef]
- Verbetsky, V.N.; Zotov, T.A.; Movlaev, E.A. Absorption of hydrogen by V–Mo and V–Mo–Ti alloys. Inorg. Mater. Appl. Res. 2014, 5, 70–74. [Google Scholar] [CrossRef]
- Tamura, T.; Kazumi, T.; Kamegawa, A.; Takamura, H.; Okada, M. Protium absorption properties and protide formations of Ti–Cr–V alloys. J. Alloy. Compd. 2003, 356, 505–509. [Google Scholar] [CrossRef]
- Okada, M.; Kuriiwa, T.; Tamura, T.; Takamura, H.; Kamegawa, A. Ti–V–Cr bcc alloys with high protium content. J. Alloy. Compd. 2002, 330, 511–516. [Google Scholar] [CrossRef]
- Kagawa, A.; Ono, E.; Kusakabe, T.; Sakamoto, Y. Absorption of Hydrogen by Vanadium-Rich V–Ti-Based Alloys. J. Less Common Metals 1991, 172, 64–70. [Google Scholar] [CrossRef]
- Tsukahara, M. Hydrogenation Properties of Vanadium-Based Alloys with Large Hydrogen Storage Capacity. Mater. Trans. 2011, 52, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Tamura, T.; Tominaga, Y.; Matsumoto, K.; Fuda, T.; Kuriiwa, T.; Kamegawa, A.; Takamura, H.; Okada, M. Protium absorption properties of Ti–V–Cr–Mn alloys with a bcc structure. J. Alloy. Compd. 2002, 330, 522–525. [Google Scholar] [CrossRef]
- Shudo, Y.; Ebisawa, T.; Itoh, H. Characterization of Ti–Zr–Mn–V-based Laves phase alloys for MH refrigeration system. J. Alloy. Compd. 2003, 356, 497–500. [Google Scholar] [CrossRef]
- Yu, X.; Wu, Z.; Xia, B.; Xu, N. Hydrogen storage performance of quenched Ti–V-based alloy. J. Alloy. Compd. 2004, 373, 134–136. [Google Scholar] [CrossRef]
- Yu, X.; Wu, Z.; Xu, N. Effects of melt-quenching rates on the hydrogen storage properties of Ti-based BCC phase alloy. Phys. B Condens. Matter 2004, 344, 456–461. [Google Scholar] [CrossRef]
- Yu, X.; Wu, Z.; Xia, B.; Xu, N. Improvement of activation performance of the quenched Ti–V-based BCC phase alloys. J. Alloy. Compd. 2005, 386, 258–260. [Google Scholar] [CrossRef]
- Nomura, K.; Akiba, E. H2 Absorbing-desorbing characterization of the TiVFe alloy system. J. Alloy. Compd. 1995, 231, 513–517. [Google Scholar] [CrossRef]
