Abstract
The extreme conditions linked with abiotic stresses have greatly affected soil and plant health. The diverse biochemical activities occurring in the soil environment have been attributed to shaping the dynamics of plant–soil microbiomes by contributing to microbial lifestyles and enhancing microbial functional properties to boost plant tolerance to abiotic-induced stresses. Soil microbiomes play crucial roles in enhancing plant nutrition and abiotic stress management through diverse mechanisms. With the current insights into the use of engineered soil microbes as single or combined inoculants, their use has contributed to plant fitness and stability under different environmental stress conditions by activating plant defense mechanisms, enzyme production (lowering free radicals resulting in plant oxidative stress), protein regulation, and the production of growth factors. The detection of certain genes involved in the growth factors can underline microbial functions in mitigating plant stress. Hence, the projections for sustainable eco-friendly agriculture with the possible exploration of beneficial rhizosphere microbes to manage the effect of abiotic stress on plant nutrition remain critical points of discussion recently, with prospects for ensuring food security. Therefore, this review focuses on the impacts of soil microbiomes in abiotic stress mitigation for enhancing plant nutrition.
1. Introduction
The availability, accessibility, production, distribution, and consumption of quality food and food products containing appropriate nutrients are essential in maintaining the sustainable healthy living of human beings [1,2,3]. Agricultural food production remains fundamental in this era, with the view of ensuring food security based on the human population increase and high demand for foods without compromising health standards [4]. The core drivers of boosting crop productivity depend on the devisable modern agricultural practices through biorational intervention in maximizing bioresources for higher crop yield and food safety [5]. Nevertheless, global food security and agricultural productivity face diverse challenges [6]. For instance, soil contamination, loss of soil biodiversity, emergence and increase in phytopathogens and pest populations, human population increase, anthropogenic activities, urbanization, degradation of arable farmlands, excessive use of chemical fertilizers, limited nutrients, climate change, biotic and abiotic factors, etc., impose pressure on agriculture crops under stress [5,7,8]. To circumvent these challenges for sustainable food production, there is a need to formulate resilient agricultural practices through the exploration and adoption of microbial inoculants that can be best integrated into agricultural management for strategic implementation to improve crop production, reduce ecological degradation, strengthen plant resilient to climate-induced changes, and ensure food security for human health protection at present and in the future.
Worldometer recently revealed that the world population is currently around 8 billion and might double by 2080 (https://www.worldometers.info, accessed on 19 July 2024) due to the incessant progressions in human populations [9]. This phenomenon has brought about an increase in demand for food globally, thus threatening food security [10]. The availability of foods with adequate nutrients for the ever-growing population in the modern era is of necessity, with the target of achieving the SDGs [4]. The reduction in crop production and food supply due to a lack of suitable agricultural practices or soil usage has adversely affected soil fertility and soil microbial diversity and functionalities, opposing the aim of meeting the SDGs by 2030 [11]. Hence, to avert this menace and meet future food demands, there is a need for more intensification in agricultural food production.
Food security, safety, and availability can be achieved by following good agricultural practices [12]. Notably, agricultural problems are critical issues that need to be addressed to ensure food availability and reduce food loss/wastage in both developed and developing countries. The incorporation of engineered food crops and hybrid cultivars stands as a major key area in boosting global agricultural productivity [13]. In addition, the microbial biotechnological approaches, which encompass the exploration of agriculturally important microorganisms can be more promising in ensuring food security due to their eco-friendly and best alternatives to chemical fertilizers usage [14]. However, this bio-rational approach has been less explored. Consequently, addressing the problem of food wastage, the use of engineered crops, and the maximum exploration of potential microorganisms can assist in managing future agricultural problems and ensure food security.
Soil remains a flourishing econiche that supports plant growth and microbial activities [15]. The beneficial soil microbes tend to establish close interactions and confer benefits to plants ranging from the enhancement of plant nutrition, disease resistance, and resilience to biotic and abiotic stresses [16]. Soil harbors diverse microorganisms in high numbers (from millions to hundreds of millions) per gram of soil, which can either be beneficial, pathogenic, or without effects [2]. The beneficial types of microorganisms support plant physiological and metabolic functions, while the pathogenic types cause diseases in plants with a negative impact on crop yield [17]. Among the soil-inhabiting microbes, bacteria are known to be more dominant, and many studies have validated their ecological services in the ecosystems [18,19,20].
Soil microorganisms contribute to plant growth by diverse mechanisms, directly or indirectly [21]. The direct mechanism summarizes plant growth promotion through phytohormone production, improvement in nutrient uptake, and enhancement of soil structure and health, while the indirect mechanism summarizes the pathogens’ control and plant abiotic stress mitigating strategies for sustainable plant health and growth. Also, the direct mechanism encompasses nutrient bioavailability and uptake for plant use, nitrogen fixation, phytohormones stimulation, and ACC deaminase production, while the indirect mechanism reveals the phytopathogens’ control through siderophores production, antibiosis, hydrogen cyanide production, enzyme production, and induction of systemic resistance [22]. The soil microbiomes with the aforementioned mechanisms can be harnessed by testing their efficacy in vivo and in vitro on plant resilience to abiotic stress under experimental conditions. Furthermore, the use of drought-tolerant soil microbiomes for plant growth promotion and phytopathogen control to reduce agricultural loss has been reported with recommendations for massive production on a large scale and for commercialization [23,24].
The plant rhizosphere accounts for a high microbial population due to the nutrient pool in the plant root environment [25]. The microbial proliferation in the rhizosphere can be linked to the profuse secretion of exudates containing certain metabolites and carbon compounds, which provide energy for microbial metabolism and fixed carbon for photosynthesis [26]. The exudates that drive the microbial activities in the rhizosphere can mediate the infiltration of plant-growth-enhancing rhizobacteria from the external root environment into the plant endosphere to become endophytic bacteria [27]. Similarly, some root-nodule-inhabiting bacteria can establish symbiotic relationships with the plant roots, thereby increasing the nitrogen pool in the soil for plant use [28].
While many recent findings and works of literature have documented the positive implications of rhizosphere microbes in plant stress mitigation [29,30,31], it is imperative to dwell more on the current status and perspectives of rhizosphere microbiomes, their recruitment, and mechanisms in alleviating abiotic stresses for plant nutrition, which can be a gateway in achieving SDGs. Also, bridging researchers’ and farmers’ knowledge to develop long-term biorational approaches for soil health maintenance and plant nutrition can assist agriculturists and policymakers in the exploration and commercialization of rhizosphere bioinoculants to ensure eco-friendly agriculture and food security, which is the rationale behind this review.
Therefore, the Section 1 provides the concept of modern agricultural practices in addressing core issues challenging food security and agricultural productivity; Section 2 dwells on the microbiomes in the rhizosphere environments, and the focus of Section 3 is on the soil fertility reduction altering the soil microbiome interactions due to climate change. Section 4 provides more insights into the rhizosphere microbiome-mediated alleviation of abiotic stresses, while abiotic stresses, such as drought, salinity, heavy metals, heat and flooding mitigation, and ecological impacts on plants, are discussed in Section 5, and Section 6 provides updated information on the impact of biotechnologically engineered soil microbiomes on plants. In the Section 7, how engineered soil microbes with desirable plant-growth-promoting traits live and compete with the autochthonous microbiomes, their selection, and application under experimental conditions, with an emphasis on genomic studies, serve as a model in plant stress management is summarized and projected.
2. Microbiomes in the Rhizosphere Environments
The rhizosphere represents the discrete region of soil depth, 2–80 mm around or adjacent to the plant roots, while the rhizoplane stoutly represents the microhabitat closer to the surface of plant roots [32]. There are usually high levels of coordination and microbial activities in the rhizosphere, which safeguard plants from deleterious or adverse abiotic and biotic stresses. The rhizosphere–rhizoplane interface remains the nutritional hub that is majorly influenced by root exudation from the plant roots into the soil environment [33]. The exudates released can serve as energy-rich metabolites that drive the proliferation of diverse microorganisms in the rhizosphere compared with the bulk soil [34]. The abundance of secondary metabolic compounds in the rhizosphere can act as signal molecules to facilitate signaling communication between the soil and plant microbes in the root vicinity, thus helping them bypass the host defense mechanisms. Also, root exudation can primarily determine the microbial community dynamics, structures, and functions in designated plants under certain environmental conditions [35]. Predominantly, nitrogen-fixing bacteria, endo-rhizobacteria, and mycorrhiza fungi have been identified to form rhizosphere microbiomes [27,36]. The level of exudate secretion can determine microbial activities, thus influencing plant growth and other physiological functions. The microbiomes inhabiting various plant niches, especially the plant roots and soil types, have been researched and documented, showing possible use in sustaining agricultural productivity [37,38]. Soil microbial communities help plants acquire nutrients, ease disease attacks, and boost stress tolerance [39]. The multidisciplinary research interests in the rhizosphere–rhizoplane microbial communities with topmost priority can potentially assist in determining their functional diversity and potential to improve crop productivity. Moreover, the soil–microbe and plant–microbe interactions exhibiting positive influence by contributing to plant growth, health, and fitness have been reported recently for enhancing plant nutrition [40].
To date, diverse research efforts have been directed toward exploring beneficial rhizosphere microbiomes, such as mycorrhiza fungi and rhizobacteria with plant-growth-promoting traits for plant nutrition [41,42]. More than 80% of plants harbor beneficial arbuscular mycorrhiza fungi with great benefits in the uptake of soil phosphorus and plant root elongation. The biological processes occurring in the belowground that shape the soil–microbe and plant–soil interactions, termed plant–microbe–soil feedback (PMS), have been recognized as key drivers mediating plant microbial community structure and soil nutrient profiling [43]. Rhizo-microbiome contributes to plant growth and the maintenance of soil health by responding to changes in environmental conditions, as these determine the composition of microbial communities belowground and plant root environments [44]. Depending on the environmental conditions, the microbiome inhabiting the rhizosphere and rhizoplane can structurally show differences in terms of diversity and functions. However, factors, such as root architecture, plant genotype, developmental stages, species, cultivars, soil parameters, and agricultural practices have been attributed to contributing to the microbial communities across rhizome systems [45].
The rhizosphere–rhizoplane should not be considered only a battleground for roots and soil pathogens but also an important region that hosts roots and beneficial microbes. Owing to the chemoattractant signal molecules ascribed to root exudates release, and root surfaces that support microbial attachment to the rhizoplane, roots are easily colonized with different microbes [44]. The rhizoplane microbiomes can partner with plant roots beneficially, neutrally, or pathogenically, thus significantly contributing to plant growth and immunity. The beneficial types contribute to plant growth and other biological activities in ecosystems, such as biogeochemical activity and the recycling of mineral elements in the soil [46].
The microorganisms found in the rhizosphere–rhizoplane can independently survive and withstand various environmental factors, thus assuring their recruitment as suitable candidates for various industrial and agricultural applications [47]. The complex nature of soil–root-associated microbiomes has been a major challenge in environmental science, and assessing their potentialities to advance agricultural productivity has necessitated research approaches to understanding their biomass in soil and their activity in alleviating abiotic stresses. Root–soil microbe interactions elicit some reactions in the rhizosphere that influence the belowground ecosphere from the diminutive up to the macro scale. The microbial dynamics in terms of space and time, as well as their functional traits, naturally influence or are induced by anthropogenic activities [48].
The activities of the rhizome microbiome depend on the chemical exudates that mediate soil microbes and plant–microbe interactions via chemo-stimulation of signal molecules produced by plants and microbes [49]. The root exudates influence rhizosphere microbial structure and functions, as well as plant selection and its beneficial rhizome–microbiome partners. The exudates secreted from plant roots have been acknowledged as key point signals that mediate root–microbe interactions and their rate of colonization to the surfaces [50]. Through research findings, it is expected that far more chemo-attractant signal molecules from plant roots need to be identified and applied to increase the rate of root colonization by beneficial microbes for improved crop yield. The induction of Bacillus subtilis root colonization by methyl salicylate secreted from the root of plants has been reported to advance microbial root colonization [51].
Nevertheless, the recent focus on rhizosphere microbial communities can help to unlock their opportunities with a broader perspective and for bioprospecting in agriculture. The pivotal means for shaping and recruiting the rhizosphere–rhizoplane microbiome from the soil can be achieved via (i) stimulation of root exudates and (ii) high rhizo-deposition adjacent to plant roots that either support or inhibit microbial processes [52]. Notably, the rhizosphere–rhizoplane microbiome does not only explore the exudates released from plant roots but also produces some signal molecules in the right proportion that enhance plant growth and plant response to abiotic stresses.
The recruitment of microorganisms in the plant root environments can be mediated by the chemo-attractants’ secretions and certain genes involved in the attachment organelles synthesis in the microbial cells. Notably, flavonoids, strigolactones, and malic acid, which act as signal molecules in plants, can facilitate recruitment and plant–microbial synergisms. The recruitment of some microorganisms with drought-tolerance potential by the production of reactive oxygen species (ROS), jasmonic acid, salicylic acid, ethylene, and defensive enzymes has been reported to support plant–root architecture under drought-induced stress [53]. The recruitment of certain arbuscular fungi in plants has been linked to the secretion of allelopathic substances, such as arachidonic acid [54]. The presence of these compounds contributes to microbial symbiosis, thereby fostering positive interactions with plant growth promoters and antibiosis against phytopathogens. Notably, the exudates from plants with antimicrobial activities have presented some plants as a model in shaping their microbial world. Certain genes have been reported to mediate root exudation in plants, and any alteration to these genes can cause major shifts in exudation patterns, thereby influencing microbial interactions in the rhizosphere [55]. Hence, exudate production can exert harmful effects on plants. Furthermore, any imbalances occurring in plant–root environments can result in soil-borne disease outbreaks, rhizosphere pathogen accumulation, root cell death, soil profiling imbalance, and uneven distribution of microbiomes in the rhizosphere.
The organic compounds that serve as a source of carbon can easily be transformed in the soil through biological and chemical processes [56]. The microbes in the root environment can respond to the magnitude of carbon deposit in the rhizosphere by acting as intermediates in the recycling of elemental compounds for improved plant nutrition and the alleviation of abiotic-induced stresses [27,57]. Also, the addition of naturally produced organic compounds to the plant roots can boost microbial efficiency and synergistic effects in the rhizosphere compartments for healthy plant growth promotion [58]. Notably, the selection of microbes in the soil–root interface containing abundant root exudates enriched with organic compounds can help shape microbial dynamics and functions in the rhizosphere [9]. The presence of certain genes involved in the production of metabolic compounds in the microbial genome can mediate rhizosphere microbial functions in various metabolic pathways necessary for plant growth, tolerance to abiotic stress, photosynthesis, and energy conservation [59].
