Abstract
Extracellular polymeric substances (EPS) are secreted by microalgae and contribute to protecting cells from damage induced by cadmium (Cd) exposure. However, the response mechanism of Chlorella sp. to Cd(II) stress as well as associated changes in the chemical properties (including functional groups and composition) of soluble EPS (SL-EPS), loosely bound EPS (LB-EPS), and tightly bound EPS (TB- EPS) in this microalga, remain unclear. This study aimed to investigate the role of EPS in enabling Chlorella sp. to resist Cd(II) stress. The results demonstrated that Cd(II) stress resulted in a significant inhibition of algal, chlorophyll a (Chl a) contents, and maximum photochemical quantum yield (Fv/Fm) of Chlorella sp., with 7 d EC30 of 6 mg/L. Nevertheless, Cd(II) exposure significantly increased both superoxide dismutase (SOD) activity and EPS content. Fourier transform infrared (FTIR) spectroscopic analysis revealed that differences existed in the functional groups involved in Cd(II) binding across algal cell density, SL-EPS, LB-EPS, and TB-EPS. The carboxyl group was identified as the most prominent functional group and were found to play a crucial role in the adsorption of Cd(II). Additionally, Tryptophan-like protein substance in EPS may be the main component binding with Cd(II) in Chlorella sp. This study indicated that Chlorella sp. resisted Cd(II) stress by increasing SOD activity and EPS content, with protein-like substance containing tryptophan proteins in EPS which could also contribute to protection against Cd stress.
1. Introduction
Cadmium (Cd) pollution has become a global challenge for environmental sustainability due to its high toxicity, bioaccumulation potential, and persistence [1]. Driven by industrial and agricultural development, Cd is continuously released into aquatic environments, posing severe threats to ecosystem stability, food safety, and ultimately human health through its accumulation in food chains. In Asia, the average concentration of Cd in surface water reaches 17.75 ± 3.96 μg/L, exceeding the World Health Organization (WHO) threshold limit of 3 μg/L. Cd [2,3]. In industrial effluents, particularly electroplating wastewater, Cd concentrations typically range from 0.03 to 102 mg/L, and in extreme cases can be as high as 1570 mg/L [4]. These findings highlight the urgent need for sustainable remediation technologies in highly contaminated regions.
Conventional remediation techniques, including adsorption, precipitation, chemical reduction, ion exchange, and evaporation, are often limited by high operational costs, secondary pollution, or inefficiency at low metal concentrations. In contrast, microalgae have been widely employed in bioremediation of heavy metals due to their high metal adsorption efficiency, environmental sustainability, and renewability [5,6,7,8]. For example, certain Scenedesmus quadricauda can achieve exceptionally rapid metal removal, with one study reporting a 100% removal rate of Cr(VI) within 4 h [9]. The high efficiency of microalgae is also evident in the removal of Pb, with Chlorella sp. achieving a 78% removal rate within 3 h [10]. These cases illustrate the remarkable potential of microalgae for rapid and effective heavy metal removal.
To mitigate the toxic effects of heavy metals, microalgae employ various strategies, such as extracellular immobilization, intracellular sequestration, and activation of antioxidant defense systems [11]. Among these, biosorption is the primary mechanism underlying extracellular immobilization, involving the binding of heavy metals to charged macromolecules on the cell surface, typically through complexation, ion exchange, surface precipitation, and redox reactions [12,13]. Due to the presence of numerous negatively charged functional groups (e.g., carboxyl, hydroxyl, amino, and sulfhydryl groups) on EPS associated with the cell wall, metal ions can be efficiently adsorbed onto the cell surface during this process [12,13,14].The efficiency of this adsorption process is further modulated by several environmental and biological factors. For instance, algae generally exhibit higher adsorption efficiencies under neutral to weakly acidic conditions, whereas efficiency decreases sharply at higher pH due to metal ion precipitation in alkaline environments [14]. Moreover, adsorption capacity tends to be greater for metal ions with higher electronegativity when comparing ions of similar size. The binding ability of EPS is strongly influenced by the abundance of negatively charged groups on its surface.
At the intracellular level, heavy metals are chelated intracellularly by specific molecules such as metallothioneins (MTs), phytochelatins (PCs), and glutathione (GSH), which constitute the primary metal-binding systems in microalgae [11]. Metallothioneins are cysteine-rich proteins that bind Cd via thiol groups, while phytochelatins are enzymatically synthesized from glutathione by phytochelatin synthase (PCS) and play a central role in intracellular Cd sequestration and detoxification. Meanwhile, oxidative damage caused by heavy-metal-induced reactive oxygen species (ROS) is further mitigated by antioxidant enzymes such as superoxide dismutase, catalase, ascorbate peroxidase, and glutathione reductase. Notably, the structure and composition of EPS in different microalgae can be altered in response to various heavy metal treatments [15,16,17]. Therefore, the specific strategies employed by microalgae to counteract the toxicity of different heavy metals warrant further investigation.
Chlorella sp. is a green algae well-known for its high production of lipids and polysaccharides [18,19]. Studies indicate that Chlorella sp. mitigates heavy metal toxicity and purifies wastewater through the secretion of large quantities of EPS [20]. However, it remains unclear which specific aspects of the chemical structure of EPS are affected by Cd(II) stress, particularly regarding the role of functional groups (e.g., carboxyl, hydroxyl, amide), the relative protein–polysaccharide composition, and the differential responses among SL-EPS, LB-EPS, and TB-EPS. In this work, we aim to illustrate the role of EPS in protecting Chlorella sp. against Cd(II) stress by analyzing biochemical generation, compositional characteristics, and functional group profiles of different types of EPS. These findings are of great significance for the application of algae in bioremediation of heavy metal.
2. Materials and Methods
2.1. Growth Conditions of Chlorella sp.
Chlorella sp. FACHB-1227 was obtained from the Freshwater Algae Culture Collection of the Institute of Hydrobiology, Chinese Academy of Sciences. The algae were cultured in Erlenmeyer flasks containing sterilized BG11 medium (pH 7.1). Cultures were maintained at 25 ± 1 °C under a 12/12 h light/dark cycle with an incident light intensity of approximately 36 μmol photons m−2 s−1. Cells used for the experiments were in the logarithmic growth phase, specifically within the range of OD680 = 0.2–0.5 (corresponding to approximately 3 × 106–8 × 106 cells mL−1). To prevent contamination from residual metals, all glassware underwent pretreatment involving a seven-day soak in 10% hydrochloric acid and was then carefully rinsed with deionized water. Prior to use, culture flasks were covered with sterile, gas-permeable sealing films and sterilized by autoclaving. During cultivation, the flasks were periodically shaken and their positions were frequently changed for ensuring uniform light exposure and avoiding adhesion of algal cells to the flask walls.
