Abstract
Tauopathies, including Alzheimer’s disease (AD), are a group of neurodegenerative disorders characterized by pathological aggregation of microtubule binding protein tau. The presence of tau neurofibrillary tangles, which are insoluble β-sheet fibrils, in the brain has been the histopathological hallmark of these diseases as their level correlates with the degree of cognitive impairment. However, recent studies suggest that tau oligomers, which are soluble proteins that are formed prior to insoluble fibrils, are the principal toxic species impairing neurons and inducing neurodegeneration. Targeting toxic tau oligomers is challenging, as they are mostly unstructured and adopting multiple conformations. The heterogeneity of tau oligomers is further illustrated by the different oligomeric species formed by various methods. The current models and technologies to study tau oligomerization represent important resources and avenues to push the forefront of elucidating the true toxic tau species. In this review, we will summarize the distinct tau oligomers generated using different strategies and discuss their conformational characteristics, neurotoxicity, relevance to pathological phenotypes, as well as their applications in drug discovery. This information will provide insights to understanding heterogeneous tau oligomers and their role as molecular targets for AD and related tauopathies.
1. Introduction
Alzheimer’s disease (AD) and related dementia are the top 7th leading causes of death in the world, afflicting more than 50 million people [1]. AD is a neurodegenerative disease and an example of tauopathies with a marked increase in the number of tau inclusions such as paired helical filaments (PHFs) and neurofibrillary tangles (NFTs) in affected brain regions of patients [2]. Tau is an intrinsically disordered protein, and its normal function is to regulate microtubule stability and axonal transport [3]. Under pathological conditions, the detachment of tau from microtubules results in misfolding and accumulation in the cytosol [4] which initiates the fibrillogenesis cascade including the formation of tau oligomers and the subsequent nucleation into PHFs and NFTs (Figure 1A) [5]. While NFTs have been the histopathological hallmark of AD and tauopathies [6], recent studies suggest that these large insoluble fibrils are not the primary toxic species and soluble tau oligomers may instead be the principal toxic tau species [7,8,9,10]. Studies, both in vitro and in vivo, have reported that tau oligomers promote neurotoxicity, and may be attributed to neurodegeneration and cognitive phenotypes in mice [11,12,13,14,15,16]. It has also been reported that promoting the growth of inert tau fibrils may be neuroprotective by sequestering toxic tau oligomers (Figure 1B) [17,18]. Furthermore, it has been suggested that tau oligomers may act as seeds that propagate between cells to induce misfolding of tau monomers and spreading of toxic tau species [19,20,21]. As a result, there is a shift of therapeutic paradigm from targeting large fibrillar aggregates to inhibiting or remodeling the formation of toxic soluble tau oligomers [22,23,24,25].
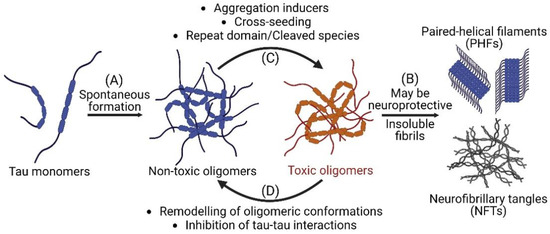
Figure 1.
Tau oligomerization and fibrillization in Alzheimer’s disease and related tauopathies. (A) Misfolded tau monomers spontaneously form into both non-toxic and toxic soluble tau oligomers which subsequently fibrillize into insoluble paired helical filaments (PHFs) and neurofibrillary tangles (NFTs). (B) Insoluble species have been proposed to potentially have neuroprotective effect by sequestering toxic oligomers. (C) Aggregation inducers, cross-seeding, and the presence of tau repeat domain or cleaved species can facilitate the transition from non-toxic to toxic oligomers. (D) Small molecules that remodel tau oligomeric conformations or inhibit tau-tau interactions can promote the conversion of toxic to non-toxic oligomers and reduce neurotoxicity. Schematics are created with BioRender.com accessed on 10 October 2022.
Currently, there are no known effective disease modifying therapies targeting AD and tauopathies [26]. Among the >100 disease-modifying therapies currently in clinical developments, there are only a limited number of them (~10%) that are tau-focused [27]. The heterogeneity of toxic tau species has been attributed to the failure of clinical trials and impeded the discovery of effective therapeutics. Importantly, small molecules such as methylene blue, which are known to target tau fibrils, have failed to obtain desirable results in the clinical trials, potentially due to the conversion of tau fibrils into toxic tau oligomers [28,29]. While the focus has shifted from targeting insoluble NFTs to soluble oligomers, the heterogeneity of toxic tau oligomers, both in vitro and in vivo, remains to be resolved. The clinical heterogeneity has been reflected through the various types of toxic tau oligomers identified and isolated in different studies [30,31,32,33,34,35,36]. Other sources of tau heterogeneity lie in molecular variations such as spontaneous formation, seeding competency, expression levels, isoform composition, truncation, use of inducers, and post-translational modifications [37,38,39,40,41,42]. Hence, there is a need to understand the different types of tau oligomers formed as well as their detailed characterizations and functions.
Tau oligomers exist as an ensemble of distinct assemblies [43,44,45,46,47] which include both toxic and non-toxic, on- and off-pathway species along the fibrillogenesis cascade [17,25,48,49,50,51,52]. Capturing this complexity in an in vitro setting is extremely difficult and established protocols for obtaining tau aggregates from purified tau proteins have, not surprisingly, been shown to produce different tau assemblies depending on aggregation conditions [31,33,53,54]. Although no specific toxic tau species has been identified to date [55,56], several studies making use of purified recombinant proteins are able to produce stable toxic tau oligomers that can be used to study their role in AD pathology. These aggregation strategies include cross-seeding approaches as well as the use of inducers and cleaved species (Figure 1C). In addition, cell-based strategies have been adopted to model tau oligomerization and the associated neurotoxicity [52]. While the cellular system has the advantage of being physiologically relevant as well as containing the cellular components such as numerous chaperone proteins [57], caspases [58], and kinases [59] that play key roles in tau oligomerization [60], it is difficult to capture the biophysical characterizations as well as direct toxicity effect of specific toxic tau oligomers in cells because they are in an ensemble of conformations [41,42]. Several rational drug discovery efforts using purified recombinant protein and cell-based assays to discover small molecules that target toxic tau oligomers have yielded efficacious, cytoprotective compounds (Figure 1D) [61,62,63,64,65,66,67], and some have further been shown to be neuroprotective in vivo [68,69]. However, there is currently no clinical trial conducted on targeting tau oligomers and it is imperative to advance successful pre-clinical studies into human trials [70,71,72].
In this review, we will describe the diverse types of tau oligomers with different conformations generated by various methods. We will also discuss the hypothesized functions and associated neurotoxicity of these tau oligomers, as well as their relevance to pathological phenotypes and applications in drug discovery. We conclude by providing future perspectives on identifying the true toxic tau species as well as delineating the mechanisms of pathogenic formation of heterogeneous tau oligomers and their role as molecular targets and potential biomarkers for AD and related tauopathies.
2. Spontaneous Formation of Tau Oligomers
Full-length wild-type (WT) tau proteins are known to be less prone to fibrillization, although excessive accumulation of these proteins can be toxic and are associated with sporadic AD [73,74]. Full-length WT tau has been shown to spontaneously form tau oligomers, rather than large fibrillar aggregates. An initial study used WT 2N4R purified tau proteins and concentrated them to create a mixture of tau monomers and granular oligomers, and the enriched granules generated β-sheet fibrils, suggesting that granular tau oligomers may be an intermediate form of tau filaments [30]. Importantly, these granular tau oligomers can be purified using sucrose step gradient centrifugation to separate from monomers and fibrils [30,75]. Although no functional or toxicity characterizations of these purified granular tau oligomers have been performed, purification of granular tau aggregates from human brains of different Braak stages suggests that granular tau aggregation precedes fibril formation [30]. These studies have proposed that tau aggregation initiates with the formation of soluble tau oligomers through disulfide binding and these oligomers would then convert into β-sheet granular tau oligomers which further interact and undergo fibrillization [30,75]. It would also be important to elucidate the initiation of the granular tau aggregation process in different brain regions to enhance the understanding of disease pathogenesis.
Another study used 1N4R and 2N4R recombinant human tau to generate monomeric, dimeric, and trimeric fractions of each isoform. The composition and height distribution of each fraction was verified by chromatography and atomic force microscopy. The toxicity of each fraction toward both human neuroblastoma cells and cholinergic-like neurons was assessed. Disulfide mediated trimeric oligomers of both splice variants, but not monomeric or dimeric species, displayed neurotoxicity at low nanomolar concentrations [36]. Interestingly, disulfide mediated oligomers have been shown to act through a nucleation effect, rather than the action of specific tau kinases, to initiate tau aggregation in mice [76]. In a separate study, an analysis of the oligomers formed by the six tau isoforms to study primary-quaternary structure relationship of tau has illustrated that 3R isoforms are more prone to oligomerization than the 4R isoforms [77].
