The Contrasting Effect of Sodium Alginate on Lysozyme and Albumin Denaturation and Fibril Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
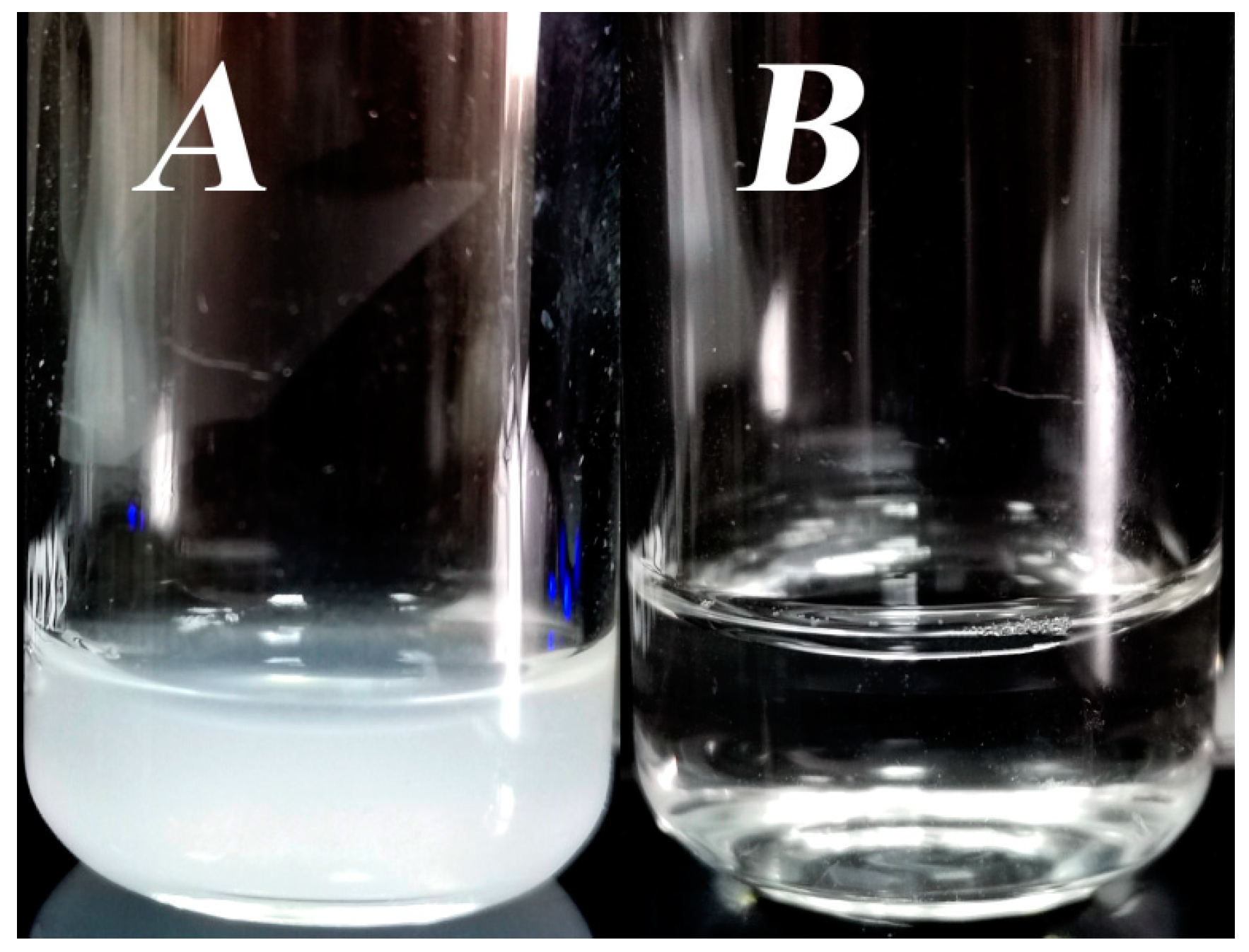
2.2. Fibril Preparation
2.3. ThT Assay
2.4. Differential Scanning Calorimetry
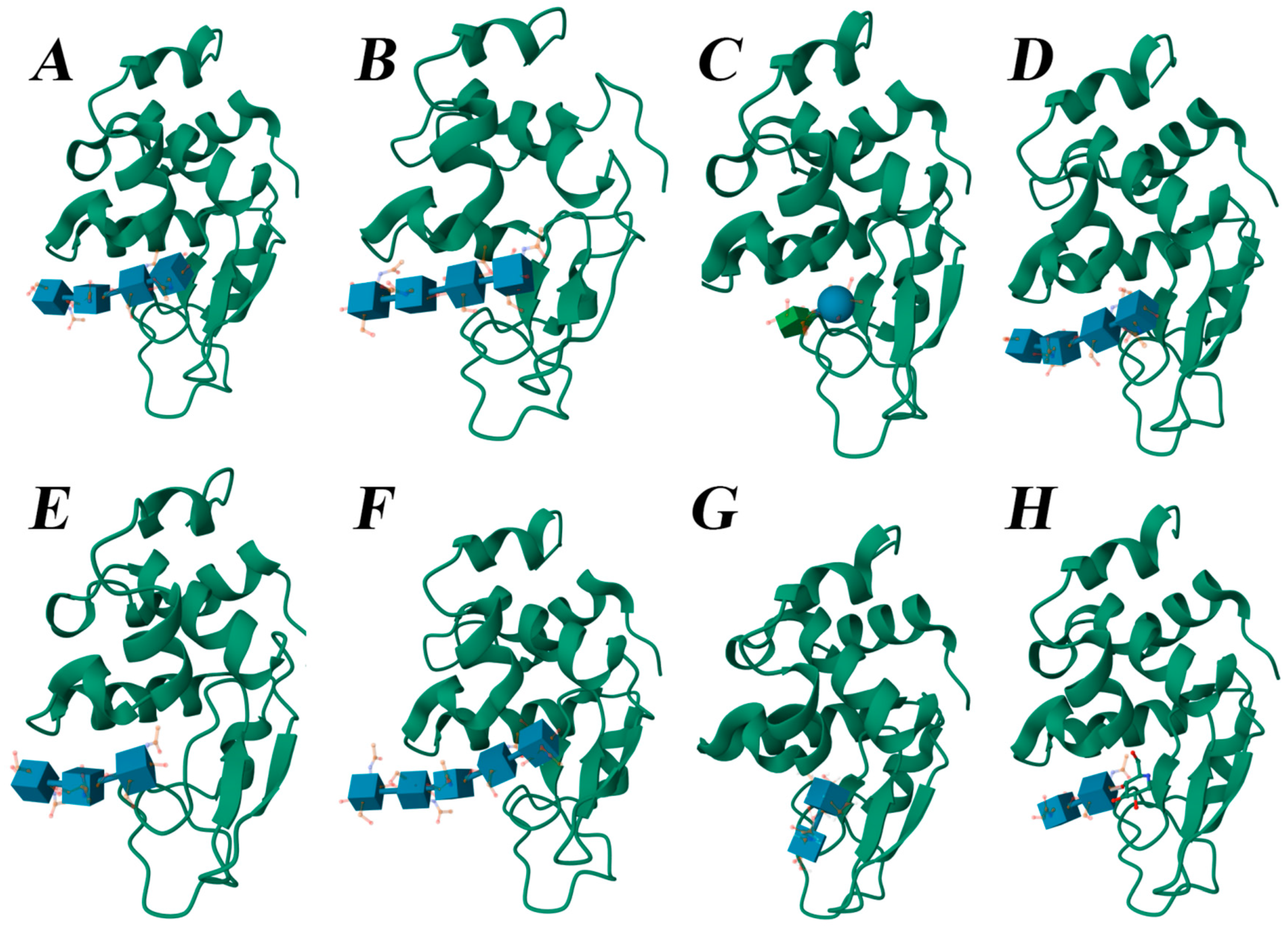
2.5. Molecular Docking
3. Results
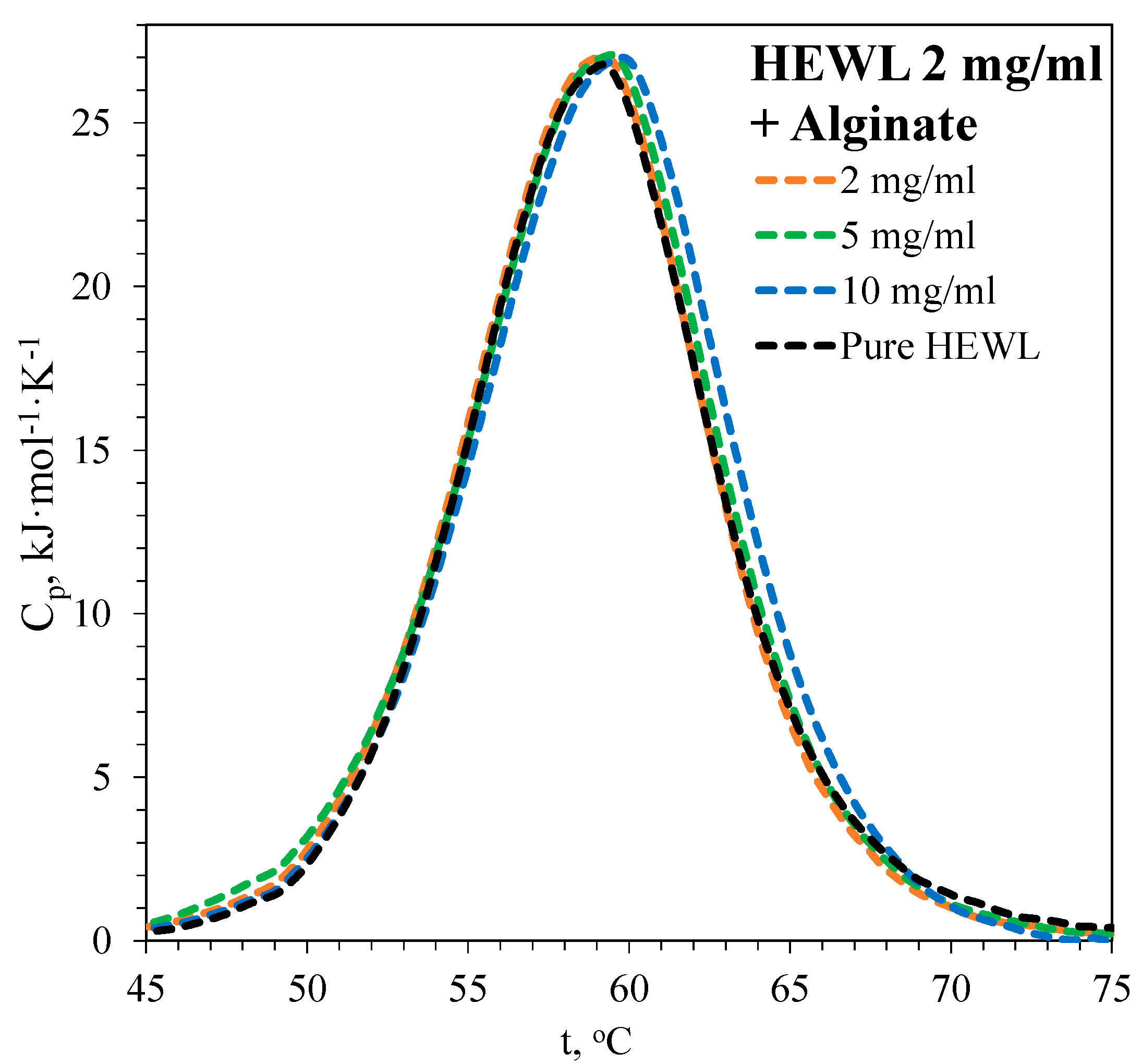
3.1. DSC Thermograms
3.1.1. Denaturation of BSA
3.1.2. Denaturation of HEWL
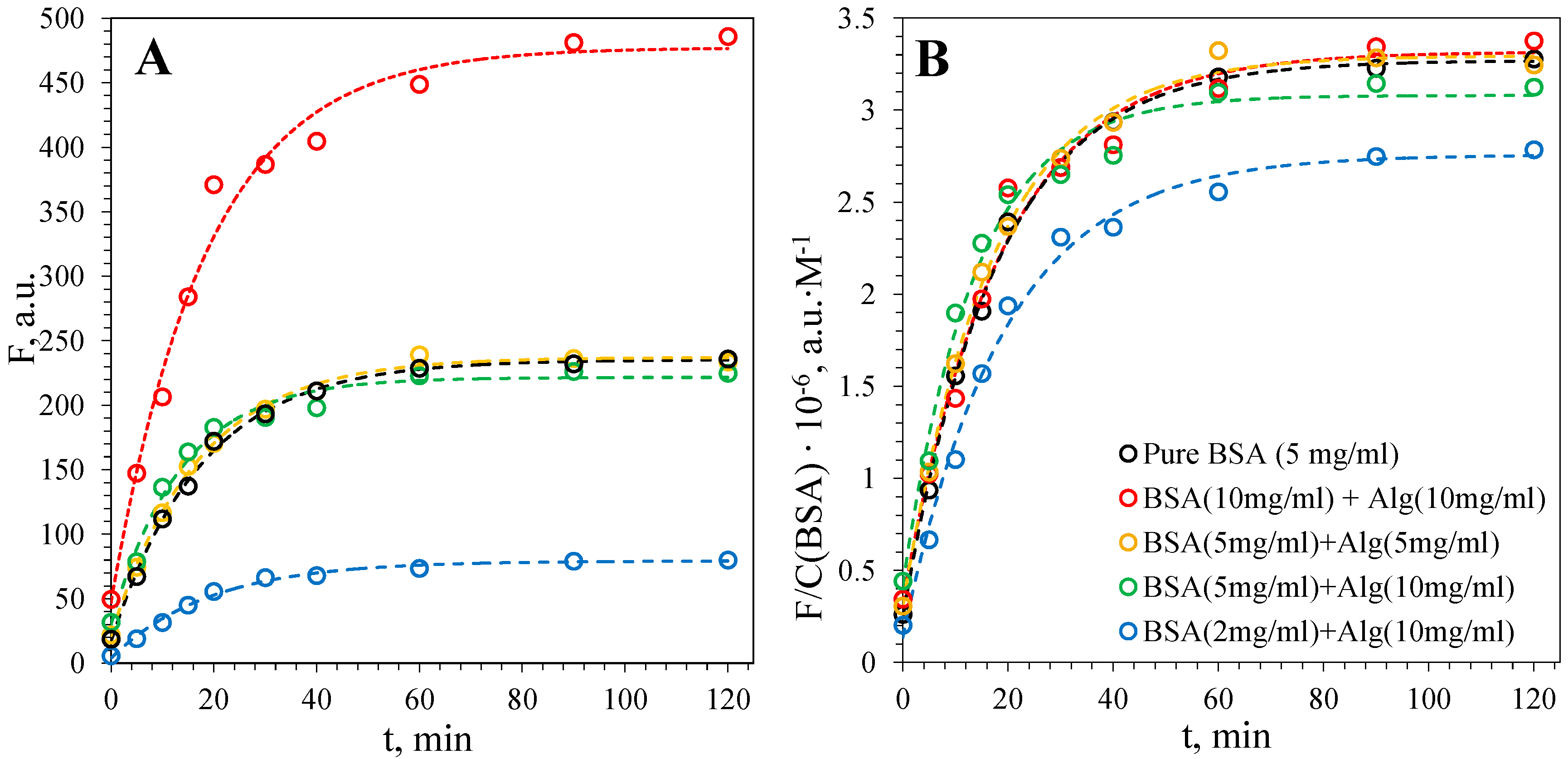
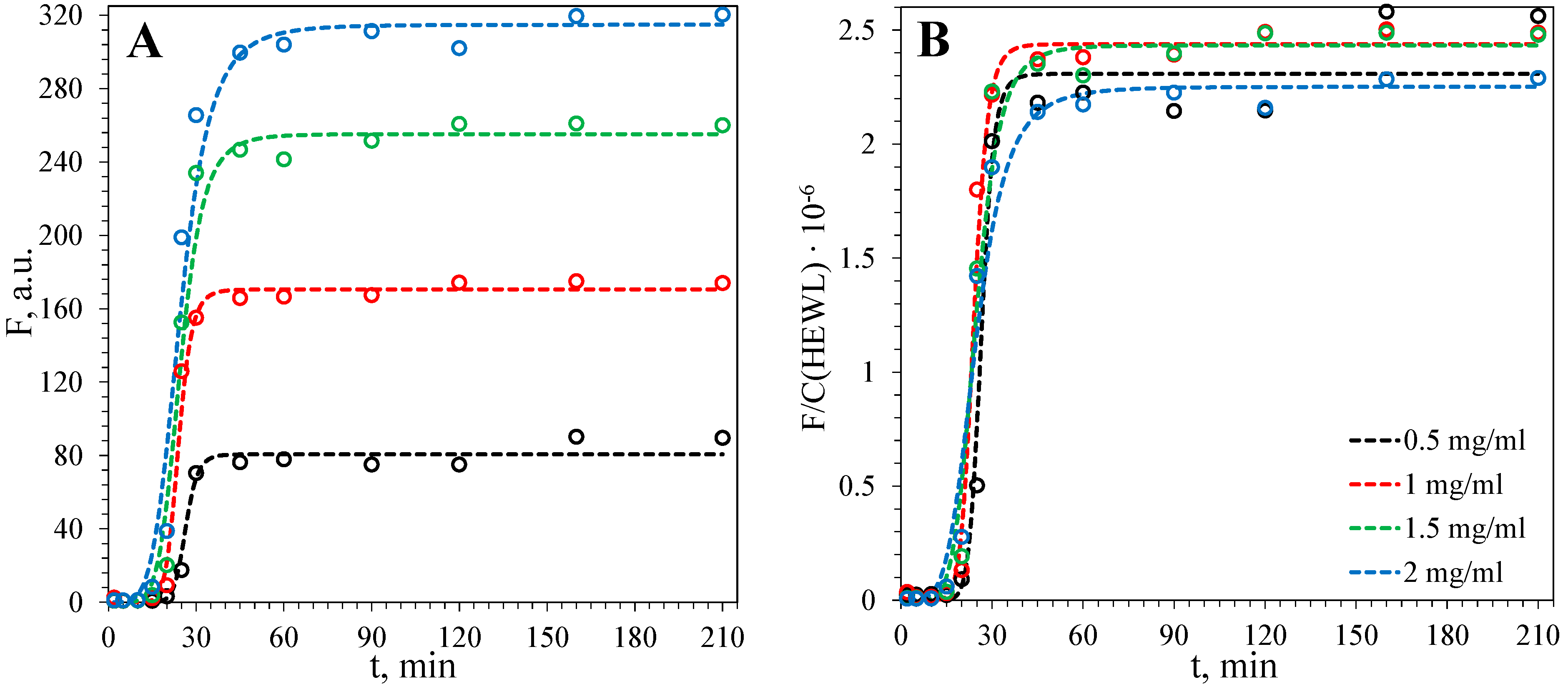
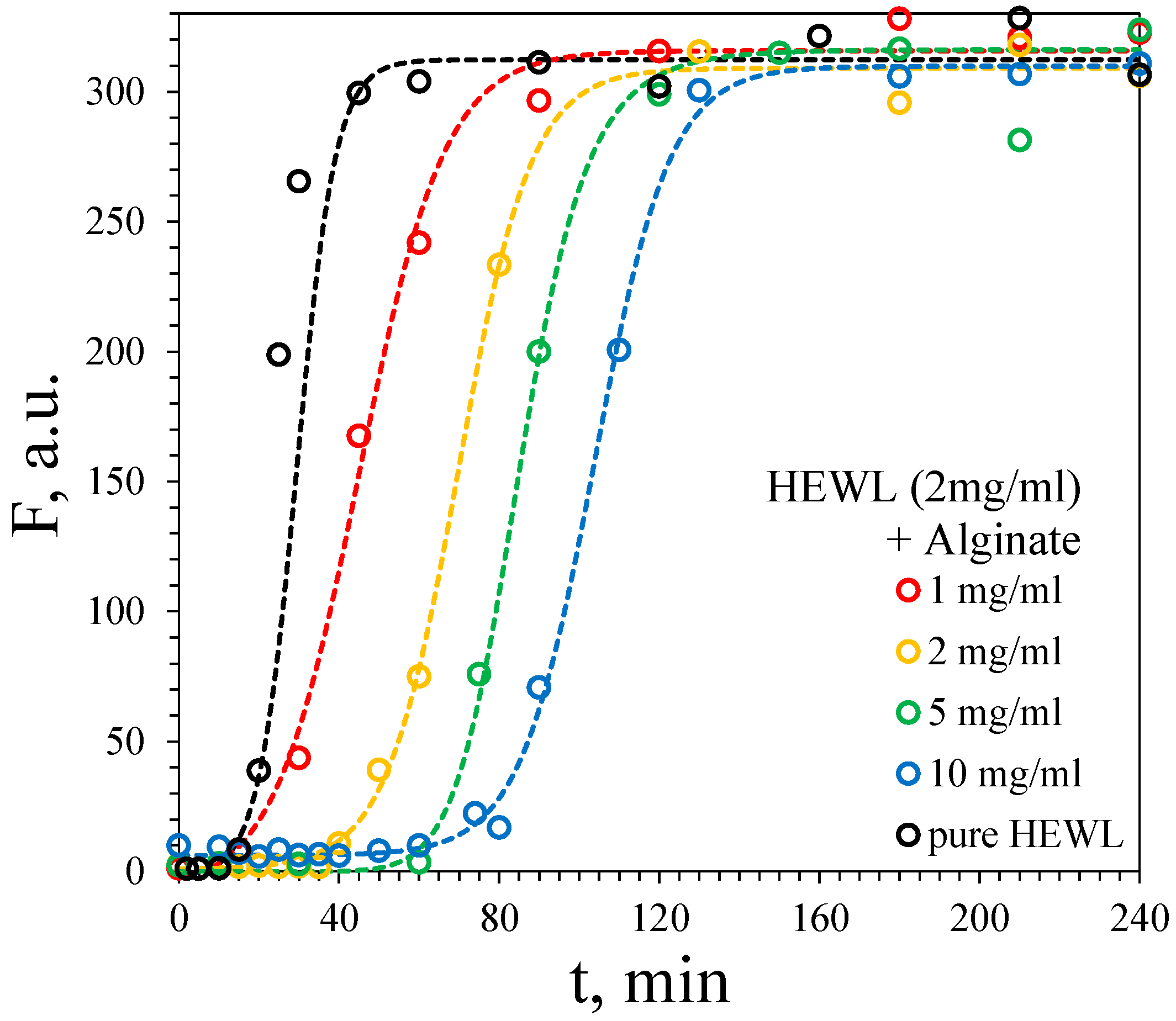
3.2. Kinetics of Fibril Formation
3.2.1. Fibril Formation of BSA
3.2.2. Fibril Formation of HEWL
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barclay, T.G.; Day, C.M.; Petrovsky, N.; Garg, S. Review of polysaccharide particle-based functional drug delivery. Carbohydr. Polym. 2019, 221, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Kirschning, A.; Dibbert, N.; Dräger, G. Chemical functionalization of polysaccharides—Towards biocompatible hydrogels for biomedical applications. Chem.–A Eur. J. 2018, 24, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-F.; Chen, X.; Tang, Y.; Wu, J.-M.; Qin, D.-L.; Yu, L.; Yu, C.-L.; Zhou, X.-G.; Wu, A.-G. The Therapeutic Potential of Plant Polysaccharides in Metabolic Diseases. Pharmaceuticals 2022, 15, 1329. [Google Scholar] [CrossRef]
- Elrosasy, A.; Zeid, M.A.; Negida, A. Sodium Oligomannate (GV-971) Safety and Efficacy in the Treatment of Alzheimer’s Disease: A Systematic Review and Meta-analysis of Randomized Controlled Trials (P11-9.005). Neurology 2024, 102, 5352. [Google Scholar] [CrossRef]
- Wozniak, M.; Bell, T.; Dénes, Á.; Falshaw, R.; Itzhaki, R. Anti-HSV1 activity of brown algal polysaccharides and possible relevance to the treatment of Alzheimer’s disease. Int. J. Biol. Macromol. 2015, 74, 530–540. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Gao, W.; Cui, Z.; Zhang, H.; Lu, F.; Liu, F. Ulvan inhibits α-synuclein fibrillation and disrupts the mature fibrils: In vitro and in vivo studies. Int. J. Biol. Macromol. 2022, 211, 580–591. [Google Scholar] [CrossRef]
