What the Owls Leave Behind: Pellet Size Variation Reflects Predator Body Size in Israel’s Owls
Abstract
1. Introduction
2. Methods
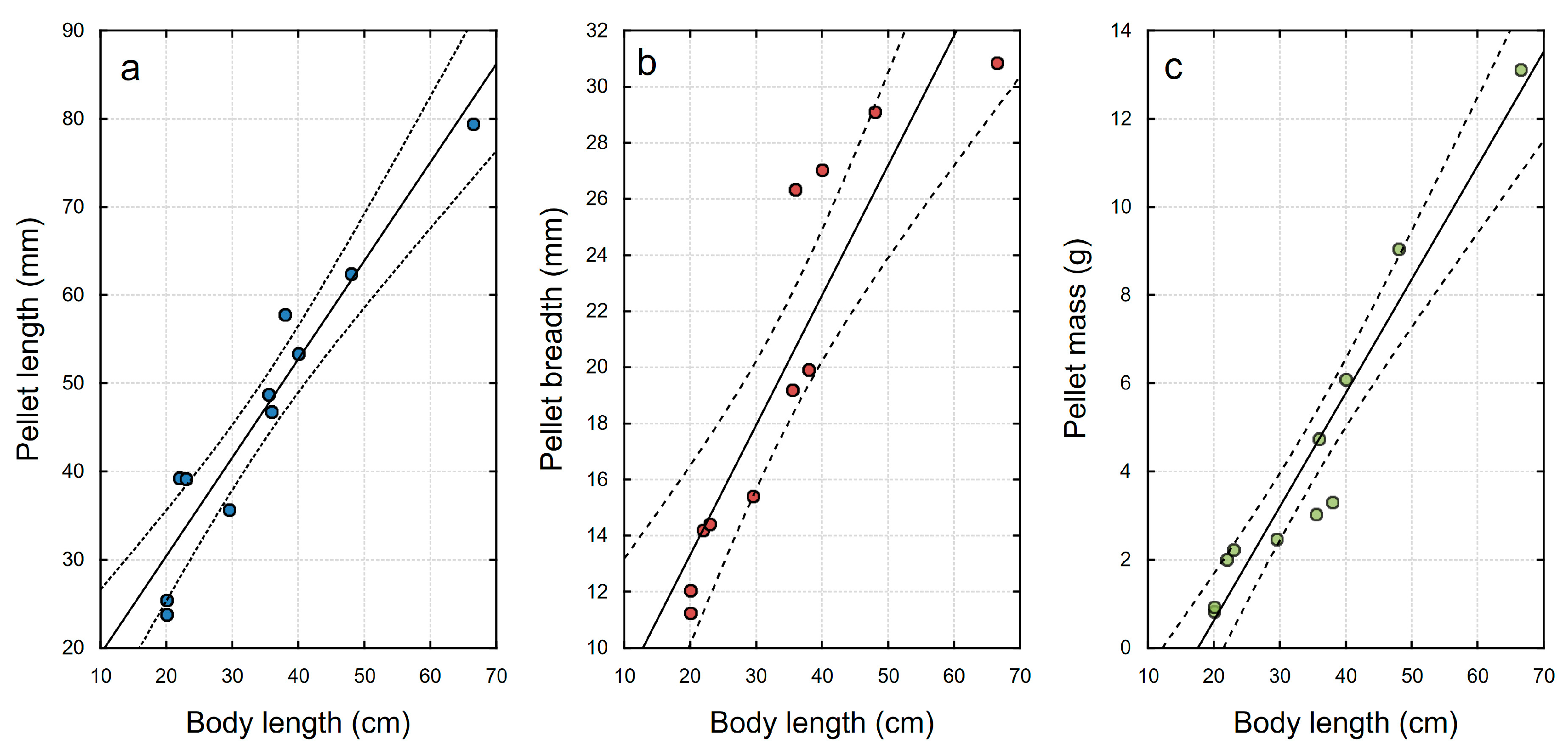
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shirihai, H. The Birds of Israel; Academic Press Limited: Bath, UK, 1996. [Google Scholar]
- Rocha, R.G.; Justino, J.; Leite, Y.L.R.; Costa, L.P. DNA from owl pellet bones uncovers hidden biodiversity. Syst. Biodivers. 2015, 13, 403–412. [Google Scholar] [CrossRef]
- Schoenefuss, P.; Kutt, A.S.; Kern, P.L.; Moffatt, K.A.; Bon, J.; Wardle, G.M.; Dickman, C.R.; Hurwood, D.A.; Baker, A.M. An investigation into the utility of eastern barn owl pellet content as a tool to monitor small mammal diversity in an arid ecosystem. Austral Ecol. 2024, 49, e13503. [Google Scholar] [CrossRef]
- Nazneen, S.; Jayakumar, S.; Albeshr, M.F.; Mahboob, S.; Manzoor, I.; Pandiyan, J.; Krishnappa, K.; Rajeswary, M.; Govindarajan, M. Analysis of toxic heavy metals in the pellets of owls: A novel approach for the evaluation of environmental pollutants. Toxics 2022, 10, 693. [Google Scholar] [CrossRef] [PubMed]
- Zduniak, P.; Wojciechowskaa, J.; Kasprzykowski, Z.; Komorowicz, I. Pellets of apex predators for monitoring metals and metalloids contamination in farmlands. Chemosphere 2025, 374, 144231. [Google Scholar] [CrossRef]
- Pietrelli, L.; Dodaro, G.; Pelosi, I.; Menegoni, P.; Battisti, C.; Coccia, C.; Scalici, M. Microplastic in an apex predator: Evidence from Barn owl (Tyto alba) pellets in two sites with different levels of anthropization. Environ. Sci. Pollut. Res. 2024, 31, 33155–33162. [Google Scholar] [CrossRef]
- Charter, M.; Izhaki, I.; Roulin, A. The relationship between intra–guild diet overlap and breeding in owls in Israel. Popul. Ecol. 2018, 60, 397–403. [Google Scholar] [CrossRef]
- Romero-Vidal, P.; Luna, Á.; Fernández-Gómez, L.; Navarro, J.; Palma, A.; Tella, J.L.; Carrete, M. Intraspecific competition and individual behaviour but not urbanization affect the dietary patterns of a generalist avian predator. Sci. Rep. 2023, 13, 10255. [Google Scholar] [CrossRef]
