Abstract
As the need for effective antiviral treatment intensifies, such as with the coronavirus disease 19 (COVID-19) infection, it is crucial to understand that while the mechanisms of action of these drugs or compounds seem apparent, they might also interact with unexplored targets, such as cell membrane ion channels in diverse cell types. In this review paper, we demonstrate that many different drugs or compounds, in addition to their known interference with viral infections, may also directly influence various types of ionic currents on the surface membrane of the host cell. These agents include artemisinin, cannabidiol, memantine, mitoxantrone, molnupiravir, remdesivir, SM-102, and sorafenib. If achievable at low concentrations, these regulatory effects on ion channels are highly likely to synergize with the identified initial mechanisms of viral replication interference. Additionally, the immediate regulatory impact of these agents on the ion-channel function may potentially result in unintended adverse effects, including changes in cardiac electrical activity and the prolongation of the QTc interval. Therefore, it is essential for patients receiving these related agents to exercise additional caution to prevent unnecessary complications.
1. Introduction
An antiviral agent refers to a substance or medication used to treat viral infections or inhibit viral growth, replication, and dissemination within the body. These drugs or compounds are designed to target and disrupt the life cycle of viruses, thereby preventing them from infecting host cells or replicating. The agents with antiviral activities can be used to manage and treat a wide range of viral infections, including those caused by viruses such as hepatitis B and C, influenza, coronavirus disease 19 (COVID-19), and more [1,2]. However, in the context of various drugs or compounds employed to interfere in, prevent, or treat infections caused by different viruses, it has become evident that they possess significant regulatory effects on transmembrane ion channels located on the cell membrane. Using advanced patch-clamp technology in conjunction with precise voltage-clamping profiles achieved through digital-to-analog conversions facilitates the accurate detection of distinctive ionic currents at the surface of various small cells [3,4]. Therefore, these alterations in ionic currents not only influence the normal functions of host cells but also assume a role in either exacerbating the progression of viral infections [5,6] or causing other adverse effects [7,8,9,10].
In this review paper, we provide descriptions of specific drugs or compounds that disturb viral replication while also exerting multiple regulatory effects on ion channels in cell membranes in various cell types. These agents are presented in alphabetical order. Table 1 showcases the two-dimensional chemical structure of each agent, while Table 2 outlines their abbreviations and documented effects on transmembrane ionic currents.

Table 1.
Two-dimensional chemical structures of the drugs or compounds mentioned in this paper. The cell type studied and the concentration range used are illustrated. These data were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/) (accessed on 4 October 2023).

Table 2.
Drugs or compounds presented in this paper. These agents can potentially disrupt viral infections but may also impact various ionic currents.
2. Summary
The agents with antiviral activities known to exert regulatory effects on transmembrane ionic currents are illustrated in Figure 1.
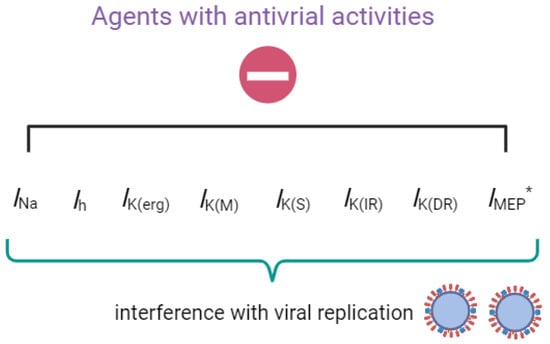
Figure 1.
Graph demonstrating that upon exposure to the compounds or drugs described in this paper, their interactions with these ionic currents (e.g., INa, Ih, IK(erg), IK(M), IK(S), IK(IR), IK(DR), and IMEP) will, in turn, interfere with the growth and replication of the virus. * indicates that IMEP is stimulated by the presence of remdesivir (RDV).
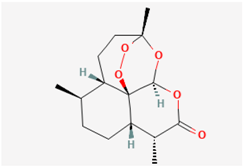
2.1. Artemisinin (ART)
For centuries, ART (qinghaosu) has been widely acknowledged as a potent antimalarial agent. This remarkable compound is derived from the sweet wormwood plant, which is scientifically known as Artemisia annua L. It features a unique chemical structure characterized by a sesquiterpene trioxane lactone containing an unusual peroxide bridge [24]. Beyond its well-established antimalarial properties, ART exhibits a diverse range of pharmacological effects. These effects encompass cytotoxicity against tumor cells, along with antiviral and antiparasitic actions [24,25]. Moreover, recent research has unveiled its promising potential in combating the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [26,27].
Notably, previous reports have shown that artemisinin can synergistically act to suppress the delayed-rectifier K+ current (IK(DR)) and voltage-gated Na+ current (INa) identified in pituitary tumor (GH3) cells [12]. The inhibitory effect of ART on INa was also observed in nodose ganglion neurons [11]. Cell exposure to ART did not simply reduce the amplitude of IK(DR). It caused a significant increase in the IK(DR) inactivation rate elicited in response to a 10 s maintained depolarizing pulse. The IC50 is the concentration of the compound at which the biological response is reduced by 50%. The IC50 value, signifying the concentration of ART required to inhibit IK(DR), was 11.2 µM, closely resembling the KD value of 14.7 µM obtained from the first-order binding scheme [12]. This value is typically interpolated from the concentration–response curve. It is commonly calculated using regression analysis. Exposure to ART caused a leftward shift in the midpoint of the steady-state inactivation curve of IK(DR), with no change in the curve’s steepness. The presence of ART also enhanced the rate of excessive accumulative inactivation of IK(DR) evoked in response to repetitive stimuli. These results suggest that the ART-induced block of IK(DR) mainly occurred after channel opening. Prior to channel activation, the ART-binding site is likely to be either in a low-affinity state or inaccessible to the compound [12].
On another note, the presence of ART has the potential to engage in interactions with voltage-gated Na+ (NaV) channels. These interactions, in turn, lead to a decrease in both the peak amplitude of voltage-gated Na+ current (INa) and the rate of inactivation of this current [12]. Consequently, the observed inhibition of INa and IK(DR) when ART is present could synergistically influence the functional activities of pituitary cells, provided that similar results are replicated in an in vivo context. The ability of ART to activate canonical transient receptor potential (TRP) channels, such as TRPC3 channels, has also been recently demonstrated [28].
Previous studies have demonstrated that ART effectively suppresses cell proliferation and hormonal secretion in various cell types, including GH3 cells [29,30]. It is pertinent to investigate the extent to which the antiviral, neurological, or ototoxic effects induced by ART [24,25,29,30] are closely linked to its influence on ionic currents.
