Safety Bubbles: A Review of the Proposed Functions of Froth Nesting among Anuran Amphibians
Abstract
1. Introduction
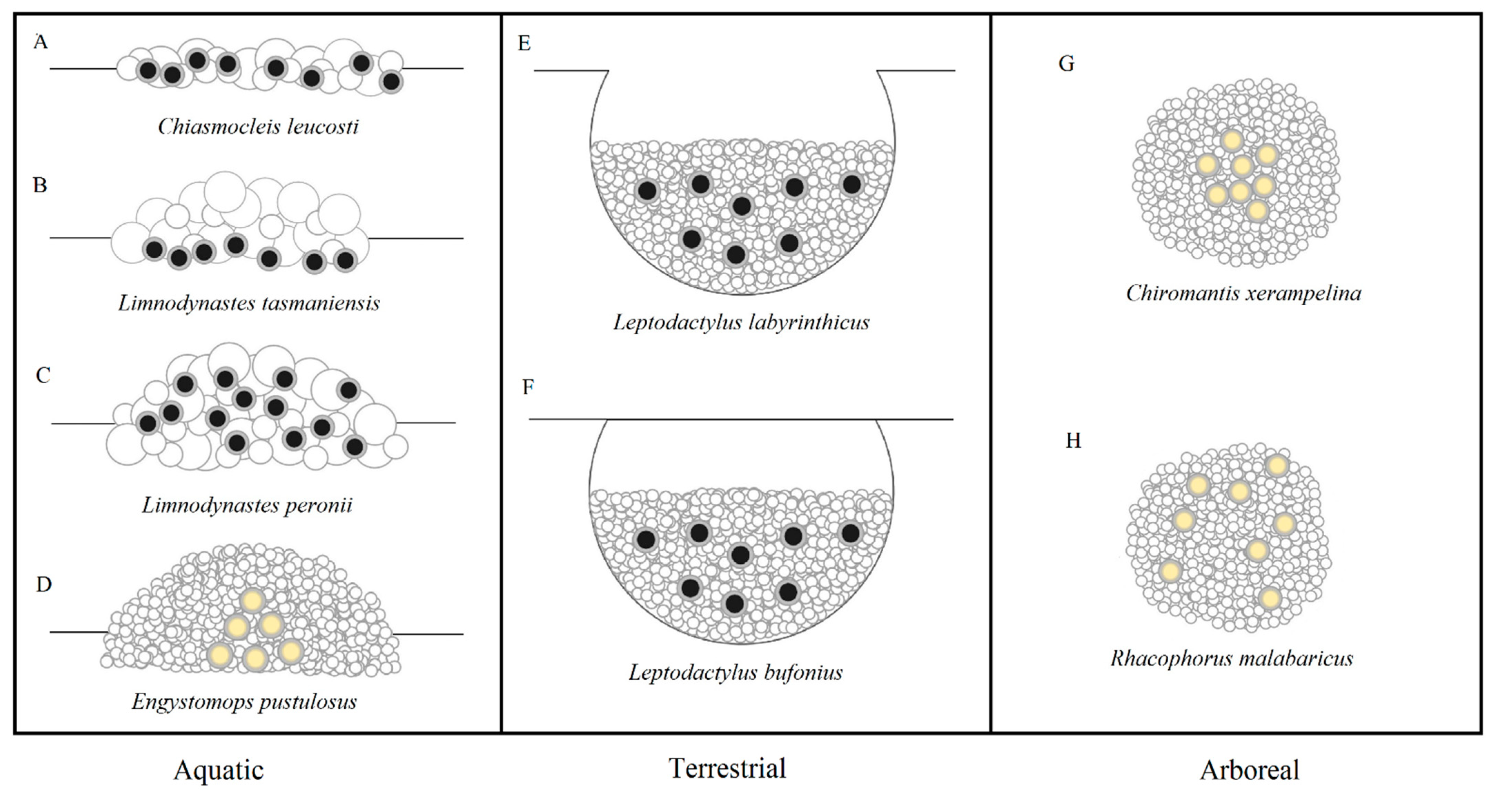
2. Froth Nest Construction
3. The Evolution of Froth Nesting
4. Benefits of Froth Nesting
4.1. Increasing Egg Temperature
4.2. Insulation against Sub-Optimal Temperatures
4.3. Environmental Control of Froth Nest Temperature
4.4. Anti-Desiccation
4.5. Predator Defence
4.6. Improved Oxygenation
5. Additional Adaptive Benefits
5.1. Post-Hatching Refuge
5.2. Insolation
5.3. Inhibition of Development
5.4. Nutrient Source
5.5. Competitive Head-Start
5.6. Temperature Acclimation
5.7. Microbial Defence
6. The Function of Froth Nest Breakdown
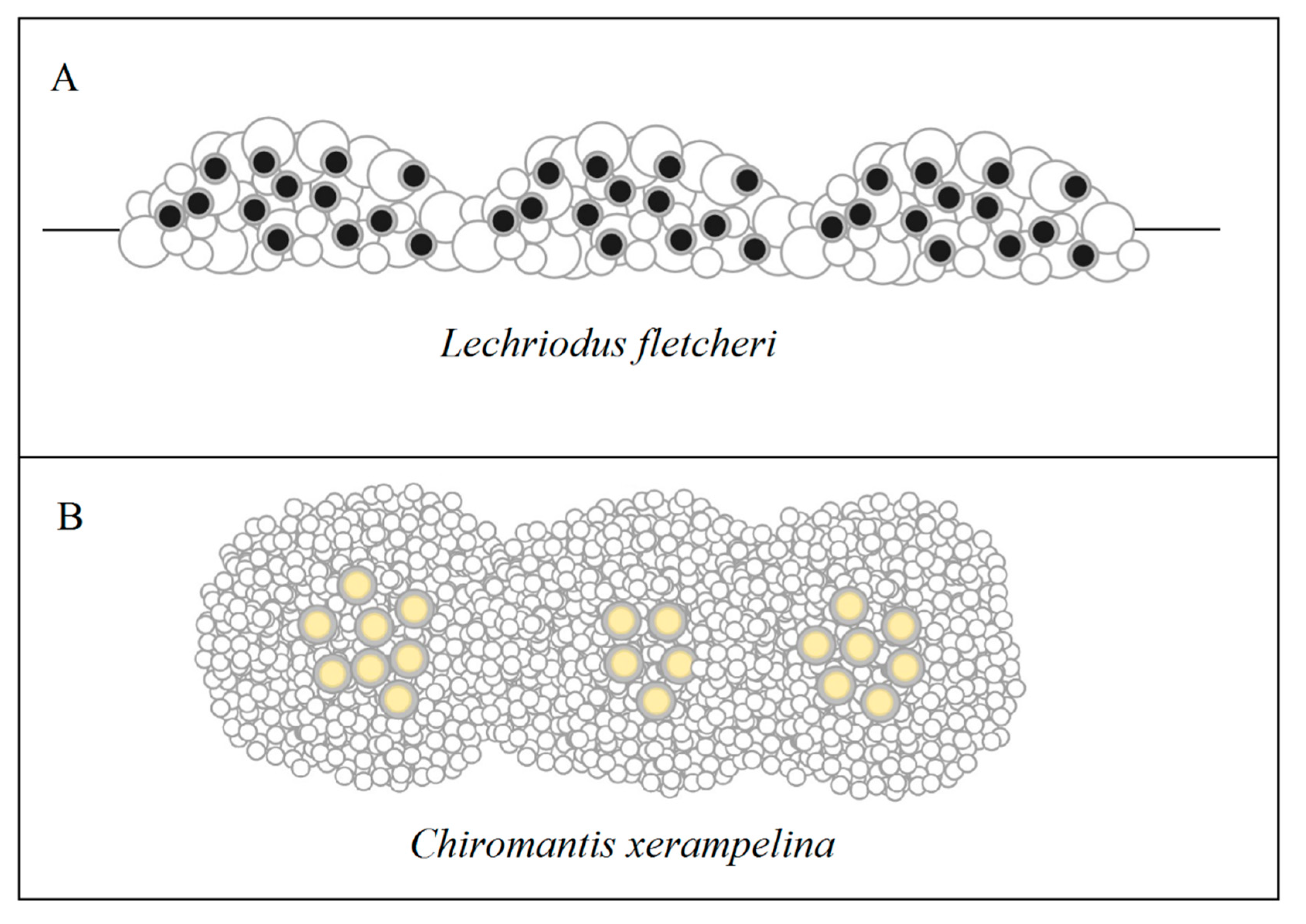
7. Communal Froth Nesting
8. Testing the Properties of the Froth Nest
8.1. Temperature
8.2. Moisture
8.3. Predation
8.4. Oxygen
8.5. Comparing Frothed and Un-Frothed Nests
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Duellman, W.; Trueb, L. Biology of Amphibians; McGrawHill: New York, NY, USA, 1986. [Google Scholar]
- Altig, R.; McDiarmid, R.W. Morphological Diversity and Evolution of Egg and Clutch Structure in Amphibians. Herpetol. Monogr. 2007, 21, 1–32. [Google Scholar] [CrossRef]
- Littlejohn, M. The breeding biology of the Baw Baw frog Philoria frosti Spencer. Proc. Linnaean Soc. N. S. Wales 1963, 88, 273–276. [Google Scholar]
- Haddad, C.F.B., Jr.; Pombal, J.P.P.; Gordo, M. Foam Nesting in a Hylid Frog (Amphibia, Anura). J. Herpetol. 1990, 24, 225–226. [Google Scholar] [CrossRef]
- Tyler, M.J.; Davies, M. Foam Nest Construction by Australian Leptodactylid Frogs (Amphibia, Anura, Leptodactylidae). J. Herpetol. 1979, 13, 509. [Google Scholar] [CrossRef]
- Hödl, W. An Analysis of Foam Nest Construction in the Neotropical Frog Physalaemus ephippifer (Leptodactylidae). Copeia 1990, 1990, 547–554. [Google Scholar] [CrossRef]
- Kadadevaru, G.G.; Kanamadi, R.D. Courtship and nesting behaviour of the Malabar gliding frog, Rhacophorus malabaricus (Jerdon, 1870). Curr. Sci. 2000, 79, 377–380. [Google Scholar]
- Frost, D.R. Amphibian Species of the World; Allen Press and Association of Systematics Collections: Lawrence, KS, USA, 1985. [Google Scholar]
- Bastos, R.P.; Haddad, C.F.; Pombal, J.P., Jr. Foam nest in Scinax rizibilis (Amphibia: Anura: Hylidae). Zoologia 2010, 27, 881–886. [Google Scholar] [CrossRef]
- Heyer, W.R. The adaptive ecology of the species groups of the genus Leptodactylus (Amphibia, Leptodactylidae). Evolution 1969, 23, 421–428. [Google Scholar] [CrossRef]
- Martin, A. Parallel evolution in the adaptive ecology of leptodactylid frogs of South America and Australia. Evolution 1970, 24, 643–644. [Google Scholar] [CrossRef]
- Fowler, J.E.; Kleinteich, T.; Franz, J.; Jaye, C.; Fischer, D.A.; Gorb, S.; Weidner, T.; Baio, J.E. Surface chemistry of the frog sticky-tongue mechanism. Biointerphases 2018, 13, 06E408. [Google Scholar] [CrossRef]
- Ramsey, J.P.; Reinert, L.K.; Harper, L.K.; Woodhams, D.C.; Rollins-Smith, L.A. Immune Defenses against Batrachochytrium dendrobatidis, a Fungus Linked to Global Amphibian Declines, in the South African Clawed Frog, Xenopus laevis. Infect. Immun. 2010, 78, 3981–3992. [Google Scholar] [CrossRef] [PubMed]