- Guimaraes, S.; Fernandez-Jalvo, Y.; Stoetzel, E.; Gorgé, O.; Bennett, E.A.; Denys, C.; Grange, T.; Geigl, E.M. Owl pellets: A wise DNA source for small mammal genetics. J. Zool. 2016, 298, 64–74. [Google Scholar] [CrossRef]
- Hadad, E.; Charter, M.; Kosicki, J.Z.; Yosef, R. Prey-base does not influence breeding success in Eagle Owls (Bubo bubo) in Judea, Israel. Animals 2022, 12, 1280. [Google Scholar] [CrossRef]
- Hadad, E. Digestion and pellets of Nocturnal Raptors of Israel. Teva HaDvarim 2024, 350, 14–21. (In Hebrew) [Google Scholar]
- Hadad, E.; Kosicki, J.Z.; Yosef, R. Habitat factors driving Long-eared Owl (Asio otus) population growth and productivity in the Judea Region. J. Raptor Res. 2024, 58, 105–113. [Google Scholar] [CrossRef]
- van Strien, A.J.; Bekker, D.L.; La Haye, M.J.; van der Meij, T. Trends in small mammals derived from owl pellet data using occupancy modelling. Mamm. Biol. 2015, 80, 340–346. [Google Scholar] [CrossRef]
- Heisler, L.M.; Somers, C.M.; Poulin, R.G. Owl pellets: A more effective alternative to conventional trapping for broad-scale studies of small mammal communities. Methods Ecol. Evol. 2016, 7, 96–103. [Google Scholar] [CrossRef]
- Gliwicz, J. Body size relationships between avian predators and their rodent prey in a North-American sagebrush community. Acta Ornithol. 2008, 43, 151–158. [Google Scholar] [CrossRef]
- Taylor, I.R. How owls select their prey: A study of Barn Owls Tyto alba and their small mammal prey. Ardea 2009, 97, 635–644. [Google Scholar] [CrossRef]
- Marti, C.D. Feeding ecology of four sympatric owls. Condor 1974, 76, 45–61. [Google Scholar] [CrossRef]
- Romanowski, J.; Lesiński, G. Comparing trophic niches of sympatric raptors in agricultural landscape in Central Poland. Pol. J. Ecol. 2020, 67, 331–338. [Google Scholar] [CrossRef]
- Ormrod, A.E.; Doyle, F.I.; Lawson, K.J.; Hodges, K.E. Niche partitioning of avian predators in northern grasslands amended by biosolids. Ecol. Evol. 2021, 11, 6248–6259. [Google Scholar] [CrossRef]