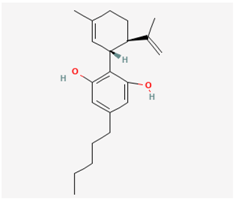
2.2. Cannabidiol (CBD)
CBD, scientifically referred to as 2-[(1R,6R)-3-methyl-6-prop-1-en-2-ylcyclohex-2-en-1-yl]-5-pentylbenzene-1,3-diol, is a non-psychoactive cannabinoid derived from the Cannabis plant renowned for its potential therapeutic applications [31]. In addition to its known attributes, recent research has highlighted CBD’s role in hindering the entry of SARS-CoV-2, including its emerging variants, and its ability to exhibit antiviral effects against a wide range of viruses, both enveloped and non-enveloped [32,33]. Moreover, recent studies have shed light on CBD’s capacity to modulate activity within the hypothalamic–pituitary–adrenal axis [34]. It has also been shown to influence various types of transmembrane ionic currents in electrically excitable cells, including INa and M-type K+ current (IK(M)) [35]. The biophysical and pharmacological characteristics of IK(M), a current encoded by KCNQ genes, have been established in previous studies [21,36,37]. Among KCNQ genes, KCNQ2 and KCNQ3 subunits heteromultimerize to form the channels responsible for IK(M) in neurons [37]. In recent research conducted on pituitary GH3 cells, it was demonstrated that exposure to CBD leads to the suppression in the amplitudes of IK(M) and the hyperpolarization-activated cation current (Ih), with the corresponding IC50 values of 3.6 and 3.3 µM, respectively [14]. The biophysical property of Ih is distinctive, marked by its slow activation kinetics during sustained hyperpolarization [38,39]. Furthermore, given CBD’s impact on ion channels situated on the cell membrane, it appears unlikely that these effects can be solely ascribed to CBD’s interaction with cannabinoid receptors. Therefore, there is a significant demand for more comprehensive research to investigate the mechanisms through which CBD affects these ion channels. This regulation, whether it results in direct or indirect interference with virus attachment or cell entry into cells, demands further investigation. Similarly, to gain a clearer understanding of how CBD modulates ionic currents and its potential impact on specific downstream signaling pathways, further in-depth studies are warranted.
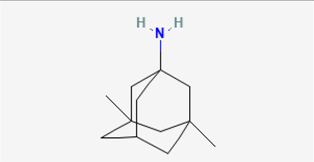
2.3. Memantine (MEM, Namenda®)
MEM (1-amino-3,5-dimethyladamantane), which is a derivative of amantadine, has found application in the management of neurological disorders characterized by excitotoxic cell death. This encompasses conditions such as Parkinson’s disease and vascular dementia [40]. MEM has been reported to be repurposed against the Chikungunya virus or to ameliorate the symptoms of long coronavirus disease 19 (COVID-19) syndrome [41,42,43]. The therapeutic effect of MEM was previously thought to be due to its ability to bind preferentially to N-methyl-D-aspartate (NMDA) receptor-operated cation channels.
Despite NMDA receptors being the primary target for MEM, several studies reported additional underlying mechanisms of action. For example, previous work has demonstrated that MEM exerted a depressant action on membrane electroporation-induced inward current (IMEP)) in a concentration-dependent manner in pituitary tumor (GH3) cells [15]. Membrane electroporation (MEP) is a well-established process known for significantly enhancing the electrical conductivity and permeability of the plasma membrane when subjected to an externally applied electrical field [44]. The electrical and pharmacological properties of IMEP in both heart cells and pituitary cells have been previously demonstrated [15,22,45].
Additionally, previous research has demonstrated that the MEM presence reduces the amplitude of the inwardly rectifying K+ current (IK(IR)) in both RAW 264.7 macrophages and BV2 microglial cells [16]. MEM exposure has been observed to decrease both the rate and extent of IK(IR) inactivation. Notably, the intracellular inclusion of spermine has been found to counteract the inhibitory effects of MEM on IK(IR). Spermine, a polyamine and polycationic compound, has been shown to block the inward rectifying K+ (Kir) channel [46]. Moreover, in single-channel recordings performed on RAW 264.7 cells, it was observed that MEM effectively decreased the open-state probability of the Kir channel while not affecting the single-channel conductance [16]. MEM-mediated reduction in Kir-channel activity was concomitant with both an increase in mean closed time and a decrease in the slow component of mean open time.
The Kir channels display strong inward rectification, meaning that they preferentially conduct K+ ions into the cell rather than out of the cell. Their gating mechanism is influenced by several factors, such as intracellular Mg2+ ions, polyamines, and phosphatidylinositol-4,5-bisphosphate [46]. The activity of these channels plays a crucial role in maintaining the resting membrane potentials of cells. They also contribute to the regulation of cell excitability, particularly in neurons and cardiac myocytes [46].
Consequently, it is expected that the reduction in IK(IR) magnitude caused by MEM may serve as a crucial mechanism by which MEM or similar compounds can disrupt the functional activities of macrophages or microglial cells, possibly contributing to their antiviral effects. However, these effects should be confirmed through further research in vivo.
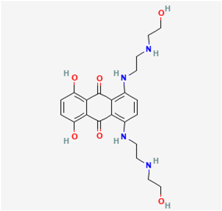
2.4. Mitoxantrone (MX, Novantrone®)
MX (1,4-dihydroxy-5,8-bis[[2-[(2-hydroxyethyl)amino]ethyl]amino]anthracene-9,10-dione) is a synthetic anthracenedione that has firmly established itself as an antineoplastic agent. It achieves this status by intercalating with DNA, thereby impeding the function of the topoisomerase II enzyme, preventing the ligation of DNA strands, and ultimately delaying cell-cycle progression. The therapeutic potential of MX has extended to a wide spectrum of malignancies, including advanced cases of prostate and breast cancers with osseous metastasis [47]. Furthermore, MX exhibits efficacy in countering viral infections, operating through similar mechanisms that disrupt viral DNA or RNA replication [48].
Earlier reports have shown that the presence of MX interacts with the activity of Kir channels to suppress the magnitude of IK(IR) in osteoclast precursor RAW 264.7 cells differentiated with lipopolysaccharide [17]. MX inhibits the amplitude of IK(IR) in a concentration-dependent manner, with an IC50 value of 6.4 μM. Doxorubicin or tertiapin also effectively suppresses the IK(IR) amplitude. Doxorubicin is another anthracycline compound, while tertiapin, a bee venom peptide, has been described as an inhibitor of acetylcholine-activated K+ current and IK(IR) in heart cells [49]. The MX-mediated decrease in Kir-channel activity is also accompanied by the shortening of the mean open time of the channel [17]. Blocking Kir channels in osteoclasts could hold significant clinical potential for the treatment of disorders characterized by disrupted mineralized tissues [50]. The suppression of these channels can also contribute to viral infections, such as the Monkeypox virus infection [48].