The organic compound production in the rhizosphere can be of importance in maintaining the root environment sustainably by serving as signaling molecules, biomass attractants, microbial stimulators, and inhibitors [60]. This also can improve nutrient bioavailability to soil–root-associated microbes by readily making nutrients available for microbial growth and metabolism [61]. Furthermore, the release of organic compounds, such as fumaric and malic acid, has been documented to facilitate the recruitment of beneficial soil microbes, with a positive impact on plant health [62].
The pool of organic compounds in the root environment can promote the colonization tendencies of beneficial plant-growth-promoting rhizobacteria (PGPR) to the plant roots and rhizosphere, which in turn can stabilize plant–soil–microbe interactions, nutrient absorption from the soil, biocontrol of phytopathogens, and contribute to a soil structure that supports microbial life [63,64]. The organic compounds in the rhizosphere can help examine (i) molecular and biochemical mechanisms in plants by understanding the response of beneficial microorganisms to organic compounds, the attraction of rhizome–microbiomes, chemotactic pathways, and abundance in root exudate; (ii) plant growth modifications through phytohormone production, abiotic stress tolerance, nutrient uptake, and solubilization; (iii) biocontrol of pathogens through suppression, competition for niche, biotic stress resistance, predation, and induce systemic resistance; (iv) synergistic approaches through the understanding of tailored approaches to rhizosphere enhancement, manual addition of organic compounds, variation in effectiveness across organic compounds, factors influencing special effectiveness contradiction and challenges; and (v) future direction through microbial engineering and molecular approaches [58].
3. Soil Fertility Reduction Alters Soil Microbiome Interactions Due to Climate Change
Climate change is a pressing global challenge resulting from the emission of greenhouse gases (GHGs), such as (N2O), methane (CH4), and carbon dioxide (CO2). The resulting alterations in temperature patterns, precipitation regimes, and extreme weather events have profound implications on terrestrial ecosystems, including soil fertility. Soil, as a fundamental component of terrestrial ecosystems, functionally supports plant growth, regulating water and nutrient cycles and sustaining biodiversity. Therefore, any changes in soil fertility induced by climate change can have far-reaching consequences for ecosystem functioning and food production [65]. Understanding the impact of environmental stressors (biotic and abiotic) on plant morphological traits is crucial for predicting how species, communities, and ecosystems respond to global climate change. In temperate deciduous trees, one such intricate trait is bud break phenology, which is primarily influenced by the interplay of high/extreme temperatures, photo/light period, and the plant’s genetic makeup. The influence of climate-induced changes on soil microbiomes alters their synergistic interactions with plants, consequently impacting indirect plant physiological traits and underscoring the intricate nature of biotic interactions within the soil environment. Various anthropogenic activities have led to soil fertility loss and decreased production [66,67].
Various alternative techniques for soil nutrient reclamation can be employed to improve soil nutrient profiling. A significant approach involves the use of microbial inoculants and organic fertilizers [2]. Different methods are effective in enhancing soil fertility through the utilization of diverse bacteria and fungi. By utilizing multifaceted microbes capable of improving the bioavailability of soil nutrients, such as nitrogen, phosphorus, potassium, and iron, soil fertility can be enhanced [68]. Additionally, certain microbes aid in nutrient mobilization from the rhizosphere to plants [69,70]. The utilization of microbial inoculants also contributes to the restoration and recovery of nutrient-limiting soil, which can be achieved through direct or indirect mechanisms [71,72].
Soil microbes can influence the circadian clock, thereby impacting the timing of plant phenological traits and the acquisition of essential nutrients [73]. The overall plant performance in response to biotic stress can be influenced by the relationship between soil and microbes. For example, the flowering time of Boechera stricta has been documented to be influenced by soil-inhabiting microbes and other biotic factors. The delay in the flowering time of Arabidopsis thaliana has also been linked to indole acetic acid (IAA) production by microorganisms and downregulation of the gene involved in the early flowering of the plant [74,75,76]. Rhizosphere microbiomes play important roles in boosting plant tolerance to environmental stressors. In one particular study, the ectomycorrhizal fungi (EMF) inoculation of Pinus edulis seedlings showed consistency in the plant growth rate under drought experimental conditions compared with normal conditions [77]. However, in drought conditions, the growth rate of drought-tolerant seedlings increased by 25%. This study confirmed the hypothesis that soil microbial communities significantly influence plant phenotypes and their responsiveness to abiotic-induced stress. Nevertheless, the precise impact of the soil-inhabiting microbes on plant physiological functions and genetic trait factors remains unclear [78,79].
Climate-induced stress also negatively affects the rate of root exudation, with elevated CO2 levels resulting in a high carbon pool in the root environment, thus causing alteration to the root exudate components (Figure 1). Consequently, the ratio of plant signaling chemical attractants and the carbon–nitrogen ratio have been influenced by climate change. Additionally, this has led to changes in the dynamics and activities of microorganisms below ground level. Therefore, evidence from the works of literature by researchers has established the implications of climate change on microbial ecological mutual interactions in the rhizosphere [20,80]. The ability of certain beneficial microorganisms to colonize the soil–root interface under environmentally induced stresses can mediate their functionalities for improving plant nutrition sustainably. The alteration in the environmental conditions can bring a change in the plant physiology and its associated microbial world [81]. The soil microbiome in the soil–root environment can influence the rhizo-compartments in response to the nutrient bio-availability and acquisition, which can, however, facilitate their colonization and penetration into the plant root endosphere, conferring beneficial effects on the host plant to improve yield [82,83].
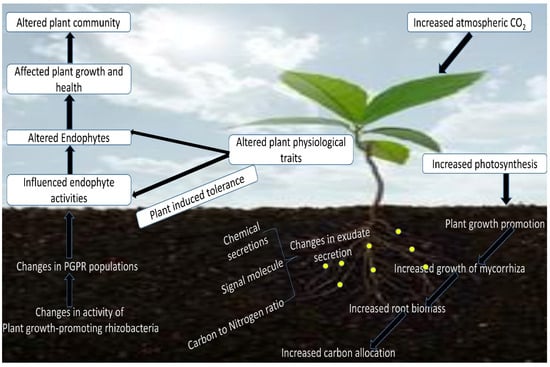
Figure 1.
Effect of increased atmospheric CO2 concentration on plant crop.
4. Rhizobiome-Mediated Alleviation of Abiotic Stresses
In environments characterized by abiotic stress, microbial interaction with plants is crucial for their survival. The phenomenon of microorganisms mediating the induction of plant response to abiotic stress is referred to as Induced Systemic Tolerance (IST). Through their metabolic and genetic capabilities, the microbiomes help plants alleviate abiotic stress [84].
Studies have shown that among the most notable inhabitants of the rhizosphere that contribute to mitigating various abiotic stresses in plants are genera such as Cyanobacteria [85], Trichoderma, Burkholderia [86], Methylobacterium [87], Bradyrhizobium [88], Enterobacter, Bacillus, Pantoea, Rhizobium, Pseudomonas [89], Azospirillium [90], and the Azotobacter group [91]. To address constraints on crop productivity, a viable approach involves selecting, screening, and applying microorganisms capable of adapting to stress conditions. One of the notable species is Trichoderma, which has been extensively researched in this context. In a particular study, Trichoderma harzianum was employed to mitigate stress induction in rice by malondialdehyde, dehydrin, and the upregulation of aquaporin [92]. T. harzianum was also utilized to increase oil production in Brassica juncea grown in a salinity environment. This application, as indicated by the results, also enhanced nutrient acquisition/uptake, increased antioxidant accumulation, and reduced Na+ uptake [93]. The application of Acinetobacter sp. and Pseudomonas sp. producing ACC-deaminase and IAA has been reported to improve barley and oat yield [94]. Furthermore, drought mitigation in maize and wheat has been linked to the use of Burkholderia phytofirmans PsJN as bioinoculants [95].
The root region’s soil microenvironment is rich in microbes, hosting a variety of nutrients, minerals, and metabolites. Plant root secretions play an important role in influencing microbial colonization in the soil–root environment. Microbes are attracted to root exudates and move towards them through chemotactic responses, which drive the establishment of microbial biomass in the root zone. PGPR acts as biofertilizers, biocontrol agents, and phytostimulators, thus leveraging the advantages of the rhizosphere compartments. PGPRs rely on their abilities, mode of interaction, and surrounding circumstances. Bacteria stimulate plant growth directly or indirectly [96]. The direct synthesis of bacterial compounds is advantageous for boosting the nutrient pool in soil. Rhizobacteria also contribute to the production of plant growth traits, such as hydrogen cyanide, ACC deaminase, and IAA, which supports plants to withstand stress. These growth-promoting factors bolster growth and mitigate the excessive inhibitory effects of stress ethylene on plant growth [97,98,99].
The root-associated endophytic fungus, Piriformospora indica has been identified to cause salt tolerance induction in barley and drought tolerance induction in Chinese cabbage, respectively. Microbes assist plants in sustaining their growth and health, particularly under harsh conditions. These functions of rhizosphere microbes render them robust and effective in helping plants combat abiotic stress. Several studies have investigated the contribution of microbiomes to alleviating abiotic stress in crop plants [22,29,100]. Certain soil-dwelling microbes, including Pseudomonas, Azospirillum, Klebsiella, Achromobacter, Variovorax, Enterobacter, Azotobacter, Aeromonas, and Bacillus, have been shown to boost plant resilience to unfavorably harsh conditions [101,102,103]. These soil-bacteria-producing indole acetic acids (IAAs) exert root initiation and growth. However, elevated auxin levels can adversely affect plant root architectural growth. The rhizobacterial mechanisms in the management of abiotic-induced stresses in plants are presented in Table 1.
5. Abiotic Stresses Mitigation and Ecological Impacts on Plants
In recent times, the need to address natural or induced abiotic stressors that threatened plant growth and crop productivity became important by finding a possible solution to avert their negative impact on the ecosystem [22]. The negative influence of nonliving components beyond normal that alters the composition and performance of living components in econiches can be responsible for abiotic stress induction. The various abiotic stressors may exert different effects on the ecosystem, such that plants may tend to develop resistance depending on the stress conditions [29]. Hence, the adequate management of abiotic-induced stresses can be the key to maintaining plant and soil health sustainably. The diverse mechanisms of plant responses to certain environmental conditions may be linked to the expression of certain genes involved in biochemical, physiological, cellular, and molecular modifications [104]. Abiotic stress factors display identical effects on plants, which include the deterioration of plant cellular and molecular components, the loss of membrane stability, nucleic acids, proteins, and lipid denaturation, production of ROS, and the alteration of enzyme activity by reducing crop yield and productivity [105].


Table 1.
Plant mechanisms to tolerate abiotic stress.
Table 1.
Plant mechanisms to tolerate abiotic stress.
| Abiotic Stress/Metal Toxicity | Microbial Inoculant | Host Plant | Tolerance Type | References |
|---|---|---|---|---|
| Zn, Pb, Cu, AS, and Cd toxicity | Pseudomonas koreensis AGB-1 | Miscanthus sinensis | IAA production and ACC deaminase | [106] |
| Hg toxicity | Photobacterium spp. | Phragmites australis | Mercury reductase and IAA activity. | [107] |
| Zn toxicity | Pseudomonas aeruginosa | Triticum aestivum | Soluble protein, N and P uptake, and improved biomass. | [108] |
| Arsenic toxicity | Staphylococcus arlettae | Brassica juncea | Phosphorus bioavailability in soil through an increased dehydrogenase and phosphatase activities | [109] |
| Heat | Bacillus amyloliquefaciens, Azospirillum brasilence | Triticum aestivum | Decreased generation of ROS, alterations in the metabolome, and preactivation of heat shock transcription factors | [110] |
| Drought | Pseudomonas chlororaphis O6 | Arabidopsis thaliana | Production of volatile compounds, i.e., 2R, 3R butanediol | [111] |
| Drought | Burkholderia phytofirmans Enterobacter sp. FD17 | Zea mays | Increased root and shoot biomass and photosynthesis under drought conditions. | [112] |
| Salinity | Azospirillum brasilense strain Cd | Phaseolus vulgaris | Production of root exudate and flavonoids | [113] |
| Salinity | Bacillus subtilis | Arabidopsis | Decreased root transcriptional expression of a high-affinity potassium ion (K+) transporter (AtHKT1) and decreasing root sodium ion (Na+) import | [114] |
| Salinity | Pantoea dispersa, Azospirillum brasilense | Capsicum annuum | High stomatal conductance and photosynthesis | [115] |
| Salt | Burkholderia, Arthrobacter Bacillus | Vitis vinifera, Capsicum Annuum | Increased accumulation of proline | [116] |
| Osmotic stress | Bacillus megaterium | Zea mays | Increased root expression, high hydraulic conductance, and ZmPIP isoforms | [117] |
| Salt | Cyanobacteria and cyanobacterial extracts | Zea mays, Oryza sativa, Triticum aestivum, Gossypium hirsutum | Production of phytohormones, eliciting molecules | [118] |
| Salt | Bacillus megaterium, Pseudomonas jaduguda, Paenibacillus cookii | Typha angustifolia | Production of growth-promoting and abiotic induction stress genes | [119] |
| Salt | Pseudomonas simiae | Glycine max | Secondary metabolite biosynthesis, i.e., 4-nitroguaiacol and quinolone and stimulation of growth factors | [120] |
| Salt | Bacillus subtilis GB03 | Arabidopsis thaliana | Upregulation of genes involved in sodium transport | [114] |
Soil microbes employ different strategies to alleviate the impact of abiotic-induced stress on plants [121]. For instance, the function of identifiable rhizobacteria in boosting soybean tolerance to drought stress has been reported to contribute to crop yield [122]. Soil microbes can infiltrate plant roots to become endophytic microbes to form endo-rhizosphere mutualists [123]. Findings have established the mutual interdependence of soil microbes with the host plants and their promise to sustain plant growth in diverse environments [19,124]. Aside from bioremediation, the expression of stress genes by endophytic microbes helps scavenge ROS and the production of antistress metabolites, as well as dissolvable solutes in plants. The phytohormone production by soil microbes and other organic acids, such as jasmonic acid, brassinosteroid, and salicylic also function to circumvent the effects of abiotic plant stress [125]. Similarly, some related findings on rhizo-endophytic microbes have proven to reduce abiotic stress in plants (Table 2). For example, Ghazi et al. [126] reported an evaluation of the plant bacterium, Klebsiella variicola, upon inoculation, to improve the physiological status and salt stress tolerance in tuberose. Also, findings by Ulrich et al. [127] on the genomic analysis of Stenotrophomonas sp., an endophytic bacterium, revealed notable genes involved in oxidative stress, which suggested the ability of the endophytic bacterium to enhance the growth of poplar trees and tolerance to abiotic drought-induced stress.