- González-Fischer, C.M.; Codesido, M.; Teta, P.; Bilenca, D. Seasonal and geographic variation in the diet of Barn Owls (Tyto alba) in temperate agroecosystems of Argentina. Ornitol. Neotrop. 2011, 22, 295–305. [Google Scholar]
- Comay, O.; Dayan, T. What determines prey selection in owls? Roles of prey traits, prey class, environmental variables, and taxonomic specialization. Ecol. Evol. 2018, 8, 3382–3392. [Google Scholar] [CrossRef]
- Yom-Tov, Y.; Wool, D. Do the contents of barn owl pellets accurately represent the proportion of prey species in the field? Condor 1997, 99, 972–976. [Google Scholar] [CrossRef]
- Meek, W.R.; Burman, P.J.; Sparks, T.H.; Nowakowski, M.; Burman, N.J. The use of Barn Owl Tyto alba pellets to assess population change in small mammals. Bird Study 2012, 59, 166–174. [Google Scholar] [CrossRef]
- Obuch, J.; Krištín, A. Prey composition of the little owl Athene noctua in an arid zone (Egypt, Syria, Iran). Folia Zool. 2004, 53, 65–79. Available online: http://www.ivb.cz/folia/53/1/65-79.pdf (accessed on 5 March 2025).
- Shehab, A.H. Diet of the Eagle Owl, Bubo bubo, in Syria. Zool. Middle East 2004, 33, 21–26. [Google Scholar] [CrossRef]
- Wink, M.; El-Sayed, A.-A.; Sauer-Gurth, H.; Gonzalez, J. Molecular phylogeny of owls (Strigiformes) inferred from DNA sequences of the mitochondrial cytochrome b and the nuclear RAG-1 gene. Ardea 2009, 97, 581–591. [Google Scholar] [CrossRef]
- Kirwan, G.M.; Schweizer, M.; Copete, J.L. Multiple lines of evidence confirm that Hume’s Owl Strix butleri (AO Hume, 1878) is two species, with description of an unnamed species (Aves: Non-Passeriformes: Strigidae). Zootaxa 2015, 3904, 28–50. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Obuch, J. On the diet of owls (Strigiformes) in Jordan. Slovak Raptor J. 2018, 12, 9–40. [Google Scholar] [CrossRef]
- van Nieuwenhuyse, D.; van Harxen, R.; Johnson, D.H. The Little Owl: Population Dynamics, Behavior and Management of Athene Noctua; Cambridge University Press: Cambridge, UK, 2023. [Google Scholar]
- Snow, D.W.; Perrins, C.M. The Birds of the Western Palearctic; Concise Edition; Oxford University Press: Oxford, UK, 1998; Volume 1 Non-Passerines. [Google Scholar]
