Abstract
An animal’s ability to navigate its home range in search of essential resources is a key aspect of its ecology. To reach these resources, animals employ varying navigational processes depending on their exocentric or egocentric view of their environment. The goal of this study was to determine if the Javan slow loris (Nycticebus javanicus), a nocturnal arboreal primate found in southeast Asia, uses some form of cognitive map and spatial memory while navigating their environment. Using behavioural and GPS data of six males and seven females collected at the Little Fireface Project field station based in West Java, Indonesia, we measured their frequency of revisiting important feeding trees, route overlap, and points where individuals significantly changed directions. We found that all individuals predominantly used four tree species while feeding and foraging. The lorises also displayed a high level of route overlap, leading us to conclude that they likely utilize a route-based cognitive map where certain landmarks are integral to their nightly movement. Few studies have specifically focused on strepsirrhine spatial cognition in the wild; here, we show the navigational mechanism used by the Javan slow loris to reach distant/out-of-sight resources. The evident reliance on spatial cognition in a strepsirrhine species suggest that it could be an important selective pressure for primates at the earliest stages of primate cognitive evolution. In addition to the importance of spatial memory in theoretical discourse, understanding slow loris movement has practical applications to conservation, particularly regarding the numerous translocations undertaken by individuals rescued from the illegal wildlife trade. We discuss the importance of considering soft release training and monitoring in such releases.
1. Introduction
An animal’s ability to navigate its home range in search of essential resources is a key aspect of its ecology [1,2]. Finding these resources requires integration of spatial, temporal, and ecological factors, which may act as an important selection pressure on the evolution of spatial cognition [3,4]. Many animals maintain large home ranges that contain numerous potential feeding locations, many of which are not within sight of one another [1]. To reach these resources, animals will employ varying navigational processes depending on their exocentric or egocentric view of their environment [5]. The investigation of foraging strategies provides insights into the cognitive capacity of animals [6,7,8].
Byrne [9] and Garber [10] identified two types of cognitive maps, or mental representations, used by primates during foraging. Route based or topological cognitive maps are based on the spatial relation between objects [9,11]. This type of map implies that individuals have a representation of space, in which they are not able to visualize distance and direction to a desired location directly, but instead they must use landmarks along a route [12]. The Euclidean or vector-based cognitive map [9,12] allows the animal to perceive distance and direction from any point in the environment and is comparable to a Euclidean representation of space [11]. The inherently complex nature of identifying the mental state of animals makes it difficult to attribute any one navigational mechanism to observed movements. We can infer what mechanism is likely to be used, under the assumption that it will be the most efficient and cognitively available option. However, there is also evidence that some animals will use both cognitive maps based on the season [13,14] and distance to the goal [15].
Studies on spatial memory and foraging conducted across primate taxa showed that route-based maps are the most used form of cognitive map [16,17], with Euclidean maps being reported in chimpanzees [12,18,19,20], bonobos [21], and to an extent gorillas [22]. Despite their importance for reconstructing primate evolution and widespread interest in primate spatial ecology and cognition, only a handful of studies focus on strepsirrhines in the wild [3,13,23] and even fewer on nocturnal strepsirrhines [24,25].
The grey mouse lemur (Microcebus murinus) is the only nocturnal strepsirrhine with published large-scale navigational data in a wild context [24,25]. These lemurs were able to return to specific feeding locations and their movement patterns fitted with the expectations of a route-based cognitive map. In a captive setting, nocturnal primates’ navigation was explored in the fat-tailed dwarf lemur (Cheirogaleus medius), grey mouse lemurs and aye-ayes (Daubentonia madagascariensis). Each showed varying degrees of exploration and heuristics based on their dietary specialization: the more frugivorous dwarf lemur relied on heuristics and the least frugivorous aye-aye explored more [26]. Like grey mouse lemurs, the Javan slow loris (Nycticebus javanicus) displays the ability to adapt to changes in their environment, which is characterized by extreme anthropogenic disturbance [27]. The species also consumes a diet containing large proportions of exudates, nectar and insects [28], but the cognitive mechanism used to find and return to key immobile resources has not been identified. Gum is important as both a primary resource and a fallback food in at least 78 primate species [29]. Aspects of the Javan slow loris feeding ecology and postural ontogeny suggest that young individuals develop adult limb proportions to enable them to gouge and feed on tree gums [30]. If individuals develop to facilitate gouging and feeding on tree gum, it is also possible that they have efficient means to return regularly to these spatially dispersed feeding resources. The Javan slow loris thus provides an opportunity to explore further factors that may shed light on the origins of primate navigational mechanisms.
In this study, we examine the travel patterns of the Javan slow loris in a montane agroforest. To determine if Javan slow lorises use some form of cognitive map and spatial memory while navigating their environment, we addressed the following research questions: does the Javan slow loris display directedness during travel? If so, which type of navigational mechanism best characterizes their spatial representation of their environment? To determine if this species uses spatial memory, we assessed their nightly movement for directedness by measuring their reuse feeding trees, how they reach these feeding trees and other behaviourally important locations, and if there are places where directional changes take place. To rule out the possibility that they are reaching goal locations randomly, we also simulate nightly routes and compare the efficiency of each route at reaching feeding trees against routes each loris travelled. We hypothesized that Javan slow loris display directed travel patterns between resources. This hypothesis is supported if their nightly routes are reused creating a route network including nodes that connect multiple routes. Furthermore, we hypothesized that the Javan slow loris uses travel patterns best categorized as route-based cognitive maps, given it is the most prevalent cognitive map in the order Primates.