- Montgomery, K.M.; Carroll, E.C.; Thwin, A.C.; Quddus, A.Y.; Hodges, P.; Southworth, D.R.; Gestwicki, J.E. Chemical Features of Polyanions Modulate Tau Aggregation and Conformational States. J. Am. Chem. Soc. 2023, 145, 3926–3936. [Google Scholar] [CrossRef]
- Braia, M.; Loureiro, D.; Tubio, G.; Lienqueo, M.E.; Romanini, D. Interaction between trypsin and alginate: An ITC and DLS approach to the formation of insoluble complexes. Colloids Surf. B Biointerfaces 2017, 155, 507–511. [Google Scholar] [CrossRef]
- Gorji, E.G.; Waheed, A.; Ludwig, R.; Toca-Herrera, J.L.; Schleining, G.; Gorji, S.G. Complex Coacervation of Milk Proteins with Sodium Alginate. J. Agric. Food Chem. 2018, 66, 3210–3220. [Google Scholar] [CrossRef] [PubMed]
- Motomura, H.; Somjit, K.; Nozaki, Y. Effect of sodium alginate on changes in the denaturation of fish myofibrillar protein and the state of water during frozen storage. Fish. Sci. 2002, 68, 1633–1634. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, Y.; Li, F.; Carvajal, M.T.; Harris, M.T. Interactions between bovine serum albumin and alginate: An evaluation of alginate as protein carrier. J. Colloid Interface Sci. 2009, 332, 345–353. [Google Scholar] [CrossRef]
- Chang, R.; Gruebele, M.; Leckband, D.E. Protein Stabilization by Alginate Binding and Suppression of Thermal Aggregation. Biomacromolecules 2022, 23, 4063–4073. [Google Scholar] [CrossRef]
- Juárez, J.; Taboada, P.; Mosquera, V. Existence of Different Structural Intermediates on the Fibrillation Pathway of Human Serum Albumin. Biophys. J. 2009, 96, 2353–2370. [Google Scholar] [CrossRef]
- Maciążek-Jurczyk, M.; Janas, K.; Pożycka, J.; Szkudlarek, A.; Rogóż, W.; Owczarzy, A.; Kulig, K. Human Serum Albumin Aggregation/Fibrillation and its Abilities to Drugs Binding. Molecules 2020, 25, 618. [Google Scholar] [CrossRef]
