Abstract
Innate immunity is the body’s first line of defense for mounting robust antiviral signaling. However, the role of cytoskeletal prestress, a hallmark of cellular mechanotransduction, in regulating innate immune pathways such as retinoic acid-inducible gene I (RIG-I) signaling remains poorly understood. Herein, we show that cells on soft vs. rigid substrates elicit cytoskeletal prestress-dependent activation of RIG-I signaling, leading to differential type-I interferon (IFN) gene expression. Cells were cultured on soft (0.6 kPa) and stiff (8.5 kPa) substrates to modulate cellular traction and prestress, followed by transfection of Poly(I:C), a synthetic viral dsRNA mimic, to measure the RIG-I-mediated innate immune response. Cells on soft substrates show minimal activation of RIG-I signaling, resulting in low expression of IFN-β1 and other IFN-stimulated genes (ISGs), compared to cells on stiff substrates. We further demonstrate that activation of TANK Binding Kinase 1 (TBK1), a downstream effector of the RIG-I pathway, is inhibited in cells on soft substrates due to the cytoplasmic sequestration of the Yes-associated protein (YAP), a HIPPO pathway effector protein. In contrast, cells on stiffer substrates experienced decreased TBK1 inhibition due to the nuclear localization of YAP and exhibited elevated TBK1 activation and heightened IFN and ISG expressions. Together, we demonstrate that cytoskeletal prestress represents a key biophysical regulator of innate immune signaling.
1. Introduction
Innate immunity is the first line of defense against pathogen infection. As an example, when a virus enters a cell, the cell detects the presence of foreign DNA or RNA and mounts robust antiviral signaling within a matter of hours. Retinoic acid-inducible gene I (RIG-I) is one of the cytosolic sensors that have a remarkable ability to detect single-stranded (ss) or double-stranded (ds) viral RNA [1,2,3,4,5]. Once the viral load or molecular pattern related to viral infection is detected, RIG-I activates downstream signaling such as the transcription factor interferon regulatory factor 3 (IRF3) gene that ultimately leads to the production of type I interferon (IFN) [6]. Following Type I IFN production, a family of genes, called interferon-stimulated genes (ISGs), are activated to counteract the viral infection, leading to adaptive immunity.
Many cell types are capable of producing type I interferon and other innate cytokines as a rapid response to a wide range of viral infections [7]. Yet not all cells within the same tissue may respond at the same level. The mechanisms governing how RIG-I–dependent interferon production is differentially regulated among cells within the same tissue in response to viral challenge remain unclear. This is not a trivial issue since identifying and clearing off viral load is very essential at the early stages of infection. Therefore, our understanding of what determines the heterogeneous cellular innate immune response remains poorly known. Among other factors, the physical properties of the tissue are not uniform throughout, and the effect of the microenvironment on cellular innate immune response remains elusive. More specifically, it is not well understood whether the cytoskeletal prestress is responsible for RIG-I activation. Here, we investigated whether changes in cytoskeletal prestress have any effect on the innate immune response of cells against viral infections. To modulate cellular prestress, we cultured HeLa cells on different substrates of varying stiffness. Consistent with our current understanding, with the increase in substrate stiffness, the traction and cytoskeletal prestress also increased [8]. To simulate viral infection, we used Poly(I:C) [6], a synthetic dsRNA analog, to trigger the RIG-I response of cells on different substrate stiffness. Our result demonstrates that RIG-I and subsequently IFN-β1 expressions are indeed higher when cells were grown on 8.5 kPa substrates compared to much softer 0.6 kPa substrates, thereby indicating that the RIG-I signaling pathway is indeed affected by changes in the cytoskeletal prestress, modulated by the underlying substrate stiffness.
2. Materials and Methods
2.1. Fabrication of Polyacrylamide Hydrogel Substrates
Polyacrylamide (PA) substrates with stiffness of 0.6 kPa and 8.5 kPa were prepared on 35 mm glass bottom dishes (Cellvis, CA, USA) following the protocol as described before [9,10,11]. Briefly, the polyacrylamide substrates with elastic Young’s modulus of 0.6 kPa (0.06% bis-acrylamide, 3% acrylamide, Bio-Rad, IL, USA) and 8.5 kPa (0.3% bis-acrylamide, 5% acrylamide) were prepared with embedded 0.2 μm carboxylate-modified yellow/green fluorospheres (Invitrogen, CA, USA) on the substrates for traction measurements. The prepared substrates were then coated with 20 μg/mL bovine plasma fibronectin (Sigma-Aldrich, MO, USA) for cell experiments.