2. Materials and Methods
2.1. Study Species
The Javan slow loris is an approximately 1 kg non-leaping nocturnal primate found on the Indonesian island of Java in insular Southeast Asia (Figure 1). As a specialized exudate feeder, it relies heavily on immobile food resources consisting of gum and sap, but with nectar from flowers also being important [31]. The Javan slow loris lives in a uni-male/uni-female family group in home ranges of 5–10 ha, but largely forages solitarily [27,28]. For this study, we included 13 adult animals (M: 6, F: 7) that had established their home ranges for a year or more, from a well-known population that has been studied since 2011 [27].
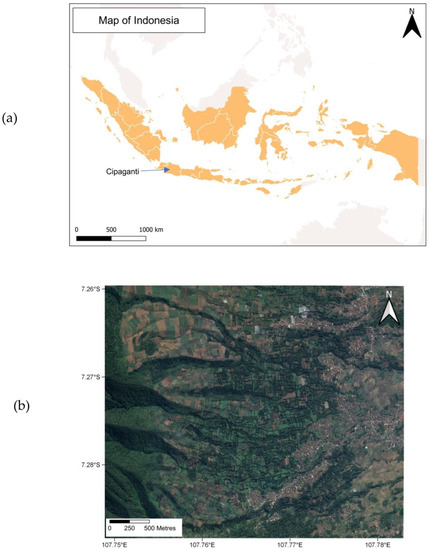
Figure 1.
(a) Map of the study location within Indonesia, and (b) a satellite image of the montane agroforest where this study population was observed. This map was reproduced from [32].
2.2. Study Site and Data Collection
As a part of a long-term research project, we collected data for this study between January and December 2016 on a population of radio-collared slow lorises in Cipaganti, Garut District, West Java (7°6′6″ 7°7′ S and 107°46′ 107°46′5″ E). The area between the adjacent village and the protected forest is primarily patches of cultivated lands, bamboo, shrubs, and tree lines. The area where we conducted nightly observations encompassed 60 ha with elevations varying between 1200–1800 m asl [31].
We followed a protocol approved by the Animal Ethics Subcommittee at Oxford Brookes University. The radio collars (BioTrack, Wareham, UK) weighed 17 g, which is on average less than 2% of the body weight of an adult Javan slow loris and less than 4% of an immature Javan slow loris. We collected data through full- and half-night follows between 17:30 and 05:00. We identified individuals by a unique radio signal emitted from the radio collars worn by each study animal. During each shift we followed one focal study animal [33] and used a five-minute instantaneous point [34,35] to collect data on (1) the animal’s behaviour (i.e., alert, feed, forage, freeze, groom, rest, sleep, social, travel, other), (2) the tree species occupied, and (3) the GPS location of the individual (Garmin 64 s).
2.3. Data Analysis
We report all values as means ± standard deviation, and we accepted statistical significance when p ≤ 0.05 in two-tailed tests. To compare differences between sexes we used a Mann–Whitney U test, and Person’s correlation was used to test the relationship between home range size, overlap, and the percentage of revisited gum trees. To calculate the frequency of revisited gum-producing trees, we first defined unique gum-tree sites as trees that were 20 m or more apart to account for clusters and GPS error (5 m). If an individual visited the same cluster of trees on different nights, we noted that as a revisited tree. If multiple sites were less than 20 m apart, this was considered one revisited site.
We calculated each lorises’ home range in hectares (ha), representing the 95% kernel density estimation (KDE) and minimum convex polygon (MCP) in QGIS. We analysed all other collected geographical data in ArcGIS version 10.3. and R Programming 10.3. We used the straight-line distance between consecutive GPS points to determine the nightly route lengths for full- and half-night follows separately. Following a modified version of the methods used by Asensio et al. [15], we determined route overlap by calculating the length of overlapping routes, divided by the total length of all included routes. We defined overlapping routes as those that fell within a 5 m buffer, for at least 20 m [15,25], and these are reported as a percentage for each individual. During the overlap analyses, we excluded routes that had discontinuous points (those collected greater than 30 min apart).
We applied the change-point test (CPT), created by Byrne et al. [36], to nightly routes that included at least 15 waypoints, in R Programming 10.3. The CPT can determine locations where each route significantly changed direction, starting from the sleep site in the early evening. We recorded the behaviour before and after each change point to identify any potential behavioural associations.