- Swaminathan, R.; Ravi, V.K.; Kumar, S.; Kumar, M.V.S.; Chandra, N. Lysozyme, In Advances in Protein Chemistry and Structural Biology; Elsevier: Amstardam, The Netherlands, 2011; pp. 63–111. [Google Scholar] [CrossRef]
- Khaibrakhmanova, D.R.; Nikifirova, A.A.; Li, Z.; Sedov, I.A. Human Serum Albumin Fibril Formation in the Presence of Ligands with Different Affinity. Russ. J. Gen. Chem. 2024, 94, 395–405. [Google Scholar] [CrossRef]
- Khaibrakhmanova, D.; Nikiforova, A.; Li, Z.; Sedov, I. Effect of ligands with different affinity on albumin fibril formation. Int. J. Biol. Macromol. 2022, 204, 709–717. [Google Scholar] [CrossRef]
- Mondal, U.S.; Paul, S. Inhibition of hen egg white lysozyme fibrillation by a self-assembled nanostructured lysozyme and graphene oxide conjugate. New J. Chem. 2023, 47, 17666–17678. [Google Scholar] [CrossRef]
- Basu, A.; Mahammad, A.; Das, A. Inhibition of the formation of lysozyme fibrillar assemblies by the isoquinoline alkaloid coralyne. New J. Chem. 2022, 46, 3258–3269. [Google Scholar] [CrossRef]
- Morshedi, D.; Rezaei-Ghaleh, N.; Ebrahim-Habibi, A.; Ahmadian, S.; Nemat-Gorgani, M. Inhibition of amyloid fibrillation of lysozyme by indole derivatives − possible mechanism of action. FEBS J. 2007, 274, 6415–6425. [Google Scholar] [CrossRef] [PubMed]
- Kolotova, D.S.; Borovinskaya, E.V.; Bordiyan, V.V.; Zuev, Y.F.; Salnikov, V.V.; Zueva, O.S.; Derkach, S.R. Phase Behavior of Aqueous Mixtures of Sodium Alginate with Fish Gelatin: Effects of pH and Ionic Strength. Polymers 2023, 15, 2253. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Lin, T.Y.; Chang, D.; Guo, Z. Thioflavin T as an amyloid dye: Fibril quantification, optimal concentration and effect on aggregation. R. Soc. Open Sci. 2017, 4, 160696. [Google Scholar] [CrossRef] [PubMed]