2.2. Cell Culture and Poly(I:C) Transfection
HeLa cell culture was maintained in DMEM culture medium supplemented with 10% fetal bovine serum (Gibco, MA, USA), 2 mM L-glutamine (Gibco), 1 mM sodium pyruvate (Gibco), and 0.1 mM penicillin/streptomycin at 37 °C with 5% CO2. The cells were seeded on PA substrates of different stiffness and cultured for 24 h at 37 °C with 5% CO2. Next, the cells were transfected with 1 μg of low-molecular-weight polyinosinic:polycytidylic acid, LMW-Poly(I:C) (Invivogen, CA, USA). 6 h post-transfection, the cells were harvested for total RNA extraction.
2.3. Cell Area and Cell Shape Index Analysis
The projected cell spreading area and perimeter were calculated using ImageJ (version 1.54m, NIH). Cell shape index (CSI) is a non-dimensional geometric quantity of circularity that was calculated using the relation, .
2.4. Real-Time Reverse Transcription PCR Analysis
Total RNA was extracted using the RNeasy Mini kit (Qiagen, USA) using the manufacturer’s protocol. 1.5 μg of the total RNA was reverse transcribed to cDNA using iScript Reverse Transcription supermix (Bio-Rad), and 1.3 ng of the cDNA was used to check for relative gene expression determined by SYBR green (Bio-Rad) incorporation using a Bio-Rad CFX Real-Time PCR detection system. Primers for genes (Supplementary Table S1) were designed using NCBI’s primer design tool. Following PCR amplification, the specificity of the product was determined by melt-curve analysis to confirm that only a single product of the correct size was present. Relative gene expressions were calculated using the 2−ΔΔCT method, described previously [12], and normalized against Rps18 housekeeping gene. Data are represented as average fold increase ± standard error of the mean (SEM).
2.5. Cellular Traction Measurement
Root-mean-square (RMS) traction quantification was performed using the protocol as previously described [10]. In brief, the HeLa cells were seeded on PA substrates with embedded green fluorospheres. The cells were grown for a period of 24 h before acquiring phase images of the cells and fluorescent images of the beads. Following this, the cells were trypsinized from the surface, and another fluorescent image of the beads was acquired. The fluorescent images of the beads before and after cell trypsinization were used to compute the displacement field, traction, and prestress of the cells using custom MATLAB (version 2024b) programs [10].
2.6. Double Immunostaining
HeLa cells grown on fibronectin-coated glass or PA substrates were washed with PBS twice and fixed with 4% paraformaldehyde (Sigma Aldrich) at 25 °C for 10 min, followed by rinsing with PBS three times. The samples are then blocked in 2% Fraction V BSA and 0.5% Triton X-100 in PBS for 1 h at room temperature. The samples were incubated with primary monoclonal anti-YAP (1:400) and primary monoclonal anti-TBK1 (1:100) for 1 h at room temperature, followed by three washes in PBS. Subsequently, cells were incubated with Alexa Fluor 488–conjugated anti-mouse IgG (Invitrogen; 1:2000) and Cy3-conjugated anti-rabbit IgG (Novus Biologicals, CO, USA; 1:5000) for 1 h at room temperature. The samples are then washed three times with PBS and counterstained with 4,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) at 1:16 dilution in PBS for 10–15 min. Coverslips were mounted with ProLong antifade mountant (Thermo Fisher Scientific, MA, USA) and examined using an inverted widefield epifluorescence microscope (Leica USA, DMi8) equipped with a live THUNDER imaging system. The acquired images were analyzed using ImageJ (NIH) to determine nuclear/cytoplasmic ratio.
2.7. In Situ Proximal Ligation Assay (PLA)
HeLa cells cultured and treated as indicated above were fixed with 4% paraformaldehyde (Sigma Aldrich) at 25 °C for 10 min and then subjected to an in situ PLA according to the manufacturer’s instructions. Primary mouse monoclonal anti-YAP and primary rabbit monoclonal anti-TBK1 were used. The Duolink PLA anti-mouse MINUS (DUO92004) and anti-rabbit PLUS (DUO92002) was used to perform the PLA. High-resolution THUNDER images were acquired in z-stacks with 1 μm z-interval. PLA dots in single cells were then analyzed and counted using ImageJ (NIH) and a custom MATLAB program [13]. Briefly, the algorithm first reads the maximum intensity projected (MIP) and the corresponding z-stack image file from the directory. For the MIP, it applies a Gaussian filter to smooth the image, corrects the background, and thresholds the MIP to begin the process of dot counting. For a spot to be counted as a “good” one, it must have the highest intensity profile (or peaks of signal) from the surrounding spots.
