Site-Based Patterns of Variation in Leaf Endophytes and Ecophysiological Performance in Sweet Birch (Betula lenta L.) in the Southern Appalachian Mountains, USA: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study System
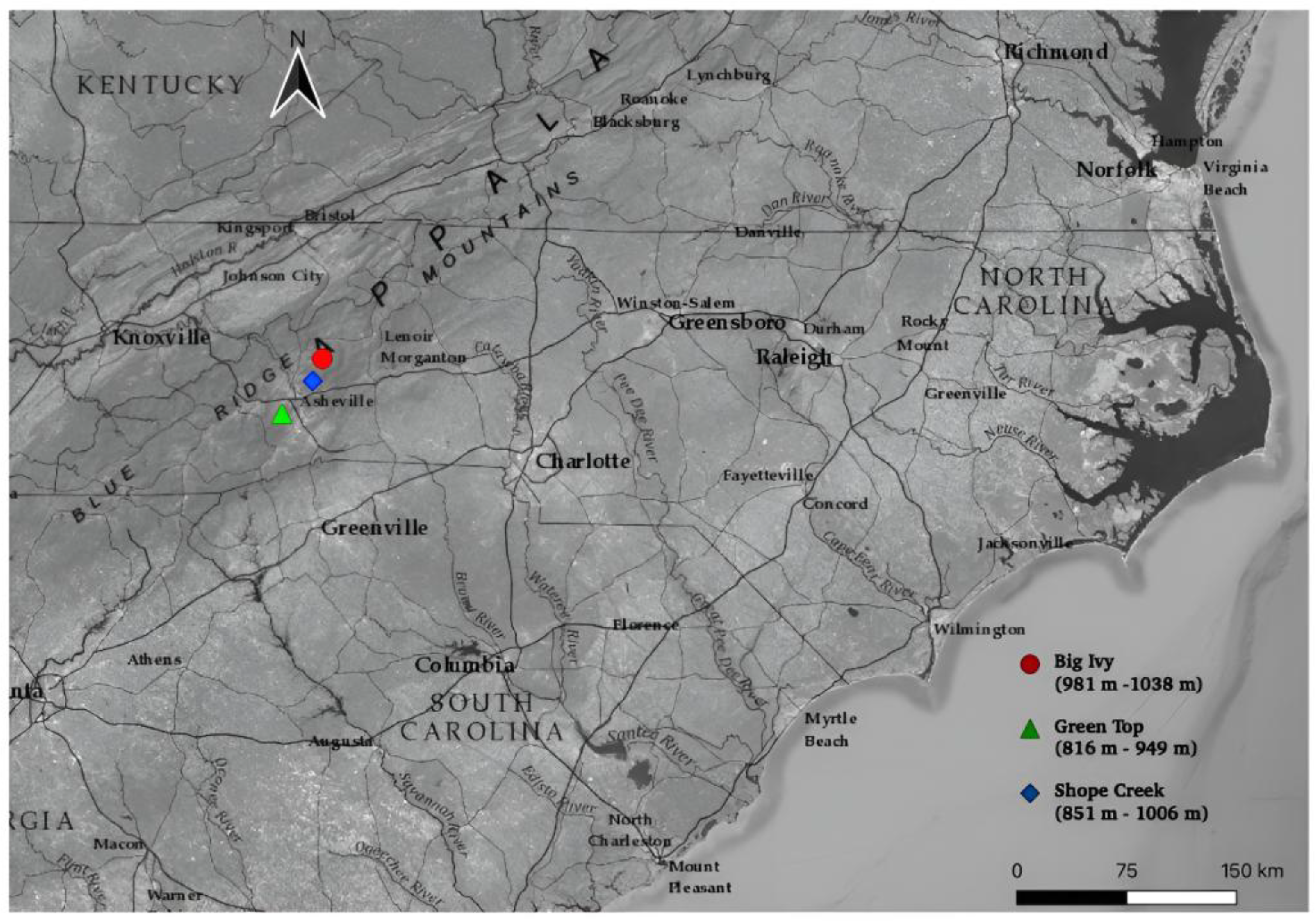
2.2. Field Data Collection
2.3. FEF Isolation and Identification
2.4. Statistical Analysis
3. Results
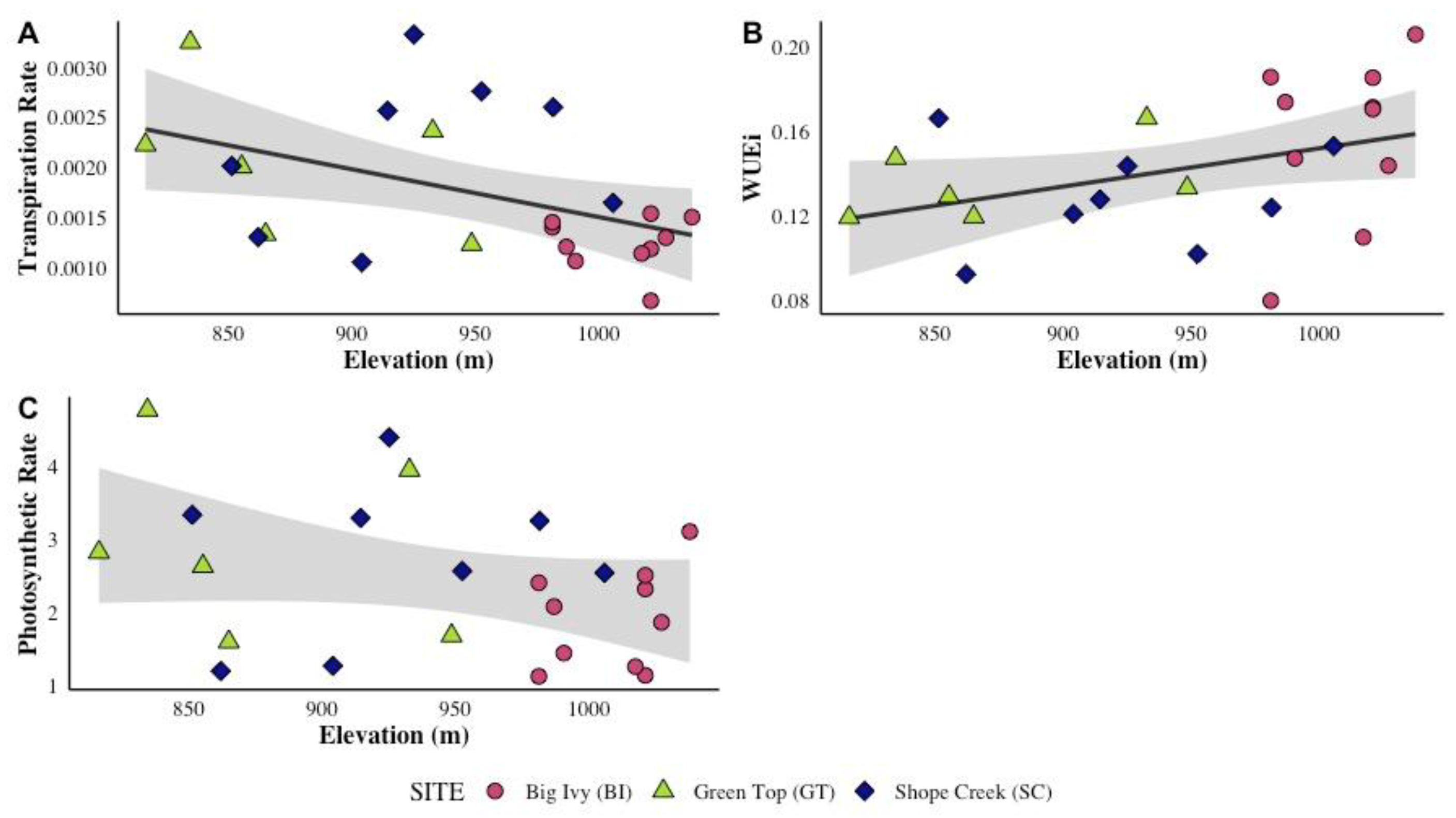
3.1. Gas Exchange Rates and Elevation
3.2. Gas Exchange Rates: Site-Level Variation
3.3. FEF Colonization and Morphotypes
3.4. Fungal OTUs Encountered
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schulz, B.; Boyle, C. The Endophytic Continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar]
- Porras-Alfaro, A.; Bayman, P. Hidden Fungi, Emergent Properties: Endophytes and Microbiomes. Annu. Rev. Phytopathol. 2011, 49, 291–315. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; White, J.F., Jr.; Arnold, A.E.; Redman, R.S. Fungal Endophytes: Diversity and Functional Roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef]
- Harrison, J.G.; Griffin, E.A. The Diversity and Distribution of Endophytes across Biomes, Plant Phylogeny and Host Tissues: How Far Have We Come and Where Do We Go from Here? Environ. Microbiol. 2020, 22, 2107–2123. [Google Scholar] [CrossRef]
- Tian, B.; Xie, J.; Fu, Y.; Cheng, J.; Li, B.; Chen, T.; Zhao, Y.; Gao, Z.; Yang, P.; Barbetti, M.J.; et al. A Cosmopolitan Fungal Pathogen of Dicots Adopts an Endophytic Lifestyle on Cereal Crops and Protects Them from Major Fungal Diseases. ISME J. 2020, 14, 3120–3135. [Google Scholar] [CrossRef]
- Arnold, A.E.; Mejía, L.C.; Kyllo, D.; Rojas, E.I.; Maynard, Z.; Robbins, N.; Herre, E.A. Fungal Endophytes Limit Pathogen Damage in a Tropical Tree. Proc. Natl. Acad. Sci. USA 2003, 100, 15649–15654. [Google Scholar] [CrossRef]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.-S.; Patra, J.K. Endophytes: A Treasure House of Bioactive Compounds of Medicinal Importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef]