- Crespo, R.; Rocha, F.A.; Damas, A.M.; Martins, P.M. A Generic Crystallization-like Model That Describes the Kinetics of Amyloid Fibril Formation. J. Biol. Chem. 2012, 287, 30585–30594. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Corso, G.; Stärk, H.; Jing, B.; Barzilay, R.; Jaakkola, T. DiffDock: Diffusion Steps, Twists, and Turns for Molecular Docking. arXiv 2022. [Google Scholar] [CrossRef]
- Sedov, I.; Nikiforova, A.; Khaibrakhmanova, D. Evaluation of the binding properties of drugs to albumin from DSC thermograms. Int. J. Pharm. 2020, 583, 119362. [Google Scholar] [CrossRef]
- Giancola, C.; De Sena, C.; Fessas, D.; Graziano, G.; Barone, G. DSC studies on bovine serum albumin denaturation Effects of ionic strength and SDS concentration. Int. J. Biol. Macromol. 1997, 20, 193–204. [Google Scholar] [CrossRef]
- Burgos, M.I.; Fernández, R.A.; Celej, M.S.; Rossi, L.I.; Fidelio, G.D.; Dassie, S.A. Binding of the Highly Toxic Tetracycline Derivative, Anhydrotetracycline, to Bovine Serum Albumin. Biol. Pharm. Bull. 2011, 34, 1301–1306. [Google Scholar] [CrossRef]
- Celej, M.S.; Dassie, S.A.; Freire, E.; Bianconi, M.L.; Fidelio, G.D. Ligand-induced thermostability in proteins: Thermodynamic analysis of ANS–albumin interaction. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2005, 1750, 122–133. [Google Scholar] [CrossRef]
- Brandts, J.F.; Lin, L.N. Study of strong to ultratight protein interactions using differential scanning calorimetry. Biochemistry 1990, 29, 6927–6940. [Google Scholar] [CrossRef]
- Vernaglia, B.A.; Huang, J.; Clark, E.D. Guanidine Hydrochloride Can Induce Amyloid Fibril Formation from Hen Egg-White Lysozyme. Biomacromolecules 2004, 5, 1362–1370. [Google Scholar] [CrossRef]
- Sulatskaya, A.I.; Sulatsky, M.I.; Stepanenko, O.V.; Povarova, O.I.; Kuznetsova, I.M.; Turoverov, K.K. Denaturing Effect of Guanidine Hydrohloride on Amyloid Fibrils. Biophys. J. 2020, 118, 509a. [Google Scholar] [CrossRef]