2.8. Statistical Testing
All statistical analyses were performed using unpaired two-tailed Student’s t-tests. A p-value of <0.05 was considered statistically significant.
3. Results
3.1. Cellular Prestress, Modulated by Substrate Rigidity, Regulates Innate Immunity
Cellular prestress refers to the preexisting tension within the highly dynamic F-actin cytoskeleton network that maintains cellular shape and stability. The prestress, in part, is regulated by the underlying substrate stiffness [14,15]. When the physical properties of the cellular microenvironment such as substrate stiffness increase, cells concomitantly increase their internal prestress to maintain cell shape via mechanical tensional integrity [16]. This cellular adaptation preserves cytoskeletal structures and influences a wide range of critical cellular functions, from migration to differentiation. The dynamic prestress adjustment in response to environmental changes has been widely studied in various model systems and cell types. Here, we showed that HeLa cells modulate cellular prestress as a function of substrate stiffness. HeLa cells exhibited a ~3.5-fold increase in cell spreading on the stiffer 8.5 kPa substrate compared to the softer 0.6 kPa substrate (Figure 1a,b). Furthermore, the cell shape index, a quantitative, dimensionless measure of cellular morphology [17,18], showed a more complex spreading pattern for cells on the stiffer substrate, while cells on the softer substrate essentially preserved a rounded shape (Figure 1c).
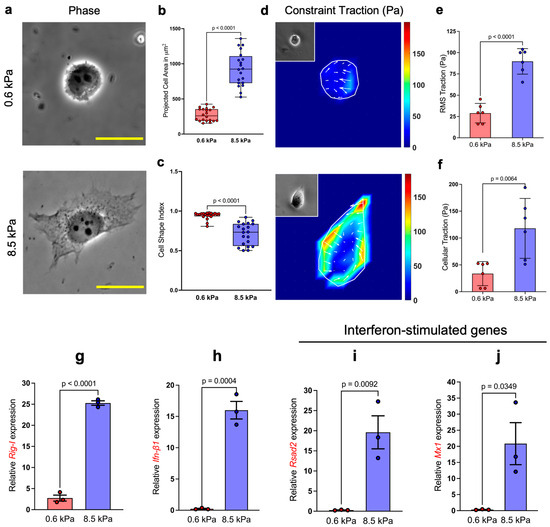
Figure 1.
Cellular prestress regulates the innate immune response: (a) Phase images of HeLa cells seeded on 0.6 kPa and 8.5 kPa PA substrates. Quantification of (b) projected cell area (n = 20) shows that cells spread significantly more on 8.5 kPa substrates compared to 0.6 kPa, and (c) cell shape index (a measure of circularity; n = 20) indicates that cells on softer substrates adopt a more rounded morphology. (d–f) Basal RMS traction (n = 6) and prestress (n = 6) demonstrate that cells on stiffer substrates generate greater contractile forces. (g–j) Relative mRNA expression of key innate immune pathway genes following LMW-Poly(I:C) transfection on substrates of differing stiffness, measured from three independent experiments. Expression levels were normalized to the housekeeping gene Rps18. Scale bar, 20 μm.
Furthermore, our results show, consistent with previous reports [8,19], that the cells experience an almost 3-fold higher RMS traction on the 8.5 kPa substrate compared to the 0.6 kPa substrate (Figure 1d,e), which also culminates in an increase in cellular prestress with substrate stiffness (Figure 1f), thereby implying that cellular prestress intensifies with increasing substrate stiffness.