The activities of endophytic bacteria, such as Pseudomonas migulae, Pantoea ananatis, Bacillus lentus, Stenotrophomonas rhizophila, and Herbaspirillum lusitanum, have been reported to stimulate plant growth upon exposure to drought stress over time [128,129,130]. Moreover, the interrelatedness of endophytic bacteria within the host plants can be greatly affected by abiotic stressors.


Table 2.
Rhizospheric microbes involved in plant abiotic stress alleviation.
Table 2.
Rhizospheric microbes involved in plant abiotic stress alleviation.
| Type of Microbes | Abiotic Stress | Plant Host | Mechanism of Action | References |
|---|---|---|---|---|
| Azospirillum lipoferum | Salinity | Cicer arietinum | It helps in the expression of genes related to antioxidants, osmolyte production, and stress | [131] |
| Bacillus sp. | Salinity | Solanum tuberosum | The bacteria increased the production of antioxidant enzymes and auxin. They also increased the uptake of K+, Ca2+, and Na+ | [132] |
| Kocuria rhizophila, Cronobacter sakazakii | Salinity | Triticum aestivum | The microbes enhanced the production of AAC deaminase and antioxidant enzymes | [133] |
| Curtobacterium albidum | Salinity | Oryza sativa | The microbe promotes photosynthetic ability and antioxidative properties | [134] |
| Enterobacter cloacae | Salinity | Brassica napus | The microbe promotes hormonal balancing in plants | [135] |
| Burkholderia sp. | Salinity | Oryza sativa | The organism reduces the production of osmolytes, ROS, and stress, while also increasing the morphological and biochemical parameters | [136] |
| Alcaligenes faecalis | Salinity | Arabidopsis thaliana | The microbes modulate ion transporters and hormonal pathways | [137] |
| Pseudomonas frederiksbergensis, Pseudomonas vancouverensis | Salinity | Capsicum annuum | The organisms modulate stress ethylene levels and increase the antioxidant enzyme activities and plant physiological properties | [138] |
| Bacillus Firmus | Salinity | Glycine max | The organism produces antioxidants and works through gene expression | [139] |
| Curtobacterium sp., Pseudomonas putida, Acinetobacter sp. | Salinity | Sulla carnosa | The microbe reduces oxidative stress and increases the acquisition of plant nutrients from the soil | [140] |
| Klebsiella sp. | Salinity | Avena sativa | The organism helps to enhance the modulation of WRKY1 and rbcL genes | [141] |
| Rhizobacter, Azotobacter | Salinity | Cicer arietinum | Boosting plant salt tolerance for improved yield | [142] |
| Bacillus | Salinity | Oryza sativa | Secretion of regulating hormones and antioxidants for plant resilience to salt stress | [143] |
| Aneurinibacillus, aneurinilyticus, Paenibacillus sp. | Salinity | Phaseolus vulgaris | Production of ACC deaminase gene for plant tolerance to salinity | [144] |
| Streptomyces spp. | Salinity | Steva rebaundia | Microbial stability against salt stress and secretion of plant growth hormones | [145] |
| Ochrobacetrum sp., Microbacterium sp., Enterobacter sp., Enterobacter cloacae | Drought | Poncirus trifoliate | The organisms help to solubilize phosphorus and are involved in ACC deaminase activity, N-fixation, siderophore and IAA production, and P-solubilization | [146] |
| Bacillus thuringiensis | Drought | Zea mays | Enhancing water and nutrient absorption, osmotic balance | [147] |
| Bacillus subtilis | Drought | Triticum aestivum L. | Augmentation of the antioxidant defense system and sugar production. Stimulation of stress-associated gene expression, synthesis of plant hormone | [148] |
| Rhizophagus clarus | Drought | Glycine max | Augmenting water and nutrient assimilation, osmotic balance | [149] |
| Pseudomonas putida | Drought | Cicer arietinum | The microbe helps in the expression of miRNAs | [150] |
| Achromobacter sp., Variovorax paradoxus, Pseudomonas sp., Ochrobactrum anthropic | Drought | Triticum aestivum and Eleusine coracana | The organisms increased the ACC deaminase activity, foliar nutrient concentrations, and enzymatic/nonenzymatic machinery antioxidants | [151] |
| Streptomyces laurentii, Penicillium sp. | Drought | Sorghum bicolor | The bacteria helped to increase the production of siderophores, ammonia, IAA, and hydrogen cyanide. They also help increase the solubilization of zinc and phosphorus | [152] |
| Pseudomonas sp. | Drought | Eleusine coracana | The microbe enhanced the nutrient concentrations and the antioxidant properties of the plants | [153] |
| Pseudomonas azotoformans | Drought | Triticum aestivum | The organism enhances ACC deaminase activity, EPS and IAA production, expression of biofilm genes, and P-solubilization | [154] |
| Pseudomonas sp., Variovorax paradoxus | Drought | Triticum aestivum | These organisms promote the upregulation of helicases and aquaporin and increase ACC deaminase activity and antioxidant properties | [155] |
| Bacillus cereus, Pseudomonas sp. | Drought | Glycine max | The microbe promotes the production of ammonia, the solubilization of phosphorus, and ACC deaminase activity | [156] |
| Arthrobacter arilaitensis, Streptomyces pseudovenezuelae | Drought | Zea mays | The organisms act by solubilizing phosphorus, producing ammonia, IAA, and siderophore through ACC deaminase activity | [157] |
| Rhizobium sp. | Drought | Glycine max | Production of ACC deaminase and drought-resistant genes | [122] |
| Alcaligenes faecalis, Bacillus cereus, Alcaligenes faecalis | Heavy metals (lead, zinc, copper, and cadmium) | Sorghum bicolor | The organisms promote the production of siderophores, proline, EPS, biosurfactants, and salicylic acid | [158] |
| Azotobacter chroococcum | Heavy metals (copper and lead) | Zea mays | The organism facilitates heavy metal chelation | [159] |
| Pseudomonas, Burkholderia | Heavy metals | Lolium perenne | Plant-growth promotion, stress reduction, and metal sequestration, promoting traits under stress | [160] |
| Bacillus | Heavy metal (chromium) | Brassica nigra | Improving plant tolerance to osmotic stress and microbial genes enhancing plant growth | [161] |
| Bacillus | Heavy metals (cadmium and lead) | Solanum nigrum | Production of plant growth hormones, notable genes involved in the removal of pollutants through various pathways | [162] |
| Alcaligenes faecalis, Bacillus Amyloliquefaciens | Heavy metal (lead) | Mentha piperita | Improvement in growth attributes, nutrient concentration, and mitigation of Pb toxicity in mint | [163] |
| Bacillus | Heavy metal (cadmium) | Triticum aestivum L. | Augmentation of the antioxidant defense system and sugar production. Stimulation of stress-associated gene expression and synthesis of plant hormones | [164] |
| Bacillus sp., Enterobacter sp., Pseudomonas fluorescens | Heat | Zea mays | Thermal stress, protein production, antioxidant synthesis | [165] |
| Bacillus safensis | Heat | Solanum lycopersicum | Secretion of thermal regulating genes and antioxidant biosynthesis | [77] |
| Bacillus safensis, Pseudomonas fluorescens | Heavy metals (zinc and lead), temperature, drought | Helianthus annus | Heavy metal binding and elimination of toxins | [166] |
The endosymbiotic relationships of bacteria with host plants under a limiting environment can facilitate microbial recruitment; however, increasing solutes in plants confers plant resistance against induced stressors and inhibits shoot growth [167]. For soil microbes to effectively impact stress tolerance in plants, two major steps have been identified, which include (a) self-stimulation of antistress metabolites and (b) stress activation mechanisms in host plants. A decrease in osmolyte and electrolyte leakage is one of the common strategies employed by a drought-surviving plant to enhance the metabolic activities of soil microbes which support the escape of plants from deleterious effects when subjected to drought responsiveness [168]. For instance, an increase in the proline and chlorophyll contents of Thlaspi arvense cultivated in metal-contaminated soils has been observed due to the synergistic effect of Pseudomonas azotoformans ASS1 inoculation [169]. The secretion of certain metabolites, alongside the effects of Azotobacter chroococcum, which is capable of nitrogen fixation under moisture deficit stress, has been reported to modulate antioxidants, growth promotion, and photosynthesis in rice [170].
Findings have also established an increase in the γ-aminobutyric acid, alanine, asparagine, glutamine, aspartic acid, and glutamic acid contents of Phleum pretense inoculated with Bacillus subtilis B26 in drought-affected regions [171]. The types of abiotic stress known may include drought, flooding, heavy metals, salinity, high winds, extreme temperatures, and toxic organics (Figure 2). Hence, the types of abiotic stresses affecting crop productivity and the amelioration effects of microorganisms are briefly discussed below.
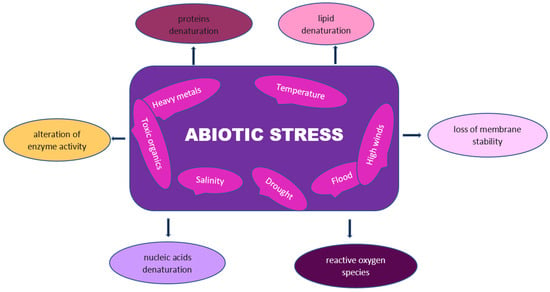
Figure 2.
Abiotic stresses and effects on plants.
5.1. Tolerance to Drought
Drought-induced stress is known to exert significant effects on plant growth and rhizosphere microbiomes [172]. Temperature and moisture remain the determining factors influencing the growth of microorganisms. The level of nutrients, such as nitrogen and carbon, which serve as energy sources for the microbes in the rhizosphere, can contribute to the loss of biodiversity and sensitivity to the soil nutrient threshold [173]. Despite the drought impacts, soil-inhabiting microbes employ diverse mechanisms in resisting drought stress and maintaining survival in the econiches. One such mechanism involves the release of osmolytes, which protect plants from drought [131].
Plant protection from water loss through irrigation systems needs to be intensified to circumvent the effect of drought stress, which negatively affects crop biomass [174]. Even though the influence of abiotic factors on some crops is known, finding biorational measures to tackle this menace by exploring potential microbiomes with the ability to stimulate drought tolerance in plants remains fundamental. Plant growth priming, relative moisture content, and the recovery of drought-affected plants are known to be mediated by the soil rhizobacteria [175], such that the pronounced effect of bacterization on some crop yield compared with stressed plants has been observed, which informs the choice of exploring these microbes in drought management. Drought affects many plants by altering water relation potential and enzyme activities, decreasing photosynthetic rate and plant weight, and enhancing oxidative stress [174]. A report by Kour et al. [176] revealed that diverse soil rhizobacteria-producing phytohormones and ACC deaminase can induce drought-stress resistance in plants based on their functional genes involved in various metabolic functions. Genes responsible for enzyme biosynthesis, such as monodehydroascorbate reductase, superoxide dismutase, hydrogen peroxidase, cytosolic ascorbate peroxidase, and peroxidase, in the genome of some rhizobacteria that enable them to degrade hydrogen peroxide and organic hydroperoxides have been detected; however, they have contributed to different behavioral plant patterns to drought tolerance in grass pea [177].
5.2. Tolerance to Salinity
The excessive disposal of wastes from various sources can result in a high accumulation of salt, which can affect plant nutrition and microbial activities in the soil [178]. Salinity stress due to the accumulation of sodium and chlorine ions has resulted in ion imbalance, toxic effects, and low water retention in plants and soil [179]. The bioavailability of soluble salts in different soils can mediate microbial functions for plant nutrition; however, excess salt pools affect plant growth [180]. The global challenges associated with salinity stress threaten the ecosystem, with a negative impact on human health. Salinity stress can be averted through crop breeding and efficient agricultural practice; nevertheless, this approach can be time-consuming and costly. Therefore, harnessing soil rhizobacteria with drought-tolerant and other genes may underline their functionalities in developing eco-friendly agriculture [17].
Plant tolerance to salinity stress contributes to their growth and improved crop productivity. The impact of salinity stress on plants due to osmotic stress and high salt concentration alters the rate of protein synthesis, metabolites synthesis, chaperones, and nonprotectants, which reduces plant performance by opposing stress to improve crop productivity in stress-associated regulation in plants [181]. Plant inoculation with abscisic-acid-producing soil rhizobacterium, Serratia marcescens DB1, has been reported to enhance salinity stress tolerance in rice by regulating the rate at which amino acids, proline, and glutamic acid are produced [182]. Sapkota et al. [183] investigated some diazotrophic bacterial genera, Enterobacter, Kosakonia, Bacillus, Pseudomonas, Sphingomonas, and Pantoea from the rhizome, which significantly alleviates salt tolerance in warm-season grasses, thus revealing their novel attributes for future exploration as bioinoculants in growing plants on marginally high saline, nutrient-limiting soils.
5.3. Tolerance to Metal Toxicity and Heat Stress
Aside from enhancing crop yield, which is not sustainable, the continuous use of agrochemicals and its impact on the ecosystems has resulted in biodiversity loss, as well as soil health deterioration and other environmental consequences in both plants and animals [184]. The accumulation of heavy metals can alter microbial diversity and functions in the soil. The intrusion of heavy metals into the soil due to human activities, such as mining, chemical fertilizer usage, industrial effluent discharge, and wastewater discharge, or natural phenomena by weathering of metal-rich rocks, can be sources of metals in the soil [185]. However, the presence of some metals, such as manganese, iron, and zinc, in trace amounts tends to support microbial growth and metabolism, while other toxic metals, such as mercury, cadmium, chromium, lead, and arsenic, affect microbial functions by disrupting cellular metabolism and damaging the cell membrane and genomic content, i.e., DNA [9].
An increase in metal toxicity in the soil and high temperatures are some of the major problems affecting plants growing in diverse climatic regions [181]. The hyperaccumulation of toxic metals into food chain systems has created major issues that affect human health. Numerous metals are present in the soil; however, some provide the required nutrients for the plant, while some prove otherwise without benefiting plants. A reduction in soil fertility coupled with high soil acidification due to heavy metal toxicity has resulted in poor root development, which limits plants’ access to soil nutrients [186]. Metal ions are made available to plants by soil microbes due to their active roles in metal ion mobilization in the soil, thus inducing the strong networking of plant defense against environmental factors [187]. Bioinoculation with copious rhizobacteria and a reduction in the toxic effect of some metals, such as lead, cadmium, zinc, copper, and nickel, in some food crops have been reported to positively enhance crop yield [188].