The spontaneous formation of tau oligomers has also been demonstrated in both 2N4R WT tau and mutant tau transfected cells. Cellular biosensors with spectroscopy probes have been used to detect the presence of tau oligomers through fluorescence resonance energy transfer (FRET), split fluorescent protein complementation (BiFC), and split luciferase complementation (SLC) assays [78]. Specifically, we have previously reported that 2N4R WT and mutant tau form oligomers in HEK293 cells as detect by FRET, but not β-sheet fibrillar species as shown by negative thioflavin-S (ThS) staining [52]. Even though both WT and mutant tau spontaneously form oligomers, there is a diverse view on their effect on cell death [73]. While some studies have suggested that WT tau is toxic to cells [79,80], several other studies have reported that WT tau overexpression does not induce cell death, despite the formation of oligomers and tau hyperphosphorylation [52,81,82,83]. On the other hand, mutant tau has a higher oligomerization propensity than WT tau [52] and is neurotoxic in both cellular and animal models of tauopathies [52,81,84]. For therapeutic targeting, we have conducted a FRET-based high-throughput screening (HTS) and identified MK-886 as a small molecule that inhibits tau oligomerization and rescues P301L mutant tau induced cell death at nanomolar concentration [52]. This FRET based HTS strategy has also been used to discover effective small molecule inhibitors targeting alpha-synuclein oligomerization [85] and huntingtin exon 1 aggregation [86] and could be generally applicable to most intrinsically disordered proteins.
3. Heparin-Induced Tau Oligomerization
Heparin has been adopted by multiple studies to induce tau oligomerization. It has also been shown that heparin induces transition from inert monomers to seed-competent monomers to initiate the aggregation cascade [37,46] and remodels tau repeat domain (tauRD) towards fibrillization prone conformations [87]. We note here that the use of heparin to induce oligomers may result in the formation of both β-sheet and non-β-sheet oligomers and it is imperative to differentiate these two types of tau oligomers, such as through thioflavin-T (ThT) staining as they represent two distinct tau species [31,88,89].
Similar to spontaneously formed disulfide mediated oligomers, heparin-induced aggregation of recombinant tau forms two distinct dimers, cysteine-dependent and cysteine-independent, that resist reduction to different extents [90,91]. These distinct types of dimeric assembly were also observed in 2N4R WT tau expressing COS-7 cells, which were abolished with a PHF6 hexapeptide deletion at residues 306–311 and C291A/C322A mutations [90]. These results suggest that the formation of higher-order oligomers could be due to PHF6 hexapeptide promoting tau oligomerization through intermolecular disulfide crosslinking both in vitro [90] and in vivo [92]. Concurrent intermolecular bridging of microtubule-binding domain through cysteine-independent mechanism may further promote tau aggregation [90]. Furthermore, oligomeric assembly containing up to six to eight tau molecules is suggested to be necessary for the detection of β-sheet structure with ThT. Importantly, disulfide-bridge formation between tau and cell membranes has been shown to facilitate tau secretion [93].
In order to target disulfide mediated tau oligomers, a rosamine derivative with tau selective thiol reactivity inhibited oligomerization and the subsequent fibrillization cascades [94]. Furthermore, compounds containing 1,2-dihydroxybenzene such as DL-isoproterenol which penetrates the brain are capable of binding to tau and capping the cysteine residues of tau to prevent tau aggregation and rescue neuronal loss in mouse models of tauopathies [32]. Antitumor drugs targeting tau cysteine 322 and PHF6 hexapeptide prevent tau seeding and propagation of tau pathology [95]. A new drug discovery strategy making use of computational based virtual oligomerization inhibition has shown that epigallocatechin gallate (EGCG) inhibits PHF6 peptide aggregation and this was validated in tau seeding assay [96]. From this study, it is also reported that a good binding affinity of the inhibitors to tau aggregates may be insufficient to perform the full function of inhibiting tau oligomerization [96].
There are several other studies focusing on understanding heparin-induced β-sheet tau oligomers. In the study, β-sheet tau oligomers were characterized by size exclusion chromatography followed by staining with A11 tau oligomer specific antibody. These tau oligomers have been reported to decrease SH-SY5Y cell viability potentially through disruption of membrane integrity [31]. In another study, β-sheet tau oligomers were prepared for aggregation and dissociation studies with phosphorylation-based antibodies [97,98]. It has also been shown that baicalein suppresses tau aggregation by sequestering tau oligomers [99,100] or by initiating the formation of non-toxic tau oligomers [49]. Furthermore, fumarprotocetraric acid [101] and rutin [102] have been shown to attenuate tau pathology in cells and in mouse models of AD. Heparin-induced β-sheet tau oligomers have also been treated to macrophages and microglia and the study shows that macrophages phagocytose these extracellular tau oligomers more efficiently than microglia which are only able to degrade internalized tau oligomers upon lipopolysaccharide (LPS) stimulation [103].
Comparing β-sheet content in heparin-induced tau oligomers and fibrils, it was reported that tau oligomers have a high hydrophobicity with a lesser extent of internal compact β-sheet structures, while mature tau fibrils contain both high content of internal compact β-sheet structures and hydrophobicity as characterized by ThS [89]. A notable transition from random coil to an increasing tendency of β-sheet formation during oligomerization has been observed by circular dichroism spectroscopy [89]. To study tau internalization and spreading, the uptake of ThT negative heparin-induced tau oligomers by human induced pluripotent stem cells (iPSCs) derived neurons has resulted in the accumulation of pathogenic tau and neuronal death [104]. Besides heparin, several other inducers such as hexafluorisopropanol [105] and arachidonic acid [106] have been used to induce formation of neurotoxic tau oligomers. Moreover, an oligomeric specific monoclonal antibody (TOC-1) has been generated using arachidonic acid induced tau aggregates as immunogens. TOC-1 has been reported to detect tau dimers and oligomeric species with little reactivity to tau fibrils [106,107,108,109].
4. Amyloid-Beta (Aβ)-Induced Tau Oligomerization
The cross-seeding potential of different amyloid species on tau aggregation has been tested on using Aβ fibrils, oligomers, and monomers as seeds to promote tau oligomerization and aggregation. It is reported that Aβ preformed oligomers, but not fibrils or monomers, convert disordered tau monomers into toxic tau oligomers with high β-sheet content. These toxic tau oligomers with spherical morphology are mostly stable as trimers in spherical morphology and they induced significantly more toxicity and neuronal dysfunctions in SH-SY5Y cells and in mice as compared to their monomeric or fibrillar counterparts [33,110,111]. Interestingly, despite containing β-sheet structure, Aβ-induced tau oligomers do not bind ThT or dyes that stain for amyloid structures, but bind strongly to 4,4′-dianilino-1,1′-binaphthyl-5,5′-disulfonic acid, dipotassium salt (bis-ANS), suggesting the presence of hydrophobic surfaces with conformational characteristics different from conventional fibrils [67]. A novel tau oligomer specific antibody (T22) has also been developed and used vastly in many in vitro cell-based studies, in vivo mouse studies as well as on human samples, suggesting the presence of this type of tau oligomeric strain in physiological relevant conditions [112,113,114,115,116,117,118,119,120,121]. The established Aβ-induced tau oligomers have also been used to study tau uptake [122], as well as seeding and propagation [19,123], where Aβ oligomers promote tau fibril uptake as seeds to potentiate intracellular tau aggregation [124]. Sonicated fibrils have also been shown to contain T22 positive oligomers which propagate with toxicity in neuronal cells, although it is unclear how the oligomers could arise from the sonication [125]. Another tau oligomer-specific monoclonal antibody (TOMA) has been developed based on Aβ-induced toxic tau oligomers. The administration of TOMA to mice expression human tau has shown to be effective in reducing tau oligomers and rescuing memory deficits [121,126].
Studies have furthered the drug discovery effort by therapeutic targeting of Aβ-induced tau oligomers, including the identification of chemicals and small molecule inhibitors [127]. Heparin oligosaccharides have been found to bind to Aβ-induced tau oligomers with nanomolar binding affinity and reduced the uptake of toxic tau oligomers, as well as rescued cell death in neuronal cells [64]. In two other studies, a derivative of Azure series of compounds such as Azure C and newly synthesized curcumin derivatives have been reported to modulate tau oligomerization and protect neuronal cell lines and primary cortical neurons from cell death [61,128]. Mechanistically, both Azure C and curcumin derivatives prevent neurotoxicity by converting toxic tau oligomers into more aggregated forms adopting non-toxic conformations and not by disrupting tau oligomers directly [61,128]. These results provide mechanistic insights into tau oligomerization and may serve as a basis for the discovery of new therapeutic agents targeting toxic tau oligomers.