- Keogh, J.P.; Allen, A.; Garner, A. Relationship between gastric mucus synthesis, secretion and surface gel erosion measured in amphibian stomach In Vitro. Clin. Exp. Pharmacol. Physiol. 1997, 24, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Toledo, R.; Jared, C. Cutaneous adaptations to water balance in amphibians. Comp. Biochem. Physiol. Part A Physiol. 1993, 105, 593–608. [Google Scholar] [CrossRef]
- Downie, J. Functions of the foam in the foam-nesting leptodactylid Physalaemus pustulosus. Herpetol. J. 1988, 1, 302–307. [Google Scholar]
- Silva, W.R.; Giaretta, A.A.; Facure, K.G. On the natural history of the South American pepper frog, Leptodactylus labyrinthicus (Spix, 1824) (Anura: Leptodactylidae). J. Nat. Hist. 2005, 39, 555–566. [Google Scholar] [CrossRef]
- Hero, J.-M.; Galatti, U. Characteristics Distinguishing Leptodactylus pentadactylus and L. knudseni in the Central Amazon Rainforest. J. Herpetol. 1990, 24, 226–228. [Google Scholar] [CrossRef]
- Meegaskumbura, M.; Senevirathne, G.; Biju, S.D.; Garg, S.; Meegaskumbura, S.; Pethiyagoda, R.; Hanken, J.; Schneider, C.J. Patterns of reproductive-mode evolution in Old World tree frogs (Anura, Rhacophoridae). Zoöl. Scr. 2015, 44, 509–522. [Google Scholar] [CrossRef]
- Shahrudin, S.; Ismail, M.N.; Kwan, S.H.; Najimudin, N. Ecology and Protein Composition of Polypedates leucomystax (Gravenhorst, 1829) (Anura: Rhacophoridae) Foam Nests from Peninsular Malaysia. Annu. Res. Rev. Biol. 2017, 14, 1–10. [Google Scholar] [CrossRef]
- Furness, A.I.; McDiarmid, R.W.; Heyer, W.R.; Zug, G.R. Oviduct Modifications in Foam-Nesting Frogs, with Emphasis on the Genus Leptodactylus (Amphibia, Leptodactylidae). S. Am. J. Herpetol. 2010, 5, 13–29. [Google Scholar] [CrossRef]
- Fleming, R.I.; MacKenzie, C.D.; Cooper, A.; Kennedy, M.W. Foam nest components of the túngara frog: A cocktail of proteins conferring physical and biological resilience. Proc. R. Soc. B Biol. Sci. 2009, 276, 1787–1795. [Google Scholar] [CrossRef]
- Seymour, R.S.; Loveridge, J.P. Embryonic and larval respiration in the arboreal foam nests of the African frog Chiromantis xerampelina. J. Exp. Biol. 1994, 197, 31–46. [Google Scholar]
- Heyer, W.R.; Rand, A.S. Foam Nest Construction in the Leptodactylid Frogs Leptodactylus pentadactylus and Physalaemus pustulosus (Amphibia, Anura, Leptodactylidae). J. Herpetol. 1977, 11, 225–228. [Google Scholar] [CrossRef][Green Version]
- Ryan, M.J. The túngara Frog: A Study in Sexual Selection and Communication; University of Chicago Press: Chicago, IL, USA, 1985. [Google Scholar]
- Coe, M. Observations on the ecology and breeding biology of the genus Chiromantis (Amphibia: Rhacophoridae). J. Zoöl. 1974, 172, 13–34. [Google Scholar] [CrossRef]
- Tyler, M. Australian Frogs; Viking O’Neil: Melbourne, Australia, 1989. [Google Scholar]
- Haddad, F.; Hödl, W. New reproductive mode in anurans: Bubble nest in Chiasmocleis leucosticta (Microhylidae). Copeia 1997, 1997, 585–588. [Google Scholar] [CrossRef]
- Cooper, A.; Kennedy, M.W.; Fleming, R.I.; Wilson, E.H.; Videler, H.; Wokosin, D.L.; Su, T.-J.; Green, R.J.; Lu, J.R. Adsorption of Frog Foam Nest Proteins at the Air-Water Interface. Biophys. J. 2005, 88, 2114–2125. [Google Scholar] [CrossRef]