1000 mg·L−1 Cd(II) stock solution was prepared using CdCl2·2.5H2O and diluted with BG11 medium to generate obtain five working concentrations: 3, 6, 10, 15, and 20 mg/L, with an initial cell density about 5 × 106 cells/mL. Each treatment group and its CdCl2-free control were prepared in three replicates. Cell growth was monitored daily by counting cell numbers using a hemocytometer. EC50 was determined via a chemical algal growth inhibition test following previously established methods [21].
2.2. Cd(II) Removal
Chlorella sp. cultures were treated with 6 mg/L Cd(II) in BG11 medium for a period of seven days. Cd(II) removal was assessed by separating the metal into three fractions: residual Cd(II) remaining in the medium, Cd(II) bound to EPS on the cell surface, and Cd(II) accumulated within the cells. The extraction procedures followed the method described by Ozturk et al. [21]. After centrifugation at 10,000× g, the supernatant was analyzed to quantify the residual Cd(II) in the medium. On the 7th day, the resulting pellets were treated with 10 mmol/L EDTA to desorb Cd(II) from the cell surface and centrifuged again at 10,000× g. The supernatant obtained at this step was examined for EPS-associated Cd(II). The remaining pellets were digested with nitric and perchloric acids to determine intracellular Cd(II). Final Cd(II) concentrations were quantified using an atomic absorption spectrophotometer (AA-6880, Shimadzu Corporation, Shimadzu, Kyoto, Japan).
2.3. Determination of Chl a and Maximal Chlorophyll Fluorescence of PSII (Fv /Fm)
Pigments analysis of algal samples was carried out according to the procedure of Sartory and Grobbelaar [22]. The algal samples were first centrifuged at 5000× g for 15 min. The resulting pellet was then extracted with 95% ethanol for 24 h in the dark at 4 °C. After a final centrifugation of the extract at 4000× g for 10 min, the resulting supernatant was collected for subsequent analysis. The Chl a concentration was then quantified by recording absorbance at 665 and 649 nm.
Meanwhile, 5 mL of Chlorella sp. suspensions were dark-adapted for 10 min, and Fv/Fm was measured using a water sample chlorophyll fluorometer (WALZ, PAM-2500, Effeltrich, Germany) with a saturating light intensity of 1500 μE·m−2 s−1.
2.4. Measurement of Malondialdehyde (MDA) and Superoxide Dismutase (SOD) Content
Algal cells exposed to 6 mg/L Cd(II) or maintained without Cd(II) were sampled at 0.5 h, 4 h, 24 h, 72 h, and 168 h. The algal suspensions were centrifuged at 3000× g for 10 min at 4 °C to obtain cell pellets. The harvested cells were then resuspended in PBS buffer and disrupted using a tissue homogenizer under the following conditions: 45 Hz, with 30 s of grinding followed by 10 s of rest, repeated for three cycles. The homogenized mixtures were subsequently centrifuged at 2000× g for 5 min at 4 °C. The resulting supernatant was used to determine the activities of MDA content and SOD activity, using corresponding commercial assay kits (Nanjing Jiancheng Bioengineering Institute, www.njjcbio.com (accessed on 15 September 2025). Measurements were strictly performed in accordance with the manufacturer’s protocol.
2.5. EPS Extraction and Determination
The algal EPS was separated into three distinct fractions: SL-EPS, LB-EPS, and TB-EPS [23,24]. Extraction of SL-EPS, LB-EPS, and TB-EPS was performed following previously described methods [24,25]. First, samples were centrifuged at 2500× g for 15 min, and the supernatant was collected to analyze SL-EPS. Next, the algal pellets obtained in the previous step were suspended in 0.05% NaCl solution with a volume equal to that of the original sample, and then centrifuged at 5000× g for 15 min. After collecting the supernatant for LB-EPS analysis, the pellets were resuspended in 0.05% NaCl, heated at 60 °C for 30 min, and centrifuged to obtain the TB-EPS fraction. Filtration of all EPS fractions through 0.45 μm PTFE membranes was performed to remove intact cells and large debris, ensuring that only extracellular polymers were collected and analyzed. Polysaccharide and protein contents in the EPS samples were quantified via the phenol-sulfuric acid method [26] and the Bradford method [27], respectively.
2.6. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
FTIR spectroscopy was used to analyze functional groups in organic matter. On 7th day, algal cultures were centrifuged at 5000× g for 15 min to collect algal biomass for further analysis. EPS fractions were subsequently isolated following the procedure outlined in Section 2.5 and dialyzed according to the method of Ge et al. [28]. Both the EPS-containing supernatants and algal pellets were lyophilized using a vacuum freeze dryer (FD-1A-50, Bio-Equip, Shanghai, China). The dried materials were then subjected to characterization by FTIR spectroscopy. (FTIR5700, Thermo Fisher Scientific, Waltham, MA, USA). Prior to FTIR analysis, all EPS subjected to identical preparation procedures, including lyophilization, fine grinding, and dialysis to remove salts and small molecules. To minimize spectral variation, the same instrumental parameters were used for all measurements (ATR mode, 4 cm−1 resolution, 32 scans per spectrum), and baseline correction and normalization were applied to each spectrum. Additionally, blank controls were measured to eliminate background interference.
2.7. Measurement of Three-Dimensional Fluorescence Excitation–Emission Matrix (3D-EEM)
3D-EEM was used to analyze compositional changes in organic matter including EPS. Due to the low EPS concentration after 7 days of culture, specific EPS components were not detectable by 3D-EEM; thus, the culture duration was extended to 14 days. On the 14th day, each type of EPS extracted using the method described in Section 2.5 was analyzed by a 3D-EEM (Lumina, Thermo Fisher Scientific, Waltham, MA, USA). This work adopted the specific parameters reported by Xu et al. [24].
2.8. Statistical Analysis
All experiments and analyses were conducted based on three independent biological replicates. Statistical analyses were performed with SPSS software (Version 22.0) via one-way analysis of variance (ANOVA) and Student’s t-test. Standard errors were calculated and displayed as error bars in figures. Differences were considered statistically significant at p < 0.05.