The use of rhizobacteria in alleviating metal toxicity and heat stress in plants contributes to plant survival in soils polluted with toxic metals [20]. Asif et al. [189] reported a reduction in the cadmium phytotoxicity in plants inoculated with rhizobacteria, which positively influences plant development under cadmium stress, thus suggesting the practical use of these bacteria, individually or in consortia for plant benefit. The alleviation of cadmium stress response in Sorghum bicolor (L) inoculated with rhizobacteria, Bacillus cereus QZS8, B. velezensis QZG6, and Enterobacter cloacae QZS3 by producing antioxidative gene that mediates the synthesis of antioxidant enzymes and other metabolites against reactive oxygen species, such as malondialdehyde, reduced glutathione, superoxide dismutase, peroxidase, flavonoid, and polyphenolic contents, polyphenol oxidase, and alcohol dehydrogenase, has also been reported to support plant growth with significant resistance to cadmium stress [29]. Wu et al. [190] demonstrated the effect of bacterial inoculation to enhance the phytoremediation performance of hybrid Pennisetum in cadmium-polluted soils, and the authors attributed the significant reduction in the cadmium toxicity on the inoculated plant to the ability of the bacterial isolate (B. megaterium BM18-2 and B. megaterium BM18) gene components that encode for heavy metal efflux pumps (BM18GM000901, BM18GM005669, and BM18GM005870), transcriptional regulators for metal stress biosensor (BM18GM003487), and replication protein (BM18GM001335). Furthermore, findings by Ahmad et al. [191] showed the ability of rhizobacteria to mitigate heat in some plants due to the expression of genes that modulate the survival and growth of tomato seedlings under high temperatures.
5.4. Flooding
Flooding is one of the abiotic-induced stresses that affect soil respiration, thus causing a reduction in the soil oxygen level [192]. Soil compaction due to flooding has also been known to affect the soil microbial community structure by altering the soil nutrient profiling and other factors, such as the pH [9]. The unfavorable soil conditions due to flooding can result in low microbial diversity and favor the growth of anaerobic microorganisms. The bacteria employ diverse mechanisms to withstand flooding under certain solute conditions due to their intracellular osmolyte adjustment and firmness of the cell wall [193].
6. Impacts of Biotechnologically Engineered Soil Microbiomes on Plants
The understanding of mechanisms underlining soil–root–microbe interactions can inform researchers on the ecological services of soil microbiomes as determinative factors for improving soil health and plant nutrition [194]. Promisingly, the use of engineered soil microbes in agricultural biotechnology and their full exploration commercially has been the major concerns of key players in agriculture in achieving the SDGs [195]. Thus, harnessing potential soil microorganisms and applying them to crops can improve plant nutrition to ensure food security [196,197]. However, the detection and selection of copious microbial strains with notable functional traits can best achieved through cultural and molecular techniques. Research findings by Gupta et al. [198] reported some identifiable bacteria, such as Burkholderia, Klebsiella, Azotobacter, Azospirillum, Enterobacter, Anthrobacter, Alcaligenes, Rhizobium, Bacillus, Serratia, and Pseudomonas, with notable functions in enhancing crop productivity under greenhouse experiments. The authors suggested the exploration of these bacteria as suitable candidates (i.e., bioinoculants) in developing eco-friendly agriculture.
The engineered soil microbes play some vital roles in effectively sustaining soil health and plant growth more than the normal soil microflora due to the expression of genes mediating their functionalities [199]. For the construction of genetically modified organisms (GMOs), conjugation, biolistic transformation, horizontal gene transfer, the transformation of protoplast, molecular cloning, and electroporation have been recognized as key molecular tools in microbial genetic engineering [81]. The selection of engineered microbes to improve plant nutrition relies on their ability to produce varieties of plant growth stimulation and secondary metabolite genes encoded in the bacterial genome. Moreover, the selection and construction of soil microbes for better performance can be best achieved through the manipulation of specific enzymes with strong affinity, gene construction, modifications of the regulatory pathway, bioproduct development and monitoring of plant growth promoting efficacy, and employing biosensor indicators, stimulating microbial functional inference to boost belowground ecological services [200]. The incorporation of novel genes into microbial genomes, either as single, combined, or consortium can mediate their functional properties by contributing to plant nutrition sustainably [201].
Soil microbiome engineering promises targets for aboveground through genotype selection, inoculation of potential plant-growth-promoting rhizobacteria, and modification of rhizosphere chemistry and for belowground through the selection and persistence of microbial population encouraged by rhizo-deposition, selection of rhizosphere microbes with improved nutrient acquisition and abiotic stress resistance, and genetic modification of rhizosphere microbes [81]. The merits attached to the use of beneficial or effective engineered soil microbes include i) the enhancement of soil nutrient acquisition, the improvement of water intake, the improvement of soil nitrogen and carbon pools, the introduction of microbial consortia into the ecosystem, improved plant growth, boosting plant resistance against biotic and abiotic stresses, plant pathogen suppression, the enhancement of soil biological properties, and the establishment of specific microbial communities [81].
The use of engineered soil microbes under laboratory and field experimental conditions might not yield the same results due to some prevailing environmental factors, such as crop types, plant cultivars, growth conditions, inoculum size, and competition against indigenous soil microbes [202]. It is therefore important to practically introduce mixed cultures of compatible genomic competence instead of using single cultures. This suggests that the mixed cultures can live and compete potentially with the autochthonous microbiome in the soil, and their influence can contribute maximally to plant growth [203]. For instance, plant inoculation with nitrogen-fixing bacteria, Bradyrhizobium, Methylobacterium, Rhizobium, Sphingomonas, isolated from the root nodules of leguminous plants showed better performance in fixing atmospheric nitrogen in the soil by inducing root nodule formation in nonhost leguminous plants [204]. The recent findings by Jiao et al. [205] reported the positive impact of identifiable rhizosphere bacteria, Pseudomonas migulae, P. poae, and P. extremaustralis in the uptake of soil nutrients and the enhancement of the seedling growth of inoculated apples. Similarly, Vigna radiata inoculated, with endophytic fungus, Aspergillus terreus CR7 isolated from the plant parts of Catharanthus roseus and also showed positive impacts on the plant yield parameters and the alleviation of salt stress on the plants [206].
However, the effects of the co-inoculation of rice soil with Bacillus licheniformis and Pseudomonas aeruginosa have been reported to mitigate heavy-metal-induced stress on plants by reducing rice arsenic accumulation and improving plant health under heavy metal stress [207]. Pandino et al. [208] reported better performance of globe artichoke productivity co-inoculated with arbuscular mycorrhizal fungi, Glomus spp., and plant-growth-promoting bacteria Bacillus spp. and Azotobacter spp. compared with the noninoculated plants. The authors’ findings provided current insights into the co-inoculation efficiency of microbial inoculants as a powerful biotechnological tool by providing a theoretical model of the real-life application of microbial co-inoculation under field experiments to increase crop productivity, reduce production costs, and decrease fertilizer usage to safeguard the environment [208]. Recent findings showing the effectiveness of co-inoculation on PGPR in the amelioration of environmentally induced plant stresses are evident in the works in [209,210,211]. However, to fully maximize the potential of rhizosphere microbiomes to ameliorate the impact of abiotic stress for improved plant protection and nutrition, it is important to devise strategic measures to understand the mechanisms underlining the design for successful microbial consortium functionalities in promoting plant growth. The mechanisms of soil–root-associated microorganisms that enable them to confer abiotic stress tolerance in plants are presented in Figure 3.
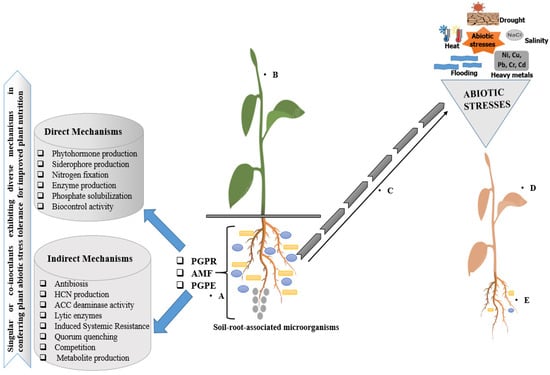
Figure 3.
Mechanisms of soil–root-associated microorganisms in conferring abiotic stress tolerance in plants: The soil microbes attach to the root surface and colonize the region to form biomass using different mechanisms (A). The lytic enzyme production by soil microbes underlines their mechanisms in boosting plant resilience to osmotic stress and the production of phytohormones stimulates nutrient uptake, plant growth, and tolerance to environmental stresses, thus improving crop yield parameters. The microbial inoculants contribute to plant growth and health by conferring abiotic tolerance for improved plant nutrition through the production of certain genes involved in biochemical, physiological, and cellular molecular modifications (B,C). The impact of abiotic stresses on the health status of plants (D) and reduction in microbial diversity, which in turn affect plant nutrition (E).
To efficiently explore rhizosphere microbiomes as bioinoculants, it is important to understand how they live and compete with autochthonous microbiomes [203]. The incorporation of genetic engineering can bring about more understanding of rhizosphere biology and insights into the ability of indigenous soil microbes to acclimatize a new niche with efficient performance in the general improvement of plant nutrition [212]. The survival and adaptation of microbes in the soil environment can be competitive due to the already established microbial communities in the same soil. Also, for microbes to establish mutual interactions in the soil, there is a need for microbial inoculants to develop strong mechanisms in competing with the soil’s normal flora and associated abiotic factors [81]. The bacterial inoculants can continue to stay in the soil for up to 1 month and 2 weeks (i.e., 6 weeks) after inoculation and successful root colonization [213]; in any case, the potentialities of this inoculum in conferring plant benefits is still obscure. Despite the persistence of microbial inoculants being influenced by soil type and species diversity [200], the co-inoculation of plants can yield more successful outcomes on crop yield than mono-inoculation. Consequently, understanding the diverse nature of soil microbes as bioinoculants, as well as screening them for plant-growth-promoting traits, remains crucial for enhancing crop productivity [214,215]. To achieve success in harnessing the engineered soil microbiomes in agricultural biotechnology, the science of autochthonous microbial communities contending with the plant-growth-promoting soil microbiomes as bioinoculants can therefore not be overemphasized.
7. Conclusions
In recent times, information on beneficial soil microbiomes and their exploration have shown their wide application in agriculture, medicine, and industry. The importance of soil microbes cannot be overemphasized due to their significance in enhancing soil and plant health and ameliorating abiotic-induced stresses for higher productivity. Nevertheless, understanding the mechanisms employed by soil microbiomes can help achieve biorational strategies for improving crop yield and ensuring maximum food production, accessibility, and availability for the ever-growing world population.
Most importantly, the insights into the molecular studies of soil microbes in unraveling the targeted genes involved in abiotic stress to boost plant resilience to stress are less studied. However, understanding the mechanisms underlining soil–plant–microbe interactions can provide insights into the use of soil microbiomes as bio-based products for plant nutrition. Hence, emphasis on genomic studies can serve as a model in the identification of soil microbes that can be incorporated into stress management with the view of addressing issues on their use under experimental conditions.
The engineered soil microbes may offer the best option for ameliorating abiotic stress by selecting potent microorganisms with desirable plant-growth-promoting traits. The targeted approaches to understanding the mechanisms underlining soil–root–microbe interactions can inform researchers of the important aspects of scientific discoveries. The real-life application of engineered soil microbes under experimental conditions and the factors mediating the inoculated microbes to compete with the autochthonous microbiome can maximize the full exploration of rhizosphere biology. Furthermore, engagement in multidisciplinary research can help bridge knowledge gaps to design frameworks for answering how engineered soil microbiomes can be selected, applied/introduced, adapted to soil-climatic conditions, live and compete with the autochthonous microbiomes, and respond to abiotic stresses, which may no doubt assure improved plant nutrition.