Elevated cytoskeletal prestress is known to cause cell stiffening, culminating in changes in gene expression and thereby affecting cellular behavior [14]. Nevertheless, the role of this cellular hallmark affecting the recognition of viral dsRNA as part of their innate immune response remains largely unknown. Herein, we investigate the potential regulatory link between cytoskeletal prestress and innate immunity. To simulate viral infection, we utilized LMW Poly(I:C) (hereafter referred to as Poly(I:C), a synthetic viral dsRNA mimic, that is known to induce type-I interferon through the activation of RIG-I signaling, a typical response of cells upon sensing the entry of viral dsRNAs in their cytoplasm [20]. We identified the optimum amount of Poly(I:C) (1000 ng) that triggers an innate immune response in HeLa cells (Supplementary Figure S1). Based on this, we transfected HeLa cells grown on the soft and stiff substrate with 1000 ng of Poly(I:C) and measured their relative expression of Rig-I, 6 h post-transfection. Surprisingly, we observed a ~9.2-fold upregulation in RIG-I expression in cells growing on the 8.5 kPa substrate compared to 0.6 kPa (Figure 1g). Alongside, we observed a 15-fold upregulation of Ifn-β1 expression (Figure 1h), thereby indicating that cells growing on the stiffer substrate tend to exhibit a significantly heightened response to Poly(I:C).
To assess if elevated Ifn-β1 expression translated into an antiviral response and is not just limited by the virtue of enhanced RIG-I, we investigated the expression of Rsad2, widely regarded as one of the ISGs. The product of Rsad2, viperin is an antiviral protein induced by lipopolysaccharide, type-I IFN, and Poly(I:C), and to a lesser extent by type-II IFN [21]. Although Rsad2 expression might be dependent or independent of IFN production, both pathways require the recognition of viral dsRNA through cytoplasmic RIG-I. Consistent with the enhanced Ifn-β1 expression, we observed a ~72-fold increase in Rsad2 expression (Figure 1i) in cells grown on 8.5 kPa substrates, indicating that the elevated response was indeed initiated by IFN-β1. Lastly, we checked the expression of Mx1, a GTPase that is a key player in antiviral response induced by type-I and type-III IFNs and is known to act against a wide range of RNA viruses by interfering with viral replication [22,23]. We found a 47.3-fold upregulation in Mx1 expression in cells grown on the stiffer substrate, corroborating the elevated IFN-β1-mediated antiviral response in these cells (Figure 1j). Taken together, our results revealed that cells experiencing high cytoskeletal prestress exhibited enhanced recognition of Poly(I:C) and mounted a more robust antiviral response compared to cells on softer 0.6 kPa substrates.
3.2. Poly(I:C)-Induced YAP Nuclear Localization Is Restricted to Cells on Soft Substrates
The Hippo signaling pathway plays a critical role in regulating cellular responses to substrate rigidity [24,25]. Earlier studies have underlined that increased actin-cytoskeleton tension inactivates the Hippo pathway, while relaxed actin tension restores it [25,26]. Interestingly, a more recent study also revealed how YAP, the effector protein of the Hippo pathway, obstructs TBK1, a decisive component of the RIG-I signaling pathway, thereby hinting a potential connection between the Hippo pathway and the innate antiviral response [27].
Building on these findings, we hypothesized that the inactive Hippo pathway in cells grown on the stiffer substrate as a result of increased cytoskeletal prestress could facilitate unrestricted TBK1 activation, leading to the enhanced antiviral response. Therefore, we examined the subcellular localization of endogenous YAP in HeLa cells grown on soft (0.6 kPa) and stiff (8.5 kPa) substrates. We observed that cells cultured on soft substrates displayed a relatively modest nuclear-to-cytoplasmic YAP ratio. Upon Poly(I:C) transfection, however, this ratio increased markedly, indicating active translocation of YAP into the nucleus in response to Poly(I:C) stimulation (Figure 2a,b), raising the possibility that nuclear translocation of YAP could be linked to the cell’s antiviral defense. However, the mechanism is not clear at this point. In contrast, cells cultured on stiffer substrates exhibited a complete absence of cytoplasmic YAP, with localization exclusively in the nucleus. Poly(I:C) transfection did not further alter this distribution, consistent with YAP being maximally nuclear under these conditions (Figure 2c,d). Our findings are consistent with earlier work by the Dupont group [25], confirming that HeLa cells grown on stiffer substrates display an inactivated Hippo pathway, as suggested by the high level of nuclear YAP.