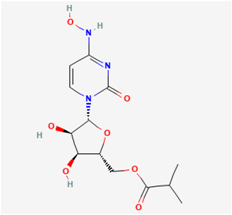
2.5. Molnupiravir (MOL, EIDD-2801, MK-4482, Lagevrio®)
MOL, an orally administered small-molecule isopropylester prodrug, is a noteworthy representative of the ribonucleoside analog β-d-N4-hydroxycytidine. This synthetic compound is esteemed for its antiviral properties, effectively hampering the replication of specific RNA viruses by inducing critical errors during viral RNA replication processes [51,52]. Its chemical nomenclature designates it as ((2R,3S,4R,5R)-3,4-dihydroxy-5-(4-(hydroxyamino)-2-oxopyrimidin-1(2H)-yl) tetrahydrofuran-2-yl) methyl isobutyrate. In light of its capacity to thwart the RNA-dependent RNA polymerase (RdRp) of SARS-CoV-2, consequently prompting RNA mutagenesis, MOL has been repurposed as a potential treatment for COVID-19 [53].
In a recent study, researchers presented findings demonstrating that MOL exerts a time-, concentration-, and frequency-dependent inhibition of INa in pituitary tumor (GH3) cells [18]. Upon exposure to MOL, both the peak and late amplitudes of INa in response to rapid membrane depolarization experienced varying degrees of suppression. The study estimated the IC50 values of MOL for inhibiting transient and late INa in GH3 cells to be 26.1 and 6.3 μM, respectively. Furthermore, MOL’s continuous presence led to cumulative inhibition of peak INa elicited throughout a series of depolarizing stimuli. Additionally, the introduction of MOL substantially attenuated the nonlinear resurgent INa evoked by the descending ramp voltage, highlighting its impact on specific electrophysiological responses. The magnitude of resurgent INa plays a crucial role in triggering action potential firing in different types of electrically excitable cells. In the presence of MOL, single-channel recordings showed a reduction in the probability of NaV-channel openings accompanied by a decrease in the mean open time of the channel; however, no change in single-channel conductance was made [18]. The voltage-activated INa detected in Neuro-2a neuroblastoma cells was also found to be responsive to inhibition by MOL [54]. It has been reported that mRNA transcripts of NaV1.1, NaV1.2, and NaV1.6 α subunits are expressed in GH3 cells [51]. It remains to be studied whether MOL can exert an influence on the activity in other isoforms of the NaV channel.
The molecular docking analysis revealed potential interactions between MOL and the RdRp of SARS-CoV-2 and NaV channels [18]. Furthermore, recent studies have linked long-term MOL usage to the emergence of additional mutations in the SARS-CoV-2 genomes [52]. Whether these unintended side effects are related to its inhibition of INa warrants further investigation. Given that ranolazine is employed in the treatment of chronic angina pectoris [55], it is worthwhile to explore the potential repurposing of MOL for the management of chronic pain [54,56].
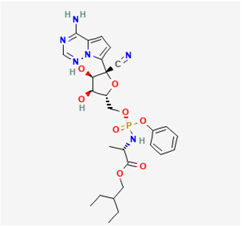
2.6. Remdesivir (RDV, GS-5734)
RDV (ethyl (2S)-2-[[[(2S,3S,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxyoxolan-2-yl]methoxy-phenoxy-phosphoryl]amino]propanoate), a potent antiviral agent with broad-spectrum activity, is acknowledged as a mono-phosphoramidate prodrug of an adenosine analog. It undergoes metabolic conversion into its active form, GS-441524, which is a C-adenosine nucleoside analog [57]. This compound, functioning as a nucleotide-analog inhibitor of RdRp, demonstrates substantial effectiveness against various coronaviruses (CoVs), including MERS-CoV, SARS-CoV-2, and the virus responsible for COVID-19 [58,59,60,61,62]. It stands out as a promising antiviral drug with potential applications against a wide spectrum of RNA viruses, primarily by targeting the viral RdRp. The active form, GS-441524, to which RDV metabolizes, exerts a less inhibitory effect on cellular RNA compared with its impact on viral polymerase [23]. Recent studies have unveiled the high efficacy of RDV in combination with chloroquine or hydroxychloroquine for controlling SARS-CoV-2 infection in in vitro settings [23]. Moreover, there is noteworthy ongoing research regarding the efficacy of RDV in treating SARS-CoV-2 infection in humans [63].
It is worth noting that prior investigators have revealed that the presence of RDV leads to a reduction in the amplitude of IK(DR) in a manner that is both time-dependent and concentration-dependent. This effect has been observed in both pituitary GH3 cells and Jurkat T-lymphocytes [19]. Additionally, the rate of IK(DR) inactivation appears to increase with higher RDV concentrations. According to data from a simplified reaction model, the dissociation constant (KD) required for RDV-induced inhibition of IK(DR) in GH3 cells was reported to be approximately 3.04 µM. This value is in close proximity to the effective IC50 value (2.8 µM) for the RDV-mediated suppression of sustained IK(DR), although it is lower than that of the IC50 (10.1 µM) for blocking the initial peak IK(DR). The exposure to RDV also suppressed the magnitude of IK(M), with an IC50 value of 2.5 µM in GH3 cells, as well as depressed the voltage-dependent hysteresis of IK(M) [23].
Furthermore, under sustained exposure to RDV, it is noteworthy that neither the addition of α,β-methylene-ATP (AMPCPP), a non-degradable ATP analog, nor the introduction of 8-cycloppentyl-1.3-dipropylxanthine (DPCPX), an antagonist of the adenosine A1 receptor, had any discernible impact on the inhibition of IK(DR) induced by RDV [64,65]. These results suggest that the altered magnitude of IK(DR) caused by RDV in GH3 cells is unlikely to be associated with its preferential binding to purinergic or adenosine receptors. This observation is significant, particularly considering that the RDV molecule was initially considered a prodrug of an adenosine nucleoside analog [57,66]. Exposure to RDV was recently discovered to reduce the amplitude of IK(erg) [20] and also to prolong QTc intervals [64]. Nonetheless, the direct suppression of IK(DR), IK(M), and IK(erg) in these cells, therefore, suggests that this compound, per se, presumably is not an inactive prodrug. Moreover, the RDV presence can activate the magnitude of IMEP [19].
It needs to be mentioned that prior studies have documented the presence of hypokalemia and, in some severe cases, lethal arrhythmia in patients with COVID-19 infection [60,65,67]. The EC50 value of RDV against SARS-CoV-2 within Vero E6 cells was significantly determined to be 1.76 µM, indicating that the concentration required for its antiviral action is likely attainable in an in vivo setting [61]. It is therefore anticipated that, apart from its effects on the viral polymerase and the proofreading exoribonuclease [57,62,66,68], the extent to which RDV-induced perturbations on ionic currents may participate in its antiviral actions has yet to be further delineated.