- Snow, D.W.; Perrins, C.M. The Birds of the Western Palearctic; Concise Edition; Oxford University Press: Oxford, UK, 1998; Volume 2 Passerines. [Google Scholar]
- Clark, R.J. Pellets of the Short-eared Owl and Marsh Hawk compared. J. Wildl. Manag. 1972, 36, 962–964. [Google Scholar] [CrossRef]
- Mikkola, H. Owls of Europe; T & A D Poyser: Calton, UK, 1983. [Google Scholar]
- Dolenec, Z.; Kiš Novak, D. Winter prey of the long–eared owl (Asio otus) in northern Croatia. Nat. Croat. Period. Musei Hist. Nat. Croat. 2010, 19, 249–252. [Google Scholar]
- Compton, E.; Daley, L.F.; Stubbings, E.M.; Büche, B.I.; Wood, M.J. Diet, ecology, and biosecurity: Analysis of owl pellets from Skomer Island. Birds Wales 2016, 13, 57–72. [Google Scholar]
- Hadad, E.; Yosef, R. Pellet Analyses of Sporadic Breeding Short-Eared Owl (Asio flammeus) in Central Israel. Isr. J. Ecol. Evol. 2025, in press. [Google Scholar]
- Holt, D.W.; Jack Lyon, L.; Hale, R. Techniques for differentiating pellets of Short-eared Owls and northern Harriers. Condor 1987, 89, 929–931. [Google Scholar] [CrossRef]
- Khaleghizadeh, A.; Arbabi, T.; Noori, G.; Javidkar, M.; Shahriari, A. Diet of wintering Long-eared Owl Asio otus in Zabol, southeastern Iran. Ardea 2009, 97, 631–633. [Google Scholar] [CrossRef]
- Selcuk, A.Y.; Özkoç, Ö.Ü.; Bilir, M.A.; Kefelioğlu, H. Diet composition of the Long-eared Owl (Asio otus) in the Eastern Anatolia (Turkey). Turk. J. For. 2019, 20, 72–75. [Google Scholar] [CrossRef]
- Abi-Said, M.; Maroun, T.M. The diet of Pharaoh Eagle Owl bubo ascalaphus (Savigny, 1809) from Al Eraiq Reserve, Qatar. Jordan J. Nat. Hist. 2023, 10, 69–73. [Google Scholar]
- Bochenski, Z.M.; ToMEK, T.; Boev, Z.; Mitev, I. Patterns of bird bone fragmentation in pellets of the Tawny Owl (Strix aluco) and the Eagle Owl (Bubo bubo) and their taphonomic implications. Acta Zool. Cracoviensia 1993, 36, 313–328. [Google Scholar]
- Mori, E.; Mazzetto, F.; Menchetti, M.; Bodino, N.; Grasso, E.; Sposimo, P. Feeding ecology of the scops owl, Otus scops (Aves: Strigiformes), in the island of Pianosa (Tuscan Archipelago, Central Italy) outside the breeding period. Ital. J. Zool. 2016, 83, 417–422. [Google Scholar] [CrossRef]