5. Tau Oligomers Formed by Repeat Domains and Cleaved Species
There is higher aggregation propensity of the repeat domains and cleaved species of tau as compared to the full-length tau protein [18,129]. Protein fragments of tauRD with the deletion of lysine 280 (tauRDΔK), which is associated with FTDP-17, have been shown to form stable and toxic tau oligomers containing β-sheet conformations [62,130]. To target these tauRDΔK tau oligomers, EGCG is capable of inhibiting tau oligomerization and rescuing toxicity in neuronal model cells [62]. In order to examine the molecular content of tauRDΔK oligomers, a study has characterized them based on 8-anilino-1-naphthalenesulfonic acid (ANS) and ThS profiles. Three main conformational states have been described with monomers having low ANS/low ThS, oligomers having high ANS/low ThS, and fibrils having high ANS/high ThS [34]. It has also been proposed that tauRDΔK oligomers do not adopt pronounced β-sheet conformation and they cause synaptotoxicity selectively without impairing overall cell viability [34]. The transient nature of tau aggregation makes it difficult to maintain the stability of preformed oligomers. The efficient labeling of tauRD with Alexa Fluor-488-C5-maleimide or N-ethyl maleimide has been shown to stabilize the resulting oligomers [35]. In terms of therapeutic targeting, curcumin and epinephrine are reported to disrupt tau oligomeric protofibrils formed by tau R3-R4 through molecular dynamics simulation [131], while a dimeric transient intermediate inhibits tau K18 fibril elongation [132]. Besides forming tau oligomers, tauRD is well known to form highly aggregated tau fibrillar species as characterized by the tau K18 FRET biosensor [133] and are capable of seeding and propagation of tau pathology [134].
Tau oligomerization from truncated species has been associated with neuronal impairments [135]. The formation of tau oligomers from caspase-cleaved species is illustrated by FRET signals between two truncated tau proteins, together with the presence of sarkosyl insoluble fractions in cell lysates [136]. Importantly, tau oligomers with hyperphosphorylation appear in mice with caspase-cleaved tau and this is associated with reduced synaptic density and rapid memory deficit [137]. Treatment with tau oligomer inhibitor or autophagy inducer reduces tau oligomers in mice with caspase-cleaved tau, attenuates tau oligomer-associated pathology, and rescues memory impairment [137,138]. Similar to caspase-cleaved tau species, calpain-mediated tau cleavage produces a 17 kDa neurotoxic fragment [76]. This fragment can be generated by inducers including Aβ oligomers, thapsigargin, and glutamate [139]. In a JNPL3 mouse model of tauopathies, overexpression of calpastatin, an inhibitor of calpain, prevents the formation of neurotoxic caspase-cleaved and calpain-cleaved tau fragments, attenuates tau oligomerization and hyperphosphorylation, delays the onset of disease, and extends survival of these mice [140].
6. Summary and Future Perspectives
With a pressing need to target AD and tauopathies which are currently untreatable, global efforts have been made to elucidate underlying disease mechanisms to facilitate the development of disease-modifying therapies as well as identify clinical phenotypes and robust biomarkers for early diagnosis [141]. In this review, we described several models of tau oligomerization using recombinant purified proteins and in cells as well as discuss the drug discovery efforts using these approaches. There are multiple other factors such as the role of phosphorylation [142,143] and liquid-liquid phase separation in tau oligomerization [144,145,146] that have to be taken into account to work towards achieving the identification of the true toxic tau species. While targeting toxic tau oligomers has been demonstrated to be effective in several pre-clinical studies [68,69,147], a caveat in specific targeting lies in need to disrupt or eliminate toxic tau oligomers, but not the non-toxic forms [148], which may aid in sequestering the toxic species. Advanced visualization and computational techniques such as machine learning-based classification [149] could further be developed and utilized to segregate these distinct tau species for more specific targeting. Besides being therapeutic targets, it has been proposed that quantifying the amount and level of tau oligomers may represent useful AD biomarkers [150]. With emerging tau-based clinical imaging techniques such as positron emission tomography (PET) [151,152,153] and magnetic resonance imaging (MRI) [154,155], the future development of tau oligomer targeted imaging modalities [156,157,158,159] would improve the diagnosis of AD and related tauopathies.
Funding
This study was supported by a Lee Kong Chian School of Medicine Dean’s Postdoctoral Fellowship to Chih Hung Lo (Grant Award Number 021207-00001) from Nanyang Technological University (NTU) Singapore.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The author would like to thank Jialiu Zeng for proofreading the manuscript.
Conflicts of Interest
The author declares no conflict of interest.
References
- World Health Organization (WHO). The Top 10 Causes of Death. 2020. Available online: https://www.who.int/news-room/fact-sheets (accessed on 15 September 2022).
- Wood, J.G.; Mirra, S.S.; Pollock, N.J.; Binder, L.I. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau). Proc. Natl. Acad. Sci. USA 1986, 83, 4040–4043. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.; Lucas, J.J.; Pérez, M.; Hernández, F. Role of Tau Protein in Both Physiological and Pathological Conditions. Physiol. Rev. 2004, 84, 361–384. [Google Scholar] [CrossRef] [PubMed]
- Bramblett, G.T.; Goedert, M.; Jakes, R.; Merrick, S.E.; Trojanowski, J.Q.; Lee, V.M.Y. Abnormal tau phosphorylation at Ser396 in alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron 1993, 10, 1089–1099. [Google Scholar] [CrossRef]
- Sahara, N.; Maeda, S.; Takashima, A. Tau Oligomerization: A Role for Tau Aggregation Intermediates Linked to Neurodegeneration. Curr. Alzheimer Res. 2008, 5, 591–598. [Google Scholar] [CrossRef]
- Ballatore, C.; Lee, V.M.Y.; Trojanowski, J.Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007, 8, 663. [Google Scholar] [CrossRef] [PubMed]
- Gerson, J.E.; Castillo-Carranza, D.L.; Kayed, R. Advances in Therapeutics for Neurodegenerative Tauopathies: Moving toward the Specific Targeting of the Most Toxic Tau Species. ACS Chem. Neurosci. 2014, 5, 752–769. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Sahara, N.; Saito, Y.; Murayama, S.; Ikai, A.; Takashima, A. Increased levels of granular tau oligomers: An early sign of brain aging and Alzheimer’s disease. Neurosci. Res. 2006, 54, 197–201. [Google Scholar] [CrossRef]
- Cárdenas-Aguayo, M.D.C.; Gómez-Virgilio, L.; Derosa, S.; Meraz-Ríos, M.A. The Role of Tau Oligomers in the Onset of Alzheimer’s Disease Neuropathology. ACS Chem. Neurosci. 2014, 5, 1178–1191. [Google Scholar] [CrossRef]
- Liang, S.-Y.; Wang, Z.-T.; Tan, L.; Yu, J.-T. Tau Toxicity in Neurodegeneration. Mol. Neurobiol. 2022, 59, 3617–3634. [Google Scholar] [CrossRef]