- Lynch, J.F.; Maeland, A.J.; Libowitz, G.G. Lattice Parameter Variation and Thermodynamics of Dihydride Formation in the Vanadium-Rich V–Ti–Fe/H2 System. Z. Phys. Chem. 1985, 145, 51–59. [Google Scholar] [CrossRef]
- Luo, L.; Li, Y.; Zhai, T.; Hu, F.; Zhao, Z.; Bian, X.; Wu, W. Microstructure and hydrogen storage properties of V48Fe12Ti15-xCr25Alx (x = 0, 1) alloys. Int. J. Hydrog. Energy 2019, 44, 25188–25198. [Google Scholar] [CrossRef]
- Akiba, E.; Iba, H. Hydrogen absorption by Laves phase related BCC solid solution. Intermetallics 1998, 6, 461–470. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, Y.; Liang, H.; Wu, C.; Tao, M.; Mingjing, T. Effect of Al on hydrogen storage properties of V30Ti35Cr25Fe10 alloy. J. Alloy. Compd. 2006, 426, 253–255. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, L.; Li, Z.; Huang, Z.; Wang, S. Improve plateau property of Ti32Cr46V22 BCC alloy with heat treatment and Ce additive. J. Alloy. Compd. 2009, 471, L36–L38. [Google Scholar] [CrossRef]
- Aoki, M.; Noritake, T.; Ito, A.; Ishikiriyama, M.; Towata, S.-I. Improvement of cyclic durability of Ti–Cr–V alloy by Fe substitution. Int. J. Hydrog. Energy 2011, 36, 12329–12332. [Google Scholar] [CrossRef]
- Towata, S.-I.; Noritake, T.; Itoh, A.; Aoki, M.; Miwa, K. Effect of partial niobium and iron substitution on short-term cycle durability of hydrogen storage Ti–Cr–V alloys. Int. J. Hydrog. Energy 2013, 38, 3024–3029. [Google Scholar] [CrossRef]
- Shen, C.-C.; Li, H.-C. Cyclic hydrogenation stability of γ-hydrides for Ti25V35Cr40 alloys doped with carbon. J. Alloy. Compd. 2015, 648, 534–539. [Google Scholar] [CrossRef]
- Yang, S.; Yang, F.; Wu, C.; Chen, Y.; Mao, Y.; Luo, L. Hydrogen storage and cyclic properties of (VFe)60(TiCrCo)40−xZrx (0≤ x ≤ 2) alloys. J. Alloy. Compd. 2016, 663, 460–465. [Google Scholar] [CrossRef]
- Nayebossadri, S.; Book, D. Development of a high-pressure Ti–Mn based hydrogen storage alloy for hydrogen compression. Renew. Energy 2019, 143, 1010–1021. [Google Scholar] [CrossRef]
