Polyplacophoran Assemblages in Shallow Waters of the West Antarctic Peninsula: Patterns of Diversity, Composition and Abundance
Abstract
1. Introduction
2. Materials and Methods
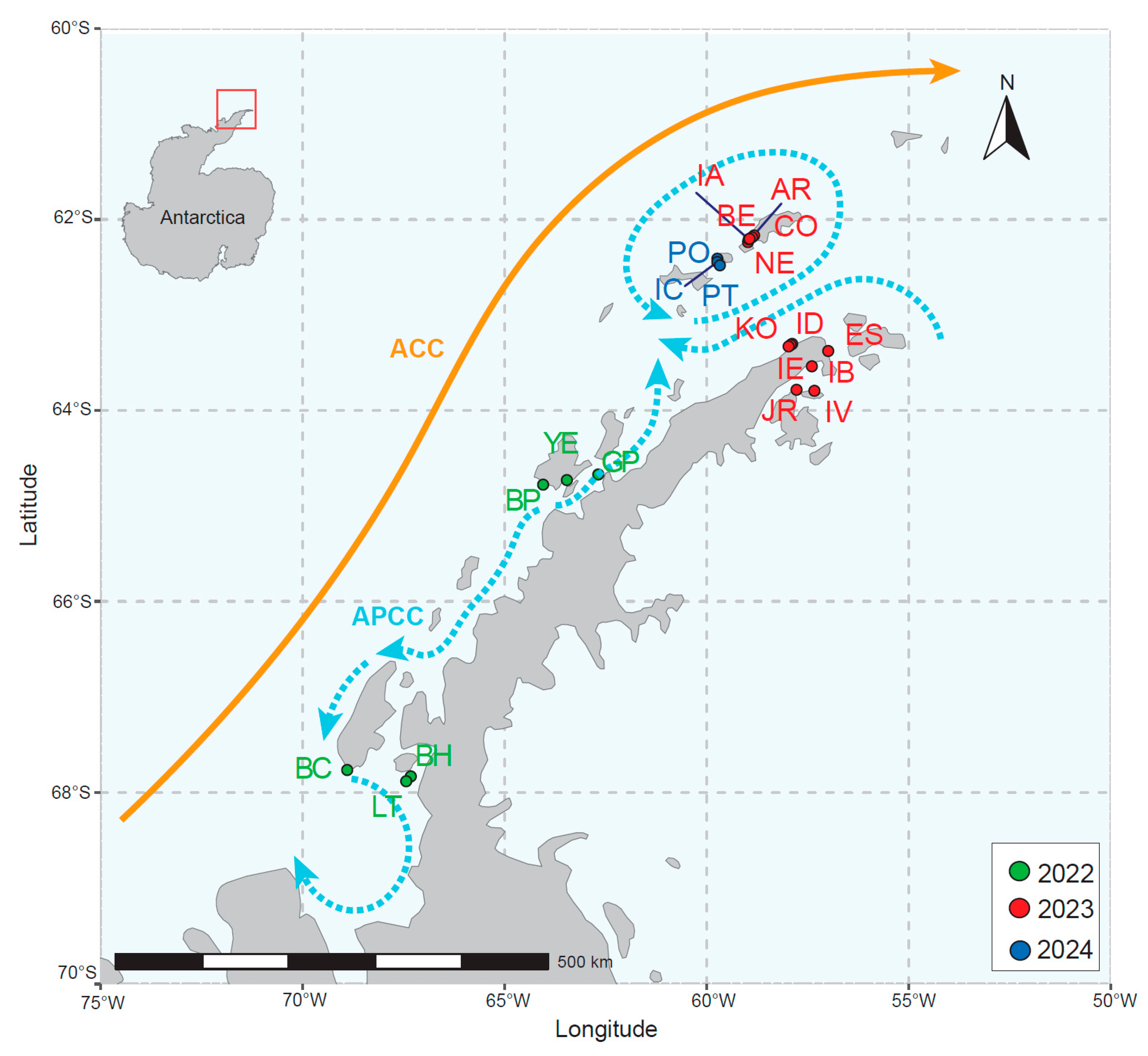
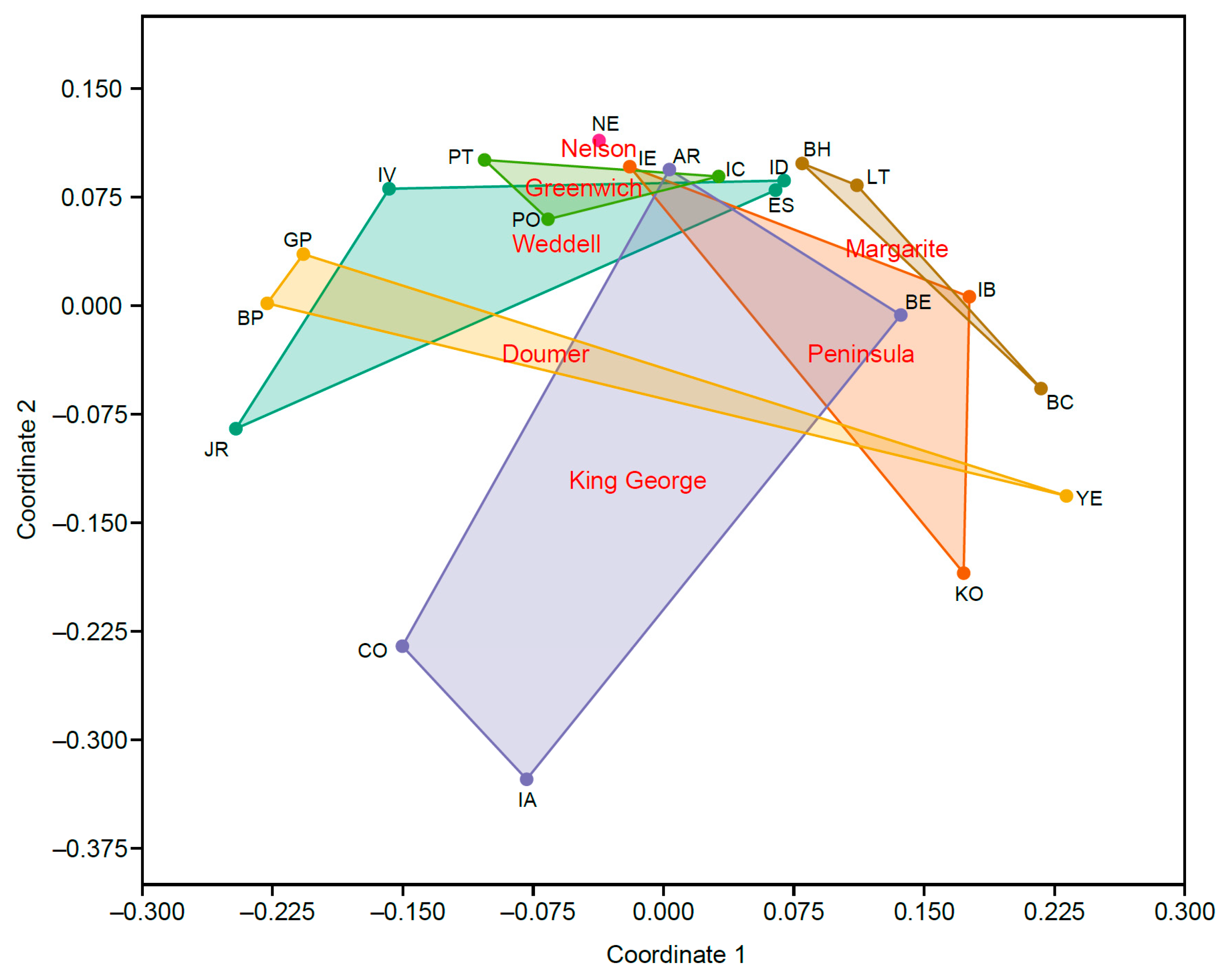
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Broyer, C.; Danis, B. How many species in the Southern Ocean? Towards a dynamic inventory of the Antarctic marine species. Deep-Sea Res. II Top. Stud. Oceanogr. 2011, 58, 5–17. [Google Scholar] [CrossRef]
- Griffiths, H.J.; Danis, B.; Clarke, A. Quantifying Antarctic marine biodiversity: The SCAR-MarBIN data portal. Deep. Sea Res. Part II: Top. Stud. Oceanogr. 2011, 58, 18–29. [Google Scholar] [CrossRef]
- Clarke, A.; Johnston, N.M. Antarctic marine benthic diversity. Oceanogr. Mar. Biol. 2003, 41, 47–114. [Google Scholar]
- Linse, K.; Griffiths, H.J.; Barnes, D.K.; Clarke, A. Biodiversity and biogeography of Antarctic and sub-Antarctic mollusca. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2006, 53, 985–1008. [Google Scholar] [CrossRef]
- Brandt, A.; DeBroyer, C.; DeMesel, I.; Ellingsen, K.E.; Gooday, A.J.; Hilbig, B.; Linse, K.; Thomson, M.R.A.; Tyler, P.A. The biodiversity of the deep Southern Ocean benthos. Philos. Trans. R. Soc. B 2007, 362, 39–66. [Google Scholar] [CrossRef]
- Ellingsen, K.E.; Brandt, A.; Ebbe, B.; Linse, K. Diversity and species distribution of polychaetes, isopods and bivalves in the Atlantic sector of the deep Southern Ocean. Polar Biol. 2007, 30, 1265–1273. [Google Scholar] [CrossRef]
- Schrödl, M.; Bohn, J.M.; Brenke, N.; Rolán, E.; Schwabe, E. Abundance, diversity, and latitudinal gradients of southeastern Atlantic and Antarctic abyssal gastropods. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2011, 58, 49–57. [Google Scholar] [CrossRef]
- Aldea, C.; Olabarria, C.; Troncoso, J.S. Bathymetric zonation and diversity gradient of gastropods and bivalves in West Antarctica from the South Shetland Islands to the Bellingshausen Sea. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2008, 55, 350–368. [Google Scholar] [CrossRef]
- Clarke, A.; Crame, J.A. The origin of the Southern Ocean marine fauna. In Origins and Evolution of the Antarctic Biota; Crame, J.A., Ed.; Geological Society Special Publications: London, UK, 1989; pp. 254–268. [Google Scholar]