Author Contributions
Conceptualization, B.S.A.; writing—original draft preparation, B.S.A., P.C., M.S.A., F.M.O., S.M.E., V.K.U., A.I.A. and S.A.A.; writing—review and editing, B.S.A. and M.S.A. B.S.A. revised and formatted the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors acknowledge the affiliated institutions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pérez-Escamilla, R. Food security and the 2015–2030 sustainable development goals: From human to planetary health. Curr. Dev. Nutr. 2017, 1, e000513. [Google Scholar] [CrossRef] [PubMed]
- Akanmu, A.O.; Babalola, O.O.; Venturi, V.; Ayilara, M.S.; Adeleke, B.S.; Amoo, A.E.; Sobowale, A.A.; Fadiji, A.E.; Glick, B.R. Plant disease management: Leveraging on the plant-microbe-soil interface in the biorational use of organic amendments. Front. Plant Sci. 2021, 12, 1590. [Google Scholar] [CrossRef]
- Varzakas, T.; Smaoui, S. Global food security and sustainability issues: The road to 2030 from nutrition and sustainable healthy diets to Food systems change. Foods 2024, 13, 306. [Google Scholar] [CrossRef] [PubMed]
- Lokko, Y.; Heijde, M.; Schebesta, K.; Scholtès, P.; Van Montagu, M.; Giacca, M. Biotechnology and the bioeconomy—Towards inclusive and sustainable industrial development. New Biotechnol. 2018, 40, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, P.; Krishanan, A.; Ramasamy, P.; Chandrasekaran, K.; Rajendran, J.; Paramasivam, S.; Chinnaiyan, U.; Panneerselvam, C.; Aziz, A.T.; Alasmari, A. Biorational methods for effective pest control management in stored products for agricultural sustainability. Entomol. Res. 2024, 54, e12697. [Google Scholar] [CrossRef]
- Wakweya, R.B. Challenges and prospects of adopting climate-smart agricultural practices and technologies: Implications for food security. J. Agric. Food Res. 2023, 14, 100698. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Adeleke, B.S.; Babalola, O.O. Bioprospecting and challenges of plant microbiome research for sustainable agriculture, a review on soybean endophytic bacteria. Microb. Ecol. 2022, 85, 1113–1135. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.; Pereira, J.A.; Benhadi, J.; Lino-Neto, T.; Baptista, P. Endophytic and epiphytic phyllosphere fungal communities are shaped by different environmental factors in a Mediterranean ecosystem. Microb. Ecol. 2018, 76, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Glick, B.R. Root Exudate metabolites alter food crops microbiomes, impacting plant biocontrol and growth. Crops 2024, 4, 43–54. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, S.K.; Singh, M.K.; Singh, V.K.; Modi, A.; Singh, P.K.; Kumar, A. Chapter 7—Plant growth–promoting rhizobacteria and their functional role in salinity stress management. In Abatement of Environmental Pollutants; Singh, P., Kumar, A., Borthakur, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 151–160. [Google Scholar]
- Verzeaux, J.; Hirel, B.; Dubois, F.; Lea, P.J.; Tétu, T. Agricultural practices to improve nitrogen use efficiency through the use of arbuscular mycorrhizae: Basic and agronomic aspects. Plant Sci. 2017, 264, 48–56. [Google Scholar] [CrossRef]
- Mrabet, R. Sustainable agriculture for food and nutritional security. In Sustainable Agriculture and the Environment; Elsevier: Amsterdam, The Netherlands, 2023; pp. 25–90. [Google Scholar]
- Turrini, A.; Sbrana, C.; Giovannetti, M. Belowground environmental effects of transgenic crops: A soil microbial perspective. Res. Microbiol. 2015, 166, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Xu, M.; Ahamad, L.; Chaudhary, A.; Kumar, G.; Adeleke, B.S.; Verma, K.K.; Hu, D.-M.; Širić, I.; Kumar, P. Application of synthetic consortia for improvement of soil fertility, pollution remediation, and agricultural productivity: A review. Agronomy 2023, 13, 643. [Google Scholar] [CrossRef]
- Meena, V.S.; Meena, S.K.; Verma, J.P.; Kumar, A.; Aeron, A.; Mishra, P.K.; Bisht, J.K.; Pattanayak, A.; Naveed, M.; Dotaniya, M.L. Plant beneficial rhizospheric microorganism (PBRM) strategies to improve nutrients use efficiency: A review. Ecol. Eng. 2017, 107, 8–32. [Google Scholar] [CrossRef]
- Gupta, A.; Gopal, M.; Thomas, G.V.; Manikandan, V.; Gajewski, J.; Thomas, G.; Seshagiri, S.; Schuster, S.C.; Rajesh, P.; Gupta, R. Whole genome sequencing and analysis of plant growth promoting bacteria isolated from the rhizosphere of plantation crops coconut, cocoa and arecanut. PLoS ONE 2014, 9, e104259. [Google Scholar] [CrossRef]
- Ercole, T.G.; Kava, V.M.; Aluizio, R.; Pauletti, V.; Hungria, M.; Galli-Terasawa, L.V. Co-inoculation of Bacillus velezensis and Stenotrophomonas maltophilia strains improves growth and salinity tolerance in maize (Zea mays L.). Rhizosphere 2023, 27, 100752. [Google Scholar] [CrossRef]
- Newcombe, G.; Marlin, M.; Barge, E.; Heitmann, S.; Ridout, M.; Busby, P.E. Plant seeds commonly host Bacillus spp., potential antagonists of phytopathogens. Microb. Ecol. 2022, 85, 1356–1366. [Google Scholar] [CrossRef]
- Swarnalakshmi, K.; Yadav, V.; Tyagi, D.; Dhar, D.W.; Kannepalli, A.; Kumar, S. Significance of plant growth promoting rhizobacteria in grain legumes: Growth promotion and crop production. Plants 2020, 9, 1596. [Google Scholar] [CrossRef]
- Khan, N.; Mehmood, A. Revisiting climate change impacts on plant growth and its mitigation with plant growth promoting rhizobacteria. South Afr. J. Bot. 2023, 160, 586–601. [Google Scholar] [CrossRef]
- Lapsansky, E.R.; Milroy, A.M.; Andales, M.J.; Vivanco, J.M. Soil memory as a potential mechanism for encouraging sustainable plant health and productivity. Curr. Opin. Biotechnol. 2016, 38, 137–142. [Google Scholar] [CrossRef]
- Kaur, M.; Karnwal, A. Screening of endophytic bacteria from stress-tolerating plants for abiotic stress tolerance and plant growth-promoting properties: Identification of potential strains for bioremediation and crop enhancement. J. Agric. Food Res. 2023, 14, 100723. [Google Scholar] [CrossRef]
- Filgueiras, L.; Silva, R.; Almeida, I.; Vidal, M.; Baldani, J.I.; Meneses, C.H.S.G. Gluconacetobacter diazotrophicus mitigates drought stress in Oryza sativa L. Plant Soil 2020, 451, 57–73. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hossain, M.S.; Akter, M. Challenges faced by plant growth-promoting bacteria in field-level applications and suggestions to overcome the barriers. Physiol. Mol. Plant Pathol. 2023, 126, 102029. [Google Scholar]
- Shi, S.; Nuccio, E.E.; Shi, Z.J.; He, Z.; Zhou, J.; Firestone, M.K. The interconnected rhizosphere: High network complexity dominates rhizosphere assemblages. Ecol. Lett. 2016, 19, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dippold, M.A.; Kuzyakov, Y.; Razavi, B.S. Spatial pattern of enzyme activities depends on root exudate composition. Soil Biol. Biochem. 2019, 133, 83–93. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O.; Glick, B.R. Plant growth-promoting root-colonizing bacterial endophytes. Rhizosphere 2021, 20, 100433. [Google Scholar] [CrossRef]
- Debnath, S.; Chakraborty, S.; Langthasa, M.; Choure, K.; Agnihotri, V.; Srivastava, A.; Rai, P.K.; Tilwari, A.; Maheshwari, D.; Pandey, P. Non-rhizobial nodule endophytes improve nodulation, change root exudation pattern and promote the growth of lentil, for prospective application in fallow soil. Front. Plant Sci. 2023, 14, 1152875. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, X.; Lin, Z.; Teng, D.; Zhao, Y.; Chen, S.; Hu, X. Screening of cadmium resistant bacteria and their growth promotion of Sorghum bicolor (L.) Moench under cadmium stress. Ecotoxicol. Environ. Saf. 2024, 272, 116012. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.X.; Hernández, A.G.; Glick, B.R.; Rossi, M.J. Plant growth-promoting activities and genomic analysis of the stress-resistant Bacillus megaterium STB1, a bacterium of agricultural and biotechnological interest. Biotechnol. Rep. 2020, 25, e00406. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-M.; Asaf, S.; Khan, A.L.; Khan, A.; Mun, B.-G.; Khan, M.A.; Gul, H.; Lee, I.-J. Complete genome sequence of Pseudomonas psychrotolerans CS51, a plant growth-promoting bacterium, under heavy metal stress conditions. Microorganisms 2020, 8, 382. [Google Scholar] [CrossRef]
- Mwajita, M.R.; Murage, H.; Tani, A.; Kahangi, E.M. Evaluation of rhizosphere, rhizoplane and phyllosphere bacteria and fungi isolated from rice in Kenya for plant growth promoters. SpringerPlus 2013, 2, 606. [Google Scholar] [CrossRef]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef]
- Odelade, K.A.; Babalola, O.O. Bacteria, fungi and archaea domains in rhizospheric soil and their effects in enhancing agricultural productivity. Int. J. Environ. Res. Public Health 2019, 16, 3873. [Google Scholar] [CrossRef] [PubMed]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Pieterse, C.M.; Bakker, P.A.; Berendsen, R.L. Beneficial microbes going underground of root immunity. Plant Cell Environ. 2019, 42, 2860–2870. [Google Scholar] [CrossRef] [PubMed]
- Babalola, O.O.; Adeleke, B.S.; Ayangbenro, A.S. Whole genome sequencing of sunflower root-associated Bacillus cereus. Evol. Bioinform. 2021, 17, 11769343211038948. [Google Scholar] [CrossRef] [PubMed]
- Ngoma, L.; Esau, B.; Babalola, O.O. Isolation and characterization of beneficial indigenous endophytic bacteria for plant growth promoting activity in Molelwane Farm, Mafikeng, South Africa. Afr. J. Biotechnol. 2013, 12, 4104–4114. [Google Scholar]
- Chen, Y.; Ding, Q.; Chao, Y.; Wei, X.; Wang, S.; Qiu, R. Structural development and assembly patterns of the root-associated microbiomes during phytoremediation. Sci. Total Environ. 2018, 644, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lang, D.; Wang, J.; Zhang, W.; Zhang, X. Plant-beneficial Streptomyces dioscori SF1 potential biocontrol and plant growth promotion in saline soil within the arid and semi-arid areas. Environ. Sci. Pollut. Res. 2023, 30, 70194–70212. [Google Scholar] [CrossRef] [PubMed]
- Fasusi, O.A.; Amoo, A.E.; Babalola, O.O. Propagation and characterization of viable arbuscular mycorrhizal fungal spores within maize plant (Zea mays L.). J. Sci. Food Agric. 2021, 101, 5834–5841. [Google Scholar] [CrossRef] [PubMed]
- Sritongon, N.; Boonlue, S.; Mongkolthanaruk, W.; Jogloy, S.; Riddech, N. The combination of multiple plant growth promotion and hydrolytic enzyme producing rhizobacteria and their effect on Jerusalem artichoke growth improvement. Sci. Rep. 2023, 13, 5917. [Google Scholar] [CrossRef]
- Hu, L.; Robert, C.A.; Cadot, S.; Zhang, X.; Ye, M.; Li, B.; Manzo, D.; Chervet, N.; Steinger, T.; Van Der Heijden, M.G. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 2018, 9, 2738. [Google Scholar] [CrossRef]
- Tsunoda, T.; van Dam, N.M. Root chemical traits and their roles in belowground biotic interactions. Pedobiologia 2017, 65, 58–67. [Google Scholar] [CrossRef]
- Postma, J.A.; Schurr, U.; Fiorani, F. Dynamic root growth and architecture responses to limiting nutrient availability: Linking physiological models and experimentation. Biotechnol. Adv. 2014, 32, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, M.; Sandhya, S.; Prasad, M.N.V.; Freitas, H. Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol. Adv. 2012, 30, 1562–1574. [Google Scholar] [CrossRef] [PubMed]
- Flandroy, L.; Poutahidis, T.; Berg, G.; Clarke, G.; Dao, M.-C.; Decaestecker, E.; Furman, E.; Haahtela, T.; Massart, S.; Plovier, H.; et al. The impact of human activities and lifestyles on the interlinked microbiota and health of humans and of ecosystems. Sci. Total Environ. 2018, 627, 1018–1038. [Google Scholar] [CrossRef] [PubMed]
- Nannipieri, P.; Ascher-Jenull, J.; Ceccherini, M.T.; Pietramellara, G.; Renella, G.; Schloter, M. Beyond microbial diversity for predicting soil functions: A mini review. Pedosphere 2020, 30, 5–17. [Google Scholar] [CrossRef]
- Sasse, J.; Martinoia, E.; Northen, T. Feed your friends: Do plant exudates shape the root microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, S.A.; Griffiths, J.; Ton, J. Crying out for help with root exudates: Adaptive mechanisms by which stressed plants assemble health-promoting soil microbiomes. Curr. Opin. Microbiol. 2019, 49, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K. Plant methyl salicylate induces defense responses in the rhizobacterium Bacillus subtilis. Environ. Microbiol. 2015, 17, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Korenblum, E.; Dong, Y.; Szymanski, J.; Panda, S.; Jozwiak, A.; Massalha, H.; Meir, S.; Rogachev, I.; Aharoni, A. Rhizosphere microbiome mediates systemic root metabolite exudation by root-to-root signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 3874–3883. [Google Scholar] [CrossRef]
- Camaille, M.; Fabre, N.; Clément, C.; Ait Barka, E. Advances in wheat physiology in response to drought and the role of plant growth promoting rhizobacteria to trigger drought tolerance. Microorganisms 2021, 9, 687. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Z.; Li, X.; Tan, J.; Liu, F.; Wu, J. Mycorrhizal fungi alter root exudation to cultivate a beneficial microbiome for plant growth. Funct. Ecol. 2023, 37, 664–675. [Google Scholar] [CrossRef]
- Wu, S.; Wu, K.; Shi, L.; Sun, X.; Tan, Q.; Hu, C. Recruitment of specific microbes through exudates affects cadmium activation and accumulation in Brassica napus. J. Hazard. Mater. 2023, 442, 130066. [Google Scholar] [CrossRef] [PubMed]
- Paul, E.A. The nature and dynamics of soil organic matter: Plant inputs, microbial transformations, and organic matter stabilization. Soil Biol. Biochem. 2016, 98, 109–126. [Google Scholar] [CrossRef]