| Material | H Density | Energy Density | ||
|---|---|---|---|---|
| wt% | kg m−3 | MJ kg−1 | MJ dm−3 | |
| Gas H2, 700 bar | 100 | 42 | 120.0 | 5 |
| Liquid H2 (20 K) | 100 | 71 | 120.0 | 8.5 |
| LaNi5H6 | 1.4 | 90 | 1.7 | 10.8 |
| TiFeH2 | 1.9 | 105 | 2.3 | 12.6 |
| MgH2 | 7.6 | 110 | 9.2 | 13.3 |
| Intermetallic Compound | Reference Alloy | Structure |
|---|---|---|
| AB5 | LaNi5 | Haucke phase, hexagonal |
| AB2 | TiMn2 | Laves phase, hexagonal or cubic |
| AB | TiFe | Cubic, CsCl-type or orthorhombic, CrB-type |
| AB3 | CeNi3 | Hexagonal, NbBe3-type |
| Solid solutions | V, Ti–V | Body centered cubic |
| Alloy | Temperature (K) | Structure Type | Lattice Parameters (nm) | Maximum/Reversible Capacity (wt%) | Pa/Pd (atm) | Remarks on the Effects of Partial Substitution |
|---|---|---|---|---|---|---|
| LaNi5 [74] | 303 | CaCu5 | a = 0.5020 c = 0.3980 | 1.56/1.25 | 2.90/2.50 | - |
| MmNi5 [75]. | 273 | CaCu5 | a = 0.4934 c = 0.3998 | 1.29/1.00 | -/5.20 | Easy activation process, increased hysteresis and maximum storage capacity ~20% lower than that of LaNi5. |
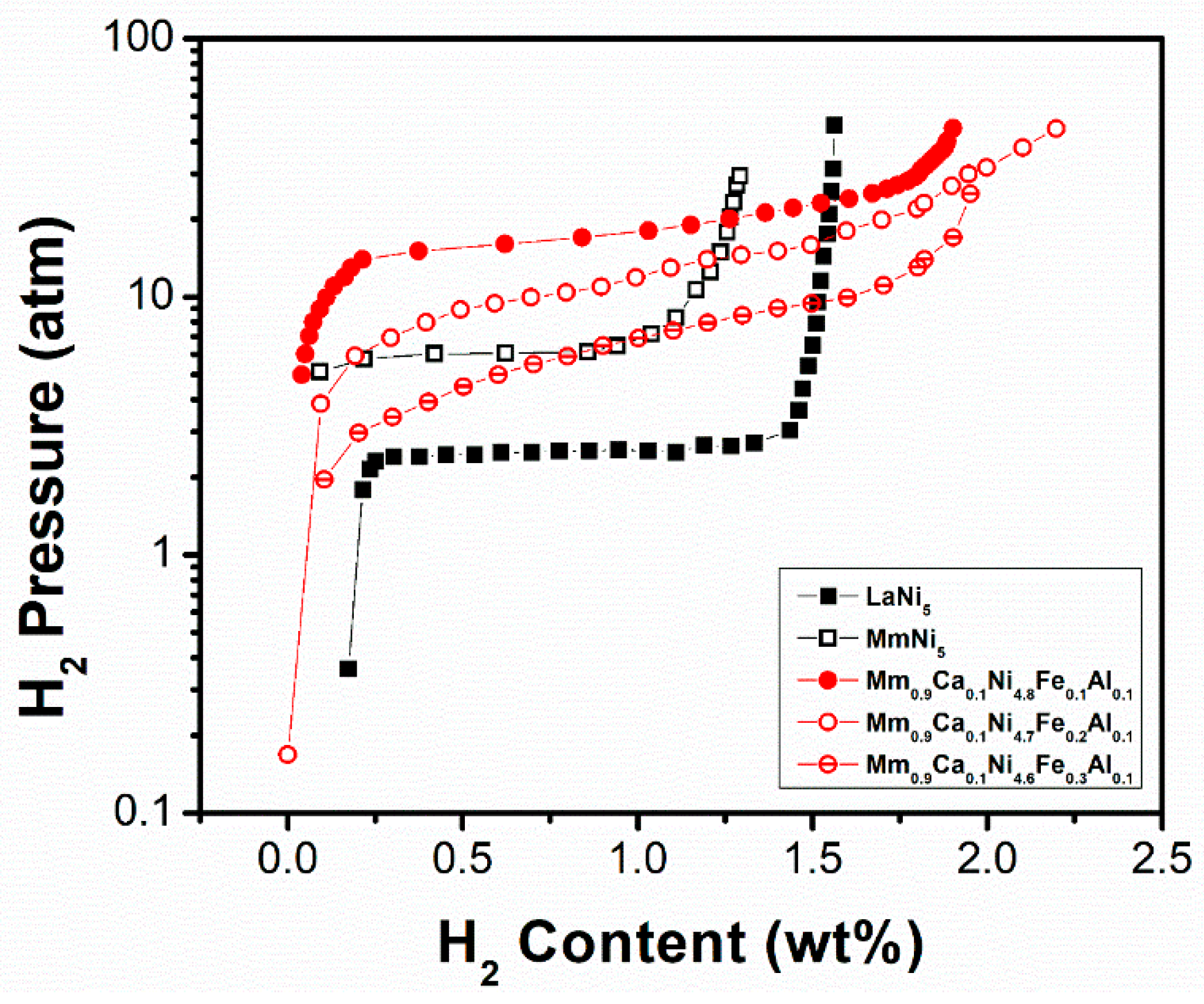
| Mm0.9Ca0.1Ni4.8Fe0.1Al0.1 [67] | 300 | CaCu5 | a = 0.4949 c = 0.4013 | 1.90/1.25 | ~24.00/18.00 | Ca enhances the hydrogen storage capacity, reduces the incubation time of the first hydrogenation, increases the absorption rate (with increasing Ca concentration), and reduces the hysteresis. Al reduces the plateau pressure, but also the maximum hydrogen storage capacity. Fe increases the storage capacity and reduces sloping and hysteresis. |
| Mm0.9Ca0.1Ni4.7Fe0.2Al0.1 [67] | 300 | CaCu5 | a = 0.4952 c = 0.4019 | 2.20/1.50 | ~16.00/13.00 | |
| Mm0.9Ca0.1Ni4.6Fe0.3Al0.1 [67] | 300 | CaCu5 | a = 0.4962 c = 0.4018 | 1.96/1.65 | ~10.00/5.00 |
| Alloy | Temperature (K) | Structure Type | Lattice Parameters (nm) | Maximum/Reversible Capacity (wt%) | Pa/Pd (atm) | Remarks on the Effects of Partial Substitution |