- Raihan, T.; Azad, A.K.; Ahmed, J.; Shepon, M.R.; Dey, P.; Chowdhury, N.; Aunkor, T.H.; Ali, H.; Suhani, S. Extracellular Metabolites of Endophytic Fungi from Azadirachta Indica Inhibit Multidrug-Resistant Bacteria and Phytopathogens. Future Microbiol. 2021, 16, 557–576. [Google Scholar] [CrossRef]
- Bowman, E.A.; Arnold, A.E. Distributions of Ectomycorrhizal and Foliar Endophytic Fungal Communities Associated with Pinus ponderosa along a Spatially Constrained Elevation Gradient. Am. J. Bot. 2018, 105, 687–699. [Google Scholar] [CrossRef]
- Rolón, B.A.; Arnold, A.E.; Juliá, M.S.; Van Bael, S.A. Evaluating Endophyte-rich Leaves and Leaf Functional Traits for Protection of Tropical Trees against Natural Enemies. Funct. Ecol. 2024, 39, 371–389. [Google Scholar] [CrossRef]
- Redman, R.S.; Dunigan, D.D.; Rodriguez, R.J. Fungal Symbiosis from Mutualism to Parasitism: Who Controls the Outcome, Host or Invader? New Phytol. 2001, 151, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, F.S.; Villarreal, R.M.; Torres, V.R.; Gallou, A. Monosporic Inoculation of Economically Important Horticultural Species with Native Endomycorrhizae under Greenhouse Conditions. Agronomy 2019, 9, 130. [Google Scholar] [CrossRef]
- Nashat, L.H.; Haleem, R.A.; Ali, S.H. Molecular Identification and Antimicrobial Potential of Endophytic Fungi against Some Grapevine Pathogens. PLoS ONE 2024, 19, e0309041. [Google Scholar] [CrossRef]
- Van Bael, S.A.; Valencia, M.C.; Rojas, E.I.; Gómez, N.; Windsor, D.M.; Herre, E.A. Effects of Foliar Endophytic Fungi on the Preference and Performance of the Leaf Beetle Chelymorpha alternans in Panama. Biotropica 2009, 41, 221–225. [Google Scholar] [CrossRef]
- Sieber, T.N. Endophytic Fungi in Forest Trees: Are They Mutualists? Fungal Biol. Rev. 2007, 21, 75–89. [Google Scholar] [CrossRef]
- Cline, L.C.; Schilling, J.S.; Menke, J.; Groenhof, E.; Kennedy, P.G. Ecological and Functional Effects of Fungal Endophytes on Wood Decomposition. Funct. Ecol. 2018, 32, 181–191. [Google Scholar] [CrossRef]
- Pinto, L.S.R.C.; Azevedo, J.L.; Pereira, J.O.; Vieira, M.L.C.; Labate, C.A. Symptomless Infection of Banana and Maize by Endophytic Fungi Impairs Photosynthetic Efficiency. New Phytol. 2000, 147, 609–615. [Google Scholar] [CrossRef]