We used simulated heuristic routes to test travel efficiency and to rule out that the slow lorises were randomly moving within their environment. The simulated routes started at the beginning of an actual travel bout and continued in a random direction until they encountered a gum-producing tree or the edge of the home range [15,25]. As part of the long-term study site, the locations of all the gum-producing trees previously visited by a slow loris as well as trees with visible gouge marks were recorded, regardless of size. To account for the varying distances an individual is able to identify and travel to a goal location through their senses (instead of using spatial memory alone), i.e., primarily vision, we added 5 m, 10 m, and 20 m buffers around each goal location while testing the efficiency of the simulated routes [25]. We defined the efficiency index as the number of goal locations visited by an individual subtracted by the number of goal locations found by the model, divided by the total number of goal locations visited within the home range. A negative value indicated that the model performed more efficiently than the study animal in finding goal locations, and a positive value indicated that the study animal was more efficient than the model [15]. We used a Kruskal–Wallis test to compare the efficiency index across detection radii.
3. Results
3.1. Nightly Route Length
Across all 13 individuals, the mean full-night route length (NPL) was 541 + 194 m, and the mean half-night NPL was 239 ± 53 m (Table 1). Males and females travelled comparable distances each night.

Table 1.
Full- and half-night route lengths (meters), homerange size using kernel density estimates and minimum convex polygon (hectare) in each adult male and female Javan slow loris denoted by their initial.
3.2. Visited Tree Diversity
Over the 12-month study period, slow lorises visited more than 20 types of trees within the area, though they predominantly revisited only four tree types: green wattle (Acacia decurrens), fairy duster (Calliandra spp.), bamboo (Bambusa spp.), and weeping paperbark (Melaleuca leucadendra). Within the proportion of sample points spent feeding and foraging, most of these points were spent at gum-producing trees, with 22.6 ± 12.6% (N = 565) spent in green wattle trees and 24.0 ± 10.3% (N = 502) in weeping paperbark. Additionally, 16.2 ± 7.0% (N = 347) of their time was spent in fairy duster trees, a nectar-producing site, and 18.0 ± 10.7% (N = 335) was spent in bamboo.
3.3. Revisited Gum-Producing Trees
Within each slow loris home range, individuals revisited gum-producing trees. They revisited 56.7 ± 13% of the unique gum trees visited throughout 2016 (Table 2). There was a significant positive correlation between the number of gum trees revisited and the home range size (r = 0.554, p = 0.0491).

Table 2.
The number of change-points present in the nightly routes, the percentage of their route that overlapped, and the number of unique gum-producing trees visited and revisited for each Javan slow loris included in the study.
3.4. Directional Changes and Route Overlap
We identified 140 change-points used during nightly travel (Table 2). Most change- points were associated with feeding (87%), sleeping (7%), and social (4%) behaviours. For two change-points, we were unable to identify any behaviourally meaningful associations. Of the 122 change-points associated with feeding behaviours, 104 were at gum-producing tree species and 18 were associated with nectar-producing fairy duster trees. Route overlap averaged 29 ± 8% per individual and ranged from 16% in individual TO to 44% in individual TE (Figure 2). Though there was no statistically significant difference between males and females, there was a trend where females displayed more overlap than males.
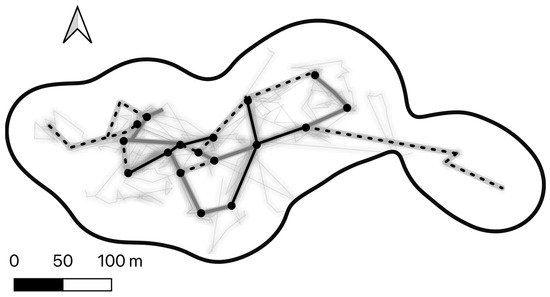
Figure 2.
Line density heatmap of the nightly routes for one female Javan slow loris (TE) throughout the study period, within their home range (grey 95% KDE). The overlapping route network is presented in black (routes travelled more than five times), grey (three to four times), and dotted (twice). The black circles are junctions or nodes where multiple routes intersected over multiple days. In grey are all the recorded routes.
3.5. The Efficiency Index (EI)
The EI varied significantly across the detection radii (H = 569.5, df = 2, p < 0.0001); Javan slow lorises were more efficient than the model at the 5 m detection radius, performed similarly at 10 m, but were less efficient at the 20 m detection radius (Figure 3). Figure 4 presents an example of an actual and simulated route in one male Javan slow loris (AL).
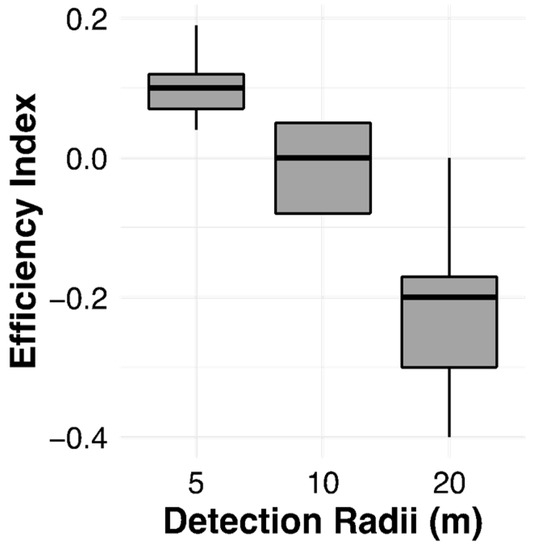
Figure 3.