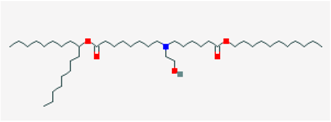
2.7. SM-102
SM-102, with its complex chemical name hepatodecan-9-yl 8-((2-hydroxyethyl)(6-oxo-6-(undecyloxy)hexyl)amino)octanoate, 4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate, stands as a synthetic and ionizable amino lipid. It has found extensive application alongside other lipids in the formulation of lipid nanoparticles [69,70,71]. These formulations incorporating SM-102 have been notably instrumental in the creation of lipid nanoparticles for delivering mRNA-based vaccines. This highly efficient transfection method relies on compacted lipopolyamine-coated plasmids and has seen progressive improvements over time [13,72]. However, it is important to note that recent reports have linked COVID-19 vaccinations to instances of myocarditis [73,74,75,76,77]. After receiving COVID-19 mRNA vaccination, there have been reported cases of acute myocarditis [74,75].
In a recent study, it was observed that, at concentrations of 100 or 300 μM, SM-102 caused a decrease in the amplitude of IK(erg) and an elevation in the deactivation rate of the current in both GH3 cells and Leydig MA-10 cells [78]. The inclusion of SM-102 resulted in a decrease in both the magnitude of IK(erg) and a shift in the current-to-voltage relationship of sustained IK(erg) towards less negative potentials. Additionally, the exposure of cells to SM-102 effectively reduced the magnitude of the voltage-dependent hysteretic strength of IK(erg) when activated by an isosceles-triangular ramp voltage.
In GH3 cells subjected to dialysis with SM-102, TurboFectinTM, or spermine, there was a gradual reduction in the magnitude of IK(erg) [78]. TurboFectinTM is a proprietary blender of a wide-ranging protein/polyamine mixture, along with histones and lipids, designated as a known transfection reagent [79]. The sensitivity of IK(IR) in microglial BV2 cells to suppression was also observed when exposed to SM-102 or spermine, as reported previously [16,78]. The extent to which SM-102-induced changes in membrane ionic currents contribute to the adverse effects of mRNA-based vaccines, such as ModernaTM, requires further investigation. An earlier report found that an individual experienced long QTc interval and syncope after receiving a single dose of COVID-19 vaccination [80]. It is, therefore, essential to determine whether the concentrations of SM-102 or TurboFectinTM used for directly altering ionic currents could be achieved in both in vitro and in vivo settings.
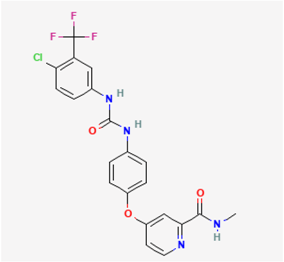
2.8. Sorafenib (SOR, Nexavar®)
SOR, scientifically designated as 4-(4-(((4-chloro-3-(trifluoromethyl)phenyl)amino)carbonyl)phenoxy)-N-methylpyridine-2-carboxamide, belongs to a distinctive class of multi-targeted, active small-molecule tyrosine kinase inhibitors that are presently employed in the treatment of hematological and oncological malignancies. Strategies for dose escalation have been contemplated for their clinical use, given that many malignancies are believed to be instigated by aberrant tyrosine kinase activity [81]. Tyrosine kinase inhibitors, such as sunitinib and SOtR, have recently been noticed to exert antiviral action [82,83,84].
A previous report has demonstrated the ability of SOR to suppress the amplitude of both the slowly activating delayed-rectifier K+ current (IK(S)) and IK(erg) identified in H9c2 cardiomyocytes [19,22]. The KV7.1-type IK(S), known as the KV7.1 (or KCNQ1)-cloned K+ channel, has been noted to be suppressed during H9c2-cell exposure to SOR. Additionally, it is worth noting that the suppression of IK(S) caused by SOR takes time to manifest during long-lasting membrane depolarization and is not an instantaneous response upon channel opening. These findings thus imply the existence of a time-dependent binding site for SOR, possibly situated in or near the IK(S)-channel pore, specifically when the channel becomes active. The SOR presence likewise resulted in a reduction in the amplitude of IK(IR), as observed in neonatal rat ventricular myocytes. This inhibition of ionic currents during SOR exposure may constitute an unintended yet crucial mechanism, contributing to alterations in QTc intervals or possibly influencing antiviral effects [9,19,82,83,84]. These findings could offer insights into the occurrence of patients exhibiting QTc prolongation and, in some cases, experiencing subsequent fatal arrhythmias following treatment with SOR [10,85].
3. Conclusions
It is acknowledged that viruses lack a complete cellular membrane structure and are not considered living organisms. They require a host cell to replicate, which necessitates their initial contact with the membrane of an infected cell. Therefore, when these antiviral medications discussed here are able to affect transmembrane ion channels in that cell, it can potentially disrupt the virus’s attachment, insertion, and entry into host cells (Figure 1). However, it should be noted that most of the experimental observations are derived from tumor cell lines, such as GH3 cells, neuroblastoma N2a cells, microglial BV2 cells, and RAW 264.7 macrophages. Further research is, therefore, needed to determine the applicability of these findings to different types of native excitable cells in vivo. Alternatively, whether there is specificity in their action on ion channels linked to antiviral activity and whether it exhibits selectivity for anti-SARS-CoV-2 activity still warrant further investigation. While certain antiviral agents influence ionic currents with low selectivity, the highlighted off-target effects are crucial considerations for disease treatment. Alternatively, further investigation is required to determine the impact of ion channel modulation by agens with antiviral activities on practical outcomes or consequences at various stages of disease development or maintenance over time.
We also need to clarify that certain antiviral agents mentioned in this paper are indeed clinically approved for use. These drugs, such as remdesivir (RDV), have been reported to intervene in transmembrane ion currents. However, substances such as cannabidiol (CBD) or arteminisin (ART), while exhibiting antiviral activity, are inappropriately classified as antiviral agents. Additionally, ART is an antimalarial drug.
It is also important to emphasize that the majority of the ion currents highlighted in Figure 1 are observed on the cardiac cell membrane. If the concentrations used in this context were to influence cardiac cells, it could disrupt their electrical activity, possibly resulting in the prolongation of ventricular action potential and QTc interval (Figure 2) [65,85,86]. It could thus pose a significant risk of causing severe arrhythmias, such as torsade de pointes arrhythmia, or even sudden cardiac death [7,8,9,10,64,67,85,86]. These antiviral suppressants display a diverse array of chemical structures (Table 1) and exhibit various regulatory effects on manifold types of ionic currents (Table 2). Therefore, when it is indeed necessary to use antiviral therapies, it is essential to exercise caution and closely monitor to mitigate unforeseen events [7,8,10,67,87]. Although a thorough examination of electrophysiological studies was conducted with these antiviral agents, further investigation is required to determine the extent of specific focus on organs that bear significance in terms of ion channel functionality. Moreover, whether these agents with antiviral activities also affect the transcriptional or translational expression levels of these ion channels is worth further investigation in the future.