3. Results and Discussion
3.1. Growth Inhibition and Cd(II) Removal by Chlorella sp.
The effects of different Cd(II) concentrations on the growth of Chlorella sp. are shown in Figure 1a. The cell density decreased significantly with increasing Cd(II) concentration (p < 0.05). Based on the growth inhibition results, the 2, 3, 4, and 7d EC50 were calculated to be 3.873, 5.874, 7.126, and 9.547 mg/L, respectively. When the Cd(II) concentration increased from 6 mg/L to 10 mg/L, a significant decrease in biomass was observed. Based on growth inhibition data, an EC30 of 6 mg/L over 7 days was selected for subsequent experiments. The inhibitory effects of Cd(II) on microalgal growth have been reported in previous studies [29,30]. The decrease in growth observed at higher Cd(II) concentrations in our study may be attributed to Cd(II)-induced damage to photosynthetic activity and the triggering of oxidative stress [29,30].
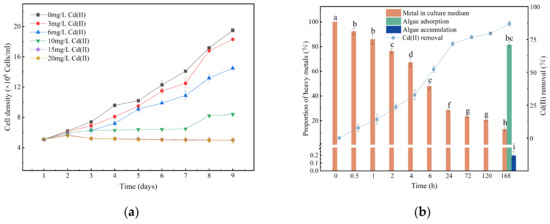
Figure 1.
Growth curve (a), and Cd(II) removal efficiency when Chlorella sp. was exposed to 6 mg/L Cd(II) (b). Data are presented as mean ± SD (n = 3). Columns sharing the same letter are not significantly different (p > 0.05); columns with different letters indicate significant differences (p < 0.05); the same applies below.
Biosorption is characterized by faster kinetics compared to bioaccumulation, which requires the activation of intracellular metal transport systems [31]. Figure 1b shows the removal efficiency of Cd(II) by Chlorella sp. at a concentration of 6 mg/L Cd(II). Within the first 24 h, the adsorption of Cd(II) was a rapid process and Cd(II) removal efficiency was 71.6%. The removal efficiency of Cd(II) increased gradually within 24 h, reaching 71.6%, after which the rate of increase slowed. By the conclusion of the·7th day, 13.2% of Cd(II) remained in the culture medium, while 86.8% was adsorbed by the cells. Concurrently, the proportions of Cd(II) remaining in the medium, adsorbed onto the cell surface EPS, and accumulated within the cells were determined to be 13.2%, 82.6%, and 3.8%, respectively. The fast binding of Cd(II) within 24 h implies that metal removal occurs primarily through interactions with surface functional groups [32,33]. Heavy ions can be effectively adsorbed by the cell surface, a finding consistent with previous studies [16,17]. This suggests that B-EPS plays a major role in the Cd(II) adsorption. Cd(II) adsorption is a rapid process, probably because Cd(II) mainly interacts with acidic sites on the cell wall, such as uronic acid and carboxyl groups [32,33].
The concentration of Cd(II) in natural water systems is typically below 1 µg/L [34], and the presence of Cd(II) in municipal and typical electroplating wastewaters is typically less than 2 mg/L [16]. As illustrated in Figure 1a, exposure to 3 mg/L Cd(II) resulted in growth of Chlorella sp. comparable to that of the control group, indicating that Chlorella sp. may not suffer significant harm from Cd(II) in actual wastewater. The removal efficiency of Cd(II) was 71.6% within 24 h, indicating that Chlorella sp. may also serve as a promising candidate for removing Cd(II) from wastewater [5,16].
3.2. Change in Chl a Content and Photosynthetic Activity
The Chl a content in the Cd(II)-treated group decreased with prolonged cultivation time (Figure 2a). This observation is consistent with previous studies. The suppression of Chl a content by Cd(II) is attributed to a decline in chlorophyllides, which act as essential precursors for the synthesis of these photosynthetic pigments [35]. Additionally, the reduction in chlorophyll levels caused by Cd may be linked to oxidative stress and thylakoid lipid peroxidation, events that can cause subsequent chlorophyll degradation [36,37].
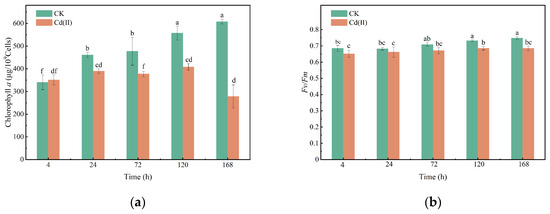
Figure 2.
Changes in Chl a (a) and Fv/Fm (b) of Chlorella sp. with or without Cd(II) exposed. (“CK” refers to the control group and “Cd(II)” refers to the treatment group exposed to 6 mg/L Cd(II). The same definition applies to all figures in this manuscript.
During the initial stage of Cd(II) stress (0–24 h), Fv/Fm showed no statistically significant difference from the control group (p > 0.05) (Figure 2b). However, a progressive decline in Fv/Fm was observed in the Cd(II)-treated group after 120 h. Fv/Fm in Chlorella sp. was reduced by 6.33%, and 8.51% at 120 and 168 h of Cd(II) exposure, respectively. The inhibitory effect of Cd(II) on photosynthesis could be due to the competition of Cd(II) with calcium, magnesium, manganese, and iron ions in the photosynthetic apparatus, thereby damaging the photosynthetic electron transport chain [31,38,39,40]. On the other hand, Cd(II) may damage the photosystems by regulating the genes involved in chlorophyll and light-harvesting antenna protein synthesis, resulting in decreased Chl a contents and photosynthetic activity [40].
3.3. Change in the MDA Content and SOD Activity
MDA serves as a key indicator of oxidative damage, reflecting the extent of lipid peroxidation induced by environmental stress [35]. Significant increases in MDA content at 4 h (by 24.5%) and 24 h (by 27.9%) were observed in the 6 mg/L Cd(II) group compared with the control group (Figure 3a). It has been established in many former studies that heavy metals can induce oxidative stress [17,35]. In this work, the increased MDA content can be attributed to Cd(II)-induced oxidative stress, which triggers the accumulation of ROS, causing lipid peroxidation and membrane damage [41,42]. However, MDA levels exhibited no significant difference from the control group as exposure time prolonged (p > 0.05). This finding demonstrates the metabolic adaptability of Chlorella sp., which can alleviate lipid peroxidation by enhancing its intrinsic antioxidant mechanisms [43].
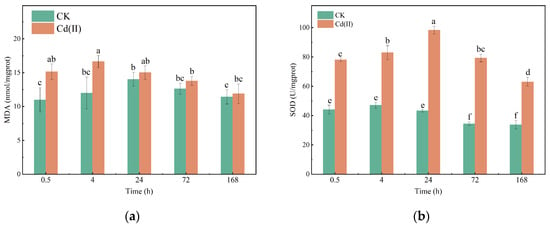
Figure 3.