- Gerson, J.; Castillo-Carranza, D.L.; Sengupta, U.; Bodani, R.; Prough, D.S.; Dewitt, D.S.; Hawkins, B.E.; Kayed, R. Tau Oligomers Derived from Traumatic Brain Injury Cause Cognitive Impairment and Accelerate Onset of Pathology in Htau Mice. J. Neurotrauma 2016, 33, 2034–2043. [Google Scholar] [CrossRef]
- Hill, E.; Karikari, T.K.; Moffat, K.G.; Richardson, M.J.E.; Wall, M.J. Introduction of Tau Oligomers into Cortical Neurons Alters Action Potential Dynamics and Disrupts Synaptic Transmission and Plasticity. eNeuro 2019, 6, ENEURO.0166-19. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Carranza, D.L.; Nilson, A.N.; Van Skike, C.E.; Jahrling, J.B.; Patel, K.; Garach, P.; Gerson, J.E.; Sengupta, U.; Abisambra, J.; Nelson, P.; et al. Cerebral Microvascular Accumulation of Tau Oligomers in Alzheimer’s Disease and Related Tauopathies. Aging Dis. 2017, 8, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, C.W.; Wszolek, M.F.; Shulman, J.M.; Salvaterra, P.M.; Lewis, J.; Hutton, M.; Feany, M.B. Tauopathy in Drosophila: Neurodegeneration without Neurofibrillary Tangles. Science 2001, 293, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Santacruz, K.; Lewis, J.; Spires, T.; Paulson, J.; Kotilinek, L.; Ingelsson, M.; Guimaraes, A.; Deture, M.; Ramsden, M.; Mcgowan, E.; et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science 2005, 309, 476–481. [Google Scholar] [CrossRef]
- Berger, Z.; Roder, H.; Hanna, A.; Carlson, A.; Rangachari, V.; Yue, M.; Wszolek, Z.; Ashe, K.; Knight, J.; Dickson, D.; et al. Accumulation of Pathological Tau Species and Memory Loss in a Conditional Model of Tauopathy. J. Neurosci. 2007, 27, 3650–3662. [Google Scholar] [CrossRef]
- Cowan, C.; Mudher, A. Are Tau Aggregates Toxic or Protective in Tauopathies? Front. Neurol. 2013, 4, 114. [Google Scholar] [CrossRef]
- Limorenko, G.; Lashuel, H.A. Revisiting the grammar of Tau aggregation and pathology formation: How new insights from brain pathology are shaping how we study and target Tauopathies. Chem. Soc. Rev. 2022, 51, 513–565. [Google Scholar] [CrossRef]
- Gerson, J.E.; Kayed, R. Formation and propagation of tau oligomeric seeds. Front. Neurol. 2013, 4, 93. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, H.; Tang, X.-Q. Tau internalization: A complex step in tau propagation. Ageing Res. Rev. 2021, 67, 101272. [Google Scholar] [CrossRef]
- Congdon, E.E.; Jiang, Y.; Sigurdsson, E.M. Targeting tau only extracellularly is likely to be less efficacious than targeting it both intra- and extracellularly. Semin. Cell Dev. Biol. 2022, 126, 125–137. [Google Scholar] [CrossRef]
- Guzmán-Martinez, L.; Farías, G.A.; Maccioni, R.B. Tau oligomers as potential targets for Alzheimer’s diagnosis and novel drugs. Front. Neurol. 2013, 4, 167. [Google Scholar] [CrossRef] [PubMed]
- Kopeikina, K.J.; Hyman, B.T.; Spires-Jones, T.L. Soluble forms of tau are toxic in Alzheimer’s disease. Transl. Neurosci. 2012, 3, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.M.; Himmelstein, D.S.; Lancia, J.K.; Binder, L.I. Tau oligomers and tau toxicity in neurodegenerative disease. Biochem. Soc. Trans. 2012, 40, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Niewiadomska, G.; Niewiadomski, W.; Steczkowska, M.; Gasiorowska, A. Tau Oligomers Neurotoxicity. Life 2021, 11, 28. [Google Scholar] [CrossRef]
- Orr, M.E.; Sullivan, A.C.; Frost, B. A Brief Overview of Tauopathy: Causes, Consequences, and Therapeutic Strategies. Trends Pharmacol. Sci. 2017, 38, 637–648. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Nahed, P.; Kambar, M.E.Z.N.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s disease drug development pipeline: 2022. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12295. [Google Scholar] [CrossRef]
- Soeda, Y.; Saito, M.; Maeda, S.; Ishida, K.; Nakamura, A.; Kojima, S.; Takashima, A. Methylene Blue Inhibits Formation of Tau Fibrils but not of Granular Tau Oligomers: A Plausible Key to Understanding Failure of a Clinical Trial for Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 68, 1677–1686. [Google Scholar] [CrossRef]
- Spires-Jones, T.L.; Friedman, T.; Pitstick, R.; Polydoro, M.; Roe, A.; Carlson, G.A.; Hyman, B.T. Methylene blue does not reverse existing neurofibrillary tangle pathology in the rTg4510 mouse model of tauopathy. Neurosci. Lett. 2014, 562, 63–68. [Google Scholar] [CrossRef]
- Maeda, S.; Sahara, N.; Saito, Y.; Murayama, M.; Yoshiike, Y.; Kim, H.; Miyasaka, T.; Murayama, S.; Ikai, A.; Takashima, A. Granular Tau Oligomers as Intermediates of Tau Filaments. Biochemistry 2007, 46, 3856–3861. [Google Scholar] [CrossRef]
- Flach, K.; Hilbrich, I.; Schiffmann, A.; Gärtner, U.; Krüger, M.; Leonhardt, M.; Waschipky, H.; Wick, L.; Arendt, T.; Holzer, M. Tau Oligomers Impair Artificial Membrane Integrity and Cellular Viability. J. Biol. Chem. 2012, 287, 43223–43233. [Google Scholar] [CrossRef]
- Soeda, Y.; Yoshikawa, M.; Almeida, O.F.X.; Sumioka, A.; Maeda, S.; Osada, H.; Kondoh, Y.; Saito, A.; Miyasaka, T.; Kimura, T.; et al. Toxic tau oligomer formation blocked by capping of cysteine residues with 1,2-dihydroxybenzene groups. Nat. Commun. 2015, 6, 10216. [Google Scholar] [CrossRef] [PubMed]
- Lasagna-Reeves, C.A.; Castillo-Carranza, D.L.; Guerrero-Muñoz, M.J.; Jackson, G.R.; Kayed, R. Preparation and Characterization of Neurotoxic Tau Oligomers. Biochemistry 2010, 49, 10039–10041. [Google Scholar] [CrossRef] [PubMed]
- Kaniyappan, S.; Chandupatla, R.R.; Mandelkow, E.-M.; Mandelkow, E. Extracellular low-n oligomers of tau cause selective synaptotoxicity without affecting cell viability. Alzheimer’s Dement. 2017, 13, 1270–1291. [Google Scholar] [CrossRef] [PubMed]
- Karikari, T.K.; Nagel, D.A.; Grainger, A.; Clarke-Bland, C.; Hill, E.J.; Moffat, K.G. Preparation of stable tau oligomers for cellular and biochemical studies. Anal. Biochem. 2019, 566, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Davidowitz, E.; Lopez, P.; Emadi, S.; Moe, J.; Sierks, M. Trimeric Tau Is Toxic to Human Neuronal Cells at Low Nanomolar Concentrations. Int. J. Cell Biol. 2013, 2013, 260787. [Google Scholar] [CrossRef] [PubMed]
- Mirbaha, H.; Chen, D.; Mullapudi, V.; Terpack, S.J.; White, C.L., 3rd; Joachimiak, L.A.; Diamond, M.I. Seed-competent tau monomer initiates pathology in a tauopathy mouse model. J. Biol. Chem. 2022, 298, 102163. [Google Scholar] [CrossRef]
- Saroja, S.R.; Sharma, A.; Hof, P.R.; Pereira, A.C. Differential expression of tau species and the association with cognitive decline and synaptic loss in Alzheimer’s disease. Alzheimer’s Dement. 2022, 18, 1602–1615. [Google Scholar] [CrossRef]
- Kovacs, G.G. Invited review: Neuropathology of tauopathies: Principles and practice. Neuropathol. Appl. Neurobiol. 2015, 41, 3–23. [Google Scholar] [CrossRef]
- Ghetti, B.; Oblak, A.L.; Boeve, B.F.; Johnson, K.A.; Dickerson, B.C.; Goedert, M. Invited review: Frontotemporal dementia caused by microtubule-associated protein tau gene (MAPT) mutations: A chameleon for neuropathology and neuroimaging. Neuropathol. Appl. Neurobiol. 2015, 41, 24–46. [Google Scholar] [CrossRef]
- Hromadkova, L.; Siddiqi, M.K.; Liu, H.; Safar, J.G. Populations of Tau Conformers Drive Prion-like Strain Effects in Alzheimer’s Disease and Related Dementias. Cells 2022, 11, 2997. [Google Scholar]