- Gazis, R.; Chaverri, P. Wild Trees in the Amazon Basin Harbor a Great Diversity of Beneficial Endosymbiotic Fungi: Is This Evidence of Protective Mutualism? Fungal Ecol. 2015, 17, 18–29. [Google Scholar] [CrossRef]
- González-Teuber, M. The Defensive Role of Foliar Endophytic Fungi for a South American Tree. AoB Plants 2016, 8, plw050. [Google Scholar] [CrossRef]
- Yang, T.; Weisenhorn, P.; Gilbert, J.A.; Ni, Y.; Sun, R.; Shi, Y.; Chu, H. Carbon Constrains Fungal Endophyte Assemblages along the Timberline. Environ. Microbiol. 2016, 18, 2455–2469. [Google Scholar] [CrossRef]
- Darcy, J.L.; Swift, S.O.I.; Cobian, G.M.; Zahn, G.L.; Perry, B.A.; Amend, A.S. Fungal Communities Living within Leaves of Native Hawaiian Dicots Are Structured by Landscape-Scale Variables as Well as by Host Plants. Mol. Ecol. 2020, 29, 3102–3115. [Google Scholar] [CrossRef]
- González-Teuber, M.; Vilo, C.; Guevara-Araya, M.J.; Salgado-Luarte, C.; Gianoli, E. Leaf Resistance Traits Influence Endophytic Fungi Colonization and Community Composition in a South American Temperate Rainforest. J. Ecol. 2020, 108, 1019–1029. [Google Scholar] [CrossRef]
- Rho, H.; Kim, S.-H. Endophyte Effects on Photosynthesis and Water Use of Plant Hosts: A Meta-Analysis. In Functional Importance of the Plant Microbiome; Doty, S.L., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 43–69. ISBN 978-3-319-65896-4. [Google Scholar]
- Ackerly, D.D.; Dudley, S.A.; Sultan, S.E.; Schmitt, J.; Coleman, J.S.; Linder, C.R.; Sandquist, D.R.; Geber, M.A.; Evans, A.S.; Dawson, T.E. The Evolution of Plant Ecophysiological Traits: Recent Advances and Future Directions: New Research Addresses Natural Selection, Genetic Constraints, and the Adaptive Evolution of Plant Ecophysiological Traits. Bioscience 2000, 50, 979–995. [Google Scholar]
- Reich, P.B.; Wright, I.J.; Cavender-Bares, J.; Craine, J.M.; Oleksyn, J.; Westoby, M.; Walters, M.B. The Evolution of Plant Functional Variation: Traits, Spectra, and Strategies. Int. J. Plant Sci. 2003, 164, S143–S164. [Google Scholar] [CrossRef]