Efficiency indices measured in 13 Javan slow lorises (Nycticebus javanicus) in relation to the detection radii.
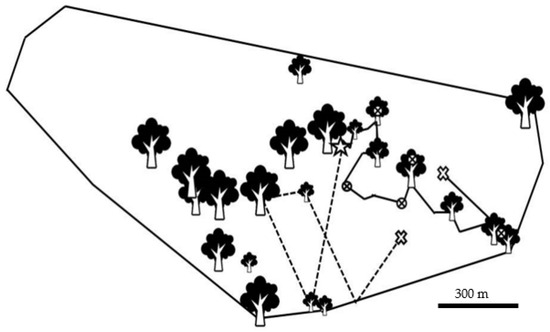
Figure 4.
Map of feeding locations visited by a male Javan slow loris (Nycticebus javanicus) and its home range (calculated using a 95% minimum convex polygon). Feeding sites are represented by tree icons, which vary in size based on the number observation points recorded in that area. The individual’s route is represented by the black line starting at the star and ending at the ‘X’. One route produced using a heuristic model at the five-metre detection radius is represented by the dotted line, starting at the star, and ending at the ‘X’. Along the actual route the encircled ‘X’ denotes change-points. The black line denotes 300 m.
4. Discussion
Based on the Javan slow loris’ frequent use of gum- and nectar-producing trees, we can see that traveling to feeding locations is an important aspect of their nightly movement. Throughout the night, individuals reused routes while traveling between goal locations and used change-points associated with those locations. The Javan slow loris outperformed the simulated routes in reaching feeding trees at the shortest detection radii, making it unlikely that they use random routes to routinely reach goal locations, but instead displays characteristics of a route-based cognitive map to reach distant immobile out-of-sight resources. Data presented here offer insight into the navigational capacity of the Javan slow loris, as resource distribution seems to structure their nightly movement.
It is important to rule out the possibility that animals are just using a random search model or happen upon needed resources [37]. The varying results produced by the efficiency index offer support towards rejecting the use of random search methods. The Javan slow loris outperformed the model in efficiency, reaching feeding resources within a 5 m detection radius; however, the model outperformed the real routes once the detection radius reached 20 m. Similar results were reported in mouse lemurs [25]. The perceptual range of slow loris species in this area is not known; thus, we were unable to assess to what degree visual, olfactory, or auditory cues may aid in reaching goal locations. However, it is known that all three of these environmental cues aid in foraging success [38,39,40,41]. Sensory cues work in concert to inform social, foraging, and movement behaviour [42].
Across primate species, including larger gregarious and small nocturnal species, the distribution of preferred feeding trees also structure the way individuals and groups move throughout their home ranges [15,25,43,44]. Tree exudates are a key food item for slow lorises [45]. Accessing tree exudates, such as gum, requires them to generate a hole and wait for the tree to repair the wound by producing the desired gums. Given the characteristics of this crucial resource, it is unlikely that slow lorises are monitoring feeding resources through visual or olfactory cues in the same ways that frugivores may [44]. The most plausible method that lorises use to revisit gum-producing trees and other behaviourally meaningful locations is to rely on their spatial memory. The fact that they revisit more than half of the unique tree sites within their home range further supports their use of spatial memory while foraging, as noted in other animals [46,47].
The Javan slow lorises in this population displayed route overlap comparable to other primates, including white-handed gibbons (Hylobates lar) (8–49%), grey mouse lemurs (9–30%), and saddleback tamarins (Saguinus fuscicollis) (32.4–74.2%) [15,25,48]. It is essential to highlight that the comparable level of overlap seen here is the result of non-consecutive observations. As a part of the 12-year study protocol, each individual was typically observed twice per month. Unlike the studies mentioned above, we did not complete consecutive multi-night observations but focused more on a longitudinal sampling method; thus, it is likely that the Javan slow loris uses a more robust route network than we detected in this study. However, we can conclude that the level of route reuse we did observe suggests that the Javan slow loris relies on the location of feeding sites and a route network to move through their home range, as is the case in other animals [49].
The presence of directional changes in association with goal resources suggests that the Javan slow loris uses the structural features of their landscape to reinforce travel decisions. When individuals reach landmark locations, they have more than one option past that point depending on the goal. When looking at cognitive map formation and use across other taxa, change-points can coincide with nodes or overlapping points within the route network where travel decisions are made routinely [1,50]. This principle is the primary assumption in justifying the importance of change-points in navigation by Byrne et al. [36]. Having a detailed cognitive map with several nodes and established routes aids movement and resource exploitation. Though highly flexible in their use and the ability to manoeuvre around their dynamic environment [27], the Javan slow loris likely uses landmarks to navigate to goal destinations, linking their survival to the landscape. In addition to the presence of a framework for their nightly travel, in a route-based map, the navigator needs the cognitive ability to integrate changing phenological data and have a sense of time to access and exploit changing resources accurately. We did not consider seasonality in this study but there is a known seasonality to diets of the slow lorises in this population [51]; therefore, further work is needed to fully understand how the route network changes with changing resources.