- Vaquer-Alicea, J.; Diamond, M.I.; Joachimiak, L.A. Tau strains shape disease. Acta Neuropathol. 2021, 142, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.; Sammalkorpi, M.; Dewitt, D.C.; Trexler, A.J.; Elbaum-Garfinkle, S.; O’hern, C.S.; Rhoades, E. The conformational ensembles of α-synuclein and tau: Combining single-molecule FRET and simulations. Biophys. J. 2012, 103, 1940–1949. [Google Scholar] [CrossRef] [PubMed]
- Gerson, J.E.; Mudher, A.; Kayed, R. Potential mechanisms and implications for the formation of tau oligomeric strains. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.M.; Thomas, T.L.; Woodard, D.R.; Kashmer, O.M.; Diamond, M.I. Tau monomer encodes strains. eLife 2018, 7, e37813. [Google Scholar] [CrossRef]
- Mirbaha, H.; Chen, D.; Morazova, O.A.; Ruff, K.M.; Sharma, A.M.; Liu, X.; Goodarzi, M.; Pappu, R.V.; Colby, D.W.; Mirzaei, H.; et al. Inert and seed-competent tau monomers suggest structural origins of aggregation. eLife 2018, 7, e36584. [Google Scholar] [CrossRef]
- Huang, R.Y.-C.; Iacob, R.E.; Sankaranarayanan, S.; Yang, L.; Ahlijanian, M.; Tao, L.; Tymiak, A.A.; Chen, G. Probing Conformational Dynamics of Tau Protein by Hydrogen/Deuterium Exchange Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2018, 29, 174–182. [Google Scholar] [CrossRef]
- Shafiei, S.S.; Guerrero-Muñoz, M.J.; Castillo-Carranza, D.L. Tau Oligomers: Cytotoxicity, Propagation, and Mitochondrial Damage. Front. Aging Neurosci. 2017, 9, 83. [Google Scholar] [CrossRef]
- Sonawane, S.K.; Uversky, V.N.; Chinnathambi, S. Baicalein inhibits heparin-induced Tau aggregation by initializing non-toxic Tau oligomer formation. Cell Commun. Signal. 2021, 19, 16. [Google Scholar] [CrossRef]
- Mamun, A.A.; Uddin, M.S.; Mathew, B.; Ashraf, G.M. Toxic tau: Structural origins of tau aggregation in Alzheimer’s disease. Neural Regen. Res. 2020, 15, 1417–1420. [Google Scholar]
- Penke, B.; Szűcs, M.; Bogár, F. Oligomerization and Conformational Change Turn Monomeric β-Amyloid and Tau Proteins Toxic: Their Role in Alzheimer’s Pathogenesis. Molecules 2020, 25, 1659. [Google Scholar] [CrossRef]
- Lo, C.H.; Lim, C.K.-W.; Ding, Z.; Wickramasinghe, S.P.; Braun, A.R.; Ashe, K.H.; Rhoades, E.; Thomas, D.D.; Sachs, J.N. Targeting the ensemble of heterogeneous tau oligomers in cells: A novel small molecule screening platform for tauopathies. Alzheimer’s Dement. 2019, 15, 1489–1502. [Google Scholar] [CrossRef] [PubMed]
- Nizynski, B.; Nieznanska, H.; Dec, R.; Boyko, S.; Dzwolak, W.; Nieznanski, K. Amyloidogenic cross-seeding of Tau protein: Transient emergence of structural variants of fibrils. PLoS ONE 2018, 13, e0201182. [Google Scholar] [CrossRef] [PubMed]
- Karikari, T.K.; Turner, A.; Stass, R.; Lee, L.C.Y.; Wilson, B.; Nagel, D.A.; Hill, E.J.; Moffat, K.G. Expression and purification of tau protein and its frontotemporal dementia variants using a cleavable histidine tag. Protein Expr. Purif. 2017, 130, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Götz, J.; Xia, D.; Leinenga, G.; Chew, Y.L.; Nicholas, H. What Renders TAU Toxic. Front. Neurol. 2013, 4, 72. [Google Scholar] [CrossRef]
- Gendron, T.F.; Petrucelli, L. The role of tau in neurodegeneration. Mol. Neurodegener. 2009, 4, 13. [Google Scholar] [CrossRef]
- Blair, L.J.; Nordhues, B.A.; Hill, S.E.; Scaglione, K.M.; O’leary, J.C., 3rd; Fontaine, S.N.; Breydo, L.; Zhang, B.; Li, P.; Wang, L.; et al. Accelerated neurodegeneration through chaperone-mediated oligomerization of tau. J. Clin. Investig. 2013, 123, 4158–4169. [Google Scholar] [CrossRef]
- Novak, P.; Cehlar, O.; Skrabana, R.; Novak, M. Tau Conformation as a Target for Disease-Modifying Therapy: The Role of Truncation. J. Alzheimer’s Dis. 2018, 64, S535–S546. [Google Scholar] [CrossRef]
- Avila, J.; Pallas, N.; Bolós, M.; Sayas, C.L.; Hernandez, F. Intracellular and extracellular microtubule associated protein tau as a therapeutic target in Alzheimer disease and other tauopathies. Expert Opin. Ther. Targets 2016, 20, 653–661. [Google Scholar] [CrossRef]
- Lauretti, E.; Praticò, D. Alzheimer’s disease: Phenotypic approaches using disease models and the targeting of tau protein. Expert Opin. Ther. Targets 2020, 24, 319–330. [Google Scholar] [CrossRef]
- Lo Cascio, F.; Kayed, R. Azure C Targets and Modulates Toxic Tau Oligomers. ACS Chem. Neurosci. 2018, 9, 1317–1326. [Google Scholar] [CrossRef]
- Wobst, H.J.; Sharma, A.; Diamond, M.I.; Wanker, E.E.; Bieschke, J. The green tea polyphenol (-)-epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios. FEBS Lett. 2015, 589, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Rane, J.S.; Bhaumik, P.; Panda, D. Curcumin Inhibits Tau Aggregation and Disintegrates Preformed Tau Filaments in vitro. J. Alzheimer’s Dis. 2017, 60, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lo Cascio, F.; Gao, J.; Kayed, R.; Huang, X. Binding and neurotoxicity mitigation of toxic tau oligomers by synthetic heparin like oligosaccharides. Chem. Commun. 2018, 54, 10120–10123. [Google Scholar] [CrossRef] [PubMed]
- Baggett, D.W.; Nath, A. The Rational Discovery of a Tau Aggregation Inhibitor. Biochemistry 2018, 57, 6099–6107. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huang, X.; Yan, L.; Zhou, L.; Yan, C.; Wu, J.; Su, Z.; Huang, Y. The Structure Biology of Tau and Clue for Aggregation Inhibitor Design. Protein J. 2021, 40, 656–668. [Google Scholar] [CrossRef]
- Giovannini, J.; Smeralda, W.; Jouanne, M.; Sopkova-De Oliveira Santos, J.; Catto, M.; Voisin-Chiret, A.S. Tau protein aggregation: Key features to improve drug discovery screening. Drug Discov. Today 2022, 27, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Umeda, T.; Sakai, A.; Shigemori, K.; Yokota, A.; Kumagai, T.; Tomiyama, T. Oligomer-Targeting Prevention of Neurodegenerative Dementia by Intranasal Rifampicin and Resveratrol Combination—A Preclinical Study in Model Mice. Front. Neurosci. 2021, 15, 763476. [Google Scholar] [CrossRef]
- Umeda, T.; Uekado, R.; Shigemori, K.; Eguchi, H.; Tomiyama, T. Nasal Rifampicin Halts the Progression of Tauopathy by Inhibiting Tau Oligomer Propagation in Alzheimer Brain Extract-Injected Mice. Biomedicines 2022, 10, 297. [Google Scholar] [CrossRef]
- Medina, M. An Overview on the Clinical Development of Tau-Based Therapeutics. Int. J. Mol. Sci. 2018, 19, 1160. [Google Scholar] [CrossRef]
- Giacobini, E.; Gold, G. Alzheimer disease therapy—Moving from amyloid-β to tau. Nat. Rev. Neurol. 2013, 9, 677. [Google Scholar] [CrossRef]
- Lee, H.E.; Lim, D.; Lee, J.Y.; Lim, S.M.; Pae, A.N. Recent tau-targeted clinical strategies for the treatment of Alzheimer’s disease. Future Med. Chem. 2019, 11, 1845–1848. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.H.; Sachs, J.N. The role of wild-type tau in Alzheimer’s disease and related tauopathies. J. Life Sci. 2020, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Tzu-Kang, S.; Hui-Yun, C. Tauopathy. In Cognitive Disorders; Foyaca, S.H., Ed.; IntechOpen: Rijeka, Croatia, 2018; Chapter 3. [Google Scholar]
- Maeda, S.; Takashima, A. Tau Oligomers. In Tau Biology; Takashima, A., Wolozin, B., Buee, L., Eds.; Springer: Singapore, 2019; pp. 373–380. [Google Scholar]