- Geber, M.A.; Griffen, L.R. Inheritance and Natural Selection on Functional Traits. Int. J. Plant Sci. 2003, 164, S21–S42. [Google Scholar] [CrossRef]
- Richardson, A.D.; Berlyn, G.P. Spectral Reflectance and Photosynthetic Properties of Betula papyrifera (Betulaceae) Leaves along an Elevational Gradient on Mt. Mansfield, Vermont, USA. Am. J. Bot. 2002, 89, 88–94. [Google Scholar] [CrossRef]
- Ramirez, H.-L.; Ivey, C.T.; Wright, J.W.; MacDonald, B.W.; Sork, V.L. Variation in Leaf Shape in a Quercus Lobata Common Garden: Tests for Adaptation to Climate and Physiological Consequences. Madroño 2020, 67, 77–84. [Google Scholar]
- Ramírez-Valiente, J.A.; López, R.; Hipp, A.L.; Aranda, I. Correlated Evolution of Morphology, Gas Exchange, Growth Rates and Hydraulics as a Response to Precipitation and Temperature Regimes in Oaks (Quercus). New Phytol. 2020, 227, 794–809. [Google Scholar] [CrossRef]
- Song, B.; Niu, S.; Wan, S. Precipitation Regulates Plant Gas Exchange and Its Long-Term Response to Climate Change in a Temperate Grassland. J. Plant Ecol. 2016, 9, 531–541. [Google Scholar]
- Mazer, S.J.; Dudley, L.S.; Hove, A.A.; Emms, S.K.; Verhoeven, A.S. Physiological Performance in Clarkia Sister Taxa with Contrasting Mating Systems: Do Early-Flowering Autogamous Taxa Avoid Water Stress Relative to Their Pollinator-Dependent Counterparts? Int. J. Plant Sci. 2010, 171, 1029–1047. [Google Scholar] [CrossRef]
- Allen, J.L.; Lendemer, J.C. Climate Change Impacts on Endemic, High-Elevation Lichens in a Biodiversity Hotspot. Biodivers. Conserv. 2016, 25, 555–568. [Google Scholar] [CrossRef]
- Cox, J.L.; McKinney, M.L.; Fitzpatrick, B.M.; Leppanen, C.; Nichols, R.J. Coniferous Conservation Supporting a Plethora of Plethodontids: Implications of Conserving Eastern Hemlock (Tsuga canadensis) on Southern Appalachian Montane Salamanders. For. Ecol. Manag. 2022, 508, 120010. [Google Scholar] [CrossRef]
- Landscape Partnership Land Use in the Appalachians. Available online: https://www.landscapepartnership.org/cooperative/our-plan/section-1/land-use-in-the-appalachians (accessed on 31 January 2025).