5. Translocation Implications
All slow lorises are globally threatened, including the Critically Endangered Javan slow loris, with a main threat to the genus being the illegal wildlife trade. For those that are confiscated, they are regularly translocated within and beyond their historical range [52,53,54,55,56]. Unfortunately, there is little data on their habitats to assist in the translocation work. In published work on slow loris translocations, habitat data is often absent [52], described in vague terms [57], or primarily focused on forest connectivity and not the spatial distribution of key resources regarding feeding and foraging [53,54,55,56]. If Javan slow lorises build a cognitive map of their environment based on the locations of key resources, characterizing resource distribution should be a key component in planning translocations. In addition, it takes time to build a cognitive map. Since post-release success is predicated on “survival” within a predetermined window, we must consider the timeframe needed for a species to familiarize themselves with their environment. Further studies on how slow lorises develop their cognitive maps could help redefine the standard timeframe by which we can measure the success of translocations. Indeed, in a study by Campera et al. [58], when comparing dispersing animals who had opportunities to familiarize themselves with potential dispersal sites with translocated ones in the same area, natal dispersers had higher survival rates and settled more quickly.
Habitat quality is an essential component of translocation success [59,60] and is most cited as the cause of translocation failures [61,62,63]. Translocations can fail when a released animal cannot acclimate itself to a new environment [64,65], or rejects a site and disperses [66,67]. Not only should we thoroughly assess the presence of essential vegetation in potential release sites, but we should also measure the spatial distribution and density of key resources. The degree to which individual trees are aggregated or dispersed is crucial to how an individual will utilize their new area. Selecting a site with an insufficient distribution of key resources may limit the number of individuals that can be translocated, especially in territorial species like the Javan slow loris. Any frequent structural changes should also be noted. As a probable route-based map user, landmarks are a crucial aspect of the way these primates envision their environment. If the potential release site is part of an anthropogenically modified area, the translocated slow lorises may face difficulty in creating a complete mental representation from which to root their umwelt, thus leading to failed translocations efforts. Despite the challenges of translocations, they can be a powerful tool for conserving wild populations if researchers can determine if the target individuals are able to find and relocate key resources to survive, especially in species that rely on spatially restricted resources.
Author Contributions
Conceptualization, S.A.P. and K.A.-I.N.; methodology, S.A.P.; software, S.A.P.; validation, S.A.P.; formal analysis, S.A.P.; investigation, K.A.-I.N. and S.A.P.; resources, K.A.-I.N.; data curation, K.A.-I.N.; writing—original draft preparation, S.A.P.; writing—review and editing, S.A.P., K.A.-I.N. and V.N.; visualization, S.A.P.; supervision, K.A.-I.N., M.A.I. and V.N.; project administration, K.A.-I.N., V.N. and M.A.I.; funding acquisition, K.A.-I.N. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Augsburg Zoo, Cleveland Zoo and Zoo Society, Conservation International Primate Action Fund and Margot Marsh Biodiversity Fund, Disney Worldwide Conservation Fund, International Primate Protection League, Lee Richardson Zoo, Memphis Zoo, Mohamed bin al Zayed Species Conservation Fund (152511813), Moody Gardens Zoo, NaturZoo Rhein, Omaha’s Henry Doorly Zoo, People’s Trust for Endangered Species, Plumploris E.V., Primate Society of Great Britain, San Francisco Zoo, Shaldon Wildlife Trust, Sophie Danforth Conservation Biology Fund and Zoo De Lille.
Institutional Review Board Statement
All research was approved by the Animal Care Subcommittee of Oxford Brookes University number OBURASC0911 and adhered to the ASAB/ABS Guidelines for the Use of Animals in Research. All necessary research permits were obtained from the Indonesian government. All research adhered to the legal and ethical guidelines of the Indonesian Institute of Sciences, Department of Wildlife and Department of Forestry.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