- Hédoux, A.; Krenzlin, S.; Paccou, L.; Guinet, Y.; Flament, M.-P.; Siepmann, J. Influence of urea and guanidine hydrochloride on lysozyme stability and thermal denaturation; a correlation between activity, protein dynamics and conformational changes. Phys. Chem. Chem. Phys. 2010, 12, 13189–13196. [Google Scholar] [CrossRef]
- Emadi, S.; Behzadi, M. A comparative study on the aggregating effects of guanidine thiocyanate, guanidine hydrochloride and urea on lysozyme aggregation. Biochem. Biophys. Res. Commun. 2014, 450, 1339–1344. [Google Scholar] [CrossRef]
- Sedov, I.; Khaibrakhmanova, D. Molecular Mechanisms of Inhibition of Protein Amyloid Fibril Formation: Evidence and Perspectives Based on Kinetic Models. Int. J. Mol. Sci. 2022, 23, 13428. [Google Scholar] [CrossRef]
- Makshakova, O.; Bogdanova, L.; Faizullin, D.; Khaibrakhmanova, D.; Ziganshina, S.; Ermakova, E.; Zuev, Y.; Sedov, I. The Ability of Some Polysaccharides to Disaggregate Lysozyme Amyloid Fibrils and Renature the Protein. Pharmaceutics 2023, 15, 624. [Google Scholar] [CrossRef]
- Wang, X.; Nian, Y.; Zhang, Z.; Chen, Q.; Zeng, X.; Hu, B. High internal phase emulsions stabilized with amyloid fibrils and their polysaccharide complexes for encapsulation and protection of β-carotene. Colloids Surf. B Biointerfaces 2019, 183, 110459. [Google Scholar] [CrossRef]
- Peydayesh, M.; Kistler, S.; Zhou, J.; Lutz-Bueno, V.; Victorelli, F.D.; Meneguin, A.B.; Spósito, L.; Bauab, T.M.; Chorilli, M.; Mezzenga, R. Amyloid-polysaccharide interfacial coacervates as therapeutic materials. Nat. Commun. 2023, 14, 1848. [Google Scholar] [CrossRef]
- Wei, S.; Li, Y.; Li, K.; Zhong, C. Biofilm-inspired Amyloid-Polysaccharide Composite Materials. Appl. Mater. Today 2022, 27, 101497. [Google Scholar] [CrossRef]
- Gorensek-Benitez, A.H.; Kirk, B.; Myers, J.K. Protein Fibrillation under Crowded Conditions. Biomolecules 2022, 12, 950. [Google Scholar] [CrossRef] [PubMed]