- Thatje, S.; Hillenbrand, C.D.; Larter, R. On the origin of Antarctic marine benthic community structure. Trends Ecol. Evol. 2005, 20, 534–540. [Google Scholar] [CrossRef]
- Allcock, A.L.; Strugnell, J.M. Southern Ocean diversity: New paradigms from molecular ecology. Trends Ecol. Evol. 2012, 27, 520–528. [Google Scholar] [CrossRef]
- Eernisse, D.J. Denny, M.W., Gaines, S., Eds.; Chitons. In Encyclopedia of Tidepools and Rocky Shores (No. 1); University of California Press: Berkeley, CA, USA, 2007. [Google Scholar]
- Granpoder, G.; Anseeuw, B.; Robin, A. Compendium of Chitons, A Guide of Recent Polyplacophora; Conch Books: Harxheim, Germany, 2023; 224p. [Google Scholar]
- Navarrete, A.H.; Sellanes, J.; Pardo-Gandarillas, M.C.; Sirenko, B.; Eernisse, D.J.; Camus, P.A.; Ojeda, F.P.; Ibáñez, C.M. Latitudinal distribution of polyplacophorans along the Southeastern Pacific coast: Unravelling biases in geographical diversity patterns. Mar. Biodivers. 2020, 50, 45. [Google Scholar] [CrossRef]
- Ibáñez, C.M.; Waldisperg, M.; Torres, F.I.; Carrasco, S.A.; Sellanes, J.; Pardo-Gandarillas, M.C.; Sigwart, J.D. Environmental and ecological factors mediate taxonomic composition and body size of polyplacophoran assemblages along the Peruvian Province. Sci. Rep. 2019, 9, 15934. [Google Scholar] [CrossRef]
- Ibáñez, C.M.; Carter, M.J.; Aguilera, M.A.; Pardo-Gandarillas, M.C.; Rezende, E.L. Body size variation in polyplacophoran mollusks: Geographic clines and community structure along the Southeastern Pacific. Glob. Ecol. Biogeogr. 2021, 30, 1781–1795. [Google Scholar] [CrossRef]
- Schwabe, E.; Kohlberg, G.; Schories, D. Chitons, Polyplacophora. In Marine Wildlife, King George Island, Antarctica; Schories, D., Kohlberg, G., Eds.; Dirk Schories Publications: Rostock, Germany, 2016; pp. 122–128. [Google Scholar]
- Sirenko, B.I. Leptochiton antarcticus (Mollusca, Polyplacophora)—A new species from the Southern Ocean. Ruthenica 2015, 25, 139–146. [Google Scholar]
- Rosenfeld, S.; Aldea, C.; Ojeda, J.; Marambio, J.; Hüne, M.; Troncoso, J.S.; Mansilla, A. Molluscan assemblages associated with Gigartina beds in the Strait of Magellan and the South Shetland Islands (Antarctica): A comparison of composition and abundance. Polar Res. 2017, 36, 1297915. [Google Scholar] [CrossRef]