- Zhang, R.; Zhou, X.-L.; Yang, L.; Long, B.; Shen, S.-K. Composition and Assembly of the Endophytic Fungal Community of Alpine Rhododendron Hosts along Elevation Gradients. Phytobiomes J. 2024, 8, 236–247. [Google Scholar] [CrossRef]
- Raudabaugh, D.B.; Matheny, P.B.; Hughes, K.W.; Iturriaga, T.; Sargent, M.; Miller, A.N. Where Are They Hiding? Testing the Body Snatchers Hypothesis in Pyrophilous Fungi. Fungal Ecol. 2020, 43, 100870. [Google Scholar]
- Rutto, L.K.; Ren, S.; Wynn, H.C.; Chamberlain, J.L. Soil and Microbe Interactions in Two Populations of Appalachian Black Cohosh (Actaea racemosa L.). J. Torrey Bot. Soc. 2021, 148, 117–131. [Google Scholar]
- Ahlholm, J.U.; Helander, M.; Henriksson, J.; Metzler, M.; Saikkonen, K. Environmental Conditions and Host Genotype Direct Genetic Diversity of Venturia Ditricha, a Fungal Endophyte of Birch Trees. Evolution 2002, 56, 1566–1573. [Google Scholar]
- Helander, M.; Neuvonen, S.; Sieber, T.; Petrini, O. Simulated Acid Rain Affects Birch Leaf Endophyte Populations. Microb. Ecol. 1993, 26, 227–234. [Google Scholar]
- United States Department of Agriculture Natural Resources Conservation Service. Betula lenta L. 2025. Available online: https://plants.usda.gov/plant-profile/BELE (accessed on 1 March 2023).
- Rossell, I.M.; Eggleston, H. Elevational Distribution of Temperate Lianas along Trails in Pisgah National Forest. Southeast. Nat. 2017, 16, 443–450. [Google Scholar] [CrossRef]
- Helander, M.; Wäli, P.; Kuuluvainen, T.; Saikkonen, K. Birch Leaf Endophytes in Managed and Natural Boreal Forests. Can. J. For. Res. 2006, 36, 3239–3245. [Google Scholar]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press, Inc.: Cambridge, MA, USA, 1990; pp. 315–322. ISBN 978-0-12-372180-8. [Google Scholar]
- Han, J.; Zhu, Y.; Chen, X.; Liao, B.; Yao, H.; Song, J.; Chen, S.; Meng, F. The Short ITS2 Sequence Serves as an Efficient Taxonomic Sequence Tag in Comparison with the Full-Length ITS. BioMed Res. Int. 2013, 2013, 741476. [Google Scholar] [CrossRef]
- Caruso, K.E.; Horton, J.L.; Hove, A.A. Assessing the Effect of Eastern Hemlock (Tsuga canadensis) Decline from Hemlock Woolly Adelgid (Adelges tsugae) Infestation on Ectomycorrhizal Colonization and Growth of Red Oak (Quercus rubra) Seedlings. Am. Midl. Nat. 2021, 186, 16–34. [Google Scholar] [CrossRef]
- Rodriguez, R.; Redman, R. More than 400 Million Years of Evolution and Some Plants Still Can’t Make It on Their Own: Plant Stress Tolerance via Fungal Symbiosis. J. Exp. Bot. 2008, 59, 1109–1114. [Google Scholar] [CrossRef]
- Oberhofer, M.; Malfent, F.; Zehl, M.; Urban, E.; Wackerlig, J.; Reznicek, G.; Vignolle, G.A.; Rückert, C.; Busche, T.; Wibberg, D.; et al. Biosynthetic Potential of the Endophytic Fungus Helotiales Sp. BL73 Revealed via Compound Identification and Genome Mining. Appl. Environ. Microbiol. 2022, 88, e02510-21. [Google Scholar] [CrossRef]
- Balasuriya, A.; Adikaram, N.K.B. Some Spatial, Temporal and Spatio-Temporal Considerations of Wood Decay of Tea (Camellia sinensis), Caused by Nemania diffusa (Syn. Hypoxylon vestitum). Crop Prot. 2009, 28, 273–279. [Google Scholar] [CrossRef]
- Okane, I.; Nonaka, K.; Kurihara, Y.; Abe, J.P.; Yamaoka, Y. A New Species of Leptobacillim, L. symbioticum, Isolated from Mites and Sori of Soybean Rust. Mycoscience 2020, 61, 165–171. [Google Scholar] [CrossRef]