- Amara, C.B.; Eghbal, N.; Oulahal, N.; Degraeve, P.; Gharsallaoui, A. Properties of lysozyme/sodium alginate complexes for the development of antimicrobial films. Food Res. Int. 2016, 89, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Raskar, T.; Koh, C.Y.; Niebling, S.; Kini, R.M.; Hosur, M.V. X-ray crystallographic analysis of time-dependent binding of guanidine hydrochloride to HEWL: First steps during protein unfolding. Int. J. Biol. Macromol. 2019, 122, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jimenez, R.; Godoy-Ruiz, R.; Ibarra-Molero, B.; Sanchez-Ruiz, J.M. The Efficiency of Different Salts to Screen Charge Interactions in Proteins: A Hofmeister Effect? Biophys. J. 2004, 86, 2414–2429. [Google Scholar] [CrossRef] [PubMed]
- Monera, O.D.; Kay, C.M.; Hodges, R.S. Protein denaturation with guanidine hydrochloride or urea provides a different estimate of stability depending on the contributions of electrostatic interactions. Protein Sci. 1994, 3, 1984–1991. [Google Scholar] [CrossRef]
- Ban, X.; Lahiri, P.; Dhoble, A.S.; Li, D.; Gu, Z.; Li, C.; Cheng, L.; Hong, Y.; Li, Z.; Kaustubh, B. Evolutionary Stability of Salt Bridges Hints Its Contribution to Stability of Proteins. Comput. Struct. Biotechnol. J. 2019, 17, 895–903. [Google Scholar] [CrossRef]
- Tokunaga, Y.; Sakakibara, Y.; Kamada, Y.; Watanabe, K.; Sugimoto, Y. Analysis of Core Region from Egg White Lysozyme Forming Amyloid Fibrils. Int. J. Biol. Sci. 2013, 9, 219–227. [Google Scholar] [CrossRef]
- Frare, E.; De Laureto, P.P.; Zurdo, J.; Dobson, C.M.A. Fontana, A Highly Amyloidogenic Region of Hen Lysozyme. J. Mol. Biol. 2004, 340, 1153–1165. [Google Scholar] [CrossRef]
- Fändrich, M.; Nyström, S.; Nilsson, K.P.R.; Böckmann, A.; LeVine, H.; Hammarström, P. Amyloid fibril polymorphism: A challenge for molecular imaging and therapy. J. Intern. Med. 2018, 283, 218–237. [Google Scholar] [CrossRef]
- Frey, L.; Zhou, J.; Cereghetti, G.; Weber, M.E.; Rhyner, D.; Pokharna, A.; Wenchel, L.; Kadavath, H.; Cao, Y.; Meier, B.H.; et al. A structural rationale for reversible vs irreversible amyloid fibril formation from a single protein. Nat. Commun. 2024, 15, 8448. [Google Scholar] [CrossRef]