We thank the Little Fireface Project field team for their help in radio tracking slow lorises, data collection, and aid in local logistics. We thank the three reviewers and the editor for their very useful comments and the Loris Lab at Oxford Brookes University for their comments on this manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Di Fiore, A.; Suarez, S.A. Route-based travel and shared routes in sympatric spider and woolly monkeys: Cognitive and evolutionary implications. Anim. Cogn. 2007, 10, 317–329. [Google Scholar] [CrossRef]
- Mueller, T.; Fagan, W.F. Search and navigation in dynamic environments–from individual behaviors to population distributions. Oikos 2008, 117, 654–664. [Google Scholar] [CrossRef]
- Lührs, M.L.; Dammhahn, M.; Kappeler, P.M.; Fichtel, C. Spatial memory in the grey mouse lemur (Microcebus murinus). Anim. Cogn. 2009, 12, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.E. Mantled howler monkey spatial foraging decisions reflect spatial and temporal knowledge of resource distributions. Anim. Cogn. 2016, 19, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.E.; Holtzer, R.; Lipton, R.B.; Hall, C.; Verghese, J. Egocentric and exocentric navigation skills in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Garber, P.A.; Dolins, F.L. Primate spatial strategies and cognition: Introduction to this special issue. Am. J. Primatol. 2014, 76, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Hills, T.T.; Butterfill, S. From foraging to autonoetic consciousness: The primal self as a consequence of embodied prospective foraging. Curr. Zool. 2015, 61, 368–381. [Google Scholar] [CrossRef]
- Gautestad, A.O.; Mysterud, A. The Lévy flight foraging hypothesis: Forgetting about memory may lead to false verification of Brownian motion. Mov. Ecol. 2013, 1, 9. [Google Scholar] [CrossRef]
- Byrne, R.W. How monkeys Find their way. Leadership, coordination, and cognitive maps of African baboons. In On the Move: How and Why Animals Travel in Groups; Boinski, S.B., Garber, P.A., Eds.; University of Chicago Press: Chicago, IL, USA, 2000; pp. 491–518. [Google Scholar]
- Garber, P.A. Evidence for the use of spatial, temporal and social information by some primate foragers. In On the Move: How and Why Animals Travel in Groups; Boinski, S.B., Garber, P.A., Eds.; University of Chicago Press: Chicago, IL, USA, 2000; pp. 261–298. [Google Scholar]
- Piaget, J.; Inhelder, B. The Child’s Concept of Space; Routledge & Paul: London, UK, 1956. [Google Scholar]
- Normand, E.; Boesch, C. Sophisticated Euclidean maps in forest chimpanzees. Anim. Behav. 2009, 77, 1195–1201. [Google Scholar] [CrossRef]
- Watkins, B.; de Guinea, M.; Poindexter, S.A.; Ganzhorn, J.U.; Donati, G.; Eppley, T.M. Routes matter: The effect of seasonality on bamboo lemur navigational strategies. Anim. Behav. 2022, 186, 137–149. [Google Scholar] [CrossRef]
- Presotto, A.; Izar, P. Spatial reference of black capuchin monkeys in Brazilian Atlantic Forest: Egocentric or allocentric? Anim. Behav. 2010, 80, 125–132. [Google Scholar] [CrossRef]
- Asensio, N.; Brockelman, W.Y.; Malaivijitnond, S.; Reichard, U.H. Gibbon travel routes are goal oriented. Anim. Cogn. 2011, 14, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Rolland, E.; Trull, S. Spatial mapping memory: Methods used to determine the existence and type of cognitive maps in arboreal mammals. Mammal Rev. 2022, 52, 96–111. [Google Scholar] [CrossRef]
- Trapanese, C.; Meunier, H.; Masi, S. What, where and when: Spatial foraging decisions in primates. Biol. Rev. 2019, 94, 483–502. [Google Scholar] [CrossRef]
- Green, S.J.; Boruff, B.J.; Grueter, C.C. From ridge tops to ravines: Landscape drivers of chimpanzee ranging patterns. Anim. Behav. 2020, 163, 51–60. [Google Scholar] [CrossRef]
- Green, S.J.; Boruff, B.J.; Bonnell, T.R.; Grueter, C.C. Chimpanzees use least-cost routes to out-of-sight goals. Curr. Biol. 2020, 30, 4528–4533. [Google Scholar] [CrossRef]
- Normand, E.; Ban, S.D.; Boesch, C. Forest chimpanzees (Pan troglodytes verus) remember the location of numerous fruit trees. Anim. Cogn. 2009, 12, 797–807. [Google Scholar] [CrossRef]
- Menzel, C.R.; Savage-Rumbaugh, E.S.; Menzel, E.W. Bonobo (Pan paniscus) spatial memory and communication in a 20-hectare forest. Int. J. Primatol. 2002, 23, 601–619. [Google Scholar] [CrossRef]
- Salmi, R.; Presotto, A.; Scarry, C.J.; Hawman, P.; Doran-Sheehy, D.M. Spatial cognition in western gorillas (Gorilla gorilla): An analysis of distance, linearity, and speed of travel routes. Anim. Cogn. 2020, 23, 545–557. [Google Scholar] [CrossRef]
- Erhart, E.M.; Overdorff, D.J. Group leadership and feeding priority in wild Propithecus diadema edwardsi and Eulemur fulvus rufus. Am. J. Primatol. 1998, 45, 178–179. [Google Scholar]