|---|---|---|---|---|---|---|
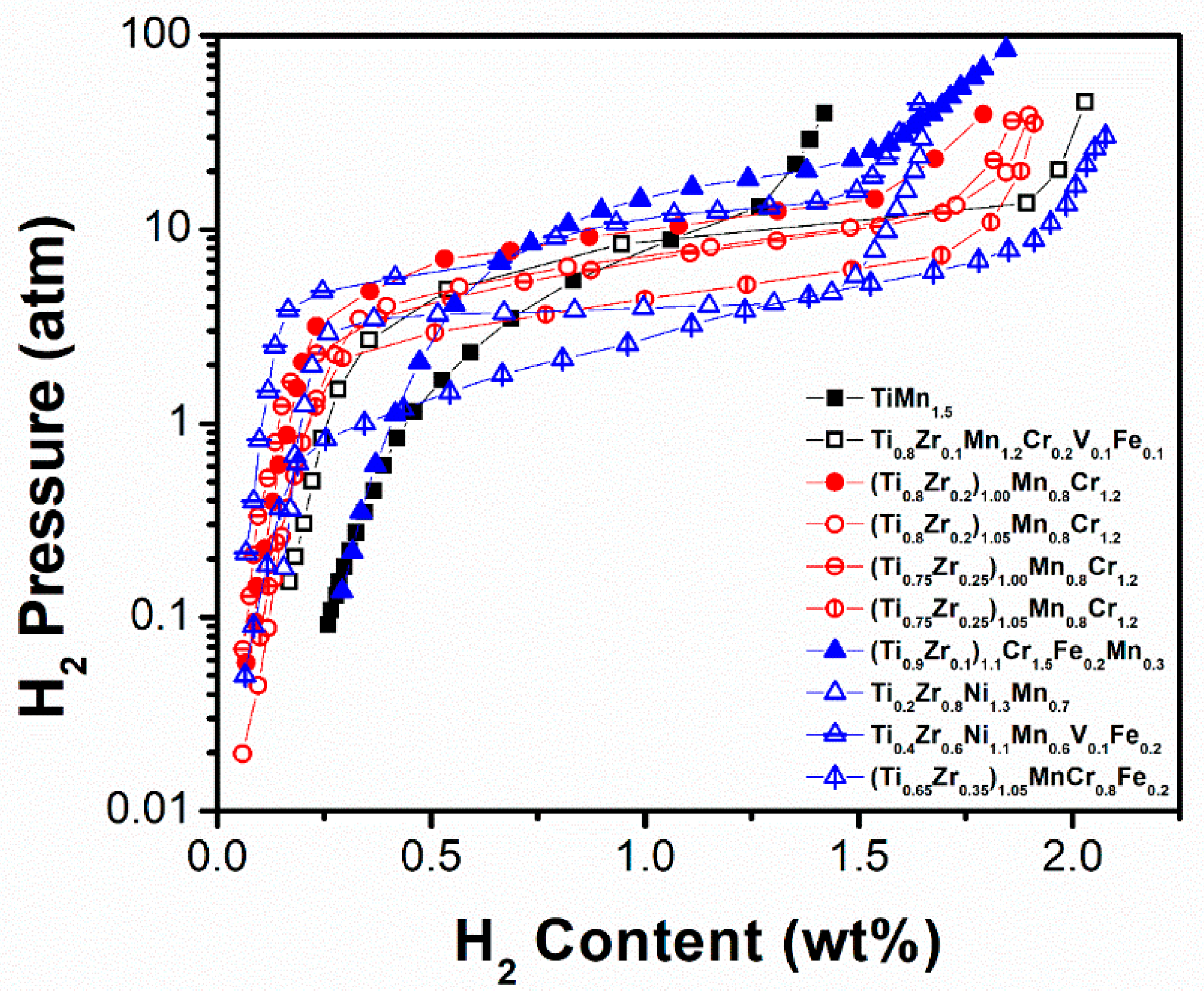
| TiMn1.5 [94] | 303 | MgZn2 | a = 0.4878 c = 0.7976 | 1.42/0.75 | -/5.45 | - |
| (Ti0.8Zr0.2)1.00Mn0.8Cr1.2 [87] | 303 | MgZn2 | a = 0.4902 c = 0.8044 | 1.79/1.35 | 12.00/10.52 | Increasing Zr content decreases the equilibrium plateau pressure, accelerates absorption kinetics, and increases the storage capacity. Cr increases hydrogen storage capacity and reduces equilibrium pressure. Lattice strain increases along with Zr/Ti ratio and partially results in important sloping |
| (Ti0.8Zr0.2)1.05Mn0.8Cr1.2 [87] | 303 | MgZn2 | - | 1.90/1.55 | 8.40/7.54 | |
| (Ti0.75Zr0.25)1.00Mn0.8Cr1.2 [87] | 303 | MgZn2 | a = 0.4912 c = 0.8064 | 1.86/1.50 | 8.72/6.99 | |
| (Ti0.75Zr0.25)1.05Mn0.8Cr1.2 [87] | 303 | MgZn2 | - | 1.91/1.60 | 5.23/4.47 | |
| (Ti0.9Zr0.1)1.1Cr1.5Fe0.2Mn0.3 [95] | 303 | MgZn2 | a = 0.4897 c = 0.8026 | 1.84/1.30 | 2.54/2.17 | Partial substitution of Mn by Fe shrinks the cell volume and increases the plateau pressure, but the hydrogen capacity does not change noticeably. |
| Ti0.2Zr0.8Ni1.3Mn0.7 [91] | 303 | MgCu2 | - | 1.65/1.35 | 1.40/0.38 | V lowers both hysteresis and plateau pressure. Ni raises the plateau pressure and reduces the plateau width, while Fe flattens and lengthens it. |
| Ti0.4Zr0.6Ni1.1Mn0.6V0.1Fe0.2 [91] | 303 | MgCu2 | - | 1.64/1.25 | 1.22/0.75 | |
| (Ti0.65Zr0.35)1.05MnCr0.8Fe0.2 [92] | 305 | MgZn2 | - | 2.2/1.75 | 5.30/2.80 | Unit cell volume and storage capacity increase, charge time reduces along with non-stoichiometry on the A site. |
| Ti0.8 Zr0.1Mn1.2Cr0.2V0.1Fe0.1 [94] | 303 | MgZn2 | - | 2.03/1.60 | 18.06/9.02 | - |
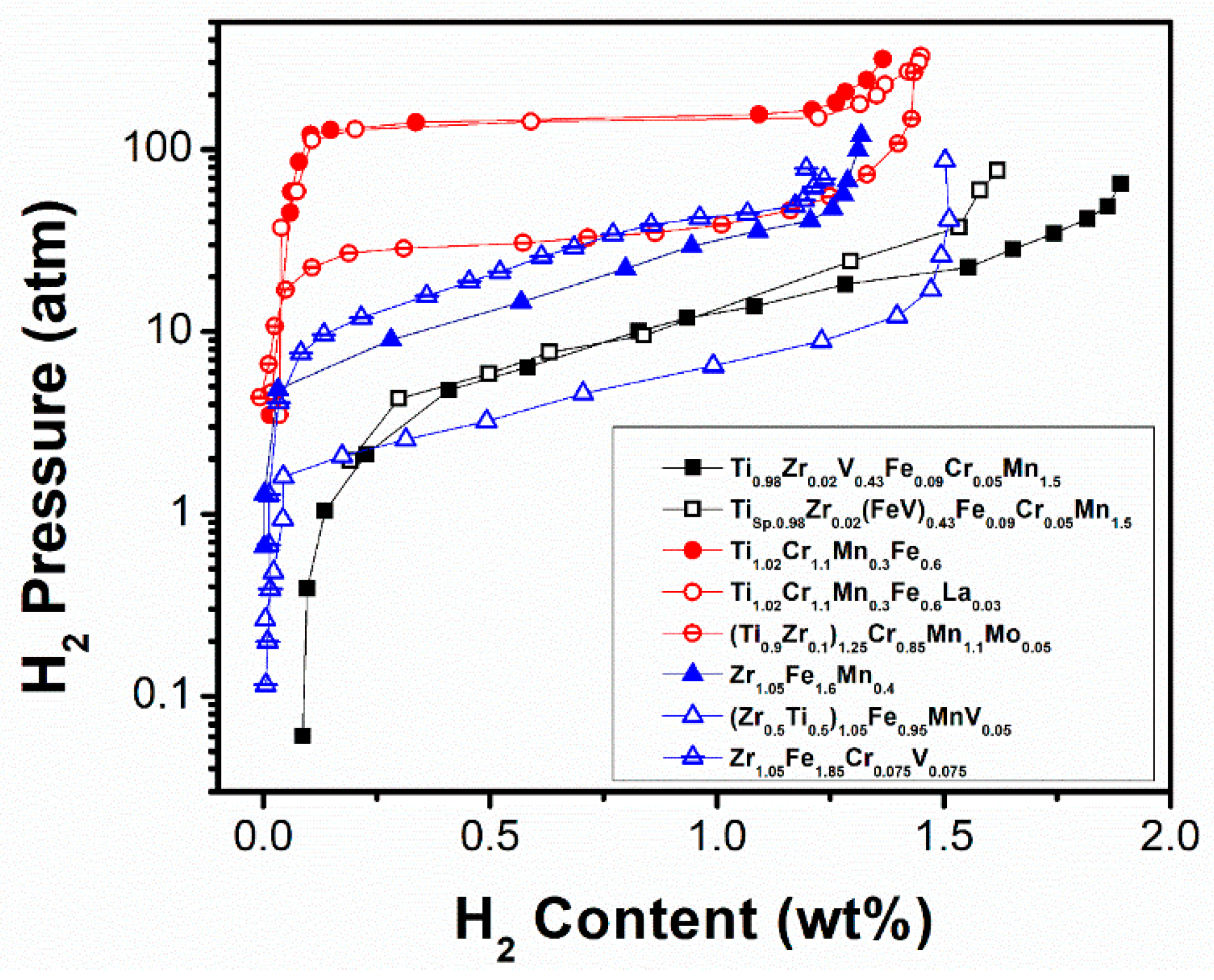
| Ti0.98Zr0.02V0.43Fe0.09Cr0.05Mn1.5 [98] | 298 | MgZn2 | a = 0.4875 c = 0.7994 | 1.89/1.36 | 23.3/12.1 | Replacing Ti by Ti sponge does not change the initial storage capacity, whereas it is reduced after substitution of V by FeV. Substitutions do not affect microstructural properties. |