Changes in MDA content (a) and SOD activity (b) of Chlorella sp. with or without Cd(II) exposed.
SOD is a key enzyme involved in ROS scavenging and regulating cellular redox homeostasis. Cd(II) significantly increased the SOD activity by 43.6%, 43.2%, 56.0%, 56.6% and 46.4% at 4 h 24 h, 72 h, 120 h and168 h, respectively, compared with the control group (Figure 3b). Elevated SOD activity in response to heavy metal exposure has been documented in various algal species, including Desmodesmus armatus [35] and Microcystis aeruginosa [14]. This phenomenon indicates the activation of a rapid antioxidant response to scavenge ROS generated by heavy ions and prevent oxidative damage [44].
3.4. The Role of EPS in Metal Sequestration
3.4.1. Changes in Polysaccharides and Proteins Contents in EPS
As shown in Figure 4, the polysaccharides contents in EPS was higher than proteins in both Cd(II)-exposed and unexposed conditions (p < 0.05). Cd(II) stress led to an accumulation of polysaccharides and proteins within the SL-EPS and B-EPS fractions. The maximum polysaccharides and proteins contents in EPS reached 4.18 and 0.686 pg/cell, respectively. This provides further support for the hypothesis that Cd(II) can induce EPS production [16,17]. EPS secretion is considered as a defensive mechanism by Microcystis aeruginosa [17] and Spirulina platensis [16] under Cd(II) stress, suggesting that Chlorella sp. may also secrete EPS to defend against the Cd(II) stress in the present study.
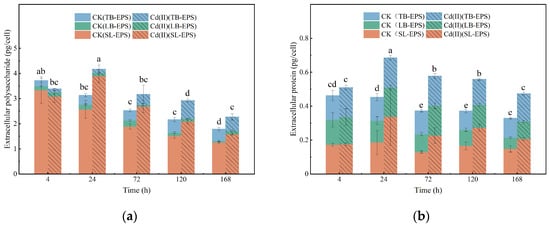
Figure 4.
Variations in the content of polysaccharides (a) and proteins (b) within the EPS of Chlorella sp. with or without Cd(II) exposed.
3.4.2. FTIR Analysis
FTIR can be used to reveal the differences in functional groups of biological materials, thereby providing further confirmation of the binding mechanism between EPS and heavy metals. FTIR spectra of algal cells and various EPS fractions (SL-EPS, LB-EPS, TB-EPS) from Chlorella sp. treated with or without 6 mg/L Cd(II) are shown in Figure 5. The functional groups responsible for the biosorption of Cd(II) by the algal cells or each EPS fraction were analyzed by FTIR spectroscopy, and these groups are listed in Table 1. Characteristic peaks were observed to shift before and after metal adsorption, suggesting the adsorption sites were occupied by metal ions [45].
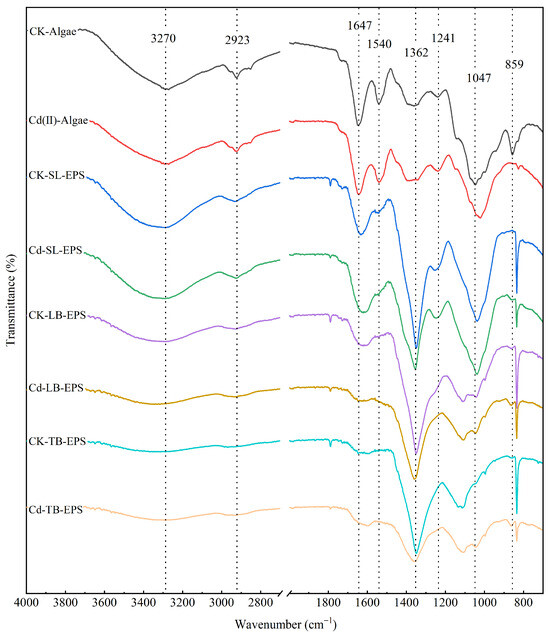
Figure 5.
FTIR spectra of cellular biomass and EPS from Chlorella. sp. treated with 0 and 6 mg/L Cd(II) exposure.
The absorption bands at 3270–3344 cm−1 are attributed to the stretching vibrations of −NH2 or −OH groups [21,46]. The strong absorption peak at 2920–2990 cm−1 can be assigned to stretching vibrations of unsaturated methyl group (−CH3) or a hydroxyl group (−OH) on benzene ring [47,48]. The broad bands at 1622–1647 cm−1 correspond to the stretching vibrations of quinone, amide, or ketone C=O bonds [49]. The peak near 1596 cm−1 corresponds C=C stretching of aromatic and the COO− stretching of deprotonated carboxylic acid [49]. The peak at 1360 cm−1 corresponds to the bending vibration of −OH groups in the carboxyl group [46]. The broad bands at 1240–1255 cm−1 correspond to the stretching vibration of P=O [14], which is a functional group present in nucleic acids, phospholipids and phosphoprotein, etc. The broad bands around 1047 cm−1 represent the stretching vibration C−O−C linkages in polysaccharides, which can be found in the dextran functional groups on algal cell walls [48]. The FTIR peak at 859 cm−1 is commonly attributed to the C−H deformation vibrations of α-pyranose rings [20].
In this study, Cd(II) exposure led to pronounced modifications in the FTIR spectra for algae cells and each type of EPS. Han et al. [17] also reported similar results in Cd(II)- treated Microcystis aeruginosa. Moreover, differences in major peaks among the various EPS fractions indicated that the functional groups involved in Cd(II) binding differed slightly between algal cells, SL-EPS, LB-EPS, and TB-EPS. Thus, each type of EPS plays a distinct role in adsorbing Cd(II). There were significant shift in peaks at around 1647 cm−1 and 1360 cm−1 in algal cells, SL-EPS, LB-EPS and TB-EPS (only at 1360 cm−1), which correspond to C=O bond and −OH bond in the carboxyl group, respectively, before and after Cd(II) adsorption. Carboxyl groups are the most common functional groups containing a C=O bond that is negatively charged and abundant in algal cell walls [50]. These results indicated that the carboxyl group served as the key functional group in the EPS, playing a primary role in Cd(II) adsorption.

Table 1.
FTIR peak wavenumber shifts in Chlorella sp. biomass and its EPS fractions before and after Cd(II) adsorption.
Table 1.