- Joly, M.; Zimmermann, E. First evidence for relocation of stationary food resources during foraging in a strepsirhine primate (Microcebus murinus). Am. J. Primatol. 2007, 69, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Joly, M.; Zimmermann, E. Do solitary foraging nocturnal mammals plan their routes? Biol. Lett. 2011, 7, 638–640. [Google Scholar] [CrossRef] [PubMed]
- Teichroeb, J.A.; Vining, A.Q. Navigation strategies in three nocturnal lemur species: Diet predicts heuristic use and degree of exploratory behavior. Anim. Cogn. 2019, 22, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Nekaris, K.A.I.; Poindexter, S.; Reinhardt, K.D.; Sigaud, M.; Cabana, F.; Wirdateti, W.; Nijman, V. Coexistence between Javan Slow Lorises (Nycticebus javanicus) and humans in a dynamic agroforestry landscape in west Java, Indonesia. Int. J. Primatol. 2017, 38, 303–320. [Google Scholar] [CrossRef]
- Nekaris, K.A.I. Extreme primates: Ecology and evolution of Asian lorises. Evol. Anthropol. Issues News Rev. 2014, 23, 177–187. [Google Scholar] [CrossRef]
- Cabana, F.; Dierenfeld, E.S.; Wirdateti, W.; Donati, G.; Nekaris, K.A.I. Exploiting a readily available but hard to digest resource: A review of exudativorous mammals identified thus far and how they cope in captivity. Integr. Zool. 2018, 13, 94–111. [Google Scholar] [CrossRef]
- Poindexter, S.A.; Nekaris, K.A.I. Vertical clingers and gougers: Rapid acquisition of adult limb proportions facilitates feeding behaviours in young Javan slow lorises (Nycticebus javanicus). Mammal Biol. 2017, 87, 40–49. [Google Scholar] [CrossRef]
- Reinhardt, K.D.; Wirdateti, W.; Nekaris, K.A.I. Climate-mediated activity of the Javan Slow Loris, Nycticebus javanicus. AIMS Environ. Sci. 2016, 3, 249–260. [Google Scholar] [CrossRef]
- Karimloo, L.; Campera, M.; Imron, M.A.; Rakholia, S.; Mehta, A.; Hedger, K.; Nekaris, K.A.I. Habitat Use, Terrestriality and Feeding Behaviour of Javan Slow Lorises in Urban Areas of a Multi-Use Landscape in Indonesia. Land 2023, 12, 1349. [Google Scholar] [CrossRef]
- Rode-Margono, E.J.; Nijman, V.; Wirdateti, N.K.; Nekaris, K.A.I. Ethology of the critically endangered Javan slow loris Nycticebus javanicus E. Geoffroy Saint-Hilaire in West Java. Asian Prim. 2014, 4, 27–41. [Google Scholar]
- Altmann, J. Observational study of behavior: Sampling methods. Behaviour 1974, 49, 227–266. [Google Scholar] [CrossRef] [PubMed]
- Nekaris, K.A.I. Activity budget and positional behavior of the Mysore slender loris (Loris tardigradus lydekkerianus): Implications for slow climbing locomotion. Folia Primatol. 2001, 72, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.W.; Noser, R.; Bates, L.A.; Jupp, P.E. How did they get here from there? Detecting changes of direction in terrestrial ranging. Anim. Behav. 2009, 77, 619–631. [Google Scholar] [CrossRef]
- Janson, C.H. Experimental evidence for spatial memory in foraging wild capuchin monkeys, Cebus apella. Anim. Behav. 1998, 55, 1229–1243. [Google Scholar] [CrossRef]
- Finnerty, P.B.; Stutz, R.S.; Price, C.J.; Banks, P.B.; McArthur, C. Leaf odour cues enable non-random foraging by mammalian herbivores. J. Anim. Ecol. 2017, 86, 1317–1328. [Google Scholar] [CrossRef]
- Kane, A.; Kendall, C.J. Understanding how mammalian scavengers use information from avian scavengers: Cue from above. J. Anim. Ecol. 2017, 86, 837–846. [Google Scholar] [CrossRef]
- Stutz, R.S.; Croak, B.M.; Proschogo, N.; Banks, P.B.; McArthur, C. Olfactory and visual plant cues as drivers of selective herbivory. Oikos 2017, 126, 259–268. [Google Scholar] [CrossRef]
- Cunningham, E.; Janson, C. Integrating information about location and value of resources by white-faced saki monkeys (Pithecia pithecia). Anim. Cogn. 2007, 10, 293–304. [Google Scholar] [CrossRef]
- Dominy, N.J.; Ross, C.F.; Smith, T.D. Evolution of the special senses in primates: Past, present, and future. Anat. Rec. Part A 2004, 281, 1078–1082. [Google Scholar] [CrossRef]
- de Guinea, M.; Estrada, A.; Nekaris, K.; Van Belle, S. Arboreal route navigation in a Neotropical mammal: Energetic implications associated with tree monitoring and landscape attributes. Mov. Ecol. 2019, 7, 39. [Google Scholar] [CrossRef]
- Janmaat, K.R.; Ban, S.D.; Boesch, C. Chimpanzees use long-term spatial memory to monitor large fruit trees and remember feeding experiences across seasons. Anim. Behav. 2013, 86, 1183–1205. [Google Scholar] [CrossRef]
- Cabana, F.; Dierenfeld, E.; Wirdateti, W.; Donati, G.; Nekaris, K.A.I. Trialling nutrient recommendations for slow lorises (Nycticebus spp.) based on wild feeding ecology. J. Anim. Physiol. Anim. Nutr. 2018, 102, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Berger-Tal, O.; Bar-David, S. Recursive movement patterns: Review and synthesis across species. Ecosphere 2015, 6, 1–12. [Google Scholar] [CrossRef]