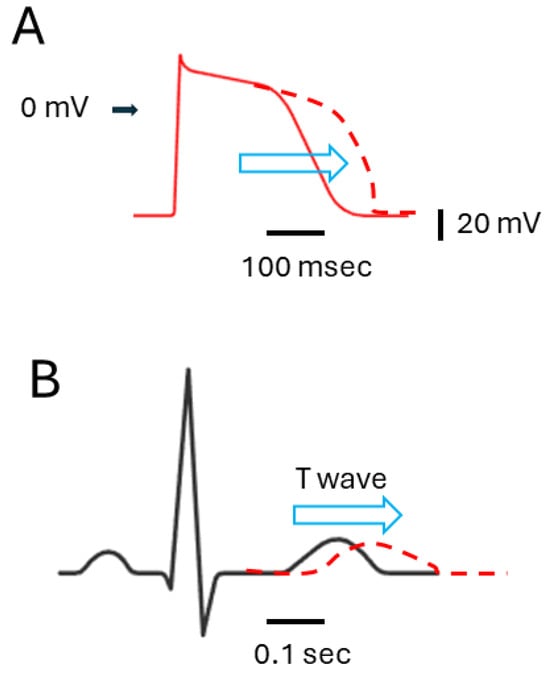
Figure 2.
Impact of agents with antiviral activities on IK(erg) or IK(S) inhibition, and their influence on ventricular action potential ((A), red) fand electrocardiogram ((B), black). The arrowhead on the left side of (A) indicates the 0 mV level. The horizontal and vertical black bars in the bottom right corner of (A,B) represent time and voltage scales, respectively. The red dashed curve in (A,B) illustrates the potential effects (denoted using horizontal blue open arrowheads) of these agents, specifically the prolongation of ventricular action potential (A) and QTc interval (B), respectively.
Author Contributions
Conceptualization, S.-N.W., R.L., V.R., E.C.S. and G.-B.L.; methodology, S.-N.W.; software, C.-L.W.; validation, G.-B.L., C.-L.S. and S.-N.W.; writing—original draft preparation, G.-B.L., and C.-L.S.; writing—review and editing, R.L., V.R., E.C.S. and C.-L.W.; visualization, G.-B.L.; supervision, S.-N.W.; funding acquisition, G.-B.L., C.-L.S. and S.-N.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly aided by the grants from the Ministry of Science and Technology (NSTC-110-2320-B-006-028, NSTC-111-2320-B-006-028, and NSTC-112-2923-B-006-001) and from An Nan Hospital (ANHRF-111-10, ANHRF-112-42, ANAR-112-43, and ANHRF-112-44), Taiwan. The funders of this study did not participate in the study design, data collection, analyses, or interpretation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
While there is a wealth of relevant papers that could be cited, we regret that, due to space limitations, we are unable to include all of them in this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| Ih | hyperpolarization-activated cation current |
| IK(erg) | erg-mediated K+ current |
| IK(IR) | inwardly rectifying K+ current |
| IK(M) | M-type K+ current |
| IK(S) | slowly activating delayed-rectifier K+ current |
| IMEP | MEP-induced inward current |
| INa | voltage-gated Na+ current |
| Kir channel | inwardly rectifying K+ channel |
| MEP | membrane electroporation |
| NaV channel | voltage-gated Na+ channel |
| QTc interval | corrected QT interval |
References
- Hong, C.M.; Liu, C.H.; Su, T.H.; Yang, H.C.; Chen, P.J.; Chen, Y.W.; Kao, J.H.; Liu, C.J. Real-world effectiveness of direct-acting antiviral agents for chronic hepatitis C in Taiwan: Real-world data. J. Microbiol. Immunol. Infect. 2020, 53, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Gorabi, A.M.; Talaei, S.; Beiraghdar, F.; Akbarzadeh, A.; Tarhriz, V.; Mellatyar, H. An overview on the treatments and prevention against COVID-19. Virol. J. 2023, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, M.J.; Clapham, D.E. Ion channels—Basic science and clinical disease. N. Engl. J. Med. 1997, 336, 1575–1586. [Google Scholar] [CrossRef] [PubMed]
- Franciolini, F. Patch clamp technique and biophysical study of membrane channels. Experientia 1986, 42, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Kausar, S.; Said Khan, F.; Ishaq Mujeeb Ur Rehman, M.; Akram, M.; Riaz, M.; Rasool, G.; Hamid Khan, A.; Saleem, I.; Shamim, S.; Malik, A. A review: Mechanism of action of antiviral drugs. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211002621. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.S.; Lai, M.C.; Huang, H.I.; Wu, S.N.; Huang, C.W. Immunity, Ion Channels and Epilepsy. Int. J. Mol. Sci. 2022, 23, 6446. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, J.; Wang, Y.; Chu, J.; Liu, Y.; Chen, X.; Chen, X. QTc prolongation during antiviral therapy in two COVID-19 patients. J. Clin. Pharm. Ther. 2020, 45, 1190–1193. [Google Scholar] [CrossRef] [PubMed]
- Santoro, F.; Monitillo, F.; Raimondo, P.; Lopizzo, A.; Brindicci, G.; Gilio, M.; Musaico, F.; Mazzola, M.; Vestito, D.; Benedetto, R.D.; et al. QTc Interval Prolongation and Life-Threatening Arrhythmias During Hospitalization in Patients with Coronavirus Disease 2019 (COVID-19): Results From a Multicenter Prospective Registry. Clin. Infect. Dis. 2021, 73, e4031–e4038. [Google Scholar] [CrossRef]
- Esmel-Vilomara, R.; Dolader, P.; Sabaté-Rotes, A.; Soriano-Arandes, A.; Gran, F.; Rosés-Noguer, F. QTc interval prolongation in patients infected with SARS-CoV-2 and treated with antiviral drugs. An. Pediatr. (Engl. Ed.) 2022, 96, 213–220. [Google Scholar] [CrossRef]
- Hu, C.H.; Wu, S.N.; So, E.C. Tyrosine kinase inhibitors, ionic currents, and cardiac arrhythmia. Front. Oncol. 2023, 13, 1218821. [Google Scholar] [CrossRef]
- Qiao, G.; Li, S.; Yang, B.; Li, B. Inhibitory effects of artemisinin on voltage-gated ion channels in intact nodose ganglion neurones of adult rats. Basic Clin. Pharmacol. Toxicol. 2007, 100, 217–224. [Google Scholar] [CrossRef] [PubMed]
- So, E.C.; Wu, S.N.; Wu, P.C.; Chen, H.Z.; Yang, C.J. Synergistic Inhibition of Delayed Rectifier K+ and Voltage-Gated Na+ Currents by Artemisinin in Pituitary Tumor (GH3) Cells. Cell. Physiol. Biochem. 2017, 41, 2053–2066. [Google Scholar] [CrossRef]
- Zhang, D.; Atochina-Vasserman, E.N.; Lu, J.; Maurya, D.S.; Xiao, Q.; Liu, M.; Adamson, J.; Ona, N.; Reagan, E.K.; Ni, H.; et al. The Unexpected Importance of the Primary Structure of the Hydrophobic Part of One-Component Ionizable Amphiphilic Janus Dendrimers in Targeted mRNA Delivery Activity. J. Am. Chem. Soc. 2022, 144, 4746–4753. [Google Scholar] [CrossRef]