| Tisp0.98Zr0.02(FeV)0.43Fe0.09Cr0.05Mn1.5 [98] | 298 | MgZn2 | a = 0.4872 c = 0.7989 | 1.62/1.12 | 23.4/13.8 | |
| Ti1.02Cr1.1Mn0.3Fe0.6 [99] | 263 | MgZn2 | a = 0.4854 c = 0.7968 | 1.38/1.05 | 208/143 | RE improves the activation behaviour, the sorption properties and storage capacity (saturation reached at RT), but decreases the hydrogen desorption plateau pressure. |
| Ti1.02Cr1.1Mn0.3Fe0.6La0.03 [99] | 263 | MgZn2 | a = 0.4862 c = 0.7975 | 1.7/1.2 | 218/137 | |
| (Ti0.9Zr0.1)1.25Cr0.85Mn1.1Mo0.05 [100] | 296 | MgZn2 | a = 0.4884 c = 0.801 | 1.45/1.25 | 54/34 | The equilibrium pressure increases with Mo amount, mostly due to the larger bulk modulus of Mo (compared to Cr) rather than the increase in cell volume. For Mo higher than x = 0.05, the hydrogen capacity greatly drops. |
| Zr1.05Fe1.6Mn0.4 [102] | 288 | MgCu2 | a = 0.7086 | 1.32/1.12 | 60/24 | V addition improves the hysteresis, while an adequate Ti amount helps to achieve low slope and high plateau pressure. |
| (Zr0.5Ti0.5)1.05Fe0.95MnV0.05 [102] | 288 | MgZn2 | a = 0.4935 c = 0.8079 | 1.64/1.32 | 23/6.7 | |
| Zr1.05Fe1.85Cr0.075V0.075 [103] | 288 | MgCu2 | a = 0.7086 | 1.54/1.08 | 61/44 | Cr and V substitutions effectively decrease the equilibrium pressure due to the enlarged unit cell, while the V substitution improves the hysteresis. Too high Cr addition induces a transition from MgCu2 to MgZn2 structure type. |
| Alloy | Temperature (K) | Structure Type | Lattice Parameters (nm) | Maximum/Reversible Capacity (wt%) | Pa/Pd (atm) | Remarks on the Effects of Partial Substitution |
|---|---|---|---|---|---|---|
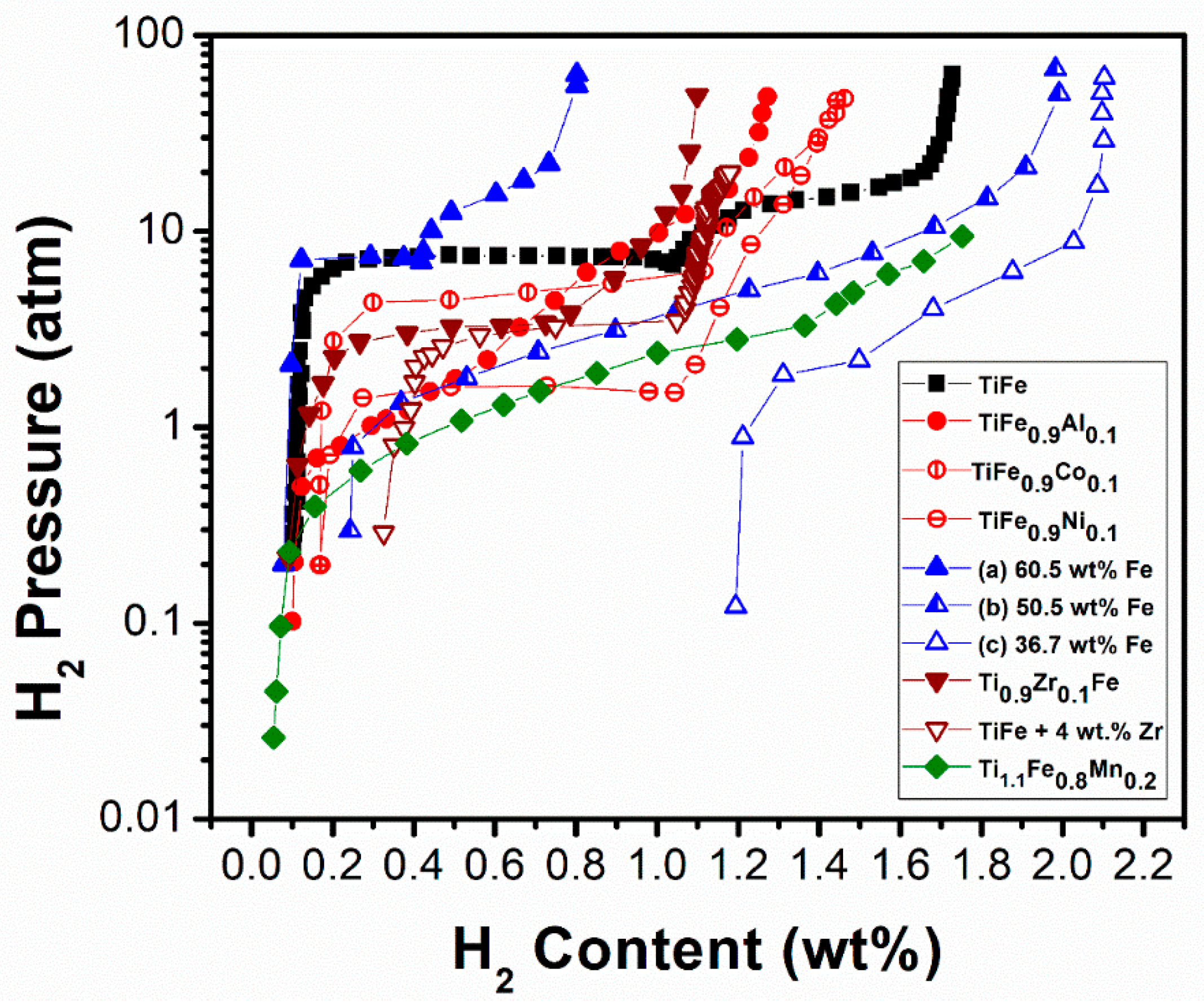
| TiFe [111] | 303 | CsCl | - | ~1.73/~1.60 | -/6 | Enthalpy of β hydride (TiFeH) formation is −5.68 kcal (mol H2)−1 and the enthalpy for hydrogen desorption is 6.31 kcal (mol H2)−1. |
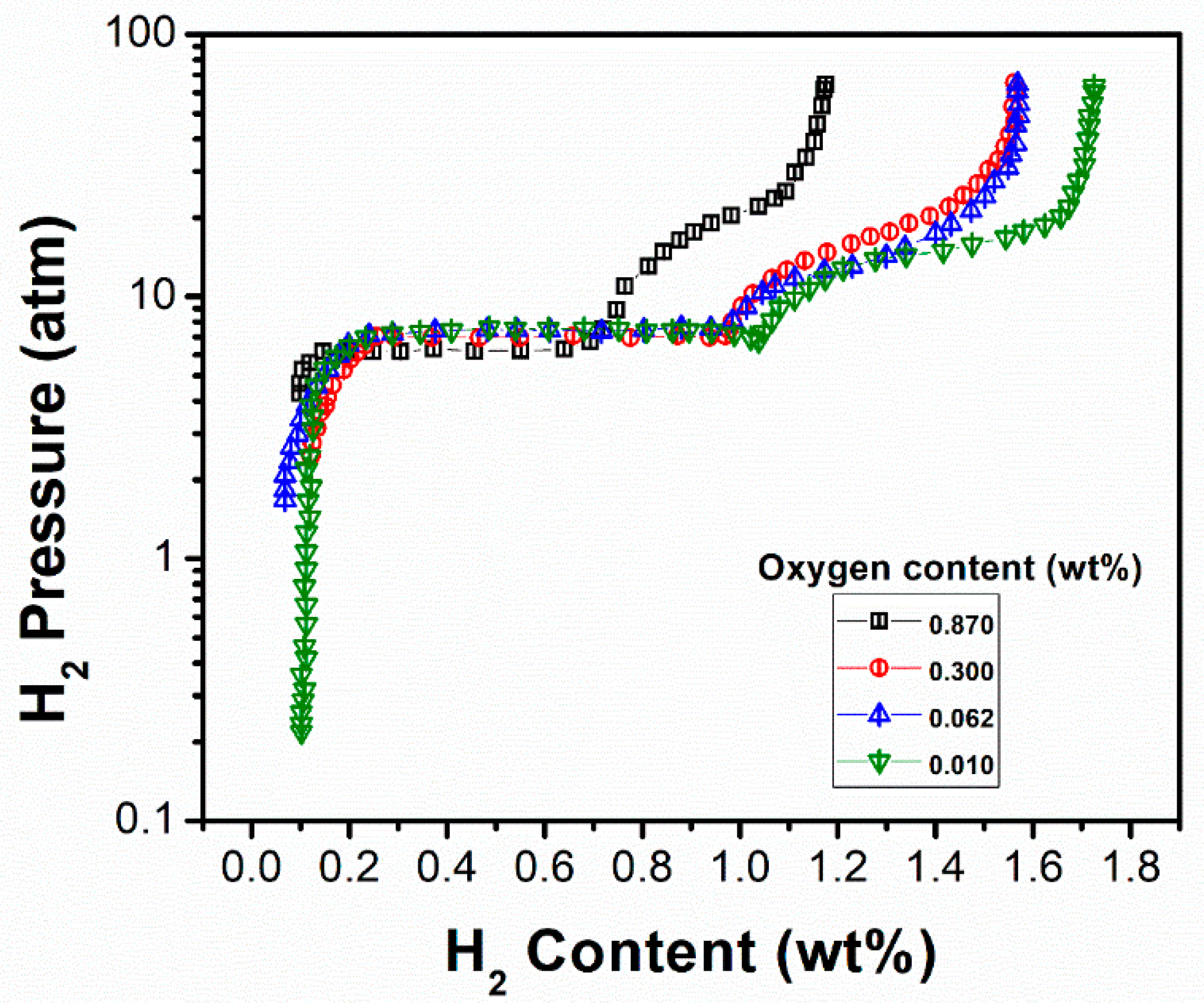