- Troncoso-Rojas, R.; Tiznado-Hernández, M.E. Alternaria alternata (Black Rot, Black Spot). Postharvest Decay Control. Strateg. 2014, 147–187. [Google Scholar] [CrossRef]
- Li, T.; Im, J.; Lee, J. Genetic Diversity of Epicoccum nigrum and Its Effects on Fusarium graminearum. Mycobiology 2022, 50, 457–466. [Google Scholar] [CrossRef]
- Darapanit, A.; Boonyuen, N.; Leesutthiphonchai, W.; Nuankaew, S.; Piasai, O. Identification, Pathogenicity and Effects of Plant Extracts on Neopestalotiopsis and Pseudopestalotiopsis Causing Fruit Diseases. Sci. Rep. 2021, 11, 22606. [Google Scholar] [CrossRef]
- Wittstein, K.; Cordsmeier, A.; Lambert, C.; Wendt, L.; Sir, E.B.; Weber, J.; Wurzler, N.; Petrini, L.E.; Stadler, M. Identification of Rosellinia Species as Producers of Cyclodepsipeptide PF1022 A and Resurrection of the Genus Dematophora as Inferred from Polythetic Taxonomy. Stud. Mycol. 2020, 96, 1–16. [Google Scholar] [CrossRef]
- Toghueo, R.M.K.; Boyom, F.F. Endophytic Penicillium Species and Their Agricultural, Biotechnological, and Pharmaceutical Applications. 3 Biotech 2020, 10, 107. [Google Scholar] [CrossRef]
- Langer, G.J.; Bußkamp, J. Fungi Associated with Woody Tissues of European Beech and Their Impact on Tree Health. Front. Microbiol. 2021, 12, 702467. [Google Scholar] [CrossRef]
- do Carmo de Souza, É.S.; do Vale, H.M.M.; de Cássia Pereira Carvalho, R.; de Oliveira Soares, W.R.; Miller, R.N.G.; Dianese, G.C. Infection by Uromyces Euphorbiae: A Trigger for the Sporulation of Endophytic Colletotrichum truncatum on the Common Host Euphorbia Hirta. Mycol. Prog. 2017, 16, 941–946. [Google Scholar] [CrossRef]
- Shahriar, S.A.; Husna, A.; Paul, T.T.; Eaty, M.N.K.; Quamruzzaman, M.; Siddique, A.B.; Rahim, M.A.; Ahmmed, A.N.F.; Uddain, J.; Siddiquee, S. Colletotrichum Truncatum Causing Anthracnose of Tomato (Solanum lycopersicum L.) in Malaysia. Microorganisms 2023, 11, 226. [Google Scholar] [CrossRef] [PubMed]
- Macías-Rubalcava, M.L.; Hernández-Bautista, B.E.; Oropeza, F.; Duarte, G.; González, M.C.; Glenn, A.E.; Hanlin, R.T.; Anaya, A.L. Allelochemical Effects of Volatile Compounds and Organic Extracts from Muscodor Yucatanensis, a Tropical Endophytic Fungus from Bursera Simaruba. J. Chem. Ecol. 2010, 36, 1122–1131. [Google Scholar] [CrossRef]
- Ju, Y.-M.; Rogers, J.D.; Hsieh, H.-M.; Vasilyeva, L. Amphirosellinia Gen. Nov. and a New Species of Entoleuca. Mycologia 2004, 96, 1393–1402. [Google Scholar]
- Martin, P.L.; Peter, K.A. Quantification of Colletotrichum fioriniae in Orchards and Deciduous Forests Indicates It Is Primarily a Leaf Endophyte. Phytopathology®® 2021, 111, 333–344. [Google Scholar] [CrossRef]
- Gauchan, D.P.; Vélëz, H.; Acharya, A.; Östman, J.R.; Lundén, K.; Elfstrand, M.; García-Gil, M.R. Annulohypoxylon Sp. Strain MUS1, an Endophytic Fungus Isolated from Taxus wallichiana Zucc., Produces Taxol and Other Bioactive Metabolites. 3 Biotech 2021, 11, 152. [Google Scholar] [CrossRef]
- U’Ren, J.M.; Miadlikowska, J.; Zimmerman, N.B.; Lutzoni, F.; Stajich, J.E.; Arnold, A.E. Contributions of North American Endophytes to the Phylogeny, Ecology, and Taxonomy of Xylariaceae (Sordariomycetes, Ascomycota). Mol. Phylogenet. Evol. 2016, 98, 210–232. [Google Scholar] [CrossRef]
- Oses, R.; Valenzuela, S.; Freer, J.; Sanfuentes, E.; Rodríguez, J. Fungal Endophytes of Healthy Chilean Trees and Their Possible Role in Early Wood Decay. Fungal Divers. 2008, 33, 77–86. [Google Scholar]