- Liu, Y.-C.; So, E.C.; Wu, S.-N. Cannabidiol Modulates M-Type K+ and Hyperpolarization-Activated Cation Currents. Biomedicines 2023, 11, 2651. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.N.; Huang, H.C.; Yeh, C.C.; Yang, W.H.; Lo, Y.C. Inhibitory effect of memantine, an NMDA-receptor antagonist, on electroporation-induced inward currents in pituitary GH3 cells. Biochem. Biophys. Res. Commun. 2011, 405, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.L.; Chang, H.F.; Wu, S.N. The inhibition of inwardly rectifying K+ channels by memantine in macrophages and microglial cells. Cell. Physiol. Biochem. 2013, 31, 938–951. [Google Scholar] [CrossRef]
- Wang, C.L.; Tsai, M.L.; Wu, S.N. Evidence for mitoxantrone-induced block of inwardly rectifying K+ channels expressed in the osteoclast precursor RAW 264.7 cells differentiated with lipopolysaccharide. Cell. Physiol. Biochem. 2012, 30, 687–701. [Google Scholar] [CrossRef]
- Shiau, A.L.; Lee, K.H.; Cho, H.Y.; Chuang, T.H.; Yu, M.C.; Wu, C.L.; Wu, S.N. Molnupiravir, a ribonucleoside antiviral prodrug against SARS-CoV-2, alters the voltage-gated sodium current and causes adverse events. Virology 2023, 587, 109865. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Liu, P.Y.; Lee, K.; Feng, Y.H.; Wu, S.N. Differential Inhibitory Actions of Multitargeted Tyrosine Kinase Inhibitors on Different Ionic Current Types in Cardiomyocytes. Int. J. Mol. Sci. 2020, 21, 1672. [Google Scholar] [CrossRef]
- Amarh, E.; Tisdale, J.E.; Overholser, B.R. Prolonged Exposure to Remdesivir Inhibits the Human Ether-A-Go-Go-Related Gene Potassium Current. J. Cardiovasc. Pharmacol. 2023, 82, 212–220. [Google Scholar] [CrossRef]
- Cho, H.Y.; Chuang, T.H.; Wu, S.N. Effective Perturbations on the Amplitude and Hysteresis of Erg-Mediated Potassium Current Caused by 1-Octylnonyl 8-[(2-hydroxyethyl)[6-oxo-6(undecyloxy)hexyl]amino]-octanoate (SM-102), a Cationic Lipid. Biomedicines 2021, 9, 1367. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.N.; Yeh, C.C.; Wu, P.Y.; Huang, H.C.; Tsai, M.L. Investigations into the correlation properties of membrane electroporation-induced inward currents: Prediction of pore formation. Cell Biochem. Biophys. 2012, 62, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Liu, P.Y.; Gao, Z.H.; Lee, S.W.; Lee, W.K.; Wu, S.N. Evidence for the Effectiveness of Remdesivir (GS-5734), a Nucleoside-Analog Antiviral Drug in the Inhibition of I (K(M)) or I (K(DR)) and in the Stimulation of I (MEP). Front. Pharmacol. 2020, 11, 1091. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Qinghaosu (artemisinin): Chemistry and pharmacology. Acta Pharmacol. Sin. 2012, 33, 1141–1146. [Google Scholar] [CrossRef]
- Ho, W.E.; Peh, H.Y.; Chan, T.K.; Wong, W.S. Artemisinins: Pharmacological actions beyond anti-malarial. Pharmacol. Ther. 2014, 142, 126–139. [Google Scholar] [CrossRef]
- Fuzimoto, A.D. An overview of the anti-SARS-CoV-2 properties of Artemisia annua, its antiviral action, protein-associated mechanisms, and repurposing for COVID-19 treatment. J. Integr. Med. 2021, 19, 375–388. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.R.; Antunes, B.S.; do Nascimento, G.O.; Kawall, J.C.S.; Oliveira, J.V.B.; Silva, K.; Costa, M.A.T.; Oliveira, C.R. Antiviral activity of medicinal plant-derived products against SARS-CoV-2. Exp. Biol. Med. 2022, 247, 1797–1809. [Google Scholar] [CrossRef] [PubMed]
- Urban, N.; Schaefer, M. Direct Activation of TRPC3 Channels by the Antimalarial Agent Artemisinin. Cells 2020, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Dillard, L.K.; Fullerton, A.M.; McMahon, C.M. Ototoxic hearing loss from antimalarials: A systematic narrative review. Travel Med. Infect. Dis. 2021, 43, 102117. [Google Scholar] [CrossRef]
- Peng, T.; Li, S.; Liu, L.; Yang, C.; Farhan, M.; Chen, L.; Su, Q.; Zheng, W. Artemisinin attenuated ischemic stroke induced cell apoptosis through activation of ERK1/2/CREB/BCL-2 signaling pathway in vitro and in vivo. Int. J. Biol. Sci. 2022, 18, 4578–4594. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Medical Use of Cannabinoids. Drugs 2018, 78, 1665–1703. [Google Scholar] [CrossRef] [PubMed]
- van Breemen, R.B.; Muchiri, R.N.; Bates, T.A.; Weinstein, J.B.; Leier, H.C.; Farley, S.; Tafesse, F.G. Cannabinoids Block Cellular Entry of SARS-CoV-2 and the Emerging Variants. J. Nat. Prod. 2022, 85, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Marquez, A.B.; Vicente, J.; Castro, E.; Vota, D.; Rodríguez-Varela, M.S.; Lanza Castronuovo, P.A.; Fuentes, G.M.; Parise, A.R.; Romorini, L.; Alvarez, D.E.; et al. Broad-Spectrum Antiviral Effect of Cannabidiol Against Enveloped and Nonenveloped Viruses. Cannabis Cannabinoid Res. 2023; ahead of print. [Google Scholar]
- Viudez-Martínez, A.; García-Gutiérrez, M.S.; Manzanares, J. Cannabidiol regulates the expression of hypothalamus-pituitary-adrenal axis-related genes in response to acute restraint stress. J. Psychopharmacol. 2018, 32, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.W.; Lin, P.C.; Chen, J.L.; Lee, M.J. Cannabidiol Selectively Binds to the Voltage-Gated Sodium Channel Na(v)1.4 in Its Slow-Inactivated State and Inhibits Sodium Current. Biomedicines 2021, 9, 1141. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Simasko, S.M. Characterization of an M-like current modulated by thyrotropin-releasing hormone in normal rat lactotrophs. J. Neurosci. 1996, 16, 1668–1678. [Google Scholar] [CrossRef] [PubMed]
- Varghese, N.; Moscoso, B.; Chavez, A.; Springer, K.; Ortiz, E.; Soh, H.; Santaniello, S.; Maheshwari, A.; Tzingounis, A.V. KCNQ2/3 Gain-of-Function Variants and Cell Excitability: Differential Effects in CA1 versus L2/3 Pyramidal Neurons. J. Neurosci. 2023, 43, 6479–6494. [Google Scholar] [CrossRef] [PubMed]