- Cooper, A.; Kennedy, M.W. Biofoams and natural protein surfactants. Biophys. Chem. 2010, 151, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Salthe, S.N.; Duellman, W.E. Quantitative constraints associated with reproductive mode in anurans. In Evolutionary Biology of the Anurans; Vial, J.L., Ed.; University of Missouri Press: Columbia, MO, USA, 1973; pp. 229–249. [Google Scholar]
- Heyer, W.R. A preliminary analysis of the intergeneric relationships of the frog family Leptodactylidae. Smithson. Contrib. Zoöl. 1975, 199, 1–55. [Google Scholar] [CrossRef]
- Méndez-Narváez, J.; Flechas, S.V.; Amézquita, A. Foam Nests Provide Context-Dependent Thermal Insulation to Embryos of Three Leptodactylid Frogs. Physiol. Biochem. Zoöl. 2015, 88, 246–253. [Google Scholar] [CrossRef]
- Pulz, R. Thermal and water relations. In Ecophysiology of Spiders; Nentwig, W., Ed.; Springer: Berlin/Heidelberg, Germany, 1987; pp. 26–55. [Google Scholar]
- Duellman, W. Reproductive modes in anuran amphibians: Phylogenetic significance of adaptive strategies. S. Afr. J. Sci. 1985, 81, 174–178. [Google Scholar]
- Zina, J. Communal nests in Physalaemus pustulosus (Amphibia: Leptodactylidae): Experimental evidence for female oviposition preferences and protection against desiccation. Amphibia-Reptilia 2006, 27, 148–150. [Google Scholar] [CrossRef]
- Muedeking, M.H.; Heyer, W.R. Descriptions of eggs and reproductive patterns of Leptodactylus pentadactylus (Amphibia: Leptodactylidae). Herpetologica 1976, 32, 137–139. [Google Scholar]
- Davis, S.; Davis, R.; James, A.; Talin, B. Reproductive behavior and larval development of Leptodactylus fallax in Dominica, West Indies. Herpetol. Rev. 2000, 31, 217–220. [Google Scholar]
- Seymour, R.S.; Mahony, M.J.; Knowles, R. Respiration of embryos and larvae of the terrestrially breeding frog Kyarranus loveridgei. Herpetologica 1995, 51, 369–376. [Google Scholar]
- Roberts, J. Call Differentiation in the Limnodynastes Tasmaniensis Complex (Anura: Leptodactylidae); University of Adelaide: Adelaide, Australia, 1976. [Google Scholar]
- Wells, K.D. The Ecology and Behavior of Amphibians; University of Chicago Press: Chicagi, IA, USA, 2007. [Google Scholar]
- Dobkin, D.S.; Gettinger, R.D. Thermal Aspects of Anuran Foam Nests. J. Herpetol. 1985, 19, 271. [Google Scholar] [CrossRef]
- Hassinger, D.D. Notes on the thermal properties of frog eggs. Herpetologica 1970, 26, 49–51. [Google Scholar]
- Salthe, S.; Mecham, J. Reproductive and courtship patterns. In Physiology of the Amphibia; Elsevier: Amsterdam, The Netherlands, 1974; Volume 2, pp. 309–521. [Google Scholar]
- Rand, A. Physalaemus pustulosus (Rana, Sapito Tingara, Foam Toad, Mud-puddle Frog). In Costa Rican Natural History; Janzen, D., Ed.; University of Chicago Press: Chicago, IA, USA, 1983; pp. 412–415. [Google Scholar]
- Gorzula, S. Foam nesting in leptodactylids: A possible function. Br. J. Herpetoloy 1977, 5, 657–659. [Google Scholar]
- Shepard, D.B.; Caldwell, J.P. From Foam to Free-living: Ecology of Larval Leptodactylus Labyrinthicus. Copeia 2005, 2005, 803–811. [Google Scholar] [CrossRef]
- Carroll, E.J., Jr. Thermal tolerance and heat shock protein synthesis during development in the anuran Lepidobatrachus laevis. Dev. Growth Differ. 1996, 38, 9–14. [Google Scholar] [CrossRef]
- Seale, D.B. Physical Factors Influencing Oviposition by the Woodfrog, Rana sylvatica, in Pennsylvania. Copeia 1982, 1982, 627–635. [Google Scholar] [CrossRef]
- Kusano, T.; Sakai, A.; Hatanaka, S. Ecological functions of the foam nests of the Japanese treefrog, Rhacophorus arboreus (Amphibia, Rhacophoridae). Herpetol. J. 2006, 16, 163–169. [Google Scholar]
- Liwanag, H.E.M.; Berta, A.; Dos Santos, D.C.; Abney, M.; Williams, T.M. Morphological and thermal properties of mammalian insulation: The evolution of fur for aquatic living. Biol. J. Linn. Soc. 2012, 106, 926–939. [Google Scholar] [CrossRef]
- Bock, W.J. Explanatory history of the origin of feathers. Am. Zool. 2000, 40, 478–485. [Google Scholar] [CrossRef]
- Boukhriss, M.; Zhani, K.; Ghribi, R. Study of thermophysical properties of a solar desalination system using solar energy. Desalination Water Treat. 2013, 51, 1290–1295. [Google Scholar] [CrossRef]
- Beattie, R.C. A physico-chemical investigation of the jelly capsules surrounding eggs of the Common frog (Rana temporaria temporaria). J. Zoöl. 1980, 190, 1–25. [Google Scholar] [CrossRef]
- Zweifel, R.G. Upper thermal tolerances of anuran embryos in relation to stage of development and breeding habits. Am. Mus. Novit. 1977, 2617, 1–21. [Google Scholar]
- Bernal, M.H.; Lynch, J.D. Thermal Tolerance in Anuran Embryos with Different Reproductive Modes: Relationship to Altitude. Sci. World J. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Philibosian, R.; Ruibal, R.; Shoemaker, V.H.; McClanahan, L.L. Nesting behavior and early larval life of the frog Leptodactylus bufonius. Herpetologica 1974, 30, 381–386. [Google Scholar]
- Touchon, J.C.; Warkentin, K.M. Reproductive mode plasticity: Aquatic and terrestrial oviposition in a treefrog. Proc. Natl. Acad. Sci. USA 2008, 105, 7495–7499. [Google Scholar] [CrossRef]
- Spieler, M.; Linsenmair, K.E. Choice of optimal oviposition sites by Hoplobatrachus occipitalis (Anura: Ranidae) in an unpredictable and patchy environment. Oecologia 1997, 109, 184–199. [Google Scholar] [CrossRef]
- Hödl, W. Foam nest construction in South American leptodactylid frogs. In Studies in Herpetology; Roek, Z., Ed.; Charles University: Prague, Czech Republic, 1986; pp. 565–570. [Google Scholar]
- Romero-Carvajal, A.; Sáenz-Ponce, N.; Venegas-Ferrín, M.; Almeida-Reinoso, D.; Lee, C.; Bond, J.; Ryan, M.J.; Wallingford, J.B.; Del Pino, E.M. Embryogenesis and laboratory maintenance of the foam-nesting túngara frogs, genus Engystomops(= Physalaemus). Dev. Dyn. 2009, 238, 1444–1454. [Google Scholar] [CrossRef]
- Narváez, A.; Santiago, R. Spawning behaviour of Engystomops pustulatus (Anura: Leptodactylidae). J. Nat. Hist. 2017, 5, 267–275. [Google Scholar] [CrossRef]
- Dalgetty, L.; Kennedy, M.W. Building a home from foam-Túngara frog foam nest architecture and three-phase construction process. Biol. Lett. 2010, 6, 293–296. [Google Scholar] [CrossRef]
- Magnusson, W.E.; Hero, J.-M. Predation and the evolution of complex oviposition behaviour in Amazon rainforest frogs. Oecologia 1991, 86, 310–318. [Google Scholar] [CrossRef]
- Roberts, J. Terrestrial Egg Deposition and Direct Development in Arenophyrne rotunda Tyler, a Myobatrachid Frog from Coastal Sand Dunes at Shark Bay, W. A. Wildl. Res. 1984, 11, 191–200. [Google Scholar] [CrossRef]
- Hartmann, P.; Hartmann, M.; Haddad, C. Visual signaling and reproductive biology in a nocturnal treefrog, genus Hyla (Anura: Hylidae). Amphibia-Reptilia 2004, 25, 395–406. [Google Scholar] [CrossRef]
- Menin, M.; Giaretta, A.A. Predation on foam nests of leptodactyline frogs (Anura: Leptodactylidae) by larvae of Beckeriella niger (Diptera: Ephydridae). J. Zoöl. 2003, 261, 239–243. [Google Scholar] [CrossRef]
- Downie, J. Functions of the foam in foam-nesting leptodactylids: The nest as a post-hatching refuge in Physalaemus pustulosus. Herpetol. J. 1993, 3, 35–42. [Google Scholar]
- Moore, J.A. The frogs of eastern New South Wales. Bull. Am. Mus. Nat. Hist. 1961, 121, 149–386. [Google Scholar]
- Jennions, M.D. Breeding Behaviour of the Foam Nest Frog, Chiromantis Xerampelina: Sperm Competition and Polyandry; University of the Witwatersrand: Johannesburg, South Africa, 1992. [Google Scholar]
- Gould, J. Build me up to break me down: Frothed spawn in the sandpaper frog, Lechriodus fletcheri, is formed by female parents and later broken down by their offspring. Aust. J. Zoöl. 2019, 67, 153–161. [Google Scholar] [CrossRef]
- Ryan, M.J.; Bartholomew, G.A.; Rand, A.S. Energetics of Reproduction in a Neotropical Frog, Physalaemus Pustulosus. Ecology 1983, 64, 1456–1462. [Google Scholar] [CrossRef]
- Barrio, A. El género Physalaemus en la Argentina (Anura, Leptodactylidae). Physis 1965, 25, 421–488. [Google Scholar]
- Wager, V. The Frogs of South Africa; Purnell and Sons: Johannesburg, South Africa, 1965. [Google Scholar]
- Crump, M.L. Parental Care among the Amphibia. Adv. Study Behav. 1996, 25, 109–144. [Google Scholar] [CrossRef]
- Lingnau, R.; Di-Bernardo, M. Predation on foam nests of two leptodactylid frogs by Solenopsis sp. (Hymenoptera, Formicidae) and Liophis miliaris (Serpentes, Colubridae). Biociências 2006, 14, 223–224. [Google Scholar]
- Waldman, B. Sibling association among schooling toad tadpoles: Field evidence and implications. Anim. Behav. 1982, 30, 700–713. [Google Scholar] [CrossRef]
- Polis, G.A.; Myers, C.A. A Survey of Intraspecific Predation among Reptiles and Amphibians. J. Herpetol. 1985, 19, 99–107. [Google Scholar] [CrossRef]
- Gould, J.; Clulow, J.; Clulow, S. Food, not friend: Tadpoles of the sandpaper frog (Lechriodus fletcheri) cannibalise conspecific eggs as a food resource in ephemeral pools. Ethology 2019, 126, 486–491. [Google Scholar] [CrossRef]
- Hödl, W. Reproductive behaviour in the neotropical foam-nesting frog Pleurodema diplolistris (Leptodactylidae). Amphibia-Reptilia 1992, 13, 263–274. [Google Scholar] [CrossRef]
- Villa, J.; McDiarmid, R.W.; Gallardo, J.M. Arthropod predators of leptodactylid frog foam nests. Brenesia 1982, 19, 577–589. [Google Scholar]
- Drewes, R.C.; Altig, R. Anuran egg predation and heterocannibalism in a breeding community of East African frogs. Trop. Zoöl. 1996, 9, 333–347. [Google Scholar] [CrossRef]
- Yorke, C.D. Survival of Embryos and Larvae of the Frog Polypedates leucomystax in Malaysia. J. Herpetol. 1983, 17, 235–241. [Google Scholar] [CrossRef]
- Davis, R.A.; Disney, R.H.L. Natural history and description of Aphiura breviceps Schmitz, a scuttle fly (Diptera: Phoridae) whose larvae prey on the eggs of frogs (Anura: Myobatrachidae) in Western Australia. Aust. J. Entomol. 2003, 42, 18–21. [Google Scholar] [CrossRef]
- Carvalho, T.; Facure, K.; Giaretta, A. Predation upon eggs of the terrestrial foam-nesting frog Leptodactylus fuscus (Leptodactylidae) by larvae of the ground beetle Loxandrus oophagus (Carabidae: Loxandrini). Herpetol. Notes 2012, 5, 319–322. [Google Scholar]
- Gould, J.; Valdez, J.W.; Clulow, J.; Clulow, S. Diving beetle offspring oviposited in amphibian spawn prey on the tadpoles upon hatching. Entomol. Sci. 2019, 22, 393–397. [Google Scholar] [CrossRef]
- Villa, J. Frogflies’ from Central and South America with notes on other organisms of the amphibian egg microhabitat. Brenesia 1980, 17, 49–68. [Google Scholar]
- Costa, C.; Vanin, S.A.; De Carvalho, T.R. A new Brazilian species of Loxandrus LeConte, 1852, with description of immatures and notes on natural history (Coleoptera: Carabidae: Loxandrini). Zootaxa 2011, 2745, 30–42. [Google Scholar] [CrossRef]
- Downie, J.; Disney, R.; Collins, L.; Hancock, E. A new species of Megaselia (Diptera, Phoridae) whose larvae prey upon the eggs of Leptodactylus fuscus (Anura, Leptodactylidae). J. Nat. Hist. 1995, 29, 993–1003. [Google Scholar] [CrossRef]
- Chen, M.-S. Inducible direct plant defense against insect herbivores: A review. Insect Sci. 2008, 15, 101–114. [Google Scholar] [CrossRef]
- Dutta, I.; Saha, P.; Majumder, P.; Sarkar, A.; Chakraborti, D.; Banerjee, S.; Das, S. The efficacy of a novel insecticidal protein, Allium sativum leaf lectin (ASAL), against homopteran insects monitored in transgenic tobacco. Plant Biotechnol. J. 2005, 3, 601–611. [Google Scholar] [CrossRef]
- Alcala, A.C.; Brown, W.C. Early life history of two Philippine frogs with notes on deposition of eggs. Herpetologica 1956, 12, 241–246. [Google Scholar]
- Licht, L.E. Unpalatability and toxicity of toad eggs. Herpetologica 1968, 24, 93–98. [Google Scholar]
- Gunzburger, M.S.; Travis, J. Critical Literature Review of the Evidence for Unpalatability of Amphibian Eggs and Larvae. J. Herpetol. 2005, 39, 547–571. [Google Scholar] [CrossRef]
- Vonesh, J.R. Egg predation and predator-induced hatching plasticity in the African reed frog, Hyperolius spinigularis. Oikos 2005, 110, 241–252. [Google Scholar] [CrossRef]
- Seymour, R.S. Respiration of Aquatic and Terrestrial Amphibian Embryos. Am. Zoöl. 1999, 39, 261–270. [Google Scholar] [CrossRef][Green Version]
- Seymour, R.S. Oxygen diffusion through the jelly capsules of amphibian eggs. Isr. J. Zool. 1994, 40, 493–506. [Google Scholar]
- Seymour, R.S.; Roberts, J.D. Embryonic Respiration and Oxygen Distribution in Foamy and Nonfoamy Egg Masses of the Frog Limnodynastes tasmaniensis. Physiol. Zoöl. 1991, 64, 1322–1340. [Google Scholar] [CrossRef]
- Downie, J.; Nokhbatolfoghahai, M. Presence and absence of the cement gland in foamnesting leptodactylids (Anura: Leptodactylidae): Implications for the transition to terrestrial development. Herpetol. J. 2006, 16, 77–81. [Google Scholar]
- Downie, J. Developmental arrest in Leptodactylus fuscus tadpoles (Anura: Leptodactylidae). I: Descriptive analysis. Herpetol. J. 1994, 4, 29–38. [Google Scholar]
- Blaustein, A.R.; Hoffman, P.D.; Hokit, D.G.; Kiesecker, J.M.; Walls, S.C.; Hays, J.B. UV repair and resistance to solar UV-B in amphibian eggs: A link to population declines? Proc. Natl. Acad. Sci. USA 1994, 91, 1791–1795. [Google Scholar] [CrossRef]
- Pisano, A.; Del Rio, A. New biological properties in the foamy jelly of amphibians. Arch. Zool. Ital. 1968, 53, 189–201. [Google Scholar]
- Gibson, R.C.; Buley, K.R. Maternal Care and Obligatory Oophagy in Leptodactylus fallax: A New Reproductive Mode in Frogs. Copeia 2004, 2004, 128–135. [Google Scholar] [CrossRef]
- Tanaka, S.; Nishihira, M. Foam nest as a potential food source for anuran larvae: A preliminary experiment. J. Ethol. 1987, 5, 86–88. [Google Scholar] [CrossRef]
- Kabisch, K.; Herrmann, H.-J.; Klossek, P.; Luppa, H.; Brauer, K. Foam gland and chemical analysis of the foam of Polypedates leucomystax (Gravenhorst 1829) (Anura: Rhacophoridae). Russ. J. Herpetol. 1998, 5, 10–14. [Google Scholar]
- Lee, A. Studies in Australian amphibia II. Taxonomy, ecology and evolution of the genus Heleioporus Gray (Anura: Leptodactylidae). Aust. J. Zoöl. 1967, 15, 367–439. [Google Scholar] [CrossRef]
- Crump, M.L. Reproductive strategies in a tropical anuran cominunity. Univ. Kans. Mus. Nat. Hist. Misc. Publ. 1974, 61, 1–68. [Google Scholar]
- Savage, R.M. The Ecology and Life History of the Common frog Rana Temporaria Temporaria; Pitman and Sons: London, UK, 1961. [Google Scholar]
- Brown, H.A. High Temperature Tolerance of the Eggs of a Desert Anuran, Scaphiopus hammondii. Copeia 1967, 1967, 365–370. [Google Scholar] [CrossRef]
- Turriago, J.; Parra, C.; Bernal, M. Upper thermal tolerance in anuran embryos and tadpoles at constant and variable peak temperatures. Can. J. Zoöl. 2015, 93, 267–272. [Google Scholar] [CrossRef]
- Kreil, G. Antimicrobial Peptides from Amphibian Skin: An Overview. Novartis Found. Symp. 2007, 186, 77–90. [Google Scholar] [CrossRef]
- Yang, X.; Lee, W.-H.; Zhang, Y. Extremely Abundant Antimicrobial Peptides Existed in the Skins of Nine Kinds of Chinese Odorous Frogs. J. Proteome Res. 2011, 11, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Walke, J.B.; Harris, R.N.; Reinert, L.K.; Rollins-Smith, L.A.; Woodhams, D.C. Social Immunity in Amphibians: Evidence for Vertical Transmission of Innate Defenses. Biotropica 2011, 43, 396–400. [Google Scholar] [CrossRef]
- Hissa, D.C.; Bezerra, W.M.; Freitas, C.D.T.; Ramos, M.V.; Lopes, J.L.S.; Beltramini, L.M.; Roberto, I.J.; Cascon, P.; Melo, V.M.M. Frog Foam Nest Protein Diversity and Synthesis. J. Exp. Zoöl. Part A Ecol. Genet. Physiol. 2016, 325, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Faivovich, J.; Ferraro, D.P.; Basso, N.G.; Haddad, C.F.; Rodrigues, M.T.; Wheeler, W.C.; Lavilla, E.O. A phylogenetic analysis of Pleurodema (Anura: Leptodactylidae: Leiuperinae) based on mitochondrial and nuclear gene sequences, with comments on the evolution of anuran foam nests. Cladistics 2012, 28, 460–482. [Google Scholar] [CrossRef]
- Tyler, M.J.; Crook, G.A. Reproductive biology of the frogs of the Magela Creek system, Northern Territory. Rec. S. Aust. Mus. 1983, 18, 415–440. [Google Scholar]
- Agostinho, C.; Foresti, F.; Lima, S.; Jim, J. Reproduction and population size of Leptodactylus labyrinthicus (Amphibia, Anura, Leptodactylidae). Russ. J. Herpetol. 2002, 9, 15–20. [Google Scholar]
- Salthe, S.N. Increase in Volume of the Perivitelline Chamber during Development of Rana pipiens Schreber. Physiol. Zoöl. 1965, 38, 80–98. [Google Scholar] [CrossRef]
- Urch, U.A.; Hedrick, J.L. The hatching enzyme from Xenopus laevis: Limited proteolysis of the fertilization envelope. J. Supramol. Struct. Cell. Biochem. 1981, 15, 111–117. [Google Scholar] [CrossRef]
- Yoshizaki, N.; Katagiri, C. Cellular basis for the production and secretion of the hatching enzyme by frog embryos. J. Exp. Zoöl. 1975, 192, 203–212. [Google Scholar] [CrossRef]
- Muñoz, M.J.R.; Martínez, T.A.; Acosta, J.C.; Blanco, G.M. Foam nest construction and first report of agonistic behaviour in Pleurodema tucumanum (Anura: Leptodactylidae). Neotrop. Biol. Conserv. 2019, 14, 117–128. [Google Scholar] [CrossRef]
- Breder, C.M. Amphibians and reptiles of the Rio Chucunaque drainage, Darien, Panama, with notes on their life histories and habits. Bull. Am. Mus. Nat. Hist. 1946, 86, 379–435. [Google Scholar]
- Giaretta, A.A.; Facure, K.G. Terrestrial and communal nesting in Eupemphix nattereri (Anura, Leiuperidae): Interactions with predators and pond structure. J. Nat. Hist. 2006, 40, 2577–2587. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, L.; Li, J.; Li, Y.; Wei, S.; Cai, L.; Wu, H. Transcriptomic analysis of differentially expressed genes in the oviduct of Rhacophorus omeimontis provides insights into foam nest construction. BMC Genom. 2019, 20, 1–14. [Google Scholar] [CrossRef]
- Taylor, P. Observations on the breeding habits of Chiromantis xerampelina. J. Herpetol. Assoc. Afr. 1971, 8, 7–8. [Google Scholar]
- Waldman, B.; Ryan, M.J. Thermal Advantages of Communal Egg Mass Deposition in Wood Frogs (Rana sylvatica). J. Herpetol. 1983, 17, 70–72. [Google Scholar] [CrossRef]
- Villamil, J.; Maneyro, R. Communal foam nest in Physalaemus gracilis (Boulenger 1881) (Anura: Leptodactylidae). Herpetol. Notes 2014, 7, 565–566. [Google Scholar]
- Hamilton, W. Geometry for the selfish herd. J. Theor. Biol. 1971, 31, 295–311. [Google Scholar] [CrossRef]
- Furness, A.I.; Capellini, I. The evolution of parental care diversity in amphibians. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gould, J. Safety Bubbles: A Review of the Proposed Functions of Froth Nesting among Anuran Amphibians. Ecologies 2021, 2, 112-137. https://doi.org/10.3390/ecologies2010006
Gould J. Safety Bubbles: A Review of the Proposed Functions of Froth Nesting among Anuran Amphibians. Ecologies. 2021; 2(1):112-137. https://doi.org/10.3390/ecologies2010006
Chicago/Turabian StyleGould, John. 2021. "Safety Bubbles: A Review of the Proposed Functions of Froth Nesting among Anuran Amphibians" Ecologies 2, no. 1: 112-137. https://doi.org/10.3390/ecologies2010006
APA StyleGould, J. (2021). Safety Bubbles: A Review of the Proposed Functions of Froth Nesting among Anuran Amphibians. Ecologies, 2(1), 112-137. https://doi.org/10.3390/ecologies2010006