| Ti1.1Fe0.8Mn0.2 [119] | 313 | CsCl | a = 0.2990 | ~1.75/~1.65 | -/~1.0 (mid-point)- | Dehydrogenation enthalpy is 5.65 kcal (mol H2)−1. The addition of over-stoichiometric Ti leads to reduction in hydrogen storage capacity and substitution of Mn for Fe results in more stable hydride and improved hydrogen storage capacity with easy activation. |
| Ti36Fe64 (60.5 wt% Fe and 39.5 wt% Ti) [105] | 313 | CsCl | - | ~0.80/~0.71 | -/7.0 | Second plateau is sloping. |
| Ti45.5Fe54.5 (50.5 wt% Fe and 49.2 wt% Ti) [105] | 313 | CsCl | - | ~1.98/~1.74 | -/~4 (mid-point) | Sloping plateau. |
| Ti59.6Fe40.4 (36.7 wt% Fe and 63.2 wt% Ti) [105] | 313 | CsCl | - | ~2.1/~0.90 | -/~1.5 | Second plateau is sloping. The desorption is not complete due to the presence of excess Ti, which forms very stable hydride. |
| Ti0.9Zr0.1Fe [111] | 303 | CsCl | - | ~1.1/~0.99 | -/3 | Enthalpy of β hydride formation is −6.25 kcal (mol H2)−1 and the enthalpy for hydrogen desorption is 6.91 kcal (mol H2)−1. Zr substitution increases the hydride stability and reduces the storage capacity. |
| TiFe0.9Ni0.1 [123] | 323 | CsCl | - | ~1.4/~1.27 | -/~1.0 | Enthalpy for hydrogen desorption is 8.51 kcal (mol H2)−1. Partial substitution greatly influences the stability of the monohydride, as reflected in the broad enthalpy range reported in this table. Easy activation at RT. |
| TiFe0.9Co0.1 [123] | 323 | CsCl | - | ~1.46/~1.29 | -/~5.0 | Enthalpy for hydrogen desorption is 7.32 kcal (mol H2)−1. Easy activation at RT. |
| TiFe0.9Al0.1 [123] | 323 | CsCl | a = 0.2997 | ~1.27/~1.15 | -/(sloping plateau) | Sloping plateau. Easy activation at RT. |
| TiFe + 4 wt% Zr [125] | 313 | CsCl | a = 0.2983 | ~1.2/~0.83 | -/(sloping plateau) | Addition of Zr results in multiphase alloy (formation of a Zr-rich inter-granular phase), RT activation but incomplete desorption at RT. |
| Alloy | Temperature (K) | Structure Type | Lattice Parameters (nm) | Maximum/Reversible Capacity (wt%) | Pa/Pd (atm) | Remarks on the Effects of Partial Substitution |
|---|---|---|---|---|---|---|
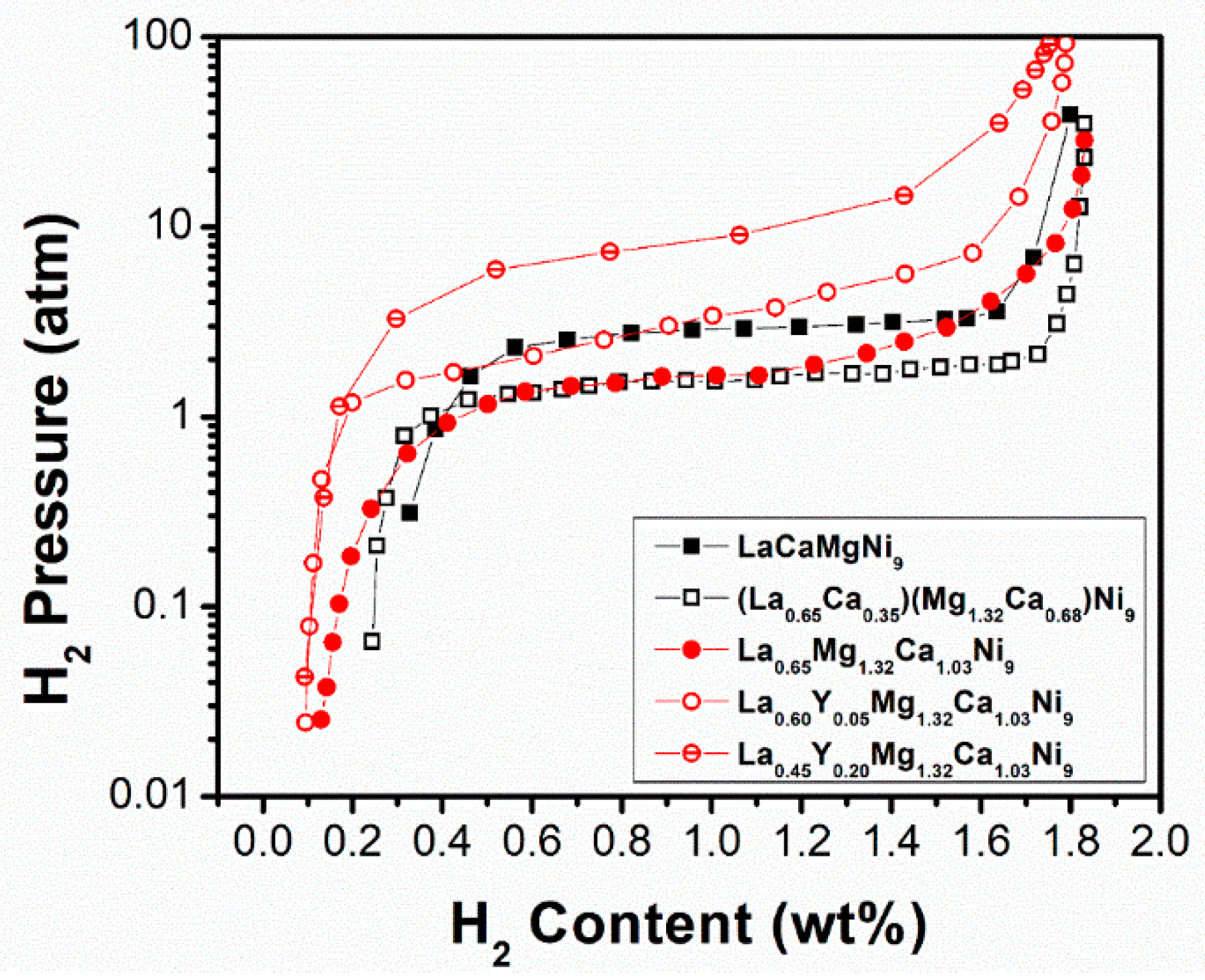