- Simasko, S.M.; Sankaranarayanan, S. Characterization of a hyperpolarization-activated cation current in rat pituitary cells. Am. J. Physiol. 1997, 272, E405–E414. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; So, E.C.; Wu, S.N. Modulating Hyperpolarization-Activated Cation Currents through Small Molecule Perturbations: Magnitude and Gating Control. Biomedicines 2023, 11, 2177. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and Management of Dementia: Review. JAMA 2019, 322, 1589–1599. [Google Scholar] [CrossRef]
- Pereira, A.; Santos, I.A.; da Silva, W.W.; Nogueira, F.A.R.; Bergamini, F.R.G.; Jardim, A.C.G.; Corbi, P.P. Memantine hydrochloride: A drug to be repurposed against Chikungunya virus? Pharmacol. Rep. 2021, 73, 954–961. [Google Scholar] [CrossRef]
- Butterworth, R.F. Adamantanes for the treatment of neurodegenerative diseases in the presence of SARS-CoV-2. Front. Neurosci. 2023, 17, 1128157. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Riederer, P.; Kuhn, W. Aminoadamantanes: From treatment of Parkinson’s and Alzheimer’s disease to symptom amelioration of long COVID-19 syndrome? Expert Rev. Clin. Pharmacol. 2023, 16, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Cheek, E.R.; Fast, V.G. Nonlinear changes of transmembrane potential during electrical shocks: Role of membrane electroporation. Circ. Res. 2004, 94, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Dyachok, O.; Zhabyeyev, P.; McDonald, T.F. Electroporation-induced inward current in voltage-clamped guinea pig ventricular myocytes. J. Membr. Biol. 2010, 238, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Hibino, H.; Inanobe, A.; Furutani, K.; Murakami, S.; Findlay, I.; Kurachi, Y. Inwardly rectifying potassium channels: Their structure, function, and physiological roles. Physiol. Rev. 2010, 90, 291–366. [Google Scholar] [CrossRef] [PubMed]
- Eklund, J.; Kozloff, M.; Vlamakis, J.; Starr, A.; Mariott, M.; Gallot, L.; Jovanovic, B.; Schilder, L.; Robin, E.; Pins, M.; et al. Phase II study of mitoxantrone and ketoconazole for hormone-refractory prostate cancer. Cancer 2006, 106, 2459–2465. [Google Scholar] [CrossRef] [PubMed]
- Preet, G.; Oluwabusola, E.T.; Milne, B.F.; Ebel, R.; Jaspars, M. Computational Repurposing of Mitoxantrone-Related Structures against Monkeypox Virus: A Molecular Docking and 3D Pharmacophore Study. Int. J. Mol. Sci. 2022, 23, 14287. [Google Scholar] [CrossRef]
- Kitamura, H.; Yokoyama, M.; Akita, H.; Matsushita, K.; Kurachi, Y.; Yamada, M. Tertiapin potently and selectively blocks muscarinic K+ channels in rabbit cardiac myocytes. J. Pharmacol. Exp. Ther. 2000, 293, 196–205. [Google Scholar] [PubMed]
- Vignani, F.; Bertaglia, V.; Buttigliero, C.; Tucci, M.; Scagliotti, G.V.; Di Maio, M. Skeletal metastases and impact of anticancer and bone-targeted agents in patients with castration-resistant prostate cancer. Cancer Treat. Rev. 2016, 44, 61–73. [Google Scholar] [CrossRef]
- Stojilkovic, S.S.; Tabak, J.; Bertram, R. Ion channels and signaling in the pituitary gland. Endocr. Rev. 2010, 31, 845–915. [Google Scholar] [CrossRef]
- Sanderson, T.; Hisner, R.; Donovan-Banfield, I.A.; Hartman, H.; Løchen, A.; Peacock, T.P.; Ruis, C. A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes. Nature 2023, 623, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Zarenezhad, E.; Marzi, M. Review on molnupiravir as a promising oral drug for the treatment of COVID-19. Med. Chem. Res. 2022, 31, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.N.; So, E.C.; Liao, Y.K.; Huang, Y.M. Reversal by ranolazine of doxorubicin-induced prolongation in the inactivation of late sodium current in rat dorsal root ganglion neurons. Pain Med. 2015, 16, 1032–1034. [Google Scholar] [CrossRef] [PubMed]
- Salazar, C.A.; Basilio Flores, J.E.; Veramendi Espinoza, L.E.; Mejia Dolores, J.W.; Rey Rodriguez, D.E.; Loza Munárriz, C. Ranolazine for stable angina pectoris. Cochrane Database Syst. Rev. 2017, 2, Cd011747. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.C.; Lewis, R.J. Sodium channels and pain: From toxins to therapies. Br. J. Pharmacol. 2018, 175, 2138–2157. [Google Scholar] [CrossRef]
- Gordon, C.J.; Tchesnokov, E.P.; Feng, J.Y.; Porter, D.P.; Götte, M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020, 295, 4773–4779. [Google Scholar] [CrossRef] [PubMed]
- Morse, J.S.; Lalonde, T.; Xu, S.; Liu, W.R. Learning from the Past: Possible Urgent Prevention and Treatment Options for Severe Acute Respiratory Infections Caused by 2019-nCoV. Chembiochem 2020, 21, 730–738. [Google Scholar] [CrossRef]
- Sheahan, T.P.; Sims, A.C.; Leist, S.R.; Schäfer, A.; Won, J.; Brown, A.J.; Montgomery, S.A.; Hogg, A.; Babusis, D.; Clarke, M.O.; et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Spinner, C.D.; Gottlieb, R.L.; Criner, G.J.; Arribas López, J.R.; Cattelan, A.M.; Soriano Viladomiu, A.; Ogbuagu, O.; Malhotra, P.; Mullane, K.M.; Castagna, A.; et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients with Moderate COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 1048–1057. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Grundeis, F.; Ansems, K.; Dahms, K.; Thieme, V.; Metzendorf, M.I.; Skoetz, N.; Benstoem, C.; Mikolajewska, A.; Griesel, M.; Fichtner, F.; et al. Remdesivir for the treatment of COVID-19. Cochrane Database Syst. Rev. 2023, 1, Cd014962. [Google Scholar] [PubMed]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Pilote, S.; Simard, C.; Drolet, B. Remdesivir (VEKLURY) for Treating COVID-19: Guinea Pig Ex Vivo and In Vivo Cardiac Electrophysiological Effects. J. Cardiovasc. Pharmacol. 2022, 80, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Fung, J.S.; Levitan, M.; Landry, S.; McIsaac, S. Torsades de pointes associated with remdesivir treatment for COVID-19 pneumonia. J. Assoc. Med. Microbiol. Infect. Dis. Can. 2023, 8, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Tchesnokov, E.P.; Feng, J.Y.; Porter, D.P.; Götte, M. Mechanism of Inhibition of Ebola Virus RNA-Dependent RNA Polymerase by Remdesivir. Viruses 2019, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Nabati, M.; Parsaee, H. Potential Cardiotoxic Effects of Remdesivir on Cardiovascular System: A Literature Review. Cardiovasc. Toxicol. 2022, 22, 268–272. [Google Scholar] [CrossRef]