- Manassero, G.; Guglielmotto, M.; Monteleone, D.; Vasciaveo, V.; Butenko, O.; Tamagno, E.; Arancio, O.; Tabaton, M. Dual Mechanism of Toxicity for Extracellular Injection of Tau Oligomers versus Monomers in Human Tau Mice. J. Alzheimer’s Dis. 2017, 59, 743–751. [Google Scholar] [CrossRef]
- Shahpasand-Kroner, H.; Portillo, J.; Lantz, C.; Seidler, P.M.; Sarafian, N.; Loo, J.A.; Bitan, G. Three-repeat and four-repeat tau isoforms form different oligomers. Protein Sci. 2022, 31, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.H. Recent advances in cellular biosensor technology to investigate tau oligomerization. Bioeng. Transl. Med. 2021, 6, e10231. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Hu, Y.; Wang, Z.H.; Luo, Y.; Zhang, Y.; Liu, X.P.; Feng, Q.; Wang, Q.; Ye, K.; Liu, G.P.; et al. Human wild-type full-length tau accumulation disrupts mitochondrial dynamics and the functions via increasing mitofusins. Sci. Rep. 2016, 6, 24756. [Google Scholar] [CrossRef]
- Ozcelik, S.; Sprenger, F.; Skachokova, Z.; Fraser, G.; Abramowski, D.; Clavaguera, F.; Probst, A.; Frank, S.; Müller, M.; Staufenbiel, M.; et al. Co-expression of truncated and full-length tau induces severe neurotoxicity. Mol. Psychiatry 2016, 21, 1790–1798. [Google Scholar] [CrossRef]
- Pickhardt, M.; Biernat, J.; Hübschmann, S.; Dennissen, F.J.A.; Timm, T.; Aho, A.; Mandelkow, E.M.; Mandelkow, E. Time course of Tau toxicity and pharmacologic prevention in a cell model of Tauopathy. Neurobiol. Aging 2017, 57, 47–63. [Google Scholar] [CrossRef]
- Hu, J.Y.; Zhang, D.L.; Liu, X.L.; Li, X.S.; Cheng, X.Q.; Chen, J.; Du, H.N.; Liang, Y. Pathological concentration of zinc dramatically accelerates abnormal aggregation of full-length human Tau and thereby significantly increases Tau toxicity in neuronal cells. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 414–427. [Google Scholar] [CrossRef]
- Tepper, K.; Biernat, J.; Kumar, S.; Wegmann, S.; Timm, T.; Hübschmann, S.; Redecke, L.; Mandelkow, E.M.; Müller, D.J.; Mandelkow, E. Oligomer formation of tau protein hyperphosphorylated in cells. J. Biol. Chem. 2014, 289, 34389–34407. [Google Scholar] [CrossRef]
- Shin, S.; Kim, D.; Song, J.Y.; Jeong, H.; Hyeon, S.J.; Kowall, N.W.; Ryu, H.; Pae, A.N.; Lim, S.; Kim, Y.K. Visualization of soluble tau oligomers in TauP301L-BiFC transgenic mice demonstrates the progression of tauopathy. Prog. Neurobiol. 2020, 187, 101782. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.R.; Liao, E.E.; Horvath, M.; Kalra, P.; Acosta, K.; Young, M.C.; Kochen, N.N.; Lo, C.H.; Brown, R.; Evans, M.D.; et al. Potent inhibitors of toxic alpha-synuclein identified via cellular time-resolved FRET biosensors. NPJ Park. Dis. 2021, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.H.; Pandey, N.K.; Lim, C.K.-W.; Ding, Z.; Tao, M.; Thomas, D.D.; Langen, R.; Sachs, J.N. Discovery of Small Molecule Inhibitors of Huntingtin Exon 1 Aggregation by FRET-Based High-Throughput Screening in Living Cells. ACS Chem. Neurosci. 2020, 11, 2286–2295. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Qi, R.; Qiao, Q.; Li, X.; Li, F.; Wan, J.; Zhang, Q.; Wei, G. Heparin remodels the microtubule-binding repeat R3 of Tau protein towards fibril-prone conformations. Phys. Chem. Chem. Phys. 2021, 23, 20406–20418. [Google Scholar] [CrossRef] [PubMed]
- Fichou, Y.; Vigers, M.; Goring, A.K.; Eschmann, N.A.; Han, S. Heparin-induced tau filaments are structurally heterogeneous and differ from Alzheimer’s disease filaments. Chem. Commun. 2018, 54, 4573–4576. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Balmik, A.A.; Chinnathambi, S. Phagocytosis of full-length Tau oligomers by Actin-remodeling of activated microglia. J. Neuroinflamm. 2020, 17, 10. [Google Scholar] [CrossRef]
- Sahara, N.; Maeda, S.; Murayama, M.; Suzuki, T.; Dohmae, N.; Yen, S.-H.; Takashima, A. Assembly of two distinct dimers and higher-order oligomers from full-length tau. Eur. J. Neurosci. 2007, 25, 3020–3029. [Google Scholar] [CrossRef]
- Chidambaram, H.; Chinnathambi, S. Role of cysteines in accelerating Tau filament formation. J. Biomol. Struct. Dyn. 2022, 40, 4366–4375. [Google Scholar] [CrossRef]
- Saito, T.; Chiku, T.; Oka, M.; Wada-Kakuda, S.; Nobuhara, M.; Oba, T.; Shinno, K.; Abe, S.; Asada, A.; Sumioka, A.; et al. Disulfide bond formation in microtubule-associated tau protein promotes tau accumulation and toxicity in vivo. Hum. Mol. Genet. 2021, 30, 1955–1967. [Google Scholar] [CrossRef]
- Hellén, M.; Bhattacharjee, A.; Uronen, R.-L.; Huttunen, H.J. Membrane interaction and disulphide-bridge formation in the unconventional secretion of Tau. Biosci. Rep. 2021, 41, BSR20210148. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.M.; Kim, D.; Yu, Y.H.; Lim, S.; Kim, D.J.; Chang, Y.-T.; Ha, H.-H.; Kim, Y.K. Inhibition of tau aggregation by a rosamine derivative that blocks tau intermolecular disulfide cross-linking. Amyloid 2014, 21, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Annadurai, N.; Malina, L.; Salmona, M.; Diomede, L.; Bastone, A.; Cagnotto, A.; Romeo, M.; Šrejber, M.; Berka, K.; Otyepka, M.; et al. Antitumour drugs targeting tau R3 VQIVYK and Cys322 prevent seeding of endogenous tau aggregates by exogenous seeds. FEBS J. 2022, 289, 1929–1949. [Google Scholar] [CrossRef] [PubMed]
- Man, V.H.; Lin, D.; He, X.; Gao, J.; Wang, J. Joint Computational/Cell-Based Approach for Screening Inhibitors of Tau Oligomerization: A Proof-of-Concept Study. J. Alzheimer’s Dis. 2022, 89, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Savanur, G.; Madhavadas, S. Passive immunization targeting the N-terminal region of phosphorylated tau (residues 68–71) improves spatial memory in okadaic acid induced tauopathy model rats. Biochem. Biophys. Res. Commun. 2017, 483, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Savanur, G. Antibodies directed to the phospho-tau peptide (residues 111–137) dissociate tau oligomers and reduce the spatial memory deficits in non-transgenic tauopathy model rats. Indian J. Exp. Biol. 2020, 58, 355–359. [Google Scholar]
- Barghorn, S.; Biernat, J.; Mandelkow, E. Purification of Recombinant Tau Protein and Preparation of Alzheimer-Paired Helical Filaments In Vitro. In Amyloid Proteins: Methods and Protocols; Sigurdsson, E.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 35–51. [Google Scholar]
- Sonawane, S.K.; Balmik, A.A.; Boral, D.; Ramasamy, S.; Chinnathambi, S. Baicalein suppresses Repeat Tau fibrillization by sequestering oligomers. Arch. Biochem. Biophys. 2019, 675, 108119. [Google Scholar] [CrossRef]
- González, C.; Cartagena, C.; Caballero, L.; Melo, F.; Areche, C.; Cornejo, A. The Fumarprotocetraric Acid Inhibits Tau Covalently, Avoiding Cytotoxicity of Aggregates in Cells. Molecules 2021, 26, 3760. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Li, L.-J.; Dong, Q.-X.; Zhu, J.; Huang, Y.-R.; Hou, S.-J.; Yu, X.-L.; Liu, R.-T. Rutin prevents tau pathology and neuroinflammation in a mouse model of Alzheimer’s disease. J. Neuroinflamm. 2021, 18, 131. [Google Scholar] [CrossRef]