| LaCaMgNi9 [137] | 293 | NbBe3 | a = 0.4924 c = 2.3875 | 1.8/1.25 | 3.20/2.64 | AB3 alloys can be tuned by different material processing methods similarly to AB2 and AB5 alloys. |
| (La0.65Ca0.35)(Mg1.32Ca0.68)Ni9 [136] | 283 | NbBe3 | a = 0.4952 c = 2.3907 | 1.87/1.35 | 2.21/1.65 | |
| La0.65Mg1.32Ca1.03Ni9 [139] | 298 | NbBe3 | a = 0.4961 c = 2.3926 | 1.83/1.3 | 2.28/1.61 | AB5 phase fraction increases with Y substitution, and becomes the dominant phase inducing a reduction of the maximum capacity. Y substitution significantly increases both the hydrogen absorption/desorption plateau pressures due to the lattice contraction. |
| La0.60Y0.05Mg1.32Ca1.03Ni9 [139] | 298 | NbBe3 | a = 0.4955 c = 2.3935 | 1.79/1.4 | 5.31/3.11 | |
| La0.45Y0.20Mg1.32Ca1.03Ni9 [139] | 298 | NbBe3 | a = 0.4948 c = 2.3942 | 1.75/1.5 | 13.32/8.37 |
| Alloy | Temperature (K) | Structure Type | Lattice Parameters (nm) | Maximum/Reversible Capacity (wt%) | Pa/Pd (atm) | Remarks on the Effects of Partial Substitution |
|---|---|---|---|---|---|---|
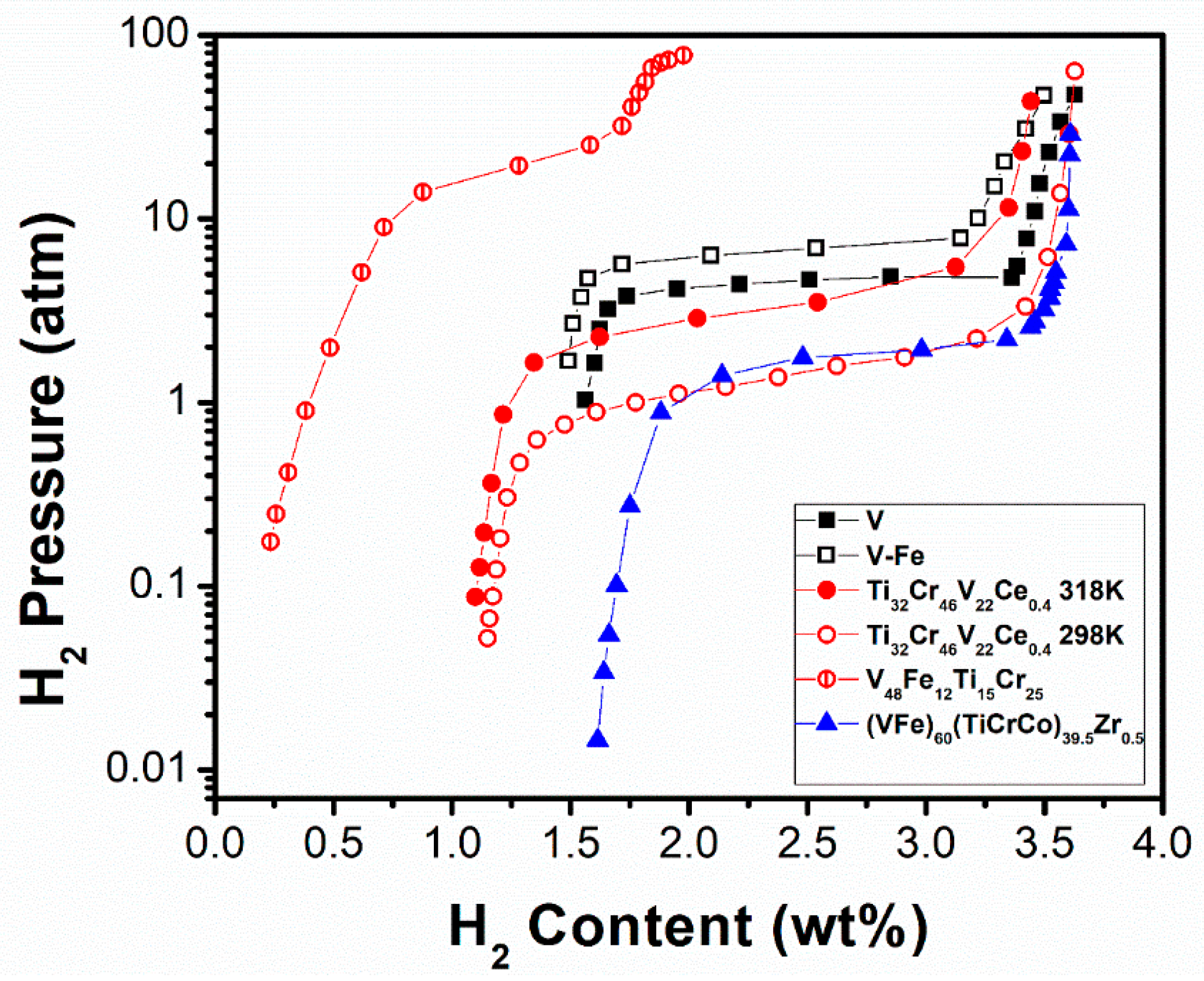
| V [146] | 313 | BCC | - | 3.63/1.8 | -/4.57 | - |
| V–Fe [146] | 313 | BCC | - | 3.50/1.6 | -/6.99 | Fe (smaller radius) increases the plateau pressure and inhibits the diffusion of hydrogen. |
| Ti32Cr46V22Ce0.4 [165] | 298 318 | BCC | - | 3.63/2.5 3.44/2.0 | -/1.41 -/3.40 | Cr and Ti additions improve cyclic stability, reaction rates, and terminal solid solubility. Ce increases the hydrogen capacity by lowering the oxygen concentration. |
| V48Fe12Ti15Cr25 [162] | 295 | BCC | a = 0.2967 | 1.98/1.1 | 1.91/1.01 | Commercial ferrovanadium substitution for V lowers the alloy costs, reduces hydrogen storage capacity, hysteresis and cycle stability, and makes higher plateau pressure. |
| (VFe)60(TiCrCo)39.5Zr0.5 [169] | 298 | BCC | a = 0.3081 | 3.61/1.6 | -/1.89 | Co and Zr enhance the storage and cyclic properties, but the hydrogen absorption/desorption capacities decrease with increasing Zr content. The rate of cyclic degradation decreases with higher Zr content and the hydriding incubation period shortens. |