- Amsler, C.D.; Miller, L.R.; Edwards, R.A.; Amsler, M.O.; Engl, W.; Mcclintock, J.B.; Baker, B.J. Gastropod assemblages associated with Himantothallus grandifolius, Sarcopeltis antarctica and other subtidal macroalgae. Antarct. Sci. 2022, 34, 246–255. [Google Scholar] [CrossRef]
- Kaas, P.; Van Belle, R. Monograph of Living Chitons (Mollusca: Polyplacophora); Volume 1. Order Neoloricata: Lepidopleurina; E.J. Brill/W. Backhuys: Leiden, The Netherlands, 1985. [Google Scholar]
- Kaas, P.; Van Belle, R.A. Monograph of Living Chitons (Mollusca: Polyplacophora); Volume 2. Suborder Ischnochitonina, Ischnochitonidae: Schizoplacinae, Callochitoninae y Lepidochitoninae; E.J. Brill/W. Backhuys: Leiden, The Netherlands, 1985. [Google Scholar]
- Hammer, Ø.; Harper, D.A. Past: Paleontological statistics software package for educaton and data anlysis. Palaeontol. Electron. 2001, 4, 1. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Assis, J.; Fernández Bejarano, S.J.; Salazar, V.W.; Schepers, L.; Gouvêa, L.; Fragkopoulou, E.; Leclercq, F.; Vanhoorne, B.; Tyberghein, L.; Serrão, E.A.; et al. Bio-ORACLE v3.0. Pushing marine data layers to the CMIP6 Earth system models of climate change research. Glob. Ecol. Biogeogr. 2024, 33, e13813. [Google Scholar] [CrossRef]
- Bernal-Durán, V.; Donoso, D.; Piñones, A.; Jonsson, P.R.; Benestan, L.; Landaeta, M.F.; Naretto, J.; Gerard, K.; Haye, P.A.; Gonzalez-Wevar, C.; et al. Combining population genomics and biophysical modelling to assess connectivity patterns in an Antarctic fish. Mol. Ecol. 2024, 33, e17360. [Google Scholar] [CrossRef]
- Clarke, K.R. Nonparametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar]
- Sirenko, B.I. Report on the present state of our knowledge with regard to the chitons (Mollusca: Polyplacophora) of the Magellan Strait and Falkland Islands. Venus 2006, 65, 81–89. [Google Scholar] [CrossRef]
- Schwabe, E.; Försterra, G.; Häussermann, V.; Melzer, R.R.; Schrödl, M. Chitons (Mollusca: Polyplaophora) from the southern Chilean Comau Fjord, with reinstatement of Tonicia calbucensis Plate, 1897. Zootaxa 2006, 1341, 1–27. [Google Scholar] [CrossRef]
- Gray, J.S. Marine diversity: The paradigms in patterns of species richness examined. Sci. Mar. 2001, 65, 41–56. [Google Scholar] [CrossRef]