- Maherali, H. Mutualism as a Plant Functional Trait: Linking Variation in the Mycorrhizal Symbiosis to Climatic Tolerance, Geographic Range, and Population Dynamics. Int. J. Plant Sci. 2020, 181, 9–19. [Google Scholar] [CrossRef]
- Oksanen, E. Birch as a Model Species for the Acclimation and Adaptation of Northern Forest Ecosystem to Changing Environment. Front. For. Glob. Change 2021, 4, 682512. [Google Scholar]
- Dawson, T.E.; Mambelli, S.; Plamboeck, A.H.; Templer, P.H.; Tu, K.P. Stable Isotopes in Plant Ecology. Annu. Rev. Ecol. Syst. 2002, 33, 507–559. [Google Scholar] [CrossRef]
- Zimmerman, N.B.; Vitousek, P.M. Fungal Endophyte Communities Reflect Environmental Structuring across a Hawaiian Landscape. Proc. Natl. Acad. Sci. USA 2012, 109, 13022–13027. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Qu, J.; Mu, H.; Sun, H.; Wu, C. Foliar Endophytic Fungi: Diversity in Species and Functions in Forest Ecosystems. Symbiosis 2020, 80, 103–132. [Google Scholar]
- Kress, W.J.; García-Robledo, C.; Uriarte, M.; Erickson, D.L. DNA Barcodes for Ecology, Evolution, and Conservation. Trends Ecol. Evol. 2015, 30, 25–35. [Google Scholar] [CrossRef]
- Terhonen, E.; Blumenstein, K.; Kovalchuk, A.; Asiegbu, F.O. Forest Tree Microbiomes and Associated Fungal Endophytes: Functional Roles and Impact on Forest Health. Forests 2019, 10, 42. [Google Scholar] [CrossRef]
- Griffin, E.A.; Carson, W.P. Tree Endophytes: Cryptic Drivers of Tropical Forest Diversity. In Endophytes of Forest Trees; Pirttilä, A.M., Frank, A.C., Eds.; Forestry Sciences; Springer International Publishing: Cham, Switzerland, 2018; Volume 86, pp. 63–103. ISBN 978-3-319-89832-2. [Google Scholar]
- Sáez-Sandino, T.; García-Palacios, P.; Maestre, F.T.; Plaza, C.; Guirado, E.; Singh, B.K.; Wang, J.; Cano-Díaz, C.; Eisenhauer, N.; Gallardo, A. The Soil Microbiome Governs the Response of Microbial Respiration to Warming across the Globe. Nat. Clim. Change 2023, 13, 1382–1387. [Google Scholar]




| Photosynthesis | Transpiration | WUEi | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | df | SS | MS | F | p-Value | SS | MS | F | p-Value | SS | MS | F | p-Value |
| Site | 2 | 1.551 | 0.7756 | 3.572 | 0.0353 | 3.16 | 1.58 | 12.158 | 4.81 × 10−5 | 0.01227 | 0.006136 | 3.166 | 0.0506 |
| Tree | 1 | 0.02 | 0.0203 | 0.094 | 0.7608 | 0.008 | 0.0085 | 0.065 | 0.7996 | 0.0004 | 0.000397 | 0.205 | 0.6527 |
| Leaf number | 1 | 0.019 | 0.0192 | 0.088 | 0.7675 | 0.029 | 0.0288 | 0.221 | 0.6401 | 0.00175 | 0.001753 | 0.904 | 0.3461 |
| Tree (site) | 2 | 1.185 | 0.5926 | 2.729 | 0.0748 | 1.305 | 0.6523 | 5.019 | 0.0102 | 0.00049 | 0.000244 | 0.126 | 0.8818 |
| Tree (leaf number) | 1 | 0.111 | 0.1108 | 0.51 | 0.4783 | 0.203 | 0.2032 | 1.563 | 0.2169 | 0.00057 | 0.000571 | 0.295 | 0.5896 |
| Leaf number (tree, site) | 2 | 0.326 | 0.1631 | 0.751 | 0.4769 | 0.092 | 0.0459 | 0.353 | 0.7039 | 0.00548 | 0.002741 | 1.414 | 0.2524 |
| Residuals | 51 | 11.073 | 0.2171 | 6.628 | 0.13 | 0.09883 | 0.001938 | ||||||
| Sample No. | Site | Family | OTU | Proposed Ecological Guild | Morphotype | Reference |
|---|---|---|---|---|---|---|
| 1 | BI | Glomerellaceae | Colletotrichum sp. | E, P | MT42: streaky black | [46] |
| 2 | BI | Leotiomycetes | Helotiales sp. | E | MT58: white billows | [47] |