FTIR peak wavenumber shifts in Chlorella sp. biomass and its EPS fractions before and after Cd(II) adsorption.
| Wavenumber (cm−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Algae | SL-EPS | LB-EPS | TB-EPS | Functional Group | References | ||||
| CK | Cd | CK | Cd | CK | Cd | CK | Cd | ||
| - | - | 3290 | 3304 | 3288 | 3344 | 3318 | 3287 | Amino group (−NH2) | [46] |
| 2923 | 2926 | 2934 | 2928 | 2932 | 2930 | 2987 | 2931 | Methyl group (−CH3) or Hydroxyl group attached to aromatic rings (X−OH) | [47,48] |
| 1647 | 1645 | 1633 | 1622 | 1622 | 1646 | Quinone, amide, or ketone C=O bond stretching | [51] | ||
| - | - | 1594 | 1601 | C=C and the COO− stretching | [49] | ||||
| 1362 | 1386 | 1350 | 1354 | 1350 | 1360 | 1350 | 1362 | Bending−OH (−COOH) | [46] |
| - | - | 1255 | 1249 | Phosphate group (P=O stretching vibration) | [14] | ||||
| 1111 | 1108 | - | - | Ether (C−O−C) in polysaccharides | [52] | ||||
| 1047 | 1023 | - | - | The elongation of bonds C−C and C−O in polysaccharides | [53] | ||||
| 859 | 833 | - | - | - | - | - | - | furanose and pyranose rings of saccharides α-glycosidic bonds | [20] |
-: no significant shift peak point.
3.4.3. 3D-EEM Analysis
As shown in Figure 6 and Table 2, the 3D-EEM contours of the EPS contained two peaks. Peak A (Ex/Em = 280/325 nm) and Peak B (Ex/Em = 365/440 nm) were attributed to tryptophan-like protein substance and humus-like substances originating from the decomposition of macromolecular organics [54,55]. The results indicate that tryptophan-like protein substance and humus-like substances were identified in the EPS under both CdCl2 exposure conditions (0 and 6 mg/L). The organic composition of the different EPS fractions was remarkably similar between the control and 6 mg/L Cd(II)-exposed groups (Figure 6), indicating that Cd(II) did not alter their organic composition.
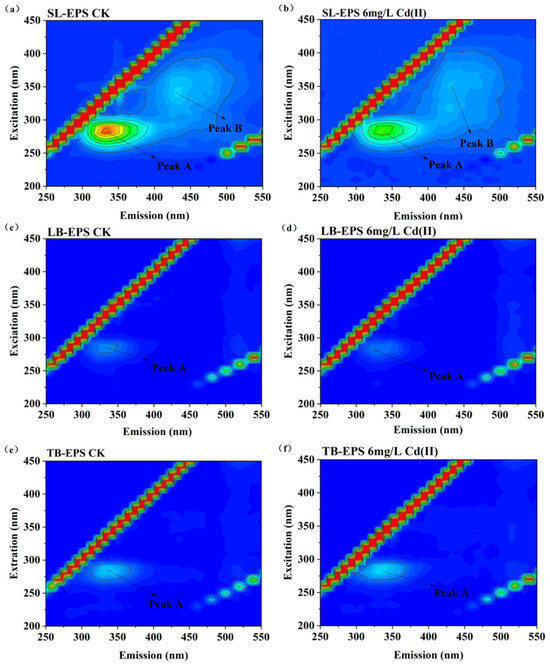
Figure 6.
3D-EEM contour plots of EPS fractions from Chlorella sp. with and without 6 mg/L Cd(II). Peaks A (Ex/Em 280/325 nm) and B (Ex/Em 365/440 nm) are indicated. (a) SL-EPS control (CK); (b) SL-EPS under Cd(II) treatment; (c) LB-EPS control (CK); (d) LB-EPS under Cd(II) treatment; (e) TB-EPS control (CK); (f) TB-EPS under Cd(II) treatment.

Table 2.
Fluorescence spectral parameters of EPS fractions obtained from Chlorella sp. under Cd(II) exposure and Cd(II)-free conditions.
SL-EPS exhibited two distinct fluorescence peaks (Peak A and Peak B), whereas LB-EPS and TB-EPS showed only Peak A (Figure 6). These findings indicate that tryptophan-containing substances are a shared component of the EPS in Chlorella sp., present consistently across all its EPS fractions. For the SL-EPS, due to its direct exposure to the external environment, it tends to accumulate a greater amount of soluble organics secreted through metabolism (e.g., humic-like substances). This accumulation, in turn, enables Chlorella sp. to respond effectively to external environmental stimuli. The treatment group showed no marked changes in EPS fluorescence regions compared to the control, whereas the fluorescence intensity of SL-EPS was significantly reduced. This finding aligns with previous studies, which have demonstrated that CdCl2 and light intensity do not alter the EEM contour patterns of algal EPS, but the fluorescence intensity of peaks varies across different EPS fractions depending on the treatment [15,17,56]. Specifically, Wang et al. [15] reported that 20 mg/L CdCl2 did not modify the organic composition of EPS from Spirulina platensis; however, the fluorescence intensities of peaks in EPS were significantly lower in the 20 mg/L CdCl2 treatment group than in the control group. Notably, the carboxyl (—COOH) and amino (—NH2) groups of tryptophan are recognized as key coordination sites for heavy metals. In the present study, Cd(II) exposure reduced the fluorescence intensity of Peak A in SL-EPS relative to the control. This observation suggests that tryptophan in EPS forms highly stable complexes with heavy metal ions via functional groups including carboxyl and amino groups, leading to fluorescence quenching. Such complexation effectively reduces the toxicity of free heavy metals and prevents their entry into algal cells [14]. Collectively, these results support the inference that tryptophan-containing protein-like substances are the primary components involved in Cd(II) binding in Chlorella sp.
3.5. Correlation Analysis
Correlation analysis was utilized to assess the associations between critical physiological responses in Chlorella sp. under Cd(II) stress. As shown in Figure 7, biomass exhibited a significant negative correlation with MDA levels (p < 0.05), suggesting that oxidative stress and lipid peroxidation directly contributed to growth inhibition. In contrast, biomass exhibited a significant positive correlation with Fv/Fm (p < 0.05), indicating that the maintenance of photosynthetic efficiency was essential for sustaining algal growth under Cd stress [29,30]. Moreover, biomass exhibited a significant negative correlation with the polysaccharide contents of EPS, suggesting that Cd(II) stress induced Chlorella sp. to allocate more photosynthetic products and metabolic energy toward the synthesis of EPS and antioxidant defenses. This reallocation likely diverted resources away from cell division and growth, thereby inhibiting biomass accumulation.