- Poulin, E.; Palma, A.T.; Féral, J.P. Evolutionary versus ecological success in Antarctic benthic invertebrates. Trends Ecol. Evol. 2002, 17, 218–222. [Google Scholar] [CrossRef]
- Kauffman, T.A. Seasonality and disturbance in benthic communities. Arthur Harbour, Antarctic Peninsula. Antarct. J. USA 1974, 9, 307–310. [Google Scholar]
- Barnes, D.K.A. The influence of ice on polar nearshore benthos. J. Mar. Biol. Assoc. UK 1999, 79, 401–407. [Google Scholar] [CrossRef]
- Gutt, J. On the direct impact of ice on marine benthic communities, a review. Polar Biol. 2001, 24, 553–564. [Google Scholar] [CrossRef]
- Amsler, C.D.; Amsler, M.O.; Klein, A.G.; Galloway, A.W.; Iken, K.; McClintock, J.B.; Heiser, S.; Lowe, A.T.; Schram, J.B.; Whippo, R. Strong correlations of sea ice cover with macroalgal cover along the Antarctic Peninsula: Ramifications for present and future benthic communities. Elem. Sci. Anthr. 2023, 11, 00020. [Google Scholar] [CrossRef]
- Ibáñez, C.M.; Sellanes, J.; Pardo-Gandarillas, M.C. Diversidad de poliplacóforos tropicales del sur de la Provincia Panameña. Lat. Am. J. Aquat. Res. 2016, 44, 807–814. [Google Scholar] [CrossRef]
- Aldea, C.; Valdovinos, C. Moluscos del intermareal rocoso del centro-sur de Chile (36°–38° S): Taxonomía y clave de identificación. Gayana 2005, 69, 364–396. [Google Scholar] [CrossRef]
- García Ríos, C.I.; Álvarez Ruiz, M. Comunidades de quitones (Mollusca: Polyplacophora) de la Bahía de La Paz, Baja California Sur, México. Rev. Biol. Trop. 2007, 55, 177–182. [Google Scholar] [CrossRef]
- Jörger, K.M.; Meyer, R.; Wehrtmann, I.S. Species composition and vertical distribution of chitons (Mollusca: Polyplacophora) in a rocky intertidal zone of the Pacific coast of Costa Rica. J. Mar. Biol. Assoc. UK 2008, 88, 807–816. [Google Scholar] [CrossRef]
- Castellanos, Z.J.A. Catálogo de los poliplacóforos argentinos y de aguas vecinas al estrecho de Magallanes. Rev. Mus. Plata 1956, 6, 465–486. [Google Scholar]
- Moffat, C.; Beardsley, R.C.; Owens, B.; Van Lipzig, N. A first description of the Antarctic Peninsula Coastal Current. Deep-Sea Res. II Top. Stud. 2008, 55, 277–293. [Google Scholar] [CrossRef]
- Moffat, C.; Meredith, M. Shelf–ocean exchange and hydrography west of the Antarctic Peninsula: A review. Philos. Transact. A Math. Phys. Eng. Sci. 2018, 376, 20170164. [Google Scholar] [CrossRef] [PubMed]