| Alloy | Temperature (K) | Structure Type | Lattice Parameters (nm) | Maximum/Reversible Storage Capacity (wt%) | Pa/Pd (atm) |
|---|---|---|---|---|---|
| Mm0.9Ca0.1Ni4.6Fe0.3Al0.1 [67] | 300 | CaCu5 | a = 0.4962 c = 0.4018 | 1.96/1.65 | 10.00/5.00 |
| (Ti0.65Zr0.35)1.05MnCr0.8Fe0.2 [92] | 305 | MgZn2 | - | 2.2/1.75 | 5.30/2.80 |
| Ti1.02Cr1.1Mn0.3Fe0.6La0.03 [99] | 263 | MgZn2 | a = 0.4862 c = 0.7975 | 1.7/1.2 | 218/137 |
| Ti1.1Fe0.8Mn0.2 [119] | 313 | CsCl | a = 0.2990 | ~1.75/~1.65 | -/~1.0 (mid-point) |
| La0.60Y0.05Mg1.32Ca1.03Ni9 [139] | 298 | NbBe3 | a = 0.4955 c = 2.3935 | 1.79/1.4 | 5.31/3.11 |
| Ti32Cr46V22Ce0.4 [165] | 298 318 | BCC | - | 3.63/2.5 3.44/2.0 | -/1.41 -/3.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lys, A.; Fadonougbo, J.O.; Faisal, M.; Suh, J.-Y.; Lee, Y.-S.; Shim, J.-H.; Park, J.; Cho, Y.W. Enhancing the Hydrogen Storage Properties of AxBy Intermetallic Compounds by Partial Substitution: A Short Review. Hydrogen 2020, 1, 38-63. https://doi.org/10.3390/hydrogen1010004
Lys A, Fadonougbo JO, Faisal M, Suh J-Y, Lee Y-S, Shim J-H, Park J, Cho YW. Enhancing the Hydrogen Storage Properties of AxBy Intermetallic Compounds by Partial Substitution: A Short Review. Hydrogen. 2020; 1(1):38-63. https://doi.org/10.3390/hydrogen1010004
Chicago/Turabian StyleLys, Andrii, Julien O. Fadonougbo, Mohammad Faisal, Jin-Yoo Suh, Young-Su Lee, Jae-Hyeok Shim, Jihye Park, and Young Whan Cho. 2020. "Enhancing the Hydrogen Storage Properties of AxBy Intermetallic Compounds by Partial Substitution: A Short Review" Hydrogen 1, no. 1: 38-63. https://doi.org/10.3390/hydrogen1010004
APA StyleLys, A., Fadonougbo, J. O., Faisal, M., Suh, J.-Y., Lee, Y.-S., Shim, J.-H., Park, J., & Cho, Y. W. (2020). Enhancing the Hydrogen Storage Properties of AxBy Intermetallic Compounds by Partial Substitution: A Short Review. Hydrogen, 1(1), 38-63. https://doi.org/10.3390/hydrogen1010004