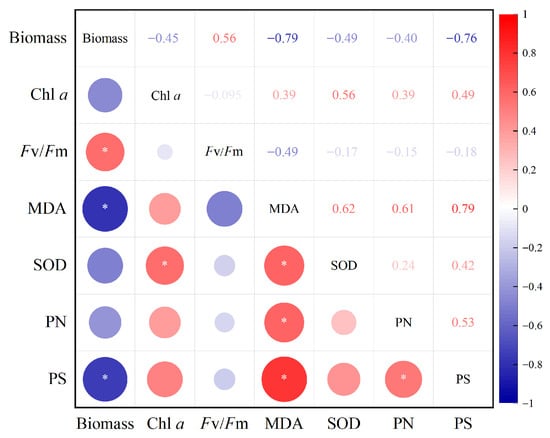
Figure 7.
Correlation of indicators of Chlorella sp. treated with 6 mg/L Cd(II). PN and PS represents proteins and polysaccharides in the EPS, respectively.
Additionally, Fv/Fm showed a negative correlation with MDA, confirming that oxidative damage impaired photosynthetic efficiency. The positive correlations observed between MDA levels and both protein (PN) and polysaccharide (PS) contents in EPS (p < 0.05) highlight that oxidative stress acts as a major trigger for EPS secretion. This suggests that Cd(II)-induced lipid peroxidation [57] not only damages cellular components but also stimulates EPS production as a protective mechanism to immobilize heavy metals and alleviate toxicity [16,17]. This may represent a self-protective response by Chlorella sp. to resist Cd(II) toxicity.
4. Conclusions
This work systematically examined the adaptive response of Chlorella sp. to Cd(II) stress. The final Cd(II) adsorption efficiency reached 86.8% when the Cd(II) concentration was 6 mg/L. Cd(II) stress significantly decreased biomass, chlorophyll content, and Fv/Fm values. Moreover, EPS played a crucial role in protecting Chlorella sp. against Cd(II) toxicity. Under Cd(II) exposure, EPS secretion increased markedly, and functional groups such as hydroxyl, carboxyl, and amide groups, together with tryptophan-containing protein-like substances, actively participated in Cd(II) binding, thereby mitigating cellular damage. These findings supply preliminary evidence for the potential application of EPS in heavy metal bioremediation.
Nevertheless, limitations of this study should be acknowledged. The experiments were conducted under controlled laboratory conditions with Cd(II) concentrations exceeding those typically observed in natural waters. Furthermore, EPS characterization was limited to FTIR and 3D-EEM analyses. Future studies should be conducted under more realistic environmental conditions to further validate the protective function of EPS in Chlorella sp. against Cd(II) stress.
Author Contributions
F.L.: Writing—original draft, Methodology, Investigation, Formal analysis; X.H.: Writing—review and editing, data curation. Methodology; Z.W.: Writing—review and editing; X.Z.: Writing—review and editing; Y.Z.: Writing—review and editing; H.G.: Conceptualization, Supervision, Writing—review and editing, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 31800457) and Open Project Funding of Hubei Key Laboratory of Environmental Geotechnology and Ecological Remediation for Lake & River, Hubei University of Technology (HJKFYP202502).
Data Availability Statement
Data contained in the study are available from the authors upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Cd | Cadmium |
| ROS | Oxygen species |
| EPS | extracellular polymeric substances |
| SL-EPS | soluble EPS |
| LB-EPS | Loosely bound EPS |
| TB- EPS | Tightly bound EPS |
| Chl a | Chlorophyll a |
| Fv/Fm | Maximum photochemical quantum yield |
| SOD | Superoxide dismutase |
| FTIR | Fourier transform infrared spectrum |
| MDA | Malondialdehyde |
| 3D-EEM | Three-dimensional fluorescence excitation–emission matrix |
| PS | Polysaccharide |
| PN | Protein |
| CK | Control group |
References
- Raza, A.; Habib, M.; Kakavand, S.N.; Zahid, Z.; Zahra, N.; Sharif, R.; Hasanuzzaman, M. Phytoremediation of Cadmium: Physiological, Biochemical, and Molecular Mechanisms. Biology 2020, 9, 177. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011; Volume 38, pp. 104–108. [Google Scholar]
- Wu, Y.Y.; Tian, W.F.; Cheng, C.X.; Yang, L.; Ye, Q.Q.; Li, W.H.; Jiang, J.Y. Effects of cadmium exposure on metabolism, antioxidant defense, immune function, and the hepatopancreas transcriptome of Cipangopaludina cathayensis. Ecotoxicol. Environ. Saf. 2023, 264, 115416. [Google Scholar] [CrossRef]
- Eniola, J.O.; Sizirici, B.; Stephen, S.; Yildiz, I.; Khaleel, A.; El Fadel, M. A new synthesis route of hydrothermally carbonized Na2CO3 activated bentonite-clay as a novel adsorbent for cadmium removal from wastewater. Sep. Purif. Technol. 2024, 350, 127960. [Google Scholar] [CrossRef]
- Yan, C.; Qu, Z.; Wang, J.; Cao, L.; Han, Q. Microalgal bioremediation of heavy metal pollution in water: Recent advances, challenges, and prospects. Chemosphere 2022, 286, 131870. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Huang, H.; Zhu, Y.; Du, Y.; Yao, L.; Jiang, X.; Gao, P. Adsorption of Pb2+ and Cd2+ onto Spirulina platensis harvested by polyacrylamide in single and binary solution systems. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123926. [Google Scholar] [CrossRef]
- Ravikumar, Y.; Razack, S.A.; Yun, J.; Zhang, G.; Zabed, H.M.; Qi, X. Recent advances in Microalgae-based distillery wastewater treatment. Environ. Technol. Innov. 2021, 24, 101839. [Google Scholar] [CrossRef]
- Gondi, R.; Kavitha, S.; Kannah, R.Y.; Karthikeyan, O.P.; Kumar, G.; Tyagi, V.K.; Banu, J.R. Algal-based system for removal of emerging pollutants from wastewater: A review. Bioresour. Technol. 2022, 344, 126245. [Google Scholar] [CrossRef]
- Daneshvar, E.; Zarrinmehr, M.J.; Kousha, M.; Hashtjin, A.M.; Saratale, G.D.; Maiti, A.; Vithanage, M.; Bhatnagar, A. Hexavalent chromium removal from water by microalgal-based materials: Adsorption, desorption and recovery studies. Bioresour. Technol. 2019, 293, 122064. [Google Scholar] [CrossRef]