- Sirenko, B.I. The enigmatic viviparous chiton Calloplax vivipara (Plate, 1899) (Mollusca: Polyplacophora) and a survey of the types of reproduction in chitons. Russ. J. Mar. Biol. 2015, 41, 24–31. [Google Scholar] [CrossRef]
- Thiel, M.; Gutow, L. The ecology of rafting in the marine environment. II. The rafting organisms and community. Oceanogr. Mar. Biol. 2005, 43, 279–418. [Google Scholar]
- Waters, J.M.; King, T.M.; Fraser, C.I.; Craw, D. An integrated ecological, genetic and geological assessment of long-distance dispersal by invertebrates on kelp rafts. Front. Biogeogr. 2018, 10. [Google Scholar] [CrossRef]
- Fraser, C.I.; Nikula, R.; Waters, J.M. Oceanic rafting by a coastal community. Proc. R. Soc. B 2011, 278, 649–655. [Google Scholar] [CrossRef] [PubMed]
- González-Wevar, C.A.; Poveda, Y.; Segovia, N.I.; Rosenfeld, S.; Maturana, C.S.; Jeldres, V.; Poulin, E. Both high and low dispersal? Apparently contradictory genetic patterns in the Antarctic littorinid gastropod Laevilacunaria antarctica. Front. Ecol. Evol. 2024, 11, 1320649. [Google Scholar] [CrossRef]


| Localities | Tonicina zschaui | Hemiarthum setulosum | Leptochiton kerguelensis | Callochiton bouveti | Total |
|---|---|---|---|---|---|
| Weddell Sea | 46.25 | 2.25 | 0.50 | 0.25 | 49.25 |
| West Peninsula | 62.25 | 40.50 | 35.50 | 138.25 | |
| King George Island | 29.75 | 30.38 | 17.63 | 0.25 | 78.00 |
| Nelson Island | 11.75 | 0.25 | 12.00 | ||
| Greenwich Island | 33.75 | 2.75 | 2.13 | 38.63 | |
| Doumer Island | 90.25 | 10.00 | 100.25 | ||
| Margarite Bay | 84.00 | 14.00 | 98.00 | ||
| Total | 358.00 | 75.88 | 79.75 | 0.75 | 514.38 |
| Localities | Richness | iChao1 | Abundance | Dominance | Simpson | Shannon |
|---|---|---|---|---|---|---|
| Weddell Sea | 4 | 4 | 49.25 | 0.8817 | 0.1183 | 0.3039 |
| West Peninsula | 3 | 3 | 138.25 | 0.3498 | 0.6502 | 1.075 |
| King George Island | 4 | 4 | 78.00 | 0.3397 | 0.6603 | 1.109 |
| Nelson Island | 2 | 2 | 12.00 | 0.9555 | 0.0445 | 0.1429 |
| Greenwich Island | 3 | 3 | 38.63 | 0.7655 | 0.2345 | 0.4915 |
| Doumer Island | 2 | 2 | 100.25 | 0.8186 | 0.1814 | 0.3295 |
| Margarite Bay | 2 | 2 | 98.00 | 0.7526 | 0.2474 | 0.4152 |
| Composition | Coefficient | S.E. | t-Value | p-Value | R2 | VIF |
|---|---|---|---|---|---|---|