- Romanowski, J.; Dudek-Godeau, D.; Lesiński, G. The Diversity of Small Mammals along a Large River Valley Revealed from Pellets of Tawny Owl Strix aluco. Biology 2023, 12, 1118. [Google Scholar] [CrossRef]
- Yatsiuk, Y.; Brusentsova, N.; Filatova, Y. Mammals in Tawny Owl (Strix aluco) pellets from Kharkiv Region, Ukraine. Biodivers. Data J. 2023, 11, e98772. [Google Scholar] [CrossRef]
- Bunn, D.S.; Warburton, A.B.; Wilson, R.D.S. The Barn Owl; T & A D Poyser: Calton, UK, 1982. [Google Scholar]
- Hernandez-Munoz, A.; Mancina, C.A. Diet of Barn Owl (Tyto alba) (Aves: Strigiformes) in natural and anthropogenic habitat in central Cuba. Rev. Mex. De Biodivers. 2011, 82, 217–226. [Google Scholar]
- Purger, J.J.; Szép, D. An attempt to determine the size of the Common Barn-owls (Tyto alba) hunting area based on its prey composition. Avian Biol. Res. 2022, 15, 41–46. [Google Scholar] [CrossRef]
- Dodson, P.; Wexlar, D. Taphonomic investigations of owl pellets. Paleobiology 1979, 5, 275–284. [Google Scholar] [CrossRef]
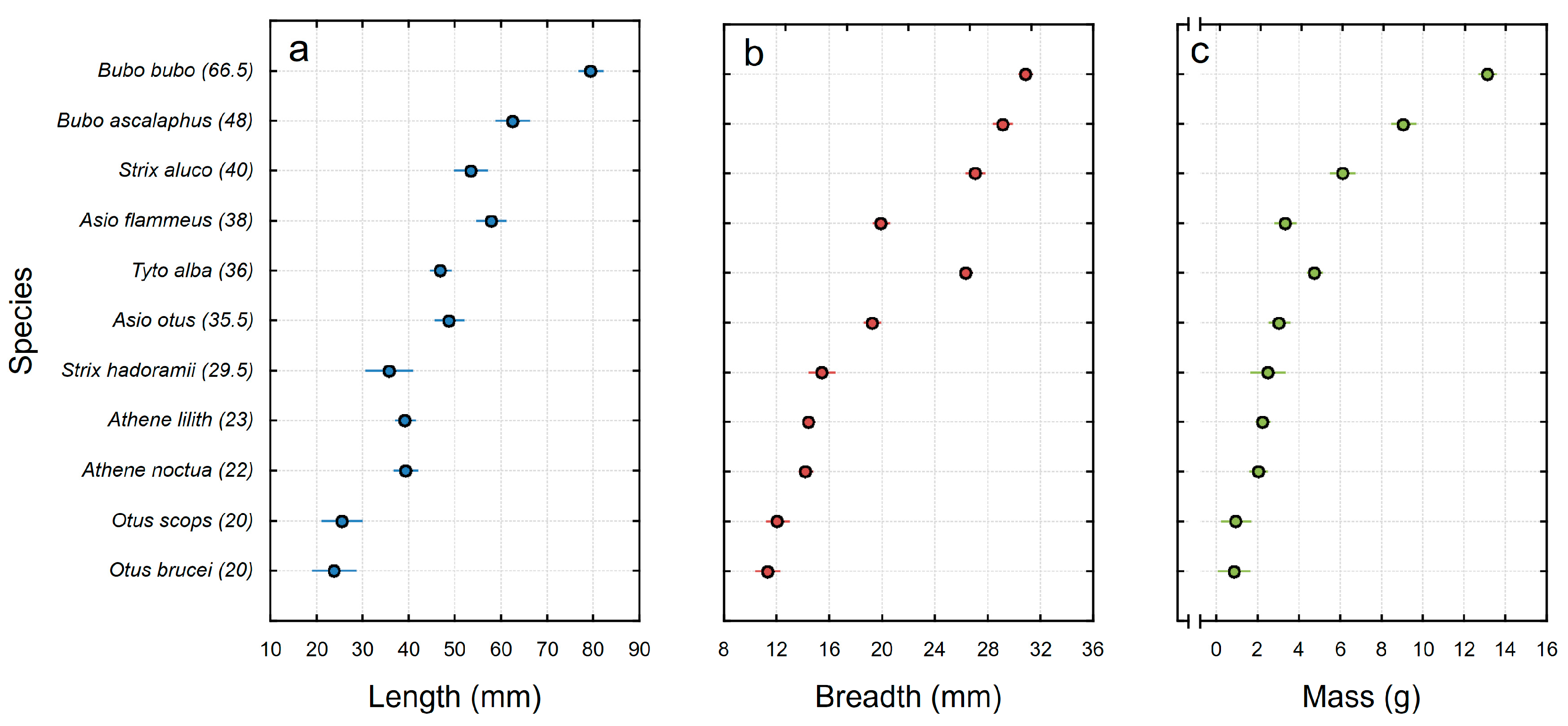
| Species | No. of Pellets | Length (mm) | Breadth (mm) | Mass (g) | Body Length (cm) | |||
|---|---|---|---|---|---|---|---|---|
| Mean (CL) | Range | Mean (CL) | Range | Mean (CL) | Range | |||
| Asio flammeus | 68 | 57.8 (53.9–61.7) | 21.8–87.8 | 19.9 (19.3–20.5) | 13.3–25.2 | 3.3 (3.0–3.6) | 1.0–4.8 | 38.0 |
| Asio otus | 70 | 48.7 (45.9–51.5) | 20.6–75.0 | 19.2 (18.6–19.9) | 11.3–25.6 | 3.0 (2.8–3.3) | 1.2–4.8 | 35.5 |
| Athene lilith | 151 | 39.2 (38.1–40.2) | 20.2–58.5 | 14.4 (14.2–14.6) | 10.5–21.1 | 2.2 (2.1–2.4) | 0.3–7.3 | 23.0 |
| Athene noctua | 102 | 39.3 (37.9–40.6) | 20.0–57.6 | 14.2 (13.9–14.5) | 10.0–18.5 | 2.0 (1.9–2.1) | 0.4–4.7 | 22.0 |
| Bubo ascalaphus | 52 | 62.4 (57.9–67.0) | 40.0–100.0 | 29.1 (28.0–30.2) | 19.5–40.3 | 9.0 (8.0–10.1) | 3.4–20.0 | 48.0 |
| Bubo bubo | 100 | 79.4 (74.1–84.7) | 48.5–160.0 | 30.9 (30.2–31.5) | 23.0–50.5 | 13.1 (12.2–14.1) | 6.7–28.0 | 66.5 |