- Molazadeh, P.; Khanjani, N.; Rahimi, M.; Nasiri, A. Adsorption of Lead by Microalgae Chaetoceros Sp. and Chlorella Sp. from Aqueous Solution. J. Community Health Res. 2015, 4, 114–127. [Google Scholar]
- Zhu, Q.; Zhang, M.; Bao, J.; Liu, J. Physiological, metabolomic, and transcriptomic analyses reveal the dynamic redox homeostasis upon extended exposure of Dunaliella salina GY-H13 cells to Cd. Ecotoxicol. Environ. Saf. 2021, 223, 112593. [Google Scholar] [CrossRef]
- Wei, L.; Li, J.; Xue, M.; Wang, S.; Li, Q.; Qin, K.; Jiang, J.; Ding, J.; Zhao, Q. Adsorption behaviors of Cu2+, Zn2+ and Cd2+ onto proteins, humic acid, and polysaccharides extracted from sludge EPS: Sorption properties and mechanisms. Bioresour. Technol. 2019, 291, 121868. [Google Scholar] [CrossRef]
- Li, M.; Ma, C.; Yin, X.; Zhang, L.; Tian, X.; Chen, Q.; Wang, L. Investigating trivalent chromium biosorption-driven extracellular polymeric substances changes of Synechocystis sp. PCC 7806 by parallel factor analysis (PARAFAC) analysis. Bioresour. Technol. Rep. 2019, 7, 100249. [Google Scholar] [CrossRef]
- Gu, S.; Lan, C.Q. Biosorption of heavy metal ions by green alga Neochloris oleoabundans: Effects of metal ion properties and cell wall structure. J. Hazard. Mater. 2021, 418, 126336. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Zhang, X.; Chen, X.; Wang, X.; Yu, D.; Ge, B. The extracellular polymeric substances (EPS) accumulation of Spirulina platensis responding to Cadmium (Cd2+) exposure. J. Hazard. Mater. 2024, 470, 134244. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tian, Q.; Zhou, H.; Kang, J.; Yu, X.; Qiu, G.; Shen, L. Physiological regulation of microalgae under cadmium stress and response mechanisms of time-series analysis using metabolomics. Sci. Total Environ. 2024, 916, 170278. [Google Scholar] [CrossRef]
- Han, X.; Liu, F.; Zhang, Y.; Cheng, K.; Wang, H.; Ge, H. Detoxification strategy of Microcystis aeruginosa to the toxicity of Cd (II): Role of EPS in alleviating toxicity. J. Oceanol. Limnol. 2024, 42, 802–815. [Google Scholar] [CrossRef]
- Mendes, A.R.; Spínola, M.P.; Lordelo, M.; Prates, J.A. Chemical compounds, bioactivities, and applications of Chlorella vulgaris in food, feed and medicine. Appl. Sci. 2024, 14, 10810. [Google Scholar] [CrossRef]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef]
- Ciempiel, W.; Czemierska, M.; Szymańska-Chargot, M.; Zdunek, A.; Wiącek, D.; Jarosz-Wilkołazka, A.; Krzemińska, I. Soluble Extracellular Polymeric Substances Produced by Parachlorella kessleri and Chlorella vulgaris: Biochemical Characterization and Assessment of Their Cadmium and Lead Sorption Abilities. Molecules 2022, 27, 7153. [Google Scholar] [CrossRef]
- Ozturk, S.; Aslim, B.; Suludere, Z.; Tan, S. Metal removal of cyanobacterial exopolysaccharides by uronic acid content and monosaccharide composition. Carbohydr. Polym. 2014, 101, 265–271. [Google Scholar] [CrossRef]
- Sartory, D.P.; Grobbelaar, J.U. Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia 1984, 114, 177–187. [Google Scholar] [CrossRef]
- Sheng, G.-P.; Yu, H.-Q.; Li, X.-Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cai, H.; Yu, G.; Jiang, H. Insights into extracellular polymeric substances of cyanobacterium Microcystis aeruginosa using fractionation procedure and parallel factor analysis. Water Res. 2013, 47, 2005–2014. [Google Scholar] [CrossRef] [PubMed]
- Christenson, L.; Sims, R. Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol. Adv. 2011, 29, 686–702. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ge, H.; Zhang, J.; Zhou, X.; Xia, L.; Hu, C. Effects of light intensity on components and topographical structures of extracellular polymeric substances from Microcoleus vaginatus (Cyanophyceae). Phycologia 2014, 53, 167–173. [Google Scholar] [CrossRef]
- Ran, Y.; Sun, D.; Liu, X.; Zhang, L.; Niu, Z.; Chai, T.; Hu, Z.; Qiao, K. Chlorella pyrenoidosa as a potential bioremediator: Its tolerance and molecular responses to cadmium and lead. Sci. Total Environ. 2024, 912, 168712. [Google Scholar] [CrossRef]
- Shin, J.; Kim, H.-S.; Bui, Q.T.N.; Kim, T.; Ki, J.-S. Photosynthesis genes modulate cadmium tolerance in the freshwater alga Closterium acutum revealed by transcriptome analysis. J. Appl. Phycol. 2025, 37, 1951–1965. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, Z.; Su, X.; Wan, F.; Zhou, Y.; Lei, Z.; Yi, L.; Dai, Z.; Li, J. Physiological of biochar and α-Fe2O3 nanoparticles as amendments of Cd accumulation and toxicity toward muskmelon grown in pots. J. Nanobiotechnol. 2021, 19, 442. [Google Scholar] [CrossRef]
- Komy, Z.R.; Gabar, R.M.; Shoriet, A.A.; Mohammed, R.M. Characterisation of acidic sites of Pseudomonas biomass capable of binding protons and cadmium and removal of cadmium via biosorption. World J. Microbiol. Biotechnol. 2006, 22, 975–982. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef] [PubMed]
- Bouida, L.; Rafatullah, M.; Kerrouche, A.; Qutob, M.; Alosaimi, A.M.; Alorfi, H.S.; Hussein, M.A. A review on cadmium and lead contamination: Sources, fate, mechanism, health effects and remediation methods. Water 2022, 14, 3432. [Google Scholar] [CrossRef]
- Piotrowska-Niczyporuk, A.; Bonda-Ostaszewska, E.; Bajguz, A. Mitigating effect of trans-zeatin on cadmium toxicity in Desmodesmus armatus. Cells 2024, 13, 686. [Google Scholar] [CrossRef]