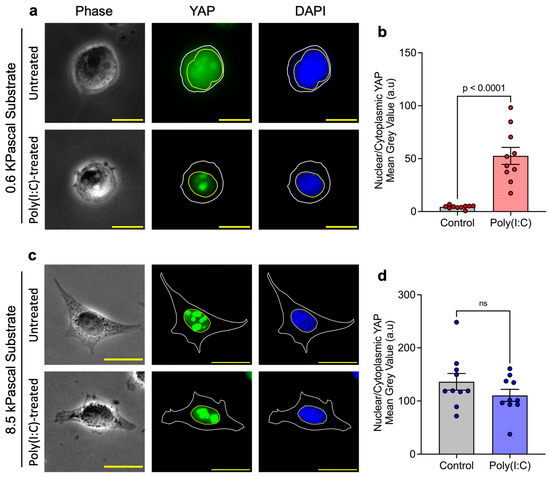
Figure 2.
Substrate stiffness regulates YAP localization in response to Poly(I:C): Representative images of YAP localization in untreated and Poly(I:C)-treated HeLa cells on (a) soft 0.6 kPa and (c) stiff 8.5 kPa substrates. (b) On 0.6 kPa substrates, Poly(I:C) treatment significantly increased the nuclear-to-cytoplasmic YAP ratio, indicating enhanced nuclear translocation. (d) On 8.5 kPa substrates, YAP was localized exclusively to the nucleus under basal conditions and was not further altered by Poly(I:C) treatment (ns = non significant). Data represent three independent experiments (n = 3)., Scale bar, 20 μm.
3.3. Cells Experience Enhanced TBK1 Inhibition by YAP on Soft Substrates
Next, we investigated the cytoplasmic expression of TBK1 in cells subjected to Poly(I:C) treatment across different substrate stiffnesses and our observations reveal that the expression levels of cytoplasmic TBK1 remain unchanged in cells grown on both soft (Figure 3a,b) and stiff (Figure 3c,d) substrates following Poly(I:C) treatment. This finding indicates that the modulation of TBK1 expression does not significantly differ with changes in substrate stiffness. Because TBK1 expression remained unaltered across substrates of varying stiffness, and building on previous findings that YAP directly inhibits TBK1 [27], we investigated whether YAP contributes to regulating TBK1 activity under these conditions using a proximity ligation assay (PLA) to visualize and quantify their interactions. We observed that Poly(I:C)-transfected cells cultured on soft substrates exhibited a higher frequency of YAP–TBK1 interactions compared to those on stiff substrates. Notably, cells on 0.6 kPa substrates exceeded an average threshold of 20 interactions per 100 μm2 of cell projected area, whereas cells on 8.5 kPa substrates show statistically less interactions (Figure 4a,b). This quantitative difference highlights the extent of crosstalk between the Hippo and RIG-I signaling pathways under varying substrate stiffness conditions. Cumulatively, these findings suggest a potential mechanism through which substrate stiffness influences innate immune responses, not by altering TBK1 expression directly, but rather through modulating the interaction between TBK1 and its inhibitor, YAP. The enhanced interaction in cells on soft substrates suggests a heightened inhibitory effect of YAP on TBK1, potentially leading to a modulation of the cells’ innate immune response through a mechanical cue-dependent pathway.
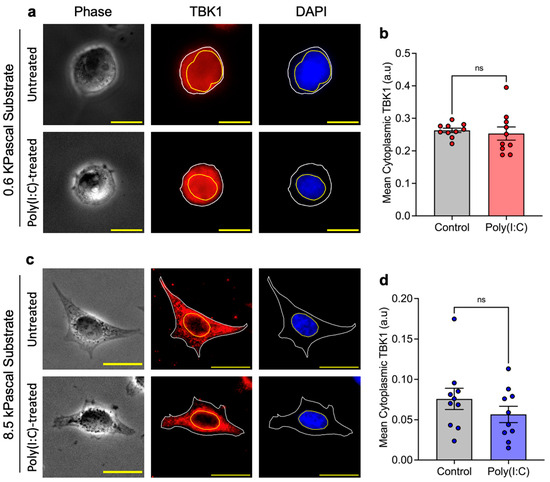
Figure 3.
Substrate stiffness influences TBK1 localization in response to Poly(I:C): Representative images of TBK1 localization in untreated and Poly(I:C)-treated HeLa cells cultured on (a) soft 0.6 kPa and (c) stiff 8.5 kPa substrates. (b,d) Quantification of cytoplasmic TBK1 intensity shows no significant difference between untreated and Poly(I:C)-treated cells on either substrate (ns = non significant). Data represent three independent experiments (n = 3). Scale bar, 20 μm.