- Ward, E.M.; Zamora, C.Y.; Schocker, N.S.; Ghosh, S.; Kizer, M.E.; Imperiali, B. Engineered Glycan-Binding Proteins for Recognition of the Thomsen–Friedenreich Antigen and Structurally Related Disaccharides. ACS Chem. Biol. 2023, 18, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Boraston, A.B.; Bolam, D.N.; Gilbert, H.J.; Davies, G.J. Carbohydrate-binding modules: Fine-tuning polysaccharide recognition. Biochem. J. 2004, 382, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liu, T.; Liu, W.; Lyu, Q. Structural insights into the substrate-binding cleft of AlyF reveal the first long-chain alginate-binding mode. Acta Crystallogr. Sect. D Struct. Biol. 2021, 77, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, O.; Szota, M.; Rakowski, K.; Prochownik, M.; Doveiko, D.; Chen, Y.; Jachimska, B. Bovine Serum Albumin as a Platform for Designing Biologically Active Nanocarriers—Experimental and Computational Studies. Int. J. Mol. Sci. 2023, 25, 37. [Google Scholar] [CrossRef]
- Bujacz, A.; Talaj, J.A.; Zielinski, K.; Pietrzyk-Brzezinska, A.J.; Neumann, P. Crystal structures of serum albumins from domesticated ruminants and their complexes with 3,5-diiodosalicylic acid. Acta Crystallogr. Sect. D Struct. Biol. 2017, 73, 896–909. [Google Scholar] [CrossRef]
- Bi, D.; Xiao, S.; Lin, Z.; Yao, L.; Fang, W.; Wu, Y.; Xu, H.; Lu, J.; Xu, X. Alginate-Derived Mannuronate Oligosaccharide Attenuates Tauopathy through Enhancing Autophagy. J. Agric. Food Chem. 2021, 69, 4438–4445. [Google Scholar] [CrossRef]
- Pleyer, C.; Flesche, J.; Saeed, F. Lysozyme amyloidosis—A case report and review of the literature. Clin. Nephrol. Case Stud. 2015, 3, 42–45. [Google Scholar] [CrossRef]
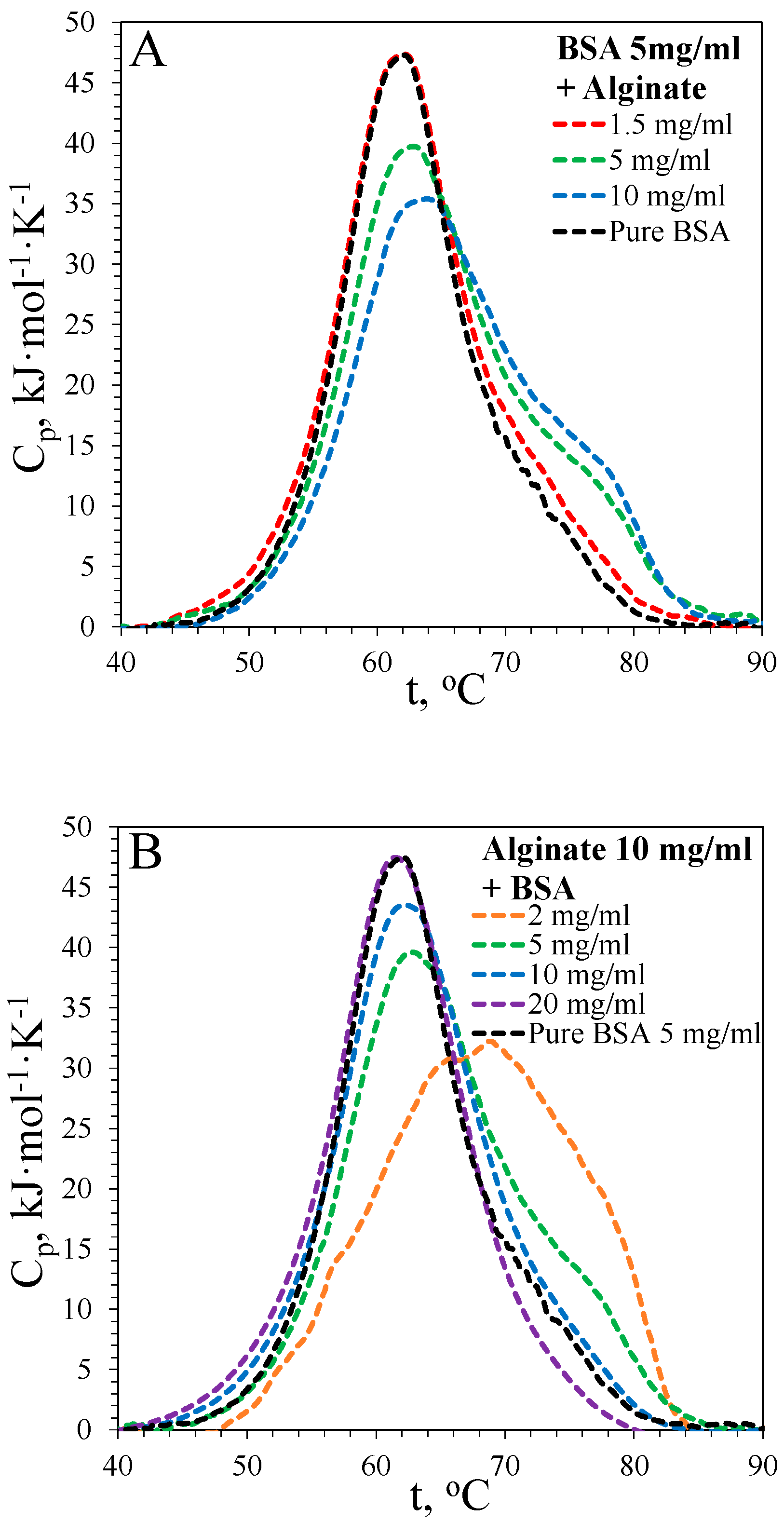

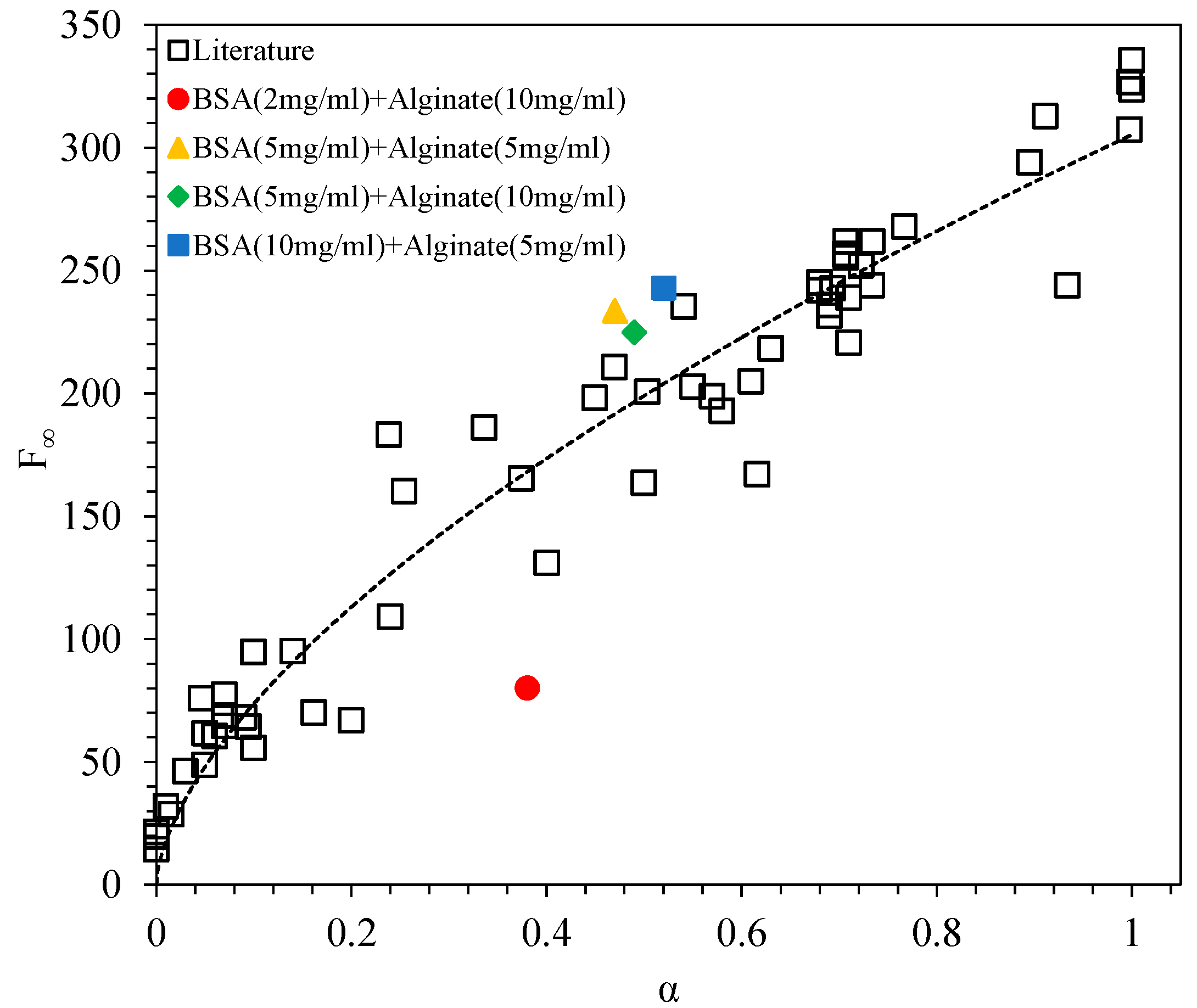


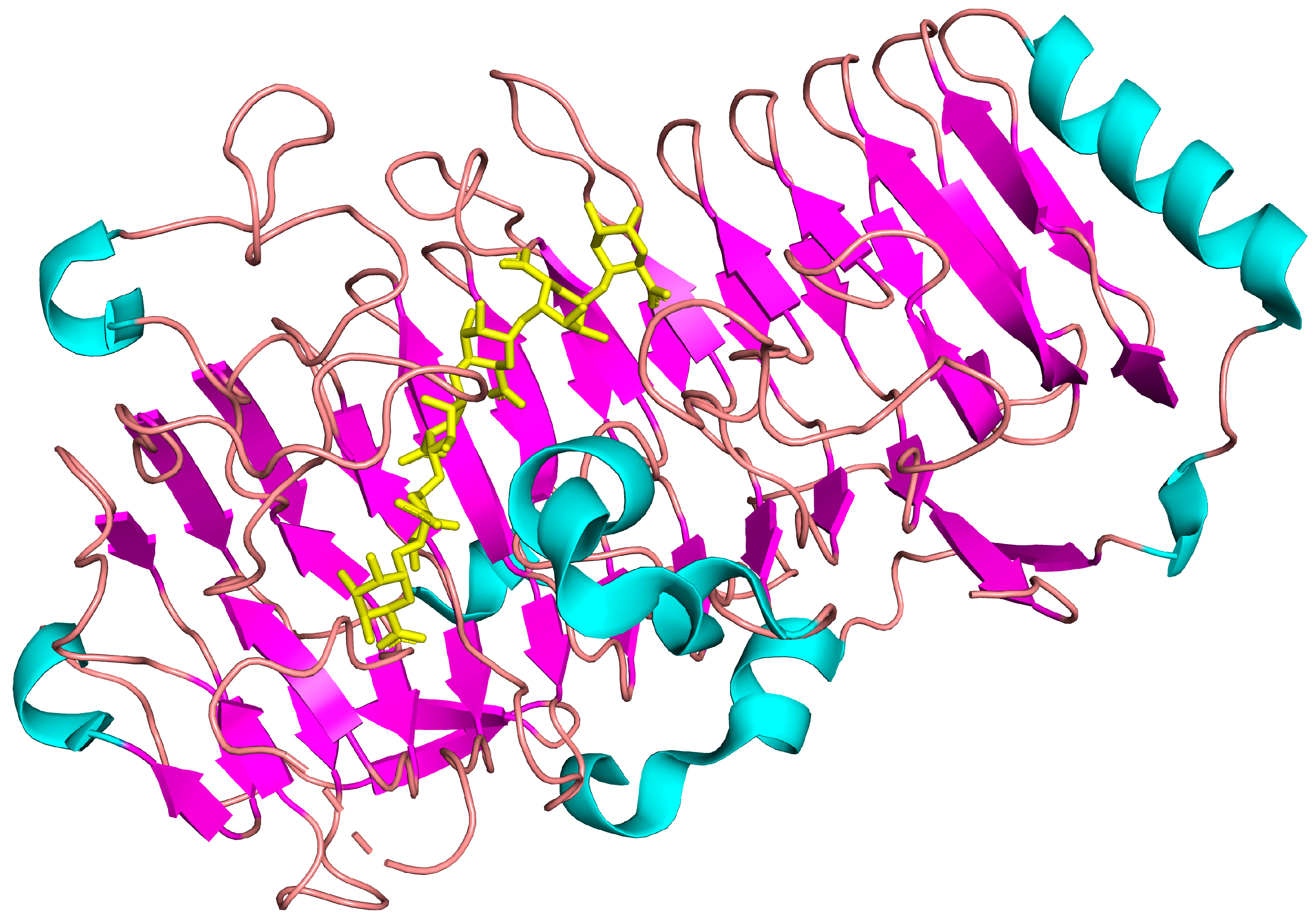
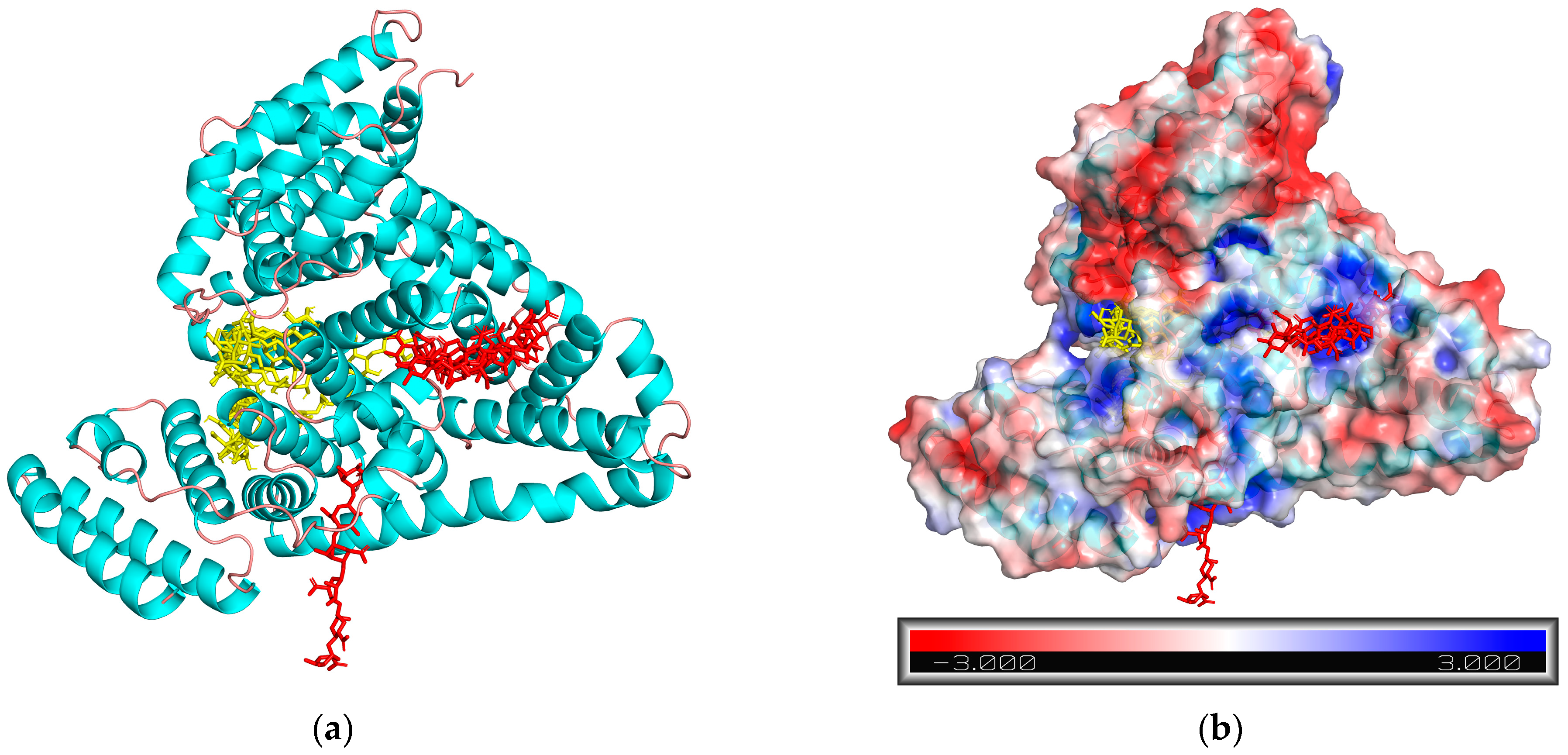
| [BSA], mg·mL−1 | [Alginate], mg·mL−1 | Td, °C | ΔHd, kJ·mol−1 | ΔH65, kJ·mol−1 | α65 |