| Latitude | 0.0680 | 0.0273 | 2.4870 | 0.0272 | 0.2372 | 1.3110 |
| Temperature | −0.0995 | 0.1955 | −0.5091 | 0.6192 | 0.0169 | 1.0172 |
| Salinity | −0.0672 | 0.1517 | −0.4426 | 0.6653 | 0.0404 | 1.0421 |
| Oxygen | 0.0123 | 0.0090 | 1.3710 | 0.1936 | 0.0342 | 1.0354 |
| Chlorophyll-a | −0.1635 | 0.1899 | −0.8610 | 0.4048 | 0.0468 | 1.0491 |
| Ice thicknes | −1.5779 | 1.6731 | −0.9431 | 0.3628 | 0.0510 | 1.0537 |
| Ice cover | 0.8676 | 2.2719 | 0.3819 | 0.7087 | 0.0342 | 1.0354 |
| SPECIES RICHNESS | ||||||
| Latitude | −0.4047 | 0.1085 | −3.7293 | 0.0025 | 0.3994 | 1.6649 |
| Temperature | 0.3675 | 0.7762 | 0.4734 | 0.6438 | 0.0081 | 1.0082 |
| Salinity | −0.0269 | 0.6024 | −0.0447 | 0.9650 | 0.1146 | 1.1294 |
| Oxygen | −0.0475 | 0.0357 | −1.3310 | 0.2061 | 0.0119 | 1.0121 |
| Chlorophyll-a | 0.6220 | 0.7538 | 0.8252 | 0.4242 | 0.0012 | 1.0012 |
| Ice thicknes | 3.6689 | 6.6427 | 0.5523 | 0.5901 | 0.1183 | 1.1341 |
| Ice cover | −2.5471 | 9.0204 | −0.2824 | 0.7821 | 0.0971 | 1.1076 |
| ABUNDANCE | ||||||
| Latitude | −1.2762 | 4.2203 | −0.3024 | 0.7671 | 0.0544 | 1.0575 |
| Temperature | 18.1320 | 30.1910 | 0.6006 | 0.5584 | 0.0539 | 1.0569 |
| Salinity | −5.0339 | 23.4320 | −0.2148 | 0.8332 | 0.0009 | 1.0009 |
| Oxygen | −0.9239 | 1.3870 | −0.6661 | 0.5170 | 0.1204 | 1.1369 |
| Chlorophyll-a | 31.7970 | 29.3190 | 1.0845 | 0.2978 | 0.1663 | 1.1994 |
| Ice thicknes | 107.9300 | 258.3700 | 0.4177 | 0.6830 | 0.0100 | 1.0101 |
| Ice cover | −224.6300 | 350.8500 | −0.6403 | 0.5331 | 0.0005 | 1.0005 |
| DOMINANCE | ||||||
| Latitude | 0.0709 | 0.0326 | 2.1787 | 0.0484 | 0.0431 | 1.0451 |
| Temperature | −0.1234 | 0.2329 | −0.5300 | 0.6051 | 0.0573 | 1.0608 |
| Salinity | 0.0665 | 0.1808 | 0.3679 | 0.7189 | 0.0439 | 1.0459 |
| Oxygen | 0.0008 | 0.0107 | 0.0791 | 0.9382 | 0.0249 | 1.0255 |
| Chlorophyll-a | −0.2867 | 0.2262 | −1.2675 | 0.2272 | 0.1079 | 1.1210 |
| Ice thicknes | −0.1851 | 1.9933 | −0.0928 | 0.9275 | 0.0215 | 1.0220 |
| Ice cover | 1.0737 | 2.7068 | 0.3967 | 0.6980 | 0.0430 | 1.0449 |
| SIMPSON INDEX | ||||||
| Latitude | −0.0709 | 0.0326 | −2.1786 | 0.0484 | 0.0431 | 1.0451 |
| Temperature | 0.1234 | 0.2329 | 0.5299 | 0.6051 | 0.0573 | 1.0608 |
| Salinity | −0.0665 | 0.1808 | −0.3679 | 0.7189 | 0.0439 | 1.0459 |
| Oxygen | −0.0008 | 0.0107 | −0.0791 | 0.9382 | 0.0249 | 1.0255 |