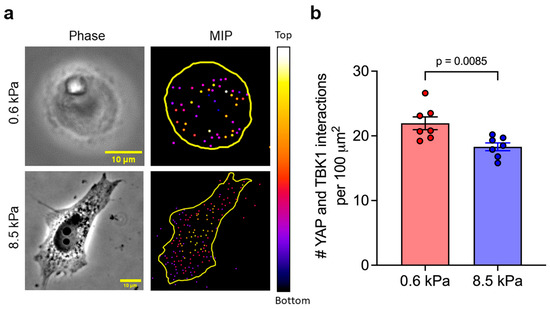
Figure 4.
Detection of YAP–TBK1 interactions by proximity ligation assay. (a) Maximum intensity Z-projections of PLA signals show YAP–TBK1 interactions within the cytoplasm. (b) Quantification of PLA signal density, normalized to cell surface area, revealed a higher frequency of YAP–TBK1 interactions in cells on 0.6 kPa substrates compared to those on 8.5 kPa substrates. Depth-encoded projections illustrate the three-dimensional spatial distribution of YAP–TBK1 interactions. The color scale indicates axial position (z-depth), with warmer colors (yellow/white) representing interactions closer to the top of the cell and cooler colors (blue/purple) corresponding to the bottom of the cell volume closer to the underlying substrate. Scale bar, 10 μm.
4. Discussion
Cellular mechanosensing of physical forces and responsive remodeling of cytoskeletal tension and prestress are known to affect a wide range of cellular behavior in terms of morphology, proliferation, differentiation, and gene expression [28,29,30]. Advancement in the field of materials engineering has paved the way for the development of inert biomaterials and bioactive designs for tissue regeneration [31]. Such developments have led to an extensive investigation into the innate response of cells toward such materials and physical cues. Nevertheless, how extracellular material properties can influence a cell’s responsiveness towards potentially harmful foreign nucleic acids has so far not been well understood. Here, we show how cellular prestress via mechanosensing of substrate rigidity can influence its innate immune responsiveness against foreign dsRNA analog.
A recent single-molecule study demonstrated that IRF3 expression, a downstream effector of RIG-I, is upregulated early during viral infection at the periphery of a cell cluster, but not at the center [32]. However, the detailed mechanism for this spatially located IRF3 upregulation around the periphery remained unclear. Perhaps, the peripheral cells experienced higher cellular prestress, thus initiated RIG-I pathway in a cellular prestress dependent way. In contrast, the cells at the center experienced low cellular prestress and did not activate RIG-I pathway. In our study, we systematically modulated cell prestress to reveal prestress-dependent differences in innate immune responses and revealed the underlying molecular mechanism. Cells under higher prestress on stiff substrates exhibited robust RIG-I signaling and generated heightened interferon responses to Poly(I:C), while this response was markedly diminished in cells with lower prestress on softer substrates. These findings demonstrate that cellular prestress governs RIG-I signaling, thus revealing a new mechanism of prestress mediated control of innate immunity.
Previous studies have proposed crosstalk between the Hippo and RIG-I signaling pathways, where YAP interacts with and negatively regulates TBK1, which essentially restricts the downstream RIG-I signaling cascade [27]. Since Hippo signaling is responsive to extracellular substrate stiffness, in our study, we investigated how this interaction influences the cellular response to foreign viral dsRNAs. To mimic the mechanical variation in substrate stiffness, we prepared soft (0.6 kPa) and stiff (8.5 kPa) polyacrylamide substrates, cultured cells on them, and quantified the cytoskeletal prestress through traction force measurements. To evaluate whether substrate stiffness modulates activation of the RIG-I pathway and the interferon response, we quantified the expression of RIG-I, Ifn-β1, and two key ISGs, namely, Rsad2 and Mx1, at 6 h post-transfection with Poly(I:C). Although the temporal dynamics of innate immune regulation are critical for a more thorough understanding, our current study was limited to a single time point. In the future, we plan to conduct a more detailed temporal investigation. In addition, YAP could potentially have additional interactions with downstream effectors such as IRF3/IRF7, which were not investigated in our study. This may further explain the severe downregulation of innate immune signaling in cells on soft substrates.