- Bracis, C.; Bildstein, K.L.; Mueller, T. Revisitation analysis uncovers spatio-temporal patterns in animal movement data. Ecography 2018, 41, 1801–1811. [Google Scholar] [CrossRef]
- Porter, L.M.; Garber, P.A. Foraging and spatial memory in wild Weddell’s saddleback tamarins (Saguinus fuscicollis weddelli) when moving between distant and out-of-sight goals. Int. J. Primatol. 2013, 28, 1035–1058. [Google Scholar] [CrossRef]
- McLean, K.A.; Trainor, A.M.; Asner, G.P.; Crofoot, M.C.; Hopkins, M.E.; Campbell, C.J.; Jansen, P.A. Movement patterns of three arboreal primates in a Neotropical moist forest explained by LiDAR-estimated canopy structure. Landsc. Ecol. 2016, 31, 1849–1862. [Google Scholar] [CrossRef]
- De Raad, A.L.; Hill, R.A. Topological spatial representation in wild chacma baboons (Papio ursinus). Anim. Cogn. 2019, 22, 397–412. [Google Scholar] [CrossRef]
- Cabana, F.; Dierenfeld, E.; Wirdateti, W.; Donati, G.; Nekaris, K.A.I. The seasonal feeding ecology of the Javan slow loris (Nycticebus javanicus). Am. J. Phys. Anthropol. 2017, 162, 768–781. [Google Scholar] [CrossRef]
- Collins, R.; Nekaris, K.A.I. Release of greater slow lorises, confiscated from the pet trade, to Batutegi Protected Forest, Sumatra, Indonesia. In Global Re-Introduction Perspectives; IUCN Reintroduction Specialist Group: Abu Dhabi, United Arab Emirates, 2008; pp. 192–195. [Google Scholar]
- Kenyon, M.; Streicher, U.; Loung, H.; Tran, T.; Tran, M.; Vo, B.; Cronin, A. Survival of reintroduced pygmy slow loris Nycticebus pygmaeus in South Vietnam. Endanger Species Res. 2014, 25, 185–195. [Google Scholar] [CrossRef]
- Fuller, G.; Eggen, W.F.; Wirdateti, W.; Nekaris, K.A.I. Welfare impacts of the illegal wildlife trade in a cohort of confiscated greater slow lorises, Nycticebus coucang. J. Appl. Anim. Wel. Sci. 2018, 21, 224–238. [Google Scholar] [CrossRef]
- Streicher, U.; Nadler, T. Re-introduction of pygmy lorises in Vietnam. Reintroduction News 2003, 23, 37–40. [Google Scholar]
- Poindexter, S.; Khoa, D.; Nekaris, K. Ranging patterns of reintroduced pygmy slow lorises (Nycticebus pygmaeus) in Cuc Phuong National Park, Vietnam. Vietnam. J. Primatol. 2017, 2, 37–49. [Google Scholar]
- Moore, R.S.; Nekaris, K.A.I. Compassionate conservation, rehabilitation and translocation of Indonesian slow lorises. Endanger Species Res. 2014, 26, 93–102. [Google Scholar] [CrossRef]
- Campera, M.; Brown, E.; Imron, M.A.; Nekaris, K.A.I. Unmonitored releases of small animals? The importance of considering natural dispersal, health, and human habituation when releasing a territorial mammal threatened by wildlife trade. Bioll. Conserv. 2020, 242, 108404. [Google Scholar] [CrossRef]
- Minckley, W.L. Translocation as a tool for conserving imperiled fishes: Experiences in western United States. Biol. Conser. 1995, 72, 297–309. [Google Scholar] [CrossRef]
- IUCN Species Survival Commission (SSC). Guidelines for Reintroductions and Other Conservation Translocations; IUCN: Gland, Switzerland, 1989; p. 57. [Google Scholar]
- Griffith, B.; Scott, J.M.; Carpenter, J.W.; Reed, C. Translocation as a species conservation tool: Status and strategy. Science 1989, 245, 477–480. [Google Scholar] [CrossRef]
- Wolf, C.M.; Garland, T., Jr.; Griffith, B. Predictors of avian and mammalian translocation success: Reanalysis with phylogenetically independent contrasts. Biol. Conserv. 1998, 86, 243–255. [Google Scholar] [CrossRef]
- Berger-Tal, O.; Blumstein, D.T.; Swaisgood, R.R. Conservation translocations: A review of common difficulties and promising directions. Anim. Conserv. 2020, 23, 121–131. [Google Scholar] [CrossRef]
- Tuberville, T.D.; Clark, E.E.; Buhlmann, K.A.; Gibbons, J.W. Translocation as a conservation tool: Site fidelity and movement of repatriated gopher tortoises (Gopherus polyphemus). In Animal Conservation Forum; Cambridge University Press: Cambridge, UK, 2005; Volume 8, pp. 349–358. [Google Scholar]
- Ebrahimi, M.; Bull, C.M. Determining the success of varying short-term confinement time during simulated translocations of the endangered pygmy bluetongue lizard (Tiliqua adelaidensis). Amphibia-Reptilia 2013, 34, 31–39. [Google Scholar] [CrossRef]
- Kenward, R.E.; Hodder, K.H. Red squirrels (Sciurus vulgaris) released in conifer woodland: The effects of source habitat, predation and interactions with grey squirrels (Sciurus carolinensis). J. Zool. 1998, 244, 23–32. [Google Scholar] [CrossRef]
- Le Gouar, P.; Mihoub, J.B.; Sarrazin, F. Dispersal and habitat selection: Behavioural and spatial constraints for animal translocations. In Reintroduction Biology: Integrating Science and Management; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 138–164. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).