Immunological Effects of Cold Atmospheric Plasma-Treated Cells in Comparison with Those of Cells Treated with Lactaptin-Based Anticancer Drugs
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Mice
2.2. Chemicals and Antibodies
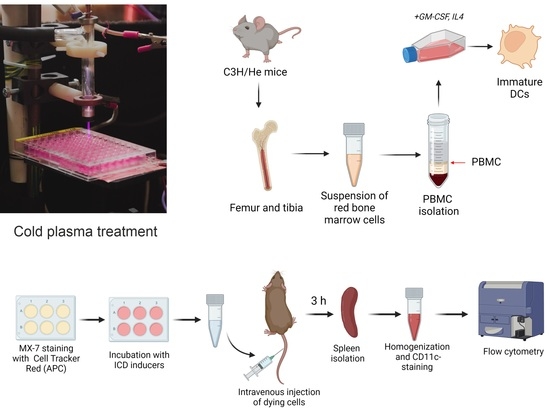
2.3. Cold Atmospheric Plasma System and Treatment Parameters
2.4. Quantitative Determination of IFN-Alpha in Culture Medium
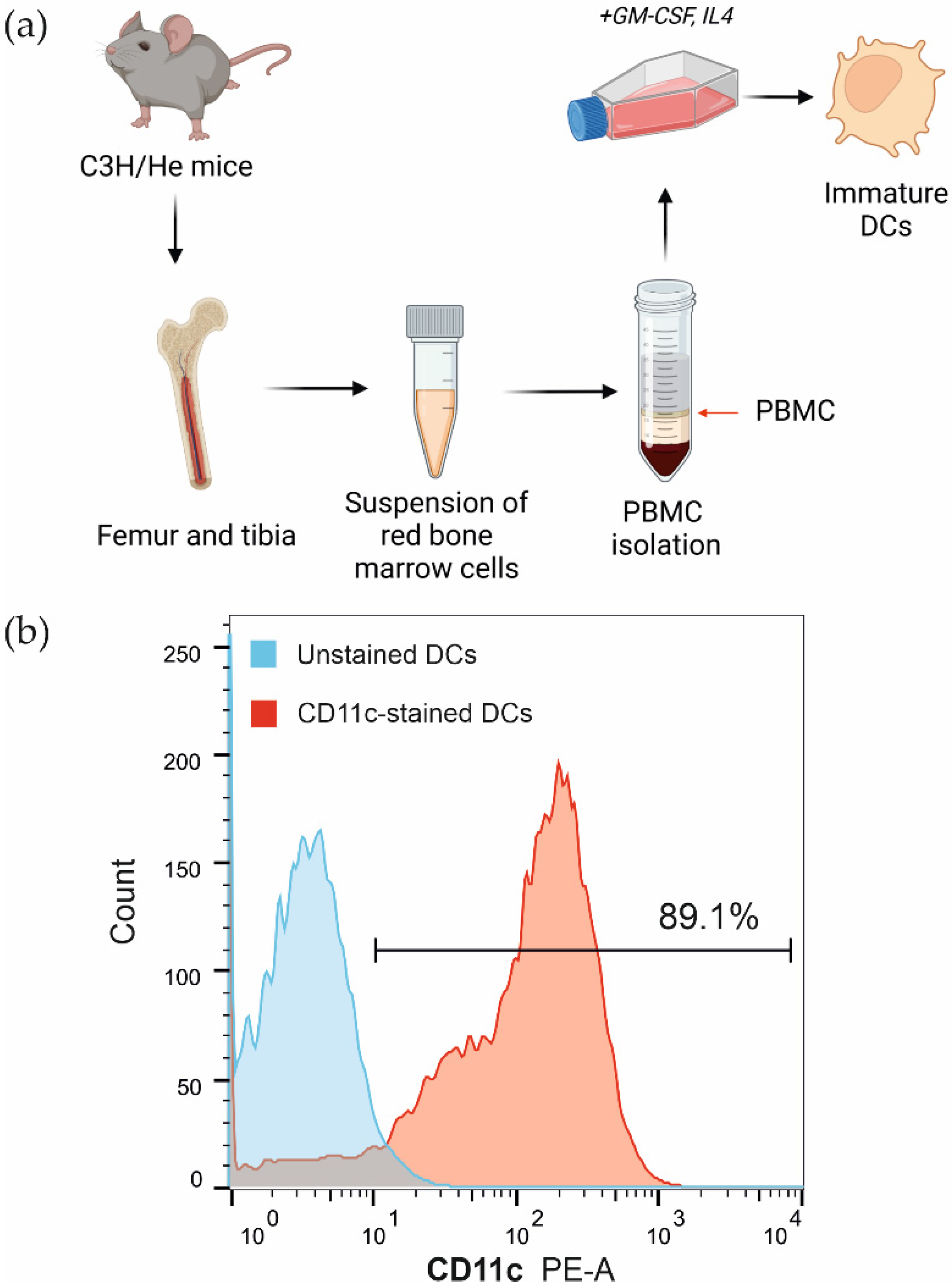
2.5. Bone Marrow-Derived Dendritic Cell Isolation