- Majerova, P.; Zilkova, M.; Kazmerova, Z.; Kovac, A.; Paholikova, K.; Kovacech, B.; Zilka, N.; Novak, M. Microglia display modest phagocytic capacity for extracellular tau oligomers. J. Neuroinflamm. 2014, 11, 161. [Google Scholar] [CrossRef]
- Usenovic, M.; Niroomand, S.; Drolet, R.E.; Yao, L.; Gaspar, R.C.; Hatcher, N.G.; Schachter, J.; Renger, J.J.; Parmentier-Batteur, S. Internalized Tau Oligomers Cause Neurodegeneration by Inducing Accumulation of Pathogenic Tau in Human Neurons Derived from Induced Pluripotent Stem Cells. J. Neurosci. 2015, 35, 14234–14250. [Google Scholar] [CrossRef]
- Gómez-Ramos, A.; Díaz-Hernández, M.; Cuadros, R.; Hernández, F.; Avila, J. Extracellular tau is toxic to neuronal cells. FEBS Lett. 2006, 580, 4842–4850. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.R.; Remmers, C.; Fu, Y.; Brooker, S.; Kanaan, N.M.; Vana, L.; Ward, S.; Reyes, J.F.; Philibert, K.; Glucksman, M.J.; et al. Characterization of prefibrillar Tau oligomers in vitro and in Alzheimer disease. J. Biol. Chem. 2011, 286, 23063–23076. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.M.; Himmelstein, D.S.; Lancia, J.K.; Fu, Y.; Patterson, K.R.; Binder, L.I. TOC1: Characterization of a Selective Oligomeric Tau Antibody. J. Alzheimer’s Dis. 2013, 37, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Mufson, E.J.; Ward, S.; Binder, L. Prefibrillar Tau Oligomers in Mild Cognitive Impairment and Alzheimer’s Disease. Neurodegener. Dis. 2014, 13, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Akbari, M.; Schirmer, C.; Reynaert, M.-L.; Loyens, A.; Lefebvre, B.; Buée, L.; Croteau, D.L.; Galas, M.-C.; Bohr, V.A. Hippocampal tau oligomerization early in tau pathology coincides with a transient alteration of mitochondrial homeostasis and DNA repair in a mouse model of tauopathy. Acta Neuropathol. Commun. 2020, 8, 25. [Google Scholar] [CrossRef]
- Gerson, J.E.; Sengupta, U.; Kayed, R. Tau Oligomers as Pathogenic Seeds: Preparation and Propagation In Vitro and In Vivo. Methods Mol. Biol. 2017, 1523, 141–157. [Google Scholar]
- Lasagna-Reeves, C.A.; Castillo-Carranza, D.L.; Sengupta, U.; Clos, A.L.; Jackson, G.R.; Kayed, R. Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild-type mice. Mol. Neurodegener. 2011, 6, 39. [Google Scholar] [CrossRef]
- Lasagna-Reeves, C.A.; Castillo-Carranza, D.L.; Sengupta, U.; Guerrero-Munoz, M.J.; Kiritoshi, T.; Neugebauer, V.; Jackson, G.R.; Kayed, R. Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci. Rep. 2012, 2, 700. [Google Scholar] [CrossRef]
- Castillo-Carranza, D.L.; Gerson, J.E.; Sengupta, U.; Guerrero-Muñoz, M.J.; Lasagna-Reeves, C.A.; Kayed, R. Specific Targeting of Tau Oligomers in Htau Mice Prevents Cognitive Impairment and Tau Toxicity Following Injection with Brain-Derived Tau Oligomeric Seeds. J. Alzheimer’s Dis. 2014, 40, S97–S111. [Google Scholar] [CrossRef]
- Violet, M.; Chauderlier, A.; Delattre, L.; Tardivel, M.; Chouala, M.S.; Sultan, A.; Marciniak, E.; Humez, S.; Binder, L.; Kayed, R.; et al. Prefibrillar Tau oligomers alter the nucleic acid protective function of Tau in hippocampal neurons in vivo. Neurobiol. Dis. 2015, 82, 540–551. [Google Scholar] [CrossRef]
- Umeda, T.; Ono, K.; Sakai, A.; Yamashita, M.; Mizuguchi, M.; Klein, W.L.; Yamada, M.; Mori, H.; Tomiyama, T. Rifampicin is a candidate preventive medicine against amyloid-β and tau oligomers. Brain 2016, 139, 1568–1586. [Google Scholar] [CrossRef] [PubMed]
- Fá, M.; Puzzo, D.; Piacentini, R.; Staniszewski, A.; Zhang, H.; Baltrons, M.A.; Li Puma, D.D.; Chatterjee, I.; Li, J.; Saeed, F.; et al. Extracellular Tau Oligomers Produce An Immediate Impairment of LTP and Memory. Sci. Rep. 2016, 6, 19393. [Google Scholar] [CrossRef] [PubMed]
- Nilson, A.N.; English, K.C.; Gerson, J.E.; Barton Whittle, T.; Nicolas Crain, C.; Xue, J.; Sengupta, U.; Castillo-Carranza, D.L.; Zhang, W.; Gupta, P.; et al. Tau Oligomers Associate with Inflammation in the Brain and Retina of Tauopathy Mice and in Neurodegenerative Diseases. J. Alzheimer’s Dis. 2017, 55, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Kolarova, M.; Sengupta, U.; Bartos, A.; Ricny, J.; Kayed, R. Tau Oligomers in Sera of Patients with Alzheimer’s Disease and Aged Controls. J. Alzheimer’s Dis. 2017, 58, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Comerota, M.M.; Tumurbaatar, B.; Krishnan, B.; Kayed, R.; Taglialatela, G. Near Infrared Light Treatment Reduces Synaptic Levels of Toxic Tau Oligomers in Two Transgenic Mouse Models of Human Tauopathies. Mol. Neurobiol. 2019, 56, 3341–3355. [Google Scholar] [CrossRef] [PubMed]
- Puangmalai, N.; Bhatt, N.; Montalbano, M.; Sengupta, U.; Gaikwad, S.; Ventura, F.; Mcallen, S.; Ellsworth, A.; Garcia, S.; Kayed, R. Internalization mechanisms of brain-derived tau oligomers from patients with Alzheimer’s disease, progressive supranuclear palsy and dementia with Lewy bodies. Cell Death Dis. 2020, 11, 314. [Google Scholar] [CrossRef]
- Bittar, A.; Bhatt, N.; Hasan, T.F.; Montalbano, M.; Puangmalai, N.; Mcallen, S.; Ellsworth, A.; Carretero Murillo, M.; Taglialatela, G.; Lucke-Wold, B.; et al. Neurotoxic tau oligomers after single versus repetitive mild traumatic brain injury. Brain Commun. 2019, 1, fcz004. [Google Scholar] [CrossRef]
- Clavaguera, F.; Grueninger, F.; Tolnay, M. Intercellular transfer of tau aggregates and spreading of tau pathology: Implications for therapeutic strategies. Neuropharmacology 2014, 76, 9–15. [Google Scholar] [CrossRef]
- Guerrero-Muñoz, M.J.; Gerson, J.; Castillo-Carranza, D.L. Tau Oligomers: The Toxic Player at Synapses in Alzheimer’s Disease. Front. Cell. Neurosci. 2015, 9, 464. [Google Scholar] [CrossRef]
- Shin, W.S.; Di, J.; Cao, Q.; Li, B.; Seidler, P.M.; Murray, K.A.; Bitan, G.; Jiang, L. Amyloid β-protein oligomers promote the uptake of tau fibril seeds potentiating intracellular tau aggregation. Alzheimer’s Res. Ther. 2019, 11, 86. [Google Scholar] [CrossRef]
- Ghag, G.; Bhatt, N.; Cantu, D.V.; Guerrero-Munoz, M.J.; Ellsworth, A.; Sengupta, U.; Kayed, R. Soluble tau aggregates, not large fibrils, are the toxic species that display seeding and cross-seeding behavior. Protein Sci. 2018, 27, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Carranza, D.L.; Sengupta, U.; Guerrero-Muñoz, M.J.; Lasagna-Reeves, C.A.; Gerson, J.E.; Singh, G.; Estes, D.M.; Barrett, A.D.T.; Dineley, K.T.; Jackson, G.R.; et al. Passive Immunization with Tau Oligomer Monoclonal Antibody Reverses Tauopathy Phenotypes without Affecting Hyperphosphorylated Neurofibrillary Tangles. J. Neurosci. 2014, 34, 4260–4272. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Muñoz, M.J.; Castillo-Carranza, D.L.; Kayed, R. Therapeutic approaches against common structural features of toxic oligomers shared by multiple amyloidogenic proteins. Biochem. Pharmacol. 2014, 88, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Lo Cascio, F.; Puangmalai, N.; Ellsworth, A.; Bucchieri, F.; Pace, A.; Palumbo Piccionello, A.; Kayed, R. Toxic Tau Oligomers Modulated by Novel Curcumin Derivatives. Sci. Rep. 2019, 9, 19011. [Google Scholar] [CrossRef]
- Lee, S.; Shea, T.B. Caspase-Mediated Truncation of Tau Potentiates Aggregation. Int. J. Alzheimer’s Dis. 2012, 2012, 731063. [Google Scholar] [CrossRef]