- Brown, A.J.; Won, J.J.; Graham, R.L.; Dinnon, K.H., 3rd; Sims, A.C.; Feng, J.Y.; Cihlar, T.; Denison, M.R.; Baric, R.S.; Sheahan, T.P. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res. 2019, 169, 104541. [Google Scholar] [CrossRef] [PubMed]
- Hassett, K.J.; Benenato, K.E.; Jacquinet, E.; Lee, A.; Woods, A.; Yuzhakov, O.; Himansu, S.; Deterling, J.; Geilich, B.M.; Ketova, T.; et al. Optimization of Lipid Nanoparticles for Intramuscular Administration of mRNA Vaccines. Mol. Ther. Nucleic Acids 2019, 15, 1–11. [Google Scholar] [CrossRef]
- Lam, K.; Leung, A.; Martin, A.; Wood, M.; Schreiner, P.; Palmer, L.; Daly, O.; Zhao, W.; McClintock, K.; Heyes, J. Unsaturated, Trialkyl Ionizable Lipids are Versatile Lipid-Nanoparticle Components for Therapeutic and Vaccine Applications. Adv. Mater. 2023, 35, e2209624. [Google Scholar] [CrossRef]
- Naidu, G.S.; Yong, S.B.; Ramishetti, S.; Rampado, R.; Sharma, P.; Ezra, A.; Goldsmith, M.; Hazan-Halevy, I.; Chatterjee, S.; Aitha, A.; et al. A Combinatorial Library of Lipid Nanoparticles for Cell Type-Specific mRNA Delivery. Adv. Sci. 2023, 10, e2301929. [Google Scholar] [CrossRef]
- Behr, J.P.; Demeneix, B.; Loeffler, J.P.; Perez-Mutul, J. Efficient gene transfer into mammalian primary endocrine cells with lipopolyamine-coated DNA. Proc. Natl. Acad. Sci. USA 1989, 86, 6982–6986. [Google Scholar] [CrossRef] [PubMed]
- Albert, E.; Aurigemma, G.; Saucedo, J.; Gerson, D.S. Myocarditis following COVID-19 vaccination. Radiol. Case Rep. 2021, 16, 2142–2145. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis with COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Jenista, E.R.; Wendell, D.C.; Azevedo, C.F.; Campbell, M.J.; Darty, S.N.; Parker, M.A.; Kim, R.J. Patients with Acute Myocarditis following mRNA COVID-19 Vaccination. JAMA Cardiol. 2021, 6, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Vidula, M.K.; Ambrose, M.; Glassberg, H.; Chokshi, N.; Chen, T.; Ferrari, V.A.; Han, Y. Myocarditis and Other Cardiovascular Complications of the mRNA-Based COVID-19 Vaccines. Cureus 2021, 13, e15576. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.B.; Choi, J.I.; Hosseini, F.; Roberts, J.; Ramanathan, K.; Ong, K. Acute Myocarditis Following mRNA-1273 SARS-CoV-2 Vaccination. CJC Open 2021, 3, 1410–1412. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Chuang, T.H.; Wu, S.N. The Effectiveness in Activating M-Type K+ Current Produced by Solifenacin ([(3R)-1-azabicyclo[2.2.2]octan-3-yl] (1S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-carboxylate): Independent of Its Antimuscarinic Action. Int. J. Mol. Sci. 2021, 22, 12399. [Google Scholar] [CrossRef] [PubMed]
- Martín-Montañez, E.; López-Téllez, J.F.; Acevedo, M.J.; Pavía, J.; Khan, Z.U. Efficiency of gene transfection reagents in NG108-15, SH-SY5Y and CHO-K1 cell lines. Methods Find. Exp. Clin. Pharmacol. 2010, 32, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Azdaki, N.; Farzad, M. Long QT interval and syncope after a single dose of COVID-19 vaccination: A case report. Pan Afr. Med. J. 2021, 40, 67. [Google Scholar] [CrossRef]
- Abdelgalil, A.A.; Alkahtani, H.M.; Al-Jenoobi, F.I. Sorafenib. Profiles Drug Subst. Excip. Relat. Methodol. 2019, 44, 239–266. [Google Scholar]
- Cabrera, R.; Limaye, A.R.; Horne, P.; Mills, R.; Soldevila-Pico, C.; Clark, V.; Morelli, G.; Firpi, R.; Nelson, D.R. The anti-viral effect of sorafenib in hepatitis C-related hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2013, 37, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Huang, S.H.; Yan, B.Y.; Lai, H.C.; Lin, C.W. Effective Antiviral Activity of the Tyrosine Kinase Inhibitor Sunitinib Malate against Zika Virus. Infect. Chemother. 2021, 53, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Theerawatanasirikul, S.; Lueangaramkul, V.; Semkum, P.; Lekcharoensuk, P. Antiviral mechanisms of sorafenib against foot-and-mouth disease virus via c-RAF and AKT/PI3K pathways. Vet. Res. Commun. 2023, 48, 329–343. [Google Scholar] [CrossRef]
- Kloth, J.S.; Pagani, A.; Verboom, M.C.; Malovini, A.; Napolitano, C.; Kruit, W.H.; Sleijfer, S.; Steeghs, N.; Zambelli, A.; Mathijssen, R.H. Incidence and relevance of QTc-interval prolongation caused by tyrosine kinase inhibitors. Br. J. Cancer 2015, 112, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Sung, R.J.; Kuo, C.-T.; Wu, S.-N.; Lai, W.-T.; Luqman, N.; Chan, N.-Y. Sudden cardiac death syndrome: Age, gender, ethnicity, and genetics. Acta Cardiol. Sin. 2008, 24, 65–74. [Google Scholar]
- Ansone, L.; Briviba, M.; Silamikelis, I.; Terentjeva, A.; Perkons, I.; Birzniece, L.; Rovite, V.; Rozentale, B.; Viksna, L.; Kolesova, O.; et al. Amino Acid Metabolism is Significantly Altered at the Time of Admission in Hospital for Severe COVID-19 Patients: Findings from Longitudinal Targeted Metabolomics Analysis. Microbiol. Spectr. 2021, 9, e0033821. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).