- Zhang, R.; Vivanco, J.M.; Shen, Q. The unseen rhizosphere root-soil-microbe interactions for crop production. Curr. Opin. Microbiol. 2017, 37, 8–14. [Google Scholar] [CrossRef]
- Wang, D.; Wang, C.; Chen, Y.; Xie, Z. Developing Plant-Growth-Promoting Rhizobacteria: A Crucial Approach for Achieving Sustainable Agriculture. Agronomy 2023, 13, 1835. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Urón, P.; Glick, B.R.; Giachini, A.; Rossi, M.J. Genomic analysis of the 1-aminocyclopropane-1-carboxylate deaminase-producing Pseudomonas thivervalensis sc5 reveals its multifaceted roles in soil and in beneficial interactions with plants. Front. Microbiol. 2021, 12, 752288. [Google Scholar] [CrossRef]
- Pantigoso, H.A.; Yuan, J.; He, Y.; Guo, Q.; Vollmer, C.; Vivanco, J.M. Role of root exudates on assimilation of phosphorus in young and old Arabidopsis thaliana plants. PLoS ONE 2020, 15, e0234216. [Google Scholar] [CrossRef]
- Rasmann, S.; Turlings, T.C.J. Root signals that mediate mutualistic interactions in the rhizosphere. Curr. Opin. Plant Biol. 2016, 32, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Yang, C.; Mei, X.; Shen, S.; Raza, W.; Shen, Q.; Xu, Y. The effect of organic acids from tomato root exudates on rhizosphere colonization of Bacillus amyloliquefaciens T-5. Appl. Soil Ecol. 2013, 64, 15–22. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Imran, M.; Naveed, M.; Khan, M.Y.; Ahmad, M.; Zahir, Z.A.; Crowley, D.E. Synergistic use of biochar, compost and plant growth-promoting rhizobacteria for enhancing cucumber growth under water deficit conditions. J. Sci. Food Agric. 2017, 97, 5139–5145. [Google Scholar] [CrossRef]
- Rekha, K.; Ramasamy, M.; Usha, B. Root exudation of organic acids as affected by plant growth-promoting rhizobacteria Bacillus subtilis RR4 in rice. J. Crop Improv. 2020, 34, 571–586. [Google Scholar] [CrossRef]
- Ahkami, A.H.; Allen White, R.; Handakumbura, P.P.; Jansson, C. Rhizosphere engineering: Enhancing sustainable plant ecosystem productivity. Rhizosphere 2017, 3, 233–243. [Google Scholar] [CrossRef]
- da Silva, A.V.; da Silva, M.K.; Sampaio, E.B.T.; Ferreira, L.F.R.; Passarini, M.R.Z.; de Oliveira, V.M.; Rosa, L.H.; Duarte, A.W.F. Benefits of plant growth-promoting symbiotic microbes in climate change era. In Microbiome under Changing Climate; Elsevier: Amsterdam, The Netherlands, 2022; pp. 85–113. [Google Scholar]
- Babalola, O.O.; Akinola, S.A.; Ayangbenro, A.S. Shotgun metagenomic survey of maize soil rhizobiome. Microbiol. Res. Announc. 2020, 9, e00860-20. [Google Scholar] [CrossRef]
- Mathenge, C.; Thuita, M.; Masso, C.; Gweyi-Onyango, J.; Vanlauwe, B. Variability of soybean response to rhizobia inoculant, vermicompost, and a legume-specific fertilizer blend in Siaya County of Kenya. Soil Tillage Res. 2019, 194, 104290. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, E.A.; El-Keblawy, A.; Mosa, K.A.; Okoh, A.I.; Saadoun, I. Role of endophytes and rhizosphere microbes in promoting the invasion of exotic plants in arid and semi-arid areas: A review. Sustainability 2021, 13, 13081. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Adeleke, B.S.; Adebajo, M.T.; Akinola, S.A.; Fayose, C.A.; Adeyemi, U.T.; Gbadegesin, L.A.; Omole, R.K.; Johnson, R.M.; Edhemuino, M. Remediation by enhanced natural attenuation; an environment-friendly remediation approach. Front. Environ. Sci. 2023, 11, 1182586. [Google Scholar] [CrossRef]
- Gamalero, E.; Bona, E.; Todeschini, V.; Lingua, G. Saline and arid soils: Impact on bacteria, plants, and their interaction. Biology 2020, 9, 116. [Google Scholar] [CrossRef]
- Akinola, S.A.; Adeyemo, R.O.; Adebayo, I.A. Overview and Emergence of Nanobiotechnology in Plants. In Diverse Applications of Nanotechnology in the Biological Sciences; Apple Academic Press: Williston, VT, USA, 2022; pp. 173–197. [Google Scholar]
- Mainka, T.; Weirathmüller, D.; Herwig, C.; Pflügl, S. Potential applications of halophilic microorganisms for biological treatment of industrial process brines contaminated with aromatics. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab015. [Google Scholar] [CrossRef]
- Gupta, A.; Joia, J.; Sood, A.; Sood, R.; Sidhu, C.; Kaur, G. Microbes as potential tool for remediation of heavy metals: A review. Microb. Biochem. Technol. 2016, 8, 364–372. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Akinola, S.A.; Adebajo, M.T. Benefits and Drawbacks of Microbial Inoculant in Terms of Human Health and the Environment. In Microbial Inoculants: Applications for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2024; pp. 411–435. [Google Scholar]
- Oyebamiji, Y.O.; Nahar, S.; Shamsudin, N.A.; Salisu, M.A.; Akinola, S.A.; Adebayo, I.A.; Arsad, H. The role of arbuscular mycorrhizal fungi in alleviating heat and drought stress in vegetables. Egypt. J. Bot. 2024, 64, 497–505. [Google Scholar] [CrossRef]
- Mukhtar, T.; Ali, F.; Rafique, M.; Ali, J.; Afridi, M.S.; Smith, D.; Mehmood, S.; Amna; Souleimanov, A.; Jellani, G. Biochemical characterization and potential of Bacillus safensis strain scal1 to mitigate heat stress in Solanum lycopersicum L. J. Plant Growth Regul. 2023, 42, 523–538. [Google Scholar] [CrossRef]
- Mukhtar, S.; Mirza, B.S.; Mehnaz, S.; Mirza, M.S.; Mclean, J.; Malik, K.A. Impact of soil salinity on the microbial structure of halophyte rhizosphere microbiome. World J. Microbiol. Biotechnol. 2018, 34, 136. [Google Scholar] [CrossRef] [PubMed]
- Akinola, S.A.; Ayangbenro, A.S.; Babalola, O.O. Pedological factors as drivers of archaeal and fungal communities in maize rhizosphere: A shotgun metagenomic sequencing approach. SN Appl. Sci. 2023, 5, 351. [Google Scholar] [CrossRef]
- Ladau, J.; Shi, Y.; Jing, X.; He, J.-S.; Chen, L.; Lin, X.; Fierer, N.; Gilbert, J.A.; Pollard, K.S.; Chu, H. Climate change will lead to pronounced shifts in the diversity of soil microbial communities. bioRxiv 2018. [Google Scholar] [CrossRef]
- Mahmud, K.; Missaoui, A.; Lee, K.; Ghimire, B.; Presley, H.W.; Makaju, S. Rhizosphere microbiome manipulation for sustainable crop production. Curr. Plant Biol. 2021, 27, 100210. [Google Scholar] [CrossRef]
- Mukhtar, S.; Mehnaz, S.; Mirza, M.S.; Malik, K.A. Isolation and characterization of bacteria associated with the rhizosphere of halophytes (Salsola stocksii and Atriplex amnicola) for production of hydrolytic enzymes. Braz. J. Microbiol. 2019, 50, 85–97. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Adeleke, B.S.; Akinola, S.A.; Fayose, C.A.; Adeyemi, U.T.; Gbadegesin, L.A.; Omole, R.K.; Johnson, R.M.; Uthman, Q.O.; Babalola, O.O. Biopesticides as a promising alternative to synthetic pesticides: A case for microbial pesticides, phytopesticides, and nanobiopesticides. Front. Microbiol. 2023, 14, 1040901. [Google Scholar] [CrossRef] [PubMed]
- Akinola, S.A.; Babalola, O.O. The importance of adverse soil microbiomes in the light of omics: Implications for food safety. Plant Soil Environ. 2020, 66, 421–430. [Google Scholar] [CrossRef]
- Dardanelli, M.S.; de Cordoba, F.J.F.; Espuny, M.R.; Carvajal, M.A.R.; Díaz, M.E.S.; Serrano, A.M.G.; Okon, Y.; Megías, M. Effect of Azospirillum brasilense coinoculated with Rhizobium on Phaseolus vulgaris flavonoids and Nod factor production under salt stress. Soil Biol. Biochem. 2008, 40, 2713–2721. [Google Scholar] [CrossRef]
- Del Amor, F.M.; Cuadra-Crespo, P. Plant growth-promoting bacteria as a tool to improve salinity tolerance in sweet pepper. Funct. Plant Biol. 2011, 39, 82–90. [Google Scholar] [CrossRef]
- Ait Barka, E.; Nowak, J.; Clément, C. Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth-promoting rhizobacterium, Burkholderia phytofirmans strain PsJN. Appl. Environ. Microbiol. 2006, 72, 7246–7252. [Google Scholar] [CrossRef]
- Marulanda, A.; Azcón, R.; Chaumont, F.; Ruiz-Lozano, J.M.; Aroca, R. Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta 2010, 232, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Vaishnav, A.; Kumari, S.; Jain, S.; Varma, A.; Tuteja, N.; Choudhary, D.K. PGPR-mediated expression of salt tolerance gene in soybean through volatiles under sodium nitroprusside. J. Basic Microbiol. 2016, 56, 1274–1288. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. A review on possible elicitor molecules of cyanobacteria: Their role in improving plant growth and providing tolerance against biotic or abiotic stress. J. Appl. Microbiol. 2014, 117, 1221–1244. [Google Scholar] [CrossRef]
- Jha, Y.; Sablok, G.; Subbarao, N.; Sudhakar, R.; Fazil, M.T.; Subramanian, R.; Squartini, A.; Kumar, S. Bacterial-induced expression of RAB18 protein in Orzya sativa salinity stress and insights into molecular interaction with GTP ligand. J. Mol. Recognit. Interdiscip. J. 2014, 27, 521–527. [Google Scholar] [CrossRef]
- Naveed, M.; Mitter, B.; Reichenauer, T.G.; Wieczorek, K.; Sessitsch, A. Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ. Exp. Bot. 2014, 97, 30–39. [Google Scholar] [CrossRef]
- Cho, S.M.; Kang, B.R.; Han, S.H.; Anderson, A.J.; Park, J.-Y.; Lee, Y.-H.; Cho, B.H.; Yang, K.-Y.; Ryu, C.-M.; Kim, Y.C. 2R, 3R-butanediol, a bacterial volatile produced by Pseudomonas chlororaphis O6, is involved in induction of systemic tolerance to drought in Arabidopsis thaliana. Mol. Plant-Microbe Interact. 2008, 21, 1067–1075. [Google Scholar] [CrossRef]
- Lu, X.; Jin, C.; Yang, J.; Liu, Q.; Wu, S.; Li, D.; Guan, Y.; Cai, Y. Prenatal and lactational lead exposure enhanced oxidative stress and altered apoptosis status in offspring rats’ hippocampus. Biol. Trace Elem. Res. 2013, 151, 75–84. [Google Scholar] [CrossRef]
- Islam, F.; Yasmeen, T.; Ali, Q.; Ali, S.; Arif, M.S.; Hussain, S.; Rizvi, H. Influence of Pseudomonas aeruginosa as PGPR on oxidative stress tolerance in wheat under Zn stress. Ecotoxicol. Environ. Saf. 2014, 104, 285–293. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Giordano, W.; Hirsch, A.M. The expression of MaEXP1, a Melilotus alba expansin gene, is upregulated during the sweetclover-Sinorhizobium meliloti interaction. Mol. Plant-Microbe Interact. 2004, 17, 613–622. [Google Scholar] [CrossRef]
- AbuQamar, S.F.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.S.M.; Elrys, A.S.; Abd El-Mageed, T.A.; Semida, W.M.; Abdelkhalik, A.; Mosa, W.F.; Al Kafaas, S.S.; et al. Halotolerant plant growth-promoting rhizobacteria improve soil fertility and plant salinity tolerance for sustainable agriculture—A review. Plant Stress 2024, 12, 100482. [Google Scholar] [CrossRef]
- Akinola, S.A.; Babalola, O.O. The fungal and archaeal community within plant rhizosphere: A review on their contribution to crop safety. J. Plant Nutr. 2021, 44, 600–618. [Google Scholar] [CrossRef]
- Muhammad, M.; Waheed, A.; Wahab, A.; Majeed, M.; Nazim, M.; Liu, Y.-H.; Li, L.; Li, W.-J. Soil salinity and drought tolerance: An evaluation of plant growth, productivity, microbial diversity, and amelioration strategies. Plant Stress 2023, 11, 100319. [Google Scholar] [CrossRef]
- Singh, P.; Singh, R.K.; Guo, D.-J.; Sharma, A.; Singh, R.N.; Li, D.-P.; Malviya, M.K.; Song, X.-P.; Lakshmanan, P.; Yang, L.-T. Whole genome analysis of sugarcane root-associated endophyte Pseudomonas aeruginosa B18—A plant growth-promoting bacterium with antagonistic potential against Sporisorium scitamineum. Front. Microbiol. 2021, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.M.; McGroty, S.E.; Chew, T.H.; Chan, K.G.; Buckley, L.J.; Savka, M.A.; Hudson, A.O. Whole-genome sequence of Enterobacter sp. strain SST3, an endophyte isolated from Jamaican sugarcane (Saccharum sp.) stalk tissue. J. Bacteriol. 2012, 194, 5981–5982. [Google Scholar] [CrossRef]
- Viscardi, S.; Ventorino, V.; Duran, P.; Maggio, A.; De Pascale, S.; Mora, M.; Pepe, O. Assessment of plant growth promoting activities and abiotic stress tolerance of Azotobacter chroococcum strains for a potential use in sustainable agriculture. J. Soil Sci. Plant Nutr. 2016, 16, 848–863. [Google Scholar] [CrossRef]
- Yarra, R. The wheat NHX gene family: Potential role in improving salinity stress tolerance of plants. Plant Gene 2019, 18, 100178. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Ouledali, S.; Ennajeh, M.; Ferrandino, A.; Khemira, H.; Schubert, A.; Secchi, F. Influence of arbuscular mycorrhizal fungi inoculation on the control of stomata functioning by abscisic acid (ABA) in drought-stressed olive plants. South Afr. J. Bot. 2019, 121, 152–158. [Google Scholar] [CrossRef]
- Rizvi, A.; Khan, M.S. Biotoxic impact of heavy metals on growth, oxidative stress and morphological changes in root structure of wheat (Triticum aestivum L.) and stress alleviation by Pseudomonas aeruginosa strain CPSB1. Chemosphere 2017, 185, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Balestrini, R.; Lumini, E. Focus on mycorrhizal symbioses. Appl. Soil Ecol. 2018, 123, 299–304. [Google Scholar] [CrossRef]
- Kapoor, R.; Evelin, H.; Mathur, P.; Giri, B. Arbuscular mycorrhiza: Approaches for abiotic stress tolerance in crop plants for sustainable agriculture. In Plant Acclimation to Environmental Stress; Springer: Berlin/Heidelberg, Germany, 2012; pp. 359–401. [Google Scholar]