| Otus brucei | 31 | 23.8 (22.6–24.9) | 19.0–31.4 | 11.3 (10.9–11.6) | 9.6–12.8 | 0.8 (0.7–1.0) | 0.3–1.6 | 20.0 |
| Otus scops | 36 | 25.4 (24.1–26.8) | 19.2–36.2 | 12.1 (11.4–12.7) | 9.1–16.2 | 0.9 (0.8–1.0) | 0.4–1.9 | 20.0 |
| Strix aluco | 52 | 53.3 (50.1–56.5) | 28.8–73.9 | 27.0 (25.8–28.3) | 17.0–35.0 | 6.1 (5.5–6.7) | 2.5–11.4 | 40.0 |
| Strix hadoramii | 26 | 35.7 (32.7–38.6) | 23.0–46.1 | 15.4 (14.5–16.3) | 11.9–19.2 | 2.5 (2.1–2.8) | 1.1–3.9 | 29.5 |
| Tyto alba | 136 | 46.8 (45.1–48.5) | 29.7–74.0 | 26.3 (25.9–26.8) | 19.1–33.9 | 4.7 (4.5–5.0) | 1.9–8.5 | 36.0 |
| Our Study: Average Pellet Size | Others: Average Pellet Size | Source | |
|---|---|---|---|
| Asio flammeus | 58 × 20 | 49 × 22 | [32,33,34,35,36] |
| Asio otus | 49 × 19 | 75 × 30 | [12,33,37,38,39] |
| Athene lilith | 39 × 14 | No data | |
| Athene noctua | 39 × 14 | 25 × 15 | [29,35] |
| Bubo ascalaphus | 62 × 29 | 47 × 25 | [40] |
| Bubo bubo | 79 × 30 | 75 × 32 | [10,33,41] |
| Otus brucei | 24 × 11 | No data | |
| Otus scops | 25 × 12 | [42] | |
| Strix aluco | 53 × 27 | 60 × 30 | [33,41,43,44] |
| Strix hadoramii | 36 × 15 | No data | |
| Tyto alba | 45 × 26 | 50 × 30 | [33,45,46,47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadad, E.; Zduniak, P.; Yosef, R. What the Owls Leave Behind: Pellet Size Variation Reflects Predator Body Size in Israel’s Owls. Ecologies 2025, 6, 44. https://doi.org/10.3390/ecologies6020044
Hadad E, Zduniak P, Yosef R. What the Owls Leave Behind: Pellet Size Variation Reflects Predator Body Size in Israel’s Owls. Ecologies. 2025; 6(2):44. https://doi.org/10.3390/ecologies6020044
Chicago/Turabian StyleHadad, Ezra, Piotr Zduniak, and Reuven Yosef. 2025. "What the Owls Leave Behind: Pellet Size Variation Reflects Predator Body Size in Israel’s Owls" Ecologies 6, no. 2: 44. https://doi.org/10.3390/ecologies6020044
APA StyleHadad, E., Zduniak, P., & Yosef, R. (2025). What the Owls Leave Behind: Pellet Size Variation Reflects Predator Body Size in Israel’s Owls. Ecologies, 6(2), 44. https://doi.org/10.3390/ecologies6020044