|---|---|---|---|---|---|
| 5 | 0 | 62.1 | 611 ± 22 | 300 ± 12 | 0.49 |
| 5 | 1.5 | 62.2 | 620 ± 16 | 320 ± 10 | 0.52 |
| 5 | 5 | 62.9 | 616 ± 25 | 290 ± 8 | 0.47 |
| 5 | 10 | 63.9 | 613 ± 22 | 303 ± 13 | 0.49 |
| 2 | 10 | 68.8 | 659 ± 18 | 254 ± 23 | 0.38 |
| 10 | 10 | 62.2 | 635 ± 12 | 336 ± 7 | 0.52 |
| 20 | 10 | 62.1 | 639 ± 11 | 344 ± 10 | 0.53 |
| [HEWL], mg·mL−1 | [Alginate], mg·mL−1 | Td, °C | ΔHd, kJ·mol−1 |
|---|---|---|---|
| 2 | 0 | 59.1 | 260 ± 12 |
| 2 | 2 | 59.2 | 262 ± 9 |
| 2 | 5 | 59.4 | 266 ± 8 |
| 2 | 10 | 59.8 | 268 ± 8 |
| [Alginate], mg·mL−1 | τlag, min | τ50, min |
|---|---|---|
| – | 18.6 | 24.1 |
| 1 | 22.7 | 45.7 |
| 2 | 48.3 | 69.8 |
| 5 | 67.1 | 85.5 |
| 10 | 81.9 | 103.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaibrakhmanova, D.R.; Kuzivanova, P.R.; Gainutdinov, B.R.; Magsumov, T.I.; Nikiforova, A.A.; Sedov, I.A. The Contrasting Effect of Sodium Alginate on Lysozyme and Albumin Denaturation and Fibril Formation. Biophysica 2024, 4, 651-666. https://doi.org/10.3390/biophysica4040043
Khaibrakhmanova DR, Kuzivanova PR, Gainutdinov BR, Magsumov TI, Nikiforova AA, Sedov IA. The Contrasting Effect of Sodium Alginate on Lysozyme and Albumin Denaturation and Fibril Formation. Biophysica. 2024; 4(4):651-666. https://doi.org/10.3390/biophysica4040043
Chicago/Turabian StyleKhaibrakhmanova, Diliara R., Polina R. Kuzivanova, Bulat R. Gainutdinov, Timur I. Magsumov, Alena A. Nikiforova, and Igor A. Sedov. 2024. "The Contrasting Effect of Sodium Alginate on Lysozyme and Albumin Denaturation and Fibril Formation" Biophysica 4, no. 4: 651-666. https://doi.org/10.3390/biophysica4040043
APA StyleKhaibrakhmanova, D. R., Kuzivanova, P. R., Gainutdinov, B. R., Magsumov, T. I., Nikiforova, A. A., & Sedov, I. A. (2024). The Contrasting Effect of Sodium Alginate on Lysozyme and Albumin Denaturation and Fibril Formation. Biophysica, 4(4), 651-666. https://doi.org/10.3390/biophysica4040043