- Arshad, M.; Saleem, M.; Hussain, S. Perspectives of bacterial ACC deaminase in phytoremediation. Trends Biotechnol. 2007, 25, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Camerini, S.; Senatore, B.; Lonardo, E.; Imperlini, E.; Bianco, C.; Moschetti, G.; Rotino, G.L.; Campion, B.; Defez, R. Introduction of a novel pathway for IAA biosynthesis to rhizobia alters vetch root nodule development. Arch. Microbiol. 2008, 190, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.K.; Ding, Y.Z.; Feng, R.W.; Wang, R.G.; Xu, Y.M.; Chen, C.; Wei, X.L.; Chen, W.M. Burkholderia metalliresistens sp. nov., a multiple metal-resistant and phosphate-solubilising species isolated from heavy metal-polluted soil in Southeast China. Antonie Van Leeuwenhoek 2015, 107, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Patten, C.L.; Glick, B.R. Bacterial biosynthesis of indole-3-acetic acid. Can. J. Microbiol. 1996, 42, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.T.; Torky, Y.; Amin, A.; Mourad, A.-H.I.; Ayyash, M.M.; El-Keblawy, A.; Hilal-Alnaqbi, A.; AbuQamar, S.F.; El-Tarabily, K.A. Halotolerant marine rhizosphere-competent actinobacteria promote Salicornia bigelovii growth and seed production using seawater irrigation. Front. Microbiol. 2020, 11, 552. [Google Scholar] [CrossRef]
- Neubauer, U.; Furrer, G.; Schulin, R. Heavy metal sorption on soil minerals affected by the siderophore desferrioxamine B: The role of Fe (III)(hydr) oxides and dissolved Fe (III). Eur. J. Soil Sci. 2002, 53, 45–55. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; ElBaghdady, K.Z.; AlKhajeh, A.S.; Ayyash, M.M.; Aljneibi, R.S.; El-Keblawy, A.; AbuQamar, S.F. Polyamine-producing actinobacteria enhance biomass production and seed yield in Salicornia bigelovii. Biol. Fertil. Soils 2020, 56, 499–519. [Google Scholar] [CrossRef]
- Ghosh, U.D.; Saha, C.; Maiti, M.; Lahiri, S.; Ghosh, S.; Seal, A.; MitraGhosh, M. Root associated iron oxidizing bacteria increase phosphate nutrition and influence root to shoot partitioning of iron in tolerant plant Typha angustifolia. Plant Soil 2014, 381, 279–295. [Google Scholar] [CrossRef]
- Yadav, J.; Verma, J.P.; Jaiswal, D.K.; Kumar, A. Evaluation of PGPR and different concentration of phosphorus level on plant growth, yield and nutrient content of rice (Oryza sativa). Ecol. Eng. 2014, 62, 123–128. [Google Scholar]
- Ali, S.; Glick, B.R. Plant–bacterial interactions in management of plant growth under abiotic stresses. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 21–45. [Google Scholar]
- Igiehon, N.O.; Babalola, O.O.; Aremu, B.R. Genomic insights into plant growth promoting rhizobia capable of enhancing soybean germination under drought stress. BMC Microbiol. 2019, 19, 159. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Parveen, H.; Gangola, S.; Kumar, G.; Bhatt, P.; Chaudhary, A. Plant growth-promoting rhizobacteria and their application in sustainable crop production. In Microbial Technology for Sustainable Environment; Springer: Singapore, 2021; pp. 217–234. [Google Scholar]
- Fahsi, N.; Mahdi, I.; Mesfioui, A.; Biskri, L.; Allaoui, A. Plant growth-promoting rhizobacteria isolated from the Jujube (Ziziphus lotus) plant enhance wheat growth, Zn uptake, and heavy metal tolerance. Agriculture 2021, 11, 316. [Google Scholar] [CrossRef]
- Zahid, G.; Iftikhar, S.; Shimira, F.; Ahmad, H.M.; Kaçar, Y.A. An overview and recent progress of plant growth regulators (PGRs) in the mitigation of abiotic stresses in fruits: A review. Sci. Hortic. 2023, 309, 111621. [Google Scholar] [CrossRef]
- Ghazi, A.; Atia, E.; Elsakhawy, T. Evaluation of an endophytic plant growth-promoting bacterium, Klebsiella variicola, in mitigation of salt stress in tuberose (Polianthes tuberosa L.). J. Hortic. Sci. Biotechnol. 2021, 96, 770–782. [Google Scholar] [CrossRef]
- Ulrich, K.; Kube, M.; Becker, R.; Schneck, V.; Ulrich, A. Genomic analysis of the endophytic Stenotrophomonas strain 169 reveals features related to plant-growth promotion and stress tolerance. Front. Microbiol. 2021, 12, 687463. [Google Scholar] [CrossRef]
- Lu, L.; Chang, M.; Han, X.; Wang, Q.; Wang, J.; Yang, H.; Guan, Q.; Dai, S. Beneficial effects of endophytic Pantoea ananatis with ability to promote rice growth under saline stress. J. Appl. Microbiol. 2021, 131, 1919–1931. [Google Scholar] [CrossRef]
- Annapurna, K.; Govindasamy, V.; Ajit, V.; Choudhary, D. Mitigation of drought stress in wheat crop by drought tolerant endophytic bacterial isolates. Vegetos. 2019, 32, 486–493. [Google Scholar]
- Gamalero, E.; Favale, N.; Bona, E.; Novello, G.; Cesaro, P.; Massa, N.; Glick, B.R.; Orozco-Mosqueda, M.d.C.; Berta, G.; Lingua, G. Screening of bacterial endophytes able to promote plant growth and increase salinity tolerance. Appl. Sci. 2020, 10, 5767. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Alayafi, A.A. Azospirillum lipoferum FK1 confers improved salt tolerance in chickpea (Cicer arietinum L.) by modulating osmolytes, antioxidant machinery and stress-related genes expression. Environ. Exp. Bot. 2019, 159, 55–65. [Google Scholar] [CrossRef]
- Tahir, M.; Ahmad, I.; Shahid, M.; Shah, G.M.; Farooq, A.B.U.; Akram, M.; Tabassum, S.A.; Naeem, M.A.; Khalid, U.; Ahmad, S. Regulation of antioxidant production, ion uptake and productivity in potato (Solanum tuberosum L.) plant inoculated with growth promoting salt tolerant Bacillus strains. Ecotoxicol. Environ. Saf. 2019, 178, 33–42. [Google Scholar] [CrossRef]
- Afridi, M.S.; Mahmood, T.; Salam, A.; Mukhtar, T.; Mehmood, S.; Ali, J.; Khatoon, Z.; Bibi, M.; Javed, M.T.; Sultan, T. Induction of tolerance to salinity in wheat genotypes by plant growth promoting endophytes: Involvement of ACC deaminase and antioxidant enzymes. Plant Physiol. Biochem. 2019, 139, 569–577. [Google Scholar] [CrossRef]
- Vimal, S.R.; Patel, V.K.; Singh, J.S. Plant growth promoting Curtobacterium albidum strain SRV4: An agriculturally important microbe to alleviate salinity stress in paddy plants. Ecol. Indic. 2019, 105, 553–562. [Google Scholar] [CrossRef]
- Li, H.; Lei, P.; Pang, X.; Li, S.; Xu, H.; Xu, Z.; Feng, X. Enhanced tolerance to salt stress in canola (Brassica napus L.) seedlings inoculated with the halotolerant Enterobacter cloacae HSNJ4. Appl. Soil Ecol. 2017, 119, 26–34. [Google Scholar] [CrossRef]
- Sarkar, A.; Pramanik, K.; Mitra, S.; Soren, T.; Maiti, T.K. Enhancement of growth and salt tolerance of rice seedlings by ACC deaminase-producing Burkholderia sp. MTCC 12259. J. Plant Physiol. 2018, 231, 434–442. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Lee, Y.H. A cocktail of volatile compounds emitted from Alcaligenes faecalis JBCS1294 induces salt tolerance in Arabidopsis thaliana by modulating hormonal pathways and ion transporters. J. Plant Physiol. 2017, 214, 64–73. [Google Scholar] [CrossRef]
- Samaddar, S.; Chatterjee, P.; Choudhury, A.R.; Ahmed, S.; Sa, T. Interactions between Pseudomonas spp. and their role in improving the red pepper plant growth under salinity stress. Microbiol. Res. 2019, 219, 66–73. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alamri, S.A.; Ali, H.M.; Alayafi, A.A. Bacillus firmus (SW5) augments salt tolerance in soybean (Glycine max L.) by modulating root system architecture, antioxidant defense systems and stress-responsive genes expression. Plant Physiol. Biochem. 2018, 132, 375–384. [Google Scholar] [CrossRef]
- Hmaeid, N.; Wali, M.; Mahmoud, O.M.-B.; Pueyo, J.J.; Ghnaya, T.; Abdelly, C. Efficient rhizobacteria promote growth and alleviate NaCl-induced stress in the plant species Sulla carnosa. Appl. Soil Ecol. 2019, 133, 104–113. [Google Scholar] [CrossRef]
- Sapre, S.; Gontia-Mishra, I.; Tiwari, S. Klebsiella sp. confers enhanced tolerance to salinity and plant growth promotion in oat seedlings (Avena sativa). Microbiol. Res. 2018, 206, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Abdiev, A.; Khaitov, B.; Toderich, K.; Park, K.W. Growth, nutrient uptake and yield parameters of chickpea (Cicer arietinum L.) enhance by Rhizobium and Azotobacter inoculations in saline soil. J. Plant Nutr. 2019, 42, 2703–2714. [Google Scholar] [CrossRef]
- Khan, M.A.; Hamayun, M.; Asaf, S.; Khan, M.; Yun, B.-W.; Kang, S.-M.; Lee, I.-J. Rhizospheric Bacillus spp. rescues plant growth under salinity stress via regulating gene expression, endogenous hormones, and antioxidant system of Oryza sativa L. Front. Plant Sci. 2021, 12, 665590. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Gupta, S. ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French bean (Phaseolus vulgaris) plants. Front. Microbiol. 2019, 10, 1506. [Google Scholar]
- Tolba, S.T.; Ibrahim, M.; Amer, E.A.; Ahmed, D.A. First insights into salt tolerance improvement of Stevia by plant growth-promoting Streptomyces species. Arch. Microbiol. 2019, 201, 1295–1306. [Google Scholar] [CrossRef]
- Wahdan, S.F.M.; Tanunchai, B.; Wu, Y.T.; Sansupa, C.; Schädler, M.; Dawoud, T.M.; Buscot, F.; Purahong, W. Deciphering Trifolium pratense L. holobiont reveals a microbiome resilient to future climate changes. Microbiologyopen 2021, 10, e1217. [Google Scholar] [CrossRef]
- Armada, E.; Azcón, R.; López-Castillo, O.M.; Calvo-Polanco, M.; Ruiz-Lozano, J.M. Autochthonous arbuscular mycorrhizal fungi and Bacillus thuringiensis from a degraded Mediterranean area can be used to improve physiological traits and performance of a plant of agronomic interest under drought conditions. Plant Physiol. Biochem. 2015, 90, 64–74. [Google Scholar] [CrossRef]
- Lastochkina, O.; Ivanov, S.; Petrova, S.; Garshina, D.; Lubyanova, A.; Yuldashev, R.; Kuluev, B.; Zaikina, E.; Maslennikova, D.; Allagulova, C. Role of endogenous salicylic acid as a hormonal intermediate in the bacterial endophyte Bacillus subtilis-induced protection of wheat genotypes contrasting in drought susceptibility under dehydration. Plants 2022, 11, 3365. [Google Scholar] [CrossRef]
- Oliveira, T.C.; Cabral, J.S.R.; Santana, L.R.; Tavares, G.G.; Santos, L.D.S.; Paim, T.P.; Müller, C.; Silva, F.G.; Costa, A.C.; Souchie, E.L. The arbuscular mycorrhizal fungus Rhizophagus clarus improves physiological tolerance to drought stress in soybean plants. Sci. Rep. 2022, 12, 9044. [Google Scholar] [CrossRef]
- Jatan, R.; Tiwari, S.; Asif, M.H.; Lata, C. Genome-wide profiling reveals extensive alterations in Pseudomonas putida-mediated miRNAs expression during drought stress in chickpea (Cicer arietinum L.). Environ. Exp. Bot. 2019, 157, 217–227. [Google Scholar] [CrossRef]
- Chandra, D.; Srivastava, R.; Glick, B.R.; Sharma, A.K. Rhizobacteria producing ACC deaminase mitigate water-stress response in finger millet (Eleusine coracana (L.) Gaertn.). 3 Biotech 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kour, D.; Rana, K.L.; Kaur, T.; Sheikh, I.; Yadav, A.N.; Kumar, V.; Dhaliwal, H.S.; Saxena, A.K. Microbe-mediated alleviation of drought stress and acquisition of phosphorus in great millet (Sorghum bicolour L.) by drought-adaptive and phosphorus-solubilizing microbes. Biocatal. Agric. Biotechnol. 2020, 23, 101501. [Google Scholar] [CrossRef]
- Chandra, D.; Srivastava, R.; Glick, B.R.; Sharma, A.K. Drought-tolerant Pseudomonas spp. improve the growth performance of finger millet (Eleusine coracana (L.) Gaertn.) under non-stressed and drought-stressed conditions. Pedosphere 2018, 28, 227–240. [Google Scholar] [CrossRef]
- Ansari, F.; Jabeen, M.; Ahmad, I. Pseudomonas azotoformans FAP5, a novel biofilm-forming PGPR strain, alleviates drought stress in wheat plant. Int. J. Environ. Sci. Technol. 2021, 18, 3855–3870. [Google Scholar] [CrossRef]
- Chandra, D.; Srivastava, R.; Gupta, V.V.; Franco, C.M.; Sharma, A.K. Evaluation of ACC-deaminase-producing rhizobacteria to alleviate water-stress impacts in wheat (Triticum aestivum L.) plants. Can. J. Microbiol. 2019, 65, 387–403. [Google Scholar] [CrossRef]
- Dubey, A.; Saiyam, D.; Kumar, A.; Hashem, A.; Abd_Allah, E.F.; Khan, M.L. Bacterial root endophytes: Characterization of their competence and plant growth promotion in soybean (Glycine max (L.) Merr.) under drought stress. Int. J. Environ. Res. Public Health 2021, 18, 931. [Google Scholar] [CrossRef] [PubMed]
- Chukwuneme, C.F.; Babalola, O.O.; Kutu, F.R.; Ojuederie, O.B. Characterization of actinomycetes isolates for plant growth promoting traits and their effects on drought tolerance in maize. J. Plant Interact. 2020, 15, 93–105. [Google Scholar] [CrossRef]
- El-Meihy, R.M.; Abou-Aly, H.E.; Youssef, A.M.; Tewfike, T.A.; El-Alkshar, E.A. Efficiency of heavy metals-tolerant plant growth promoting bacteria for alleviating heavy metals toxicity on sorghum. Environ. Exp. Bot. 2019, 162, 295–301. [Google Scholar] [CrossRef]
- Rizvi, A.; Khan, M.S. Heavy metal induced oxidative damage and root morphology alterations of maize (Zea mays L.) plants and stress mitigation by metal tolerant nitrogen fixing Azotobacter chroococcum. Ecotoxicol. Environ. Saf. 2018, 157, 9–20. [Google Scholar] [CrossRef]
- Ke, T.; Guo, G.; Liu, J.; Zhang, C.; Tao, Y.; Wang, P.; Xu, Y.; Chen, L. Improvement of the Cu and Cd phytostabilization efficiency of perennial ryegrass through the inoculation of three metal-resistant PGPR strains. Nviron. Pollut. 2021, 271, 116314. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Ilyas, N.; Yasmin, H.; Sayyed, R.; Hasnain, Z.; Elsayed, E.A.; El Enshasy, H.A. Role of Bacillus cereus in improving the growth and phytoextractability of Brassica nigra (L.) K. Koch in chromium contaminated soil. Molecules 2021, 26, 1569. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xu, M.; Wei, Q.; Tang, M.; Guan, L.; Lou, L.; Xu, X.; Hu, Z.; Chen, Y.; Shen, Z. Promotion of growth and phytoextraction of cadmium and lead in Solanum nigrum L. mediated by plant-growth-promoting rhizobacteria. Ecotoxicol. Environ. Saf. 2020, 205, 111333. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Tahzeeb-ul-Hassan, M.; Wahid, A.; Danish, S.; Khan, M.J.; Fahad, S.; Brtnicky, M.; Hussain, G.S.; Battaglia, M.L.; Datta, R. Compost mixed fruits and vegetable waste biochar with ACC deaminase rhizobacteria can minimize lead stress in mint plants. Sci. Rep. 2021, 11, 6606. [Google Scholar] [CrossRef] [PubMed]