- Chandrashekharaiah, P.S.; Sanyal, D.; Dasgupta, S.; Banik, A. Cadmium biosorption and biomass production by two freshwater microalgae Scenedesmus acutus and Chlorella pyrenoidosa: An integrated approach. Chemosphere 2021, 269, 128755. [Google Scholar] [CrossRef]
- Qi, F.; Gao, Y.; Liu, J.; Yao, X.; Han, K.; Wu, Z.; Wang, Y. Alleviation of cadmium-induced photoinhibition and oxidative stress by melatonin in Chlamydomonas reinhardtii. Environ. Sci. Pollut. Res. 2023, 30, 78423–78437. [Google Scholar] [CrossRef]
- Faller, P.; Kienzler, K.; Krieger-Liszkay, A. Mechanism of Cd2+ toxicity: Cd2+ inhibits photoactivation of Photosystem II by competitive binding to the essential Ca2+ site. Biochim. Biophys. Acta (BBA)—Bioenerg. 2005, 1706, 158–164. [Google Scholar] [CrossRef]
- Samadani, M.; Perreault, F.; Oukarroum, A.; Dewez, D. Effect of cadmium accumulation on green algae Chlamydomonas reinhardtii and acid-tolerant Chlamydomonas CPCC 121. Chemosphere 2018, 191, 174–182. [Google Scholar] [CrossRef]
- Ruan, G.; Liu, C.; Song, G.; Qian, J.; Bao, T.; Zhao, Y.; Sun, S.; Wan, D.; Mi, W.; He, M. Sll1725, an ABC transporter in Synechocystis sp. PCC 6803 for the detoxification of cadmium ion stress. Ecotoxicol. Environ. Saf. 2025, 300, 118389. [Google Scholar] [CrossRef]
- Zhang, H.; Heal, K.; Zhu, X.; Tigabu, M.; Xue, Y.; Zhou, C. Tolerance and detoxification mechanisms to cadmium stress by hyperaccumulator Erigeron annuus include molecule synthesis in root exudate. Ecotoxicol. Environ. Saf. 2021, 219, 112359. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Jiang, W.; Xiukang, W.; Hussain, S.; Ahmad, M.; Maqsood, M.F.; Ali, N.; Ishfaq, M.; Kaleem, M.; Haider, F.U.; et al. Cadmium Phytotoxicity, Tolerance, and Advanced Remediation Approaches in Agricultural Soils; A Comprehensive Review. Front. Plant Sci. 2022, 13, 773815. [Google Scholar] [CrossRef]
- Xiao, X.; Li, W.; Jin, M.; Zhang, L.; Qin, L.; Geng, W. Responses and tolerance mechanisms of microalgae to heavy metal stress: A review. Mar. Environ. Res. 2023, 183, 105805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, M.; Chang, F.; Yi, M.; Ge, H.; Fu, J.; Dang, C. The distinct resistance mechanisms of cyanobacteria and green algae to sulfamethoxazole and its implications for environmental risk assessment. Sci. Total Environ. 2023, 854, 158723. [Google Scholar] [CrossRef] [PubMed]
- Elleuch, J.; Hmani, R.; Drira, M.; Michaud, P.; Fendri, I.; Abdelkafi, S. Potential of three local marine microalgae from Tunisian coasts for cadmium, lead and chromium removals. Sci. Total Environ. 2021, 799, 149464. [Google Scholar] [CrossRef] [PubMed]
- Cid, H.A.; Flores, M.I.; Pizarro, J.F.; Castillo, X.A.; Barros, D.E.; Moreno-Piraján, J.C.; Ortiz, C.A. Mechanisms of Cu2+ biosorption on Lessonia nigrescens dead biomass: Functional groups interactions and morphological characterization. J. Environ. Chem. Eng. 2018, 6, 2696–2704. [Google Scholar] [CrossRef]
- Ozturk, S.; Aslim, B.; Suludere, Z. Cadmium (II) sequestration characteristics by two isolates of Synechocystis sp. in terms of exopolysaccharide (EPS) production and monomer composition. Bioresour. Technol. 2010, 101, 9742–9748. [Google Scholar] [CrossRef]
- Tavana, M.; Pahlavanzadeh, H.; Zarei, M.J. The novel usage of dead biomass of green algae of Schizomeris leibleinii for biosorption of copper (II) from aqueous solutions: Equilibrium, kinetics and thermodynamics. J. Environ. Chem. Eng. 2020, 8, 104272. [Google Scholar] [CrossRef]
- Ding, L.; Luo, Y.; Yu, X.; Ouyang, Z.; Liu, P.; Guo, X. Insight into interactions of polystyrene microplastics with different types and compositions of dissolved organic matter. Sci. Total Environ. 2022, 824, 153883. [Google Scholar] [CrossRef]
- Demey, H.; Vincent, T.; Guibal, E. A novel algal-based sorbent for heavy metal removal. Chem. Eng. J. 2018, 332, 582–595. [Google Scholar] [CrossRef]
- Cheng, P.; Chang, T.; Wang, C.; Yao, C.; Zhou, C.; Liu, T.; Wang, G.; Yan, X.; Ruan, R. High cobalt exposure facilitates bioactive exopolysaccharides production with a novel molecular structure in Botryococcus braunii. Chem. Eng. J. 2022, 442, 136294. [Google Scholar] [CrossRef]
- Mecozzi, M.; Pietroletti, M.; Tornambè, A. Molecular and structural characteristics in toxic algae cultures of Ostreopsis ovata and Ostreopsis spp. evidenced by FTIR and FTNIR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 1572–1580. [Google Scholar] [CrossRef]
- Plöhn, M.; Escudero-Onate, C.; Funk, C. Biosorption of Cd (II) by Nordic microalgae: Tolerance, kinetics and equilibrium studies. Algal Res. 2021, 59, 102471. [Google Scholar] [CrossRef]
- Parlanti, E.; Wörz, K.; Geoffroy, L.; Lamotte, M. Dissolved organic matter fluorescence spectroscopy as a tool to estimate biological activity in a coastal zone submitted to anthropogenic inputs. Org. Geochem. 2000, 31, 1765–1781. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation−emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, X.; Ge, H. Effect of light intensity on bound EPS characteristics of two Microcystis morphospecies: The role of bEPS in the proliferation of Microcystis. J. Oceanol. Limnol. 2022, 40, 1706–1719. [Google Scholar] [CrossRef]
- Ruan, G.; Mi, W.; Yin, X.; Song, G.; Bi, Y. Molecular Responses Mechanism of Synechocystis sp. PCC 6803 to Cadmium Stress. Water 2022, 14, 32. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).