| 3 | BI | Xylariaceae | Nemania diffusa | P | MT54: wacky brown moss | [48] |
| 4 | BI | Cordycipitaceae | Leptobacillium symbioticum | EN | MT19: fuzzy white pile | [49] |
| 5 | BI | Pleosporaceae | Alternaria alternata | P, S | MT22: gray starburst | [50] |
| 6 | BI | Didymellaceae | Epicoccum nigrum | E, P | MT10: clear yellow w/orange nerves | [51] |
| 7 | BI | Xylariaceae | Nemania diffusa | P | MT25: off-white dust pile | [48] |
| 8 | BI | Xylariaceae | Nemania diffusa | P | MT67: white veins w/snail-shaped fruit | [48] |
| 9 | BI | Sporocadaceae | Neopestalotiopsis sp. | P | MT51: streaky yellowish cloud ring w/black fruit | [52] |
| 10 | BI | Glomerellaceae | Colletotrichum sp. | E, P | MT37: orange/yellow billows | [46] |
| 12 | BI | Xylariaceae | Rosellinia corticium | E | MT45: streaky gray | [53] |
| 13 | BI | Aspergillaceae | Penicillium sp. | E | MT64: white starbursts w/gray fuzzy centers | [54] |
| 14 | BI | Hypoxylaceae | Jackrogersella cohaerens | E, S | MT13: fragrant white mesh | [55] |
| 15 | BI | Glomerellaceae | Colletotrichum truncatum | E, P | MT40: soot sprites | [56,57] |
| 16 | BI | Xylariaceae | Nemania diffusa | P | MT66: white veins | [48] |
| 17 | SC | Xylariaceae | Muscodor yucatanensis | E | MT23: gray-green ring | [58] |
| 18 | SC | Xylariaceae | Amphirosellinia fushanensis | unspecified | MT48: streaky white | [59] |
| 20 | SC | Glomerellaceae | Colletotrichum truncatum | E, P | MT5: black blobs | [56,57] |
| 21 | SC | Xylariaceae | Nemania diffusa | P | MT41: speckled black | [48] |
| 22 | SC | Glomerellaceae | Colletotrichum fioriniae | E, P | MT45: streaky gray | [60] |
| 23 | GT | Hypoxylaceae | Annulohypoxylon sp. | E | MT27: opaque brown w/ring | [61] |
| 24 | GT | Xylariaceae | Rosellinia corticium | E | MT48: streaky white | [53] |
| 25 | GT | Xylariaceae | Whalleya microplaca | E | MT28: opaque mat | [62] |
| 26 | GT | Phanerochaetaceae | Bjerkandera adusta | E, S | MT62: white smelly fuzz | [63] |
| 27 | GT | Hypoxylaceae | Annulohypoxylon sp. | E | MT13: fragrant white mesh | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dougherty, G.A.; Zaboski, G.C.; Griffin, E.A.; Hove, A.A. Site-Based Patterns of Variation in Leaf Endophytes and Ecophysiological Performance in Sweet Birch (Betula lenta L.) in the Southern Appalachian Mountains, USA: A Preliminary Study. Ecologies 2025, 6, 30. https://doi.org/10.3390/ecologies6020030
Dougherty GA, Zaboski GC, Griffin EA, Hove AA. Site-Based Patterns of Variation in Leaf Endophytes and Ecophysiological Performance in Sweet Birch (Betula lenta L.) in the Southern Appalachian Mountains, USA: A Preliminary Study. Ecologies. 2025; 6(2):30. https://doi.org/10.3390/ecologies6020030
Chicago/Turabian StyleDougherty, Grace A., Grace C. Zaboski, Eric A. Griffin, and Alisa A. Hove. 2025. "Site-Based Patterns of Variation in Leaf Endophytes and Ecophysiological Performance in Sweet Birch (Betula lenta L.) in the Southern Appalachian Mountains, USA: A Preliminary Study" Ecologies 6, no. 2: 30. https://doi.org/10.3390/ecologies6020030
APA StyleDougherty, G. A., Zaboski, G. C., Griffin, E. A., & Hove, A. A. (2025). Site-Based Patterns of Variation in Leaf Endophytes and Ecophysiological Performance in Sweet Birch (Betula lenta L.) in the Southern Appalachian Mountains, USA: A Preliminary Study. Ecologies, 6(2), 30. https://doi.org/10.3390/ecologies6020030