- Ono, K.; Yamada, M. Low-n oligomers as therapeutic targets of Alzheimer’s disease. J. Neurochem. 2011, 117, 19–28. [Google Scholar] [CrossRef]
- Zou, Y.; Qi, B.; Tan, J.; Sun, Y.; Gong, Y.; Zhang, Q. Mechanistic insight into the disruption of Tau R3–R4 protofibrils by curcumin and epinephrine: An all-atom molecular dynamics study. Phys. Chem. Chem. Phys. 2022, 24, 20454–20465. [Google Scholar] [CrossRef]
- Kumar, H.; Udgaonkar, J.B. Elongation of Fibrils Formed by a Tau Fragment is Inhibited by a Transient Dimeric Intermediate. J. Phys. Chem. B 2022, 126, 3385–3397. [Google Scholar] [CrossRef]
- Holmes, B.B.; Furman, J.L.; Mahan, T.E.; Yamasaki, T.R.; Mirbaha, H.; Eades, W.C.; Belaygorod, L.; Cairns, N.J.; Holtzman, D.M.; Diamond, M.I. Proteopathic tau seeding predicts tauopathy in vivo. Proc. Natl. Acad. Sci. USA 2014, 111, E4376–E4385. [Google Scholar] [CrossRef]
- Annadurai, N.; Malina, L.; Malohlava, J.; Hajdúch, M.; Das, V. Tau R2 and R3 are essential regions for tau aggregation, seeding and propagation. Biochimie 2022, 200, 79–86. [Google Scholar] [CrossRef]
- Hill, E.; Karikari, T.K.; Lantero-Rodriguez, J.; Zetterberg, H.; Blennow, K.; Richardson, M.J.; Wall, M.J. Truncating tau reveals different pathophysiological actions of oligomers in single neurons. Commun. Biol. 2021, 4, 1265. [Google Scholar] [CrossRef] [PubMed]
- Chun, W.; Johnson, G.V. Activation of glycogen synthase kinase 3beta promotes the intermolecular association of tau. The use of fluorescence resonance energy transfer microscopy. J. Biol. Chem. 2007, 282, 23410–23417. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choi, H.; Lee, W.; Park, H.; Kam, T.-I.; Hong, S.-H.; Nah, J.; Jung, S.; Shin, B.; Lee, H.; et al. Caspase-cleaved tau exhibits rapid memory impairment associated with tau oligomers in a transgenic mouse model. Neurobiol. Dis. 2016, 87, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Zhu, F.; Kanaan, N.M.; Asano, R.; Shirafuji, N.; Sasaki, H.; Yamaguchi, T.; Enomoto, S.; Endo, Y.; Ueno, A.; et al. Clioquinol Decreases Levels of Phosphorylated, Truncated, and Oligomerized Tau Protein. Int. J. Mol. Sci. 2021, 22, 12063. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Timm, T.; Mandelkow, E.-M.; Mandelkow, E.; Wang, Y. Cleavage of Tau by calpain in Alzheimer’s disease: The quest for the toxic 17 kD fragment. Neurobiol. Aging 2011, 32, 1–14. [Google Scholar] [CrossRef]
- Rao, M.V.; Mcbrayer, M.K.; Campbell, J.; Kumar, A.; Hashim, A.; Sershen, H.; Stavrides, P.H.; Ohno, M.; Hutton, M.; Nixon, R.A. Specific calpain inhibition by calpastatin prevents tauopathy and neurodegeneration and restores normal lifespan in tau P301L mice. J. Neurosci. 2014, 34, 9222–9234. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, K.-M.; Yang, L.; Dong, Q.; Yu, J.-T. Tauopathies: New perspectives and challenges. Mol. Neurodegener. 2022, 17, 28. [Google Scholar] [CrossRef]
- Wegmann, S.; Biernat, J.; Mandelkow, E. A current view on Tau protein phosphorylation in Alzheimer’s disease. Curr. Opin. Neurobiol. 2021, 69, 131–138. [Google Scholar] [CrossRef]
- Iqbal, K.; Gong, C.X.; Liu, F. Hyperphosphorylation-induced tau oligomers. Front. Neurol. 2013, 4, 112. [Google Scholar] [CrossRef]
- Pradhan, A.; Mishra, S.; Surolia, A.; Panda, D. C1 Inhibits Liquid–Liquid Phase Separation and Oligomerization of Tau and Protects Neuroblastoma Cells against Toxic Tau Oligomers. ACS Chem. Neurosci. 2021, 12, 1989–2002. [Google Scholar] [CrossRef]
- Venkatramani, A.; Mukherjee, S.; Kumari, A.; Panda, D. Shikonin impedes phase separation and aggregation of tau and protects SH-SY5Y cells from the toxic effects of tau oligomers. Int. J. Biol. Macromol. 2022, 204, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Ash, P.E.A.; Lei, S.; Shattuck, J.; Boudeau, S.; Carlomagno, Y.; Medalla, M.; Mashimo, B.L.; Socorro, G.; Al-Mohanna, L.F.A.; Jiang, L.; et al. TIA1 potentiates tau phase separation and promotes generation of toxic oligomeric tau. Proc. Natl. Acad. Sci. USA 2021, 118, e2014188118. [Google Scholar] [CrossRef] [PubMed]
- Martinisi, A.; Flach, M.; Sprenger, F.; Frank, S.; Tolnay, M.; Winkler, D.T. Severe oligomeric tau toxicity can be reversed without long-term sequelae. Brain 2021, 144, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Cowan, C.M.; Quraishe, S.; Hands, S.; Sealey, M.; Mahajan, S.; Allan, D.W.; Mudher, A. Rescue from tau-induced neuronal dysfunction produces insoluble tau oligomers. Sci. Rep. 2015, 5, 17191. [Google Scholar] [CrossRef] [PubMed]
- Gyparaki, M.T.; Arab, A.; Sorokina, E.M.; Santiago-Ruiz, A.N.; Bohrer, C.H.; Xiao, J.; Lakadamyali, M. Tau forms oligomeric complexes on microtubules that are distinct from tau aggregates. Proc. Natl. Acad. Sci. USA 2021, 118, e2021461118. [Google Scholar] [CrossRef]
- Kulenkampff, K.; Wolf Perez, A.-M.; Sormanni, P.; Habchi, J.; Vendruscolo, M. Quantifying misfolded protein oligomers as drug targets and biomarkers in Alzheimer and Parkinson diseases. Nat. Rev. Chem. 2021, 5, 277–294. [Google Scholar] [CrossRef]
- Vagenknecht, P.; Luzgin, A.; Ono, M.; Ji, B.; Higuchi, M.; Noain, D.; Maschio, C.A.; Sobek, J.; Chen, Z.; Konietzko, U.; et al. Non-invasive imaging of tau-targeted probe uptake by whole brain multi-spectral optoacoustic tomography. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2137–2152. [Google Scholar] [CrossRef]
- Ayubcha, C.; Rigney, G.; Borja, A.J.; Werner, T.; Alavi, A. Tau-PET imaging as a molecular modality for Alzheimer’s disease. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 374–386. [Google Scholar]
- Maschio, C.; Ni, R. Amyloid and Tau Positron Emission Tomography Imaging in Alzheimer’s Disease and Other Tauopathies. Front. Aging Neurosci. 2022, 14, 838034. [Google Scholar] [CrossRef]
- Ni, R. Magnetic Resonance Imaging in Tauopathy Animal Models. Front. Aging Neurosci. 2022, 13, 791679. [Google Scholar] [CrossRef]
- Badachhape, A.; Parekh, P.A.; Mu, Q.; Bhavane, R.; Srivastava, M.; Stupin, I.; Bhandari, P.; Devkota, L.; Tanifum, E.; Ghaghada, K.; et al. A novel MRI contrast agent for identifying hyperphosphorylative neurons as a marker of future tau pathology. Alzheimer’s Dement. 2020, 16, e041080. [Google Scholar] [CrossRef]
- Tolar, M.; Hey, J.; Power, A.; Abushakra, S. Neurotoxic Soluble Amyloid Oligomers Drive Alzheimer’s Pathogenesis and Represent a Clinically Validated Target for Slowing Disease Progression. Int. J. Mol. Sci. 2021, 22, 6355. [Google Scholar] [CrossRef] [PubMed]
- Arora, H.; Ramesh, M.; Rajasekhar, K.; Govindaraju, T. Molecular Tools to Detect Alloforms of Aβ and Tau: Implications for Multiplexing and Multimodal Diagnosis of Alzheimer’s Disease. Bull. Chem. Soc. Jpn. 2020, 93, 507–546. [Google Scholar] [CrossRef]
- Hansson, O.; Mormino, E.C. Is longitudinal tau PET ready for use in Alzheimer’s disease clinical trials? Brain 2018, 141, 1241–1244. [Google Scholar] [CrossRef]
- Zhao, Y.; Tietz, O.; Kuan, W.-L.; Haji-Dheere, A.K.; Thompson, S.; Vallin, B.; Ronchi, E.; Tóth, G.; Klenerman, D.; Aigbirhio, F.I. A fluorescent molecular imaging probe with selectivity for soluble tau aggregated protein. Chem. Sci. 2020, 11, 4773–4778. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).