Finally, we believe that the core mechanistic axis we identified connecting substrate stiffness, cytoskeletal prestress, YAP localization, TBK1 inhibition, and RIG-I/IFN signaling is likely conserved across multiple cell types, including the HeLa cells. Any adherent primary cells capable of generating actomyosin contractility, such as muscle cells or non-muscle cells like fibroblasts, are expected to exhibit behavior similar to that observed in HeLa cells: higher matrix stiffness enhances prestress, reduces YAP–TBK1 association, and amplifies interferon responses. In contrast, suspended cells like lymphocytes may exhibit more context-dependent modulation through confinement or integrin-mediated engagement. In such systems, assessing the relationship between cellular prestress, YAP–TBK1 proximity, and interferon response, or employing microfluidic confinement assays that enable independent control of prestress, would be valuable outcomes to test the generality of this mechanistic pathway.
5. Conclusions
In conclusion, our findings show how extracellular substrate stiffness can regulate cellular innate immunity by modulating cellular prestress. These findings provide mechanistic insights into RIG-I-mediated innate immunity, revealing how cells interpret their mechanical environment to mount appropriate antiviral responses.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biophysica5040051/s1, Figure S1: Relative Rig-I and Ifn-β1 gene expression in response to varying doses of LMW-Poly(I:C); Table S1: Forward and reverse primers used in the qPCR study.
Author Contributions
F.C. and S.N. conceived the project. F.C. and A.R. designed experiments. A.R., S.S., S.N.R., J.B., S.B., S.M., and K.Y.H. performed experiments, analyzed data, and summarized data. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by National Institutes of Health grant R15GM140448 (F.C.) and the National Science Foundation (NSF) under grant number 2138459 (S.N.). Additionally, F.C., S.N., J.B., and S.B. acknowledge support from the NSF DMR REU program grant (Awards # 1757954, 2150489).
Data Availability Statement
The data are available from the corresponding author upon request.
Acknowledgments
The authors would like to thank M. Taher A. Saif for technical discussion.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Baum, A.; Garcia-Sastre, A. Induction of type I interferon by RNA viruses: Cellular receptors and their substrates. Amino Acids 2010, 38, 1283–1299. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Takeuchi, O.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Matsui, K.; Uematsu, S.; Jung, A.; Kawai, T.; Ishii, K.J.; et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 2006, 441, 101–105. [Google Scholar] [CrossRef]
- Kell, A.M.; Gale, M. RIG-I in RNA virus recognition. Virology 2015, 479–480, 110–121. [Google Scholar] [CrossRef]
- Pichlmair, A.; Schulz, O.; Tan, C.P.; Näslund, T.I.; Liljeström, P.; Weber, F.; Reis e Sousa, C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 2006, 314, 997–1001. [Google Scholar] [CrossRef]
- Ren, X.; Linehan, M.M.; Iwasaki, A.; Pyle, A.M. RIG-I Recognition of RNA Targets: The Influence of Terminal Base Pair Sequence and Overhangs on Affinity and Signaling. Cell Rep. 2019, 29, 3807–3815.e3803. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like Receptor and RIG-1-like Receptor Signaling. Ann. N. Y. Acad. Sci. 2008, 1143, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Biron, C.A. Chapter 4—Innate Immunity: Recognizing and Responding to Foreign Invaders—No Training Needed. In Viral Pathogenesis, 3rd ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 41–55. [Google Scholar] [CrossRef]
- Wang, N.; Tolic-Nørrelykke, I.M.; Chen, J.; Mijailovich, S.M.; Butler, J.P.; Fredberg, J.J.; Stamenovic, D. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am. J. Physiol. Cell Physiol. 2002, 282, C606–C616. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, F.; Li, Y.; Poh, Y.-C.; Yokohama-Tamaki, T.; Wang, N.; Tanaka, T.S. Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions. PLoS ONE 2010, 5, e15655. [Google Scholar] [CrossRef]