| Chlorophyll-a | 0.2867 | 0.2262 | 1.2675 | 0.2272 | 0.1079 | 1.1210 |
| Ice thicknes | 0.1851 | 1.9933 | 0.0928 | 0.9274 | 0.0215 | 1.0220 |
| Ice cover | −1.0737 | 2.7068 | −0.3967 | 0.6980 | 0.0430 | 1.0449 |
| SHANNON INDEX | ||||||
| Latitude | −0.1349 | 0.0488 | −2.7629 | 0.0161 | 0.10832 | 1.1215 |
| Temperature | 0.2169 | 0.3493 | 0.6211 | 0.5453 | 0.05644 | 1.0598 |
| Salinity | −0.1165 | 0.2711 | −0.4297 | 0.6745 | 0.05073 | 1.0534 |
| Oxygen | −0.0003 | 0.0160 | −0.0162 | 0.9873 | 0.01365 | 1.0138 |
| Chlorophyll-a | 0.4915 | 0.3392 | 1.4489 | 0.1711 | 0.07157 | 1.0771 |
| Ice thicknes | 0.7275 | 2.9892 | 0.2434 | 0.8115 | 0.03169 | 1.0327 |
| Ice cover | −2.3599 | 4.0592 | −0.5814 | 0.5709 | 0.04516 | 1.0473 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibáñez, C.M.; Rosenfeld, S.; Carvajal, I.; Catalán, J.; Zapata-Hernández, G.; Gacitúa-Leible, M.; Vargas, R.; Morales, P.; Díaz, A.; Carrasco, S.A.; et al. Polyplacophoran Assemblages in Shallow Waters of the West Antarctic Peninsula: Patterns of Diversity, Composition and Abundance. Ecologies 2025, 6, 23. https://doi.org/10.3390/ecologies6010023
Ibáñez CM, Rosenfeld S, Carvajal I, Catalán J, Zapata-Hernández G, Gacitúa-Leible M, Vargas R, Morales P, Díaz A, Carrasco SA, et al. Polyplacophoran Assemblages in Shallow Waters of the West Antarctic Peninsula: Patterns of Diversity, Composition and Abundance. Ecologies. 2025; 6(1):23. https://doi.org/10.3390/ecologies6010023
Chicago/Turabian StyleIbáñez, Christian M., Sebastián Rosenfeld, Ivka Carvajal, Jennifer Catalán, Germán Zapata-Hernández, Manuel Gacitúa-Leible, Rocio Vargas, Pamela Morales, Angie Díaz, Sergio A. Carrasco, and et al. 2025. "Polyplacophoran Assemblages in Shallow Waters of the West Antarctic Peninsula: Patterns of Diversity, Composition and Abundance" Ecologies 6, no. 1: 23. https://doi.org/10.3390/ecologies6010023
APA StyleIbáñez, C. M., Rosenfeld, S., Carvajal, I., Catalán, J., Zapata-Hernández, G., Gacitúa-Leible, M., Vargas, R., Morales, P., Díaz, A., Carrasco, S. A., Sellanes, J., Mills, S., & Pardo-Gandarillas, M. C. (2025). Polyplacophoran Assemblages in Shallow Waters of the West Antarctic Peninsula: Patterns of Diversity, Composition and Abundance. Ecologies, 6(1), 23. https://doi.org/10.3390/ecologies6010023