- Awan, S.A.; Ilyas, N.; Khan, I.; Raza, M.A.; Rehman, A.U.; Rizwan, M.; Rastogi, A.; Tariq, R.; Brestic, M. Bacillus siamensis reduces cadmium accumulation and improves growth and antioxidant defense system in two wheat (Triticum aestivum L.) varieties. Plants 2020, 9, 878. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Nawaz, F.; Hussain, M.B.; Ikram, R.M. Comparative effects of individual and consortia plant growth promoting bacteria on physiological and enzymatic mechanisms to confer drought tolerance in maize (Zea mays L.). J Soil Sci. Plant Nutr. 2021, 21, 3461–3476. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Motesharezadeh, B.; Hosseini, H.M.; Alikhani, H.; Zolfaghari, A.A. Root-induced changes of Zn and Pb dynamics in the rhizosphere of sunflower with different plant growth promoting treatments in a heavily contaminated soil. Ecotoxicol. Environ. Saf. 2018, 147, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.N. Agriculturally important microbiomes: Biodiversity and multifarious PGP attributes for amelioration of diverse abiotic stresses in crops for sustainable agriculture. Biomed. J. Sci. Technol. Res. 2017, 1, 861–864. [Google Scholar]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Moreno, A.; Zhang, C.; Freitas, H. Serpentine endophytic bacterium Pseudomonas azotoformans ASS1 accelerates phytoremediation of soil metals under drought stress. Chemosphere 2017, 185, 75–85. [Google Scholar] [CrossRef]
- Kumar, U.; Kaviraj, M.; Rout, S.; Chakraborty, K.; Swain, P.; Nayak, P.; Nayak, A. Combined application of ascorbic acid and endophytic N-fixing Azotobacter chroococcum Avi2 modulates photosynthetic efficacy, antioxidants and growth-promotion in rice under moisture deficit stress. Microbiol. Res. 2021, 250, 126808. [Google Scholar] [CrossRef] [PubMed]
- Gagné-Bourque, F.; Bertrand, A.; Claessens, A.; Aliferis, K.A.; Jabaji, S. Alleviation of drought stress and metabolic changes in timothy (Phleum pratense L.) colonized with Bacillus subtilis B26. Front. Plant Sci. 2016, 7, 584. [Google Scholar] [CrossRef] [PubMed]
- Cherni, M.; Ferjani, R.; Mapelli, F.; Boudabous, A.; Borin, S.; Ouzari, H.-I. Soil parameters drive the diversity of Citrus sinensis rhizosphere microbiota which exhibits a potential in plant drought stress alleviation. Appl. Soil Ecol. 2019, 135, 182–193. [Google Scholar] [CrossRef]
- Valetti, L.; Iriarte, L.; Fabra, A. Growth promotion of rapeseed (Brassica napus) associated with the inoculation of phosphate solubilizing bacteria. Appl. Soil Ecol. 2018, 132, 1–10. [Google Scholar] [CrossRef]
- Silva, E.R.; Zoz, J.; Oliveira, C.E.S.; Zuffo, A.M.; Steiner, F.; Zoz, T.; Vendruscolo, E.P. Can co-inoculation of Bradyrhizobium and Azospirillum alleviate adverse effects of drought stress on soybean (Glycine max L. Merrill.)? Arch. Microbiol. 2019, 201, 325–335. [Google Scholar] [CrossRef]
- Arora, S.; Jha, P.N. Drought-tolerant Enterobacter bugandensis WRS7 induces systemic tolerance in Triticum aestivum L. (wheat) under drought conditions. J. Plant Growth Regul. 2023, 42, 7715–7730. [Google Scholar] [CrossRef]
- Kour, D.; Khan, S.S.; Kour, H.; Kaur, T.; Devi, R.; Rai, A.K.; Yadav, A.N. ACC deaminase producing phytomicrobiomes for amelioration of abiotic stresses in plants for agricultural sustainability. J. Plant Growth Regul. 2023, 43, 963–985. [Google Scholar] [CrossRef]
- Abdelkrim, S.; Abid, G.; Chaieb, O.; Taamalli, W.; Mannai, K.; Louati, F.; Jebara, M.; Jebara, S.H. Plant growth promoting rhizobacteria modulates the antioxidant defense and the expression of stress-responsive genes providing Pb accumulation and tolerance of grass pea. Environ. Sci. Pollut. Res. 2023, 30, 10789–10802. [Google Scholar] [CrossRef]
- Noori, F.; Etesami, H.; Noori, S.; Forouzan, E.; Jouzani, G.S.; Malboobi, M.A. Whole genome sequence of Pantoea agglomerans ANP8, a salinity and drought stress–resistant bacterium isolated from alfalfa (Medicago sativa L.) root nodules. Biotechnol. Rep. 2021, 29, e00600. [Google Scholar] [CrossRef]
- Lastochkina, O.; Aliniaeifard, S.; Garshina, D.; Garipova, S.; Pusenkova, L.; Allagulova, C.; Fedorova, K.; Baymiev, A.; Koryakov, I.; Sobhani, M. Seed priming with endophytic Bacillus subtilis strain-specifically improves growth of Phaseolus vulgaris plants under normal and salinity conditions and exerts anti-stress effect through induced lignin deposition in roots and decreased oxidative and osmotic damages. J. Plant Physiol. 2021, 263, 153462. [Google Scholar]
- Nohwar, N.; Khandare, R.V.; Desai, N.S. Isolation and characterization of salinity tolerant nitrogen fixing bacteria from Sesbania sesban (L) root nodules. Biocataly. Agric. Biotechnol. 2019, 21, 101325. [Google Scholar] [CrossRef]
- Khan, M.; Asaf, S.; Khan, A.; Adhikari, A.; Jan, R.; Ali, S.; Imran, M.; Kim, K.M.; Lee, I.J. Plant growth-promoting endophytic bacteria augment growth and salinity tolerance in rice plants. Plant Biol. 2020, 22, 850–862. [Google Scholar] [CrossRef]
- Bhatta, D.; Adhikari, A.; Kang, S.-M.; Kwon, E.-H.; Jan, R.; Kim, K.-M.; Lee, I.-J. Hormones and the antioxidant transduction pathway and gene expression, mediated by Serratia marcescens DB1, lessen the lethality of heavy metals (As, Ni, and Cr) in Oryza sativa L. Ecotoxicol. Environ. Saf. 2023, 263, 115377. [Google Scholar] [CrossRef]
- Sapkota, S.; Harris-Shultz, K.R.; Strickland, T.C.; Anderson, W.F. Identification of cultured and diazotrophic bacterial endophytes in warm-season grasses. PhytoFrontiers 2023, 3, 411–419. [Google Scholar] [CrossRef]
- Pérez-García, A.; Romero, D.; de Vicente, A. Plant protection and growth stimulation by microorganisms: Biotechnological applications of Bacilli in agriculture. Curr. Opin. Biotechnol. 2011, 22, 187–193. [Google Scholar] [CrossRef]
- Lal, S.; Ratna, S.; Said, O.B.; Kumar, R. Biosurfactant and exopolysaccharide-assisted rhizobacterial technique for the remediation of heavy metal contaminated soil: An advancement in metal phytoremediation technology. Environ. Technol. Innov. 2018, 10, 243–263. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, F.; Liu, Q.; Chen, M.; Liu, X.; Wang, Y.; Sun, Y.; Zhang, L. Nanomaterials and plants: Positive effects, toxicity and the remediation of metal and metalloid pollution in soil. Sci. Total Environ. 2019, 662, 414–421. [Google Scholar] [CrossRef]
- Kaushal, M.; Wani, S.P. Plant-growth-promoting rhizobacteria: Drought stress alleviators to ameliorate crop production in drylands. Ann. Microbiol. 2016, 66, 35–42. [Google Scholar] [CrossRef]
- Oyedoh, O.P.; Yang, W.; Dhanasekaran, D.; Santoyo, G.; Glick, B.R.; Babalola, O.O. Rare rhizo-Actinomycetes: A new source of agroactive metabolites. Biotechnol. Adv. 2023, 67, 108205. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Ahmad, R.; Pervez, A.; Al Farraj, D.A.; Elshikh, M.S.; Shahzad, M.; Irshad, U.; Abbasi, A.M. Combination of melatonin and plant growth promoting rhizobacteria improved the growth of Spinacia oleracea L. under the arsenic and cadmium stresses. Physiol. Mol. Plant Pathol. 2023, 127, 102097. [Google Scholar] [CrossRef]
- Wu, J.; Kamal, N.; Hao, H.; Qian, C.; Liu, Z.; Shao, Y.; Zhong, X.; Xu, B. Endophytic Bacillus megaterium BM18-2 mutated for cadmium accumulation and improving plant growth in Hybrid Pennisetum. Biotechnol. Rep. 2019, 24, e00374. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Imtiaz, M.; Nawaz, M.S.; Mubeen, F.; Sarwar, Y.; Hayat, M.; Asif, M.; Naqvi, R.Z.; Ahmad, M.; Imran, A. Thermotolerant PGPR consortium B3P modulates physio-biochemical and molecular machinery for enhanced heat tolerance in maize during early vegetative growth. Ann. Microbiol. 2023, 73, 34. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Ayilara, M.S.; Akinola, S.A.; Babalola, O.O. Biocontrol mechanisms of endophytic fungi. Egypt. J. Biol. Pest Control 2022, 32, 46. [Google Scholar] [CrossRef]
- Tewari, S.; Arora, N. Soybean production under flooding stress and its mitigation using plant growth-promoting microbes. In Environmental Stresses in Soybean Production; Elsevier: Amsterdam, The Netherlands, 2016; pp. 23–40. [Google Scholar]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Wang, C.; Kuzyakov, Y. Rhizosphere engineering for soil carbon sequestration. Trends Plant Sci. 2024, 29, 447–468. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O. The endosphere microbial communities, a great promise in agriculture. Int. Microbiol. 2021, 24, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.; Ahmad, E.; Khan, M.S.; Saif, S.; Rizvi, A. Role of plant growth promoting rhizobacteria in sustainable production of vegetables: Current perspective. Sci. Hortic. 2015, 193, 231–239. [Google Scholar] [CrossRef]
- Gupta, G.; Parihar, S.S.; Ahirwar, N.K.; Snehi, S.K.; Singh, V. Plant growth promoting rhizobacteria (PGPR): Current and future prospects for development of sustainable agriculture. J. Microbiol. Biochem. Technol. 2015, 7, 096–102. [Google Scholar]
- Sudheer, S.; Bai, R.G.; Usmani, Z.; Sharma, M. Insights on engineered microbes in sustainable agriculture: Biotechnological developments and future prospects. Curr. Genom. 2020, 21, 321–333. [Google Scholar] [CrossRef]
- O’Callaghan, M.; Ballard, R.A.; Wright, D. Soil microbial inoculants for sustainable agriculture: Limitations and opportunities. Soil Use Manag. 2022, 38, 1340–1369. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Ayangbenro, A.S.; Babalola, O.O. Genomic assessment of Stenotrophomonas indicatrix for improved sunflower plant. Curr. Genet. 2021, 67, 891–907. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.K.; McClure, R.; Egbert, R.G. Soil microbiome engineering for sustainability in a changing environment. Nat. Biotechnol. 2023, 41, 1716–1728. [Google Scholar] [CrossRef] [PubMed]
- Moreira, Z.P.M.; Chen, M.Y.; Ortuno, D.L.Y.; Haney, C.H. Engineering plant microbiomes by integrating eco-evolutionary principles into current strategies. Curr. Opinion Plant Biol. 2023, 71, 102316. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Joshi, A.; Hur, H.-G.; Lee, J.-H. Nodulation experiment by cross-inoculation of nitrogen-fixing bacteria isolated from root nodules of several leguminous plants. J. Microbiol. Biotechnol. 2024, 34, 570. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Wang, R.; Qin, W.; Yang, J. Screening of rhizosphere nitrogen fixing, phosphorus and potassium solubilizing bacteria of Malus sieversii (Ldb.) Roem. and the effect on apple growth. J. Plant Physiol. 2024, 292, 154142. [Google Scholar] [CrossRef]
- Chauhan, P.; Singh, M.; Sharma, A.; Singh, M.; Chadha, P.; Kaur, A. Halotolerant and plant growth-promoting endophytic fungus Aspergillus terreus CR7 alleviates salt stress and exhibits genoprotective effect in Vigna radiata. Front. Microbiol. 2024, 15, 1336533. [Google Scholar] [CrossRef]
- Zeng, R.; Liu, H.; Hong, Z.; Wang, X.; Cheng, S.; Xu, J.; Dai, Z. Co-inoculation effects of B. licheniformis and P. aeruginosa on soil Cd and As availability and rice accumulation. J. Environ. Manag. 2024, 351, 119739. [Google Scholar] [CrossRef]
- Pandino, G.; Abbate, C.; Scavo, A.; Di Benedetto, D.; Mauromicale, G.; Lombardo, S. Co-inoculation of arbuscular mycorrhizal fungi and plant growth promoting bacteria improves plant growth and yield of globe artichoke (Cynara cardunculus scolymus). Sci. Hortic. 2024, 335, 113365. [Google Scholar] [CrossRef]
- Solórzano-Acosta, R.; Toro, M.; Zúñiga-Dávila, D. Effect of co-inoculation with growth-promoting bacteria and arbuscular Mycorrhizae on growth of Persea americana seedlings infected with Phytophthora cinnamomi. Microorganisms 2024, 12, 721. [Google Scholar] [CrossRef]
- Slimani, A.; Oufdou, K.; Meddich, A. Intercropping and co-inoculation of beneficial microorganisms of soils improve drought tolerance in barley and Alfalfa plants. J. Crop Health 2024, 76, 471–485. [Google Scholar] [CrossRef]
- Agunbiade, V.F.; Fadiji, A.E.; Agbodjato, N.A.; Babalola, O.O. Isolation and characterization of plant-growth-promoting, drought-tolerant rhizobacteria for improved maize productivity. Plants 2024, 13, 1298. [Google Scholar] [CrossRef] [PubMed]
- Geller, A.M.; Levy, A. “What I cannot create, I do not understand”: Elucidating microbe–microbe interactions to facilitate plant microbiome engineering. Curr. Opin. Microbiol. 2023, 72, 102283. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.; Williams, A.P.; Griffith, G.W.; Withers, P.J. Use of commercial bio-inoculants to increase agricultural production through improved phosphrous acquisition. Appl. Soil Ecol. 2015, 86, 41–54. [Google Scholar] [CrossRef]
- Xiao, J.-l.; Sun, J.-G.; Pang, B.; Zhou, X.; Gong, Y.; Jiang, L.; Zhang, L.; Ding, X.; Yin, J. Isolation and screening of stress-resistant endophytic fungus strains from wild and cultivated soybeans in cold region of China. Appl. Microbiol. Biotechnol. 2021, 105, 755–768. [Google Scholar] [CrossRef]
- Adeleke, B.; Ayangbenro, A.; Babalola, O. In vitro screening of sunflower associated endophytic bacteria with Plant growth-promoting traits. Front. Sustain. Food Syst. 2022, 6, 903114. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).