- Chowdhury, F.; Na, S.; Li, D.; Poh, Y.-C.; Tanaka, T.S.; Wang, F.; Wang, N. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat. Mater. 2009, 9, 82–88. [Google Scholar] [CrossRef]
- Pelham Jr, R.J.; Wang, Y.-l. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA 1997, 94, 13661–13665. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ren, J.; Han, K.Y. 2.5 D microscopy: Fast, high-throughput imaging via volumetric projection for quantitative subcellular analysis. ACS Photonics 2021, 8, 933–942. [Google Scholar] [CrossRef]
- Chowdhury, F.; Huang, B.; Wang, N. Cytoskeletal prestress: The cellular hallmark in mechanobiology and mechanomedicine. Cytoskeleton 2021, 78, 249–276. [Google Scholar] [CrossRef]
- Erlich, A.; Etienne, J.; Fouchard, J.; Wyatt, T. How dynamic prestress governs the shape of living systems, from the subcellular to tissue scale. Interface Focus 2022, 12, 20220038. [Google Scholar] [CrossRef]
- Ingber, D.E.; Wang, N.; Stamenović, D. Tensegrity, cellular biophysics, and the mechanics of living systems. Rep. Prog. Phys. 2014, 77, 046603. [Google Scholar] [CrossRef]
- Chowdhury, F.; Doğanay, S.; Leslie, B.J.; Singh, R.; Amar, K.; Talluri, B.; Park, S.; Wang, N.; Ha, T. Cdc42-dependent modulation of rigidity sensing and cell spreading in tumor repopulating cells. Biochem. Biophys. Res. Commun. 2018, 500, 557–563. [Google Scholar] [CrossRef]
- Yu, H.; Lim, K.P.; Xiong, S.; Tan, L.P.; Shim, W. Functional Morphometric Analysis in Cellular Behaviors: Shape and Size Matter. Adv. Healthc. Mater. 2013, 2, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Solon, J.; Levental, I.; Sengupta, K.; Georges, P.C.; Janmey, P.A. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys. J. 2007, 93, 4453–4461. [Google Scholar] [CrossRef] [PubMed]
- Linehan, M.M.; Dickey, T.H.; Molinari, E.S.; Fitzgerald, M.E.; Potapova, O.; Iwasaki, A.; Pyle, A.M. A minimal RNA ligand for potent RIG-I activation in living mice. Sci. Adv. 2018, 4, e1701854. [Google Scholar] [CrossRef] [PubMed]
- Mattijssen, S.; Pruijn, G.J. Viperin, a key player in the antiviral response. Microbes Infect. 2012, 14, 419–426. [Google Scholar] [CrossRef]
- Haller, O.; Staeheli, P.; Kochs, G. Interferon-induced Mx proteins in antiviral host defense. Biochimie 2007, 89, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Imaizumi, T.; Tsuruga, K.; Aizawa, T.; Ito, T.; Matsumiya, T.; Yoshida, H.; Joh, K.; Ito, E.; Tanaka, H. Glomerular expression of myxovirus resistance protein 1 in human mesangial cells: Possible activation of innate immunity in the pathogenesis of lupus nephritis. Nephrology 2013, 18, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Meng, Z.; Chen, R.; Guan, K.-L. The Hippo Pathway: Biology and Pathophysiology. Annu. Rev. Biochem. 2019, 88, 577–604. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Itoga, K.; Okano, T.; Yonemura, S.; Sasaki, H. Hippo pathway regulation by cell morphology and stress fibers. Development 2011, 138, 3907–3914. [Google Scholar] [CrossRef]
- Zhang, Q.; Meng, F.; Chen, S.; Plouffe, S.W.; Wu, S.; Liu, S.; Li, X.; Zhou, R.; Wang, J.; Zhao, B.; et al. Hippo signalling governs cytosolic nucleic acid sensing through YAP/TAZ-mediated TBK1 blockade. Nat. Cell Biol. 2017, 19, 362–374. [Google Scholar] [CrossRef]
- Elson, E.L. Cellular mechanics as an indicator of cytoskeletal structure and function. Annu. Rev. Biophys. Biophys. Chem. 1988, 17, 397–430. [Google Scholar] [CrossRef]
- Mammoto, A.; Ingber, D.E. Cytoskeletal control of growth and cell fate switching. Curr. Opin. Cell Biol. 2009, 21, 864–870. [Google Scholar] [CrossRef]
- Martino, F.; Perestrelo, A.R.; Vinarsky, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Cao, D.; Ding, J. Recent advances in regenerative biomaterials. Regen. Biomater. 2022, 9, rbac098. [Google Scholar] [CrossRef]
- Doganay, S.; Lee, M.Y.; Baum, A.; Peh, J.; Hwang, S.Y.; Yoo, J.Y.; Hergenrother, P.J.; Garcia-Sastre, A.; Myong, S.; Ha, T. Single-cell analysis of early antiviral gene expression reveals a determinant of stochastic IFNB1 expression. Integr. Biol. 2017, 9, 857–867. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).