Do Agrochemical-Free Paddy Fields Serve as Refuge Habitats for Odonata?
Abstract
:1. Introduction
2. Methods
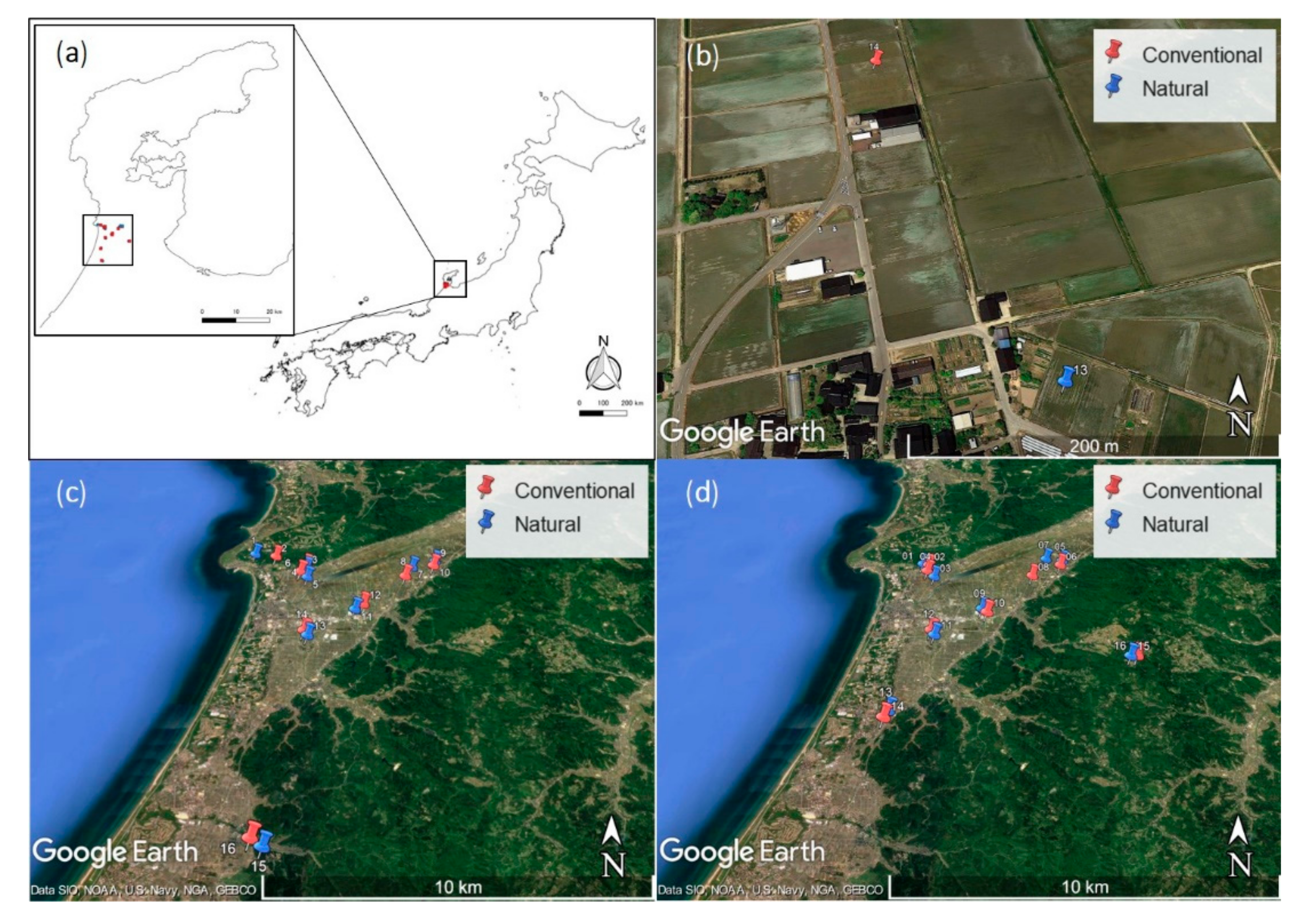
2.1. Study Sites
2.2. Farming Calendar for Conventional and Natural Practices
2.3. Life Histories of Representative Odonata
2.4. Preliminary Survey (2017)
2.5. Main Survey (2019)
2.6. Statistical Analyses
3. Results
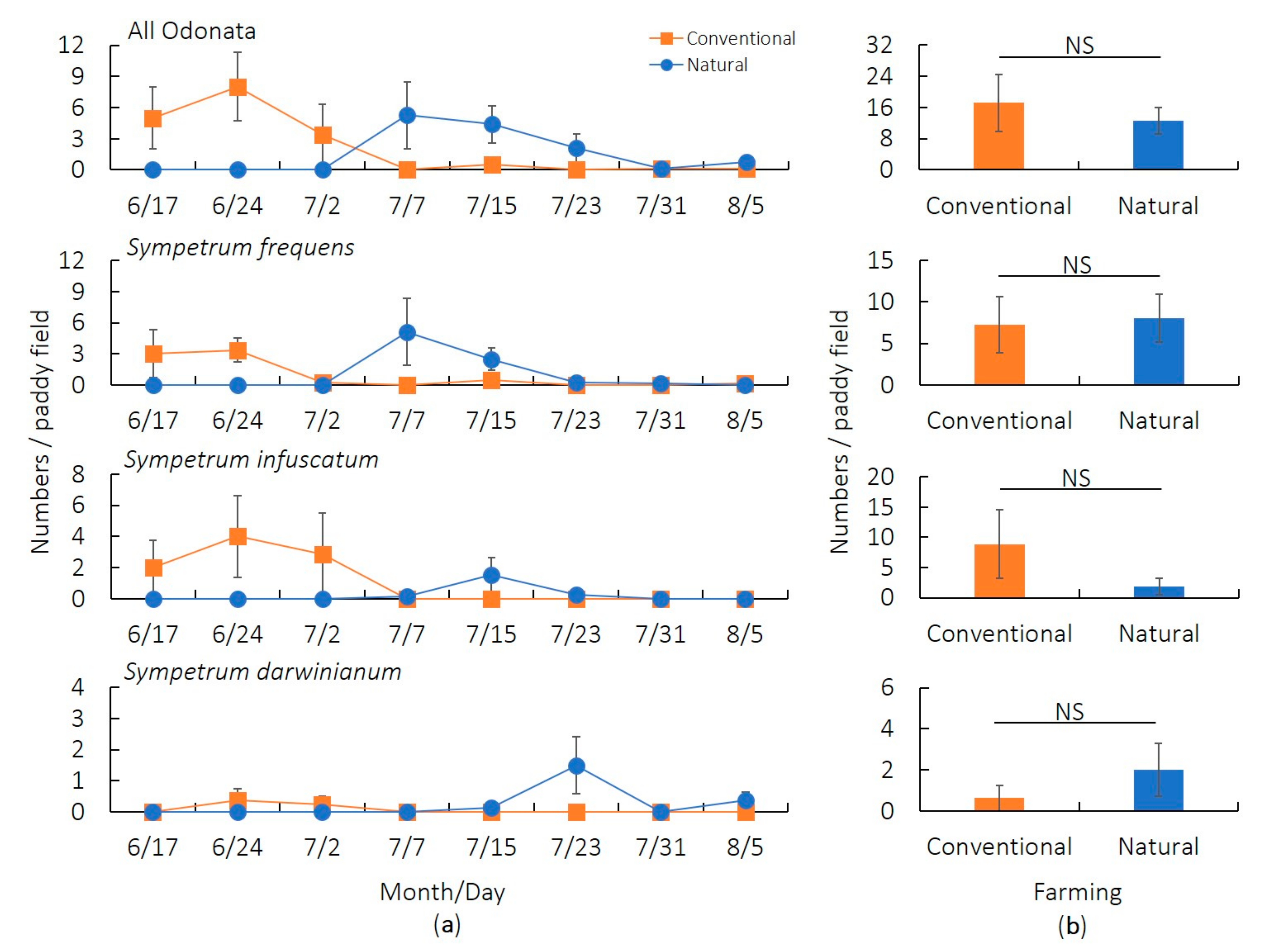
3.1. Preliminary Survey (2017)
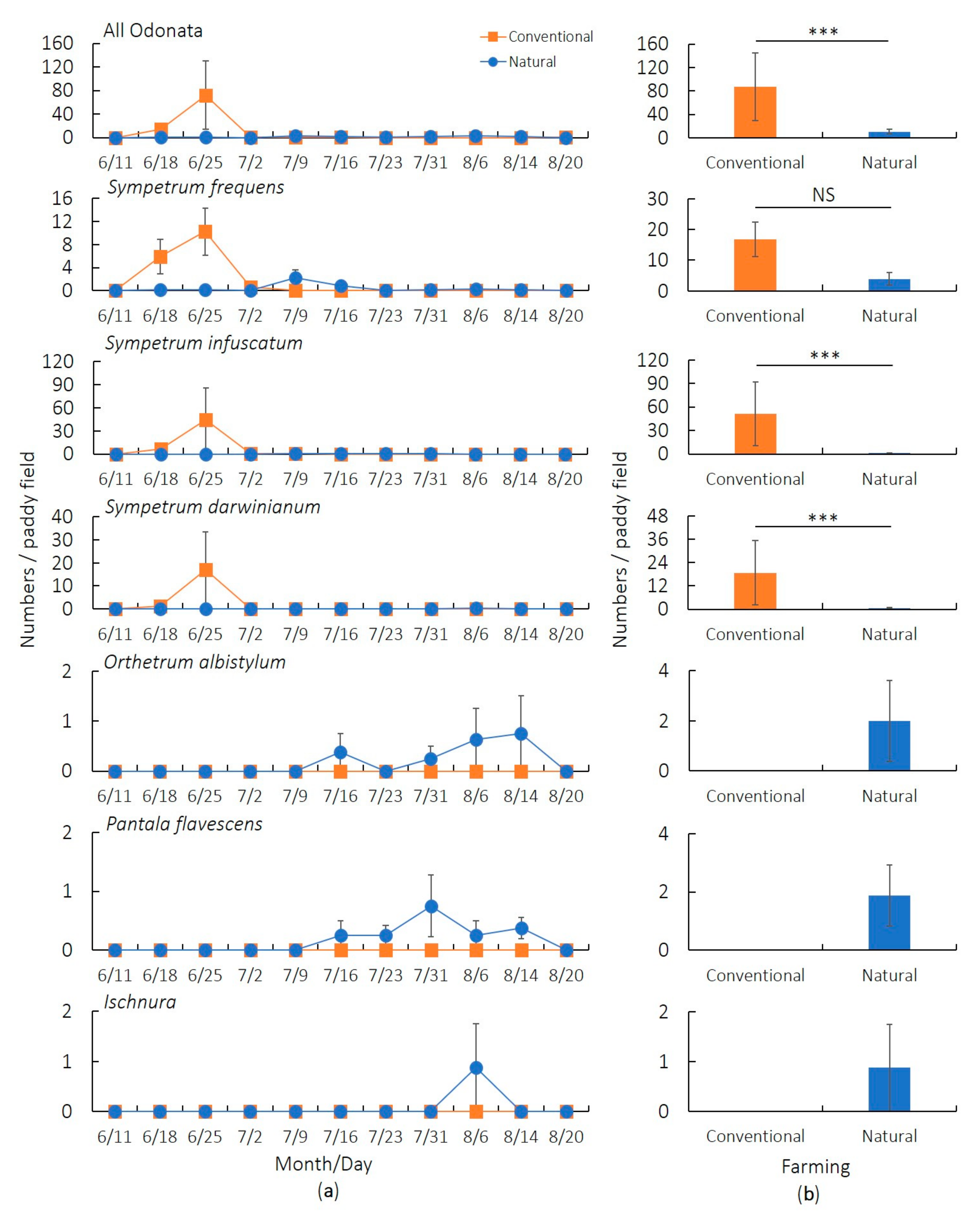
3.2. Main Survey (2019)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Figure A1 | Type of Agrochemical | Main Ingredient (Product Name in Japan) [Number of Active Ingredients] | Application Amount |
|---|---|---|---|
| Late March to late April | Seed disinfectant | Ipconazole–cupric hydroxide (Techlead C Flowable) [1] Talaromyces flavus SAY-Y-94-01 strain spore (Tafuburokku) [0] | 200 times dilution |
| Early to mid-May | Nursery box insecticide | Chlorantraniliprole–probenazole (Dr. Orize Ferterra) [2] Chlorantraniliprole–tiazinil (Buiget Ferterra) [2] | 50 g/nursery box |
| Late July to early August | Insecticide—fungicide | Ethofenprox–tricyclazole (Beam Eight Trebon Sol) [2] Echiprole (Kirappu Flowable) [1] Validamycin (Varidasin Air) [0] | 800 mL/0.1 ha † |
| Early to mid-May | Paddy herbicide | Butachlor (Marchette 1 kg granules) [1] Imazosulfuron–oxazichromephone–pyraclonil (Sarabureddo KAI 1 kg granules) [3] | 1.0 kg/0.1 ha 1.0 kg/0.1 ha |
| Late April to mid-July | Levee herbicide | Gluphosate potassium salt (Roundup Max Road) [1] Glufosinate (Basta) [1] Glyphosate isopropylamine salt (Sanfuron) [1] | 300–500 mL/0.1 ha |
| Mid-March to early April Mid-April to mid-May Mid-June Mid-July | Soil conditioner Basal fertilizer Interim fertilizer Fertilizer for ear manuring | Magnesium–aluminum–silicic acid (BB Ta-no-megumi) Magnesium–aluminum–silicic acid (Super-keisan) Nitrogen–phosphorus–potassium–organic material (BB yuuki-iri Noto-koshi-ippatsu), Nitrogen–phosphorus–potassium– magnesium–silicic acid (BB keisan-power koshi-ippatsu-kun) Phosphorus–potassium– magnesium–silicic acid–boron (PK keisan) Nitrogen–phosphorus–potassium–magnesium (BB tsuihi 550-go) | 40–60 kg/0.1 ha 30–50 kg/0.1 ha 40 kg/0.1 ha 13–20 kg/0.1 ha |


References
- Geographical Survey Institute. Changes in Wetland Area, Japan. Available online: https://www.gsi.go.jp/kankyochiri/shicchimenseki2.html (accessed on 18 November 2020). (In Japanese).
- Fujioka, M.; Don Lee, S.; Kurechi, M. Bird use of Rice Fields in Korea and Japan. Waterbirds 2011, 33, 8. [Google Scholar] [CrossRef]
- Pernollet, C.A.; Guelmami, A.; Green, A.J.; Curcó Masip, A.; Dies, B.; Bogliani, G.; Tesio, F.; Brogi, A.; Gauthier-Clerc, M.; Guillemain, M. A comparison of wintering duck numbers among European rice production areas with contrasting flooding regimes. Biol. Conserv. 2015, 186, 214–224. [Google Scholar] [CrossRef] [Green Version]
- Naito, R.; Yamasaki, M.; Lmanishi, A.; Natuhara, Y.; Morimoto, Y. Effects of water management, connectivity, and surrounding land use on habitat use by frogs in rice paddies in Japan. Zool. Sci. 2012, 29, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Holzer, K.A.; Bayers, R.P.; Nguyen, T.T.; Lawler, S.P. Habitat value of cities and rice paddies for amphibians in rapidly urbanizing Vietnam. J. Urban Ecol. 2017, 3, 3. [Google Scholar] [CrossRef]
- Katano, O.; Hosoya, K.; Iguchi, K.; Yamaguchi, M.; Aonuma, Y.; Kitano, S. Species diversity and abundance of freshwater fishes in irrigation ditches around rice fields. Environ. Biol. Fishes 2003, 66, 107–121. [Google Scholar] [CrossRef]
- Amilhat, E.; Lorenzen, K. Habitat use, migration pattern and population dynamics of chevron snakehead Channa striata in a rainfed rice farming landscape. J. Fish Biol. 2005, 67, 23–34. [Google Scholar] [CrossRef]
- Miyashita, T.; Chishiki, Y.; Takagi, S.R. Landscape heterogeneity at multiple spatial scales enhances spider species richness in an agricultural landscape. Popul. Ecol. 2012, 54, 573–581. [Google Scholar] [CrossRef]
- Watanabe, K.; Koji, S.; Hidaka, K.; Nakamura, K. Abundance, diversity, and seasonal population dynamics of aquatic Coleoptera and Heteroptera in rice fields: Effects of direct seeding management. Environ. Entomol. 2013, 42, 841–850. [Google Scholar] [CrossRef] [Green Version]
- Usio, N.; Miyashita, T. (Eds.) Social-Ecological Restoration in Paddy-Dominated Landscapes; Springer: Tokyo, Japan, 2014. [Google Scholar]
- AFFRC; NIAES; NARO (Eds.) Manual for Biodiversity Survey/Evaluation in Agriculture Using Biodiversity Indicators—I. Survey and Evaluation Methods; Sato Press Inc.: Tsukuba, Japan, 2012; Available online: http://www.naro.affrc.go.jp/archive/niaes/techdoc/shihyo/ (accessed on 18 November 2020). (In Japanese)
- NIAES. Biodiversity Survey/Evaluation Manual for Understanding Bird-Friendly Paddy Fields; NIAES: Tsukuba, Japan, 2018; Available online: http://www.naro.affrc.go.jp/publicity_report/publication/pamphlet/tech-pamph/080832.html (accessed on 18 November 2020). (In Japanese)
- Hayasaka, D.; Korenaga, T.; Suzuki, K.; Saito, F.; Sánchez-Bayo, F.; Goka, K. Cumulative ecological impacts of two successive annual treatments of imidacloprid and fipronil on aquatic communities of paddy mesocosms. Ecotoxicol. Environ. Saf. 2012, 80, 355–362. [Google Scholar] [CrossRef]
- Aoda, T.; Katano, K.; Toyama, K.; Jinguji, H. Assessment of paddy environment using emergence husks of red-dragonflies with civic participation. Bull. Facul. Agric. Niigata Univ. 2013, 65, 131–135. (In Japanese) [Google Scholar]
- Jinguji, H.; Ohtsu, K.; Ueda, T.; Goka, K. Effects of short-term, sublethal fipronil and its metabolite on dragonfly feeding activity. PLoS ONE 2018, 13, e0200299. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, K.; Yokomizo, H.; Hayashi, T.I. Were the sharp declines of dragonfly populations in the 1990s in Japan caused by fipronil and imidacloprid? An analysis of Hill’s causality for the case of Sympetrum frequens. Environ. Sci. Pollut. Res. 2018, 25, 35352–35364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, A.L.; Watts, R.J.; Stevens, M.M. Effects of different management regimes on aquatic macroinvertebrate diversity in Australian rice fields. Ecol. Res. 2008, 23, 565–572. [Google Scholar] [CrossRef]
- Hamasaki, K.; Tanaka, K.; Nakatani, Y.; Yoshitake, H.; Tabata, J. Effects of organic cultivation practices on arthropod assemblages in paddy fields in Tochigi prefecture, Japan. In Proceedings of the Biodiversity and Agro-Ecosystem in Rice Paddy Landscape in Monsoon Asia; NIAES: Tsukuba, Japan, 2009; p. P4-05. [Google Scholar]
- Tsutsui, M.H.; Kobayashi, K.; Miyashita, T. Temporal trends in arthropod abundances after the transition to organic farming in paddy fields. PLoS ONE 2018, 13, e0190946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baba, Y.G.; Kusumoto, Y.; Tanaka, K. Positive effect of environmentally friendly farming on paddy field odonate assemblages at a small landscape scale. J. Insect Conserv. 2019, 23, 467–474. [Google Scholar] [CrossRef]
- Dewan, S.; Darnal, N.; Kumar Acharya, B.; Subramanian, K.A.; Chettri, B.; Jins, V.J. Effectiveness of organic terrace rice cultivation in conservation of odonates in Sikkim, Eastern Himalaya, India. Int. J. Odonatol. 2019, 22, 207–222. [Google Scholar] [CrossRef]
- Giuliano, D.; Bogliani, G. Odonata in rice agroecosystems: Testing good practices for their conservation. Agric. Ecosyst. Environ. 2019, 275, 65–72. [Google Scholar] [CrossRef]
- Katayama, N.; Osada, Y.; Mashiko, M.; Baba, Y.G.; Tanaka, K.; Kusumoto, Y.; Okubo, S.; Ikeda, H.; Natuhara, Y. Organic farming and associated management practices benefit multiple wildlife taxa: A large-scale field study in rice paddy landscapes. J. Appl. Ecol. 2019, 56, 1970–1981. [Google Scholar] [CrossRef]
- Naito, K.; Sagawa, S.; Ohsako, Y. Using the Oriental White Stork as an Indicator Species for Farmland Restoration. In Social-Ecological Restoration in Paddy-Dominated Landscapes; Usio, N., Miyashita, T., Eds.; Springer: Tokyo, Japan, 2014; pp. 123–138. [Google Scholar] [CrossRef]
- Orihara, K.; Kamiyama, K.; Fujiwara, S. Characteristics of the heavy metal content in animal manure compost. Jpn. J. Soil Sci. Plant Nutr. 2002, 73, 403–409. (In Japanese) [Google Scholar] [CrossRef]
- Sato, T.; Yoshida, S.; Shigemori, I. The phytotoxicity of clopyralid contaminated compost to sensitive plants. Jpn. J. Soil Sci. Plant Nutr. 2010, 81, 158–161. (In Japanese) [Google Scholar] [CrossRef]
- ARIC Suido-No-Ishizue. Available online: https://suido-ishizue.jp/kokuei/hokuriku/Prefectures/1702/1702.html (accessed on 14 December 2020). (In Japanese).
- Ozono, A.; Kawashima, I.; Futahashi, R. Dragonflies of Japan; Bunichi-Sogo Syuppan: Hillerød, Denmark, 2012. (In Japanese) [Google Scholar]
- Ishida, S.; Ishida, K.; Kojima, K.; Sugimura, M. Illustrated Guide for Identification of the Japanese Odonata; Tokai University Press: Tokyo, Japan, 1988. (In Japanese) [Google Scholar]
- Hobson, K.A.; Jinguji, H.; Ichikawa, Y.; Kusack, J.W.; Anderson, R.C. Long-Distance Migration of the Globe Skimmer Dragonfly to Japan Revealed Using Stable Hydrogen (δ 2H) Isotopes. Environ. Entomol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, M.; Ishida, S.; Kojima, K.; Ishida, K.; Aoki, T. Dragonflies of the Japanese Archipelago in Color; Hokkaido University Press: Sapporo, Japan, 2001. (In Japanese) [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Team R. RStudio Team RStudio: Integrated Development for R; RStudio: Boston, MA, USA, 2020. [Google Scholar]
- Benke, A. Interactions among coexisting predators—A field experiment with dragonfly larvae. J. Anim. Ecol. 1978, 47, 335–350. [Google Scholar] [CrossRef]
- Nakanishi, K.; Uéda, T.; Yokomizo, H.; Hayashi, T.I. Effects of systemic insecticides on the population dynamics of the dragonfly Sympetrum frequens in Japan: Statistical analyses using field census data from 2009 to 2016. Sci. Total Environ. 2020, 703, 134499. [Google Scholar] [CrossRef] [PubMed]
- Usio, N.; Saito, R.; Akanuma, H.; Watanabe, R. Effectiveness of Wildlife-Friendly Farming on Aquatic Macroinvertebrate Diversity on Sado Island in Japan. In Social-Ecological Restoration in Paddy-Dominated Landscapes; Usio, N., Miyashita, T., Eds.; Springer: Tokyo, Japan, 2014; pp. 95–113. [Google Scholar] [CrossRef]
- Baba, Y.G.; Kusumoto, Y.; Tanaka, K. Effects of agricultural practices and fine-scale landscape factors on spiders and a pest insect in Japanese rice paddy ecosystems. BioControl 2018, 63, 265–275. [Google Scholar] [CrossRef]
- Nagy, H.B.; László, Z.; Szabó, F.; Szőcs, L.; Dévai, G.; Tóthmérész, B. Landscape-scale terrestrial factors are also vital in shaping Odonata assemblages of watercourses. Sci. Rep. 2019, 9, 18196. [Google Scholar] [CrossRef]
- Miyashita, T.; Yamanaka, M.; Tsutsui, M. Distribution and Abundance of Organisms in Paddy-Dominated Landscapes with Implications for Wildlife-Friendly Farming. In Social-Ecological Restoration in Paddy-Dominated Landscapes; Springer: Tokyo, Japan, 2014; pp. 45–65. ISBN 978-4-431-55329-8. [Google Scholar] [CrossRef]
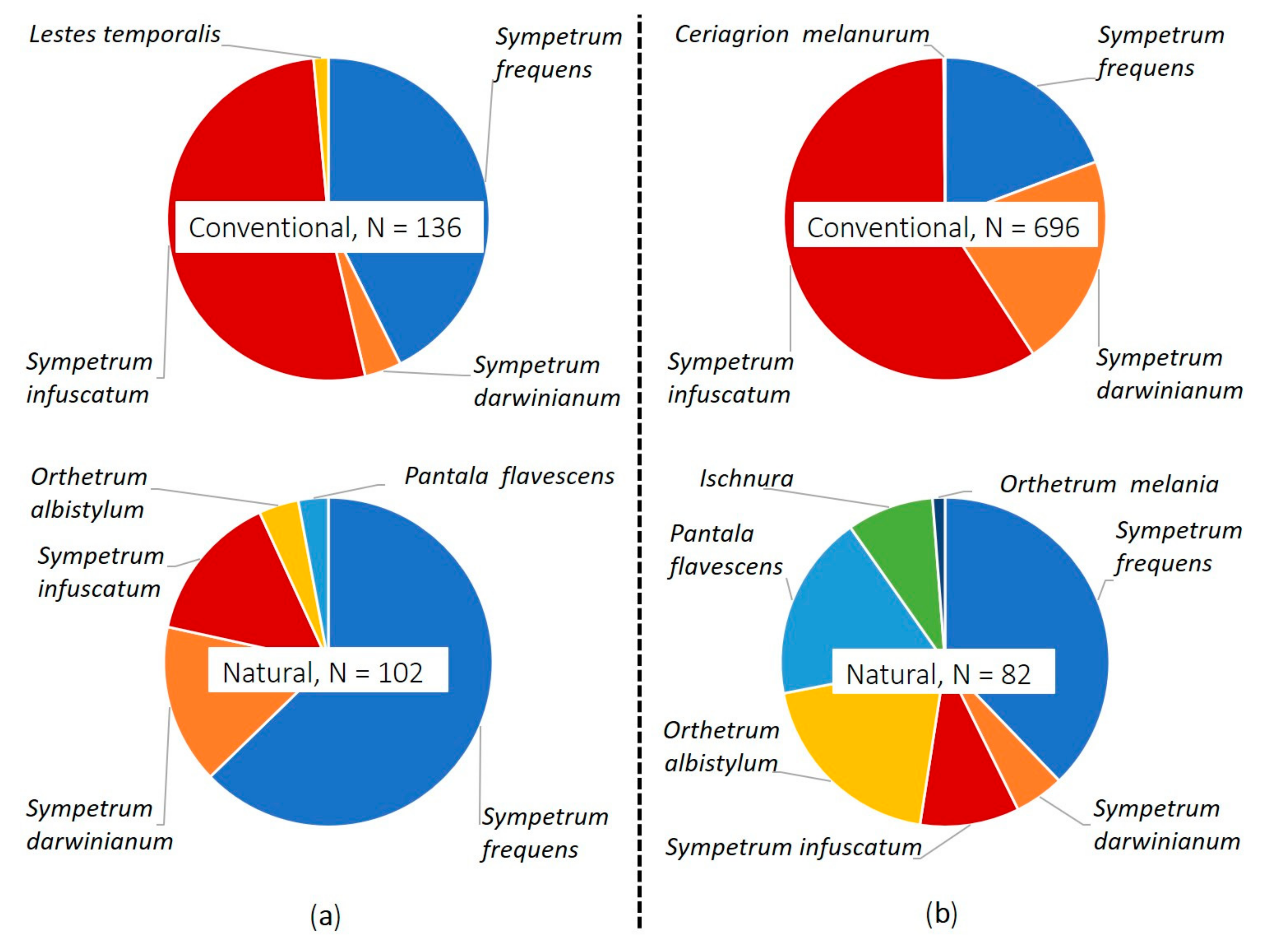
| Taxon | Conventional Farming | Natural Farming | ||||
|---|---|---|---|---|---|---|
| Exuviae (Number) | Cont. (%) | Prev. (%) | Exuviae (Number) | Cont. (%) | Prev. (%) | |
| Lestes temporalis | 2 | 1.5 | 12.5 | - | - | - |
| Sympetrum darwinianum | 5 | 3.7 | 12.5 | 16 | 15.7 | 37.5 |
| Sympetrum infuscatum | 71 | 52.2 | 37.5 | 15 | 14.7 | 37.5 |
| Sympetrum frequens | 58 | 42.6 | 75.0 | 64 | 62.7 | 100.0 |
| Pantala flavescens | - | - | - | 3 | 2.9 | 12.5 |
| Orthetrum albistylum | - | - | - | 4 | 3.9 | 25.0 |
| Response Variable | Covariate | Estimate | SE | z | p |
|---|---|---|---|---|---|
| Total Odonata | Intercept | 2.586 | 0.439 | 5.888 | <0.001 |
| Farming † | −0.852 | 0.835 | −1.020 | 0.308 | |
| Mean water depth | 0.336 | 0.345 | 0.974 | 0.330 | |
| Sympetrum frequens | Intercept | 2.008 | 0.477 | 4.214 | <0.001 |
| Farming † | 0.551 | 0.779 | 0.707 | 0.480 | |
| Mean water depth | −0.233 | 0.328 | −0.709 | 0.478 | |
| Sympetrum infuscatum | Intercept | 1.520 | 0.913 | 1.665 | 0.096 |
| Farming † | −3.620 | 3.324 | −1.089 | 0.276 | |
| Mean water depth | 1.005 | 1.230 | 0.817 | 0.414 | |
| Sympetrumdarwinianum | Intercept | 1.520 | 0.913 | 1.665 | 0.096 |
| Farming † | −3.620 | 3.324 | −1.089 | 0.276 | |
| Mean water depth | 1.005 | 1.230 | 0.817 | 0.414 |
| Taxon | Conventional Farming | Natural Farming | ||||
|---|---|---|---|---|---|---|
| Exuviae (Number) | Cont. (%) | Prev. (%) | Exuviae (Number) | Cont. (%) | Prev. (%) | |
| Ceriagrion melanurum | 1 | 0.1 | 12.5 | - | - | - |
| Ischnura | - | - | - | 7 | 8.5 | 12.5 |
| Sympetrum darwinianum | 150 | 21.6 | 62.5 | 4 | 4.9 | 37.5 |
| Sympetrum infuscatum | 411 | 59.1 | 62.5 | 8 | 9.8 | 37.5 |
| Sympetrum frequens | 134 | 19.3 | 62.5 | 31 | 37.8 | 62.5 |
| Pantala flavescens | - | - | - | 15 | 18.3 | 50.0 |
| Orthetrum albistylum | - | - | - | 16 | 19.5 | 25.0 |
| Orthetrum melania | - | - | - | 1 | 1.2 | 12.5 |
| Response Variable | Covariate | Estimate | SE | z | p |
|---|---|---|---|---|---|
| Total Odonata | Intercept | 3.089 | 0.725 | 4.263 | <0.001 |
| Farming † | −2.850 | 0.837 | −3.406 | <0.001 | |
| Mean water depth | 0.839 | 0.436 | 1.925 | 0.054 | |
| Sympetrum frequens | Intercept | 2.204 | 1.085 | 2.032 | 0.042 |
| Farming † | −2.012 | 1.269 | −1.585 | 0.113 | |
| Mean water depth | 0.459 | 0.750 | 0.612 | 0.540 | |
| Sympetrum infuscatum | Intercept | 2.438 | 1.153 | 2.115 | 0.034 |
| Farming † | −4.567 | 1.323 | −3.452 | <0.001 | |
| Mean water depth | 0.866 | 0.667 | 1.299 | 0.194 | |
| Sympetrumdarwinianum | Intercept | −0.556 | 1.272 | −0.437 | 0.662 |
| Farming † | −3.935 | 0.626 | −6.286 | <0.001 | |
| Mean water depth | 0.687 | 0.429 | 1.603 | 0.109 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huynh, T.Q.; Oyabu, A.; Nomura, S.; Takashima, T.; Usio, N. Do Agrochemical-Free Paddy Fields Serve as Refuge Habitats for Odonata? Ecologies 2021, 2, 1-15. https://doi.org/10.3390/ecologies2010001
Huynh TQ, Oyabu A, Nomura S, Takashima T, Usio N. Do Agrochemical-Free Paddy Fields Serve as Refuge Habitats for Odonata? Ecologies. 2021; 2(1):1-15. https://doi.org/10.3390/ecologies2010001
Chicago/Turabian StyleHuynh, Thien Quang, Aisha Oyabu, Shinya Nomura, Tadao Takashima, and Nisikawa Usio. 2021. "Do Agrochemical-Free Paddy Fields Serve as Refuge Habitats for Odonata?" Ecologies 2, no. 1: 1-15. https://doi.org/10.3390/ecologies2010001
APA StyleHuynh, T. Q., Oyabu, A., Nomura, S., Takashima, T., & Usio, N. (2021). Do Agrochemical-Free Paddy Fields Serve as Refuge Habitats for Odonata? Ecologies, 2(1), 1-15. https://doi.org/10.3390/ecologies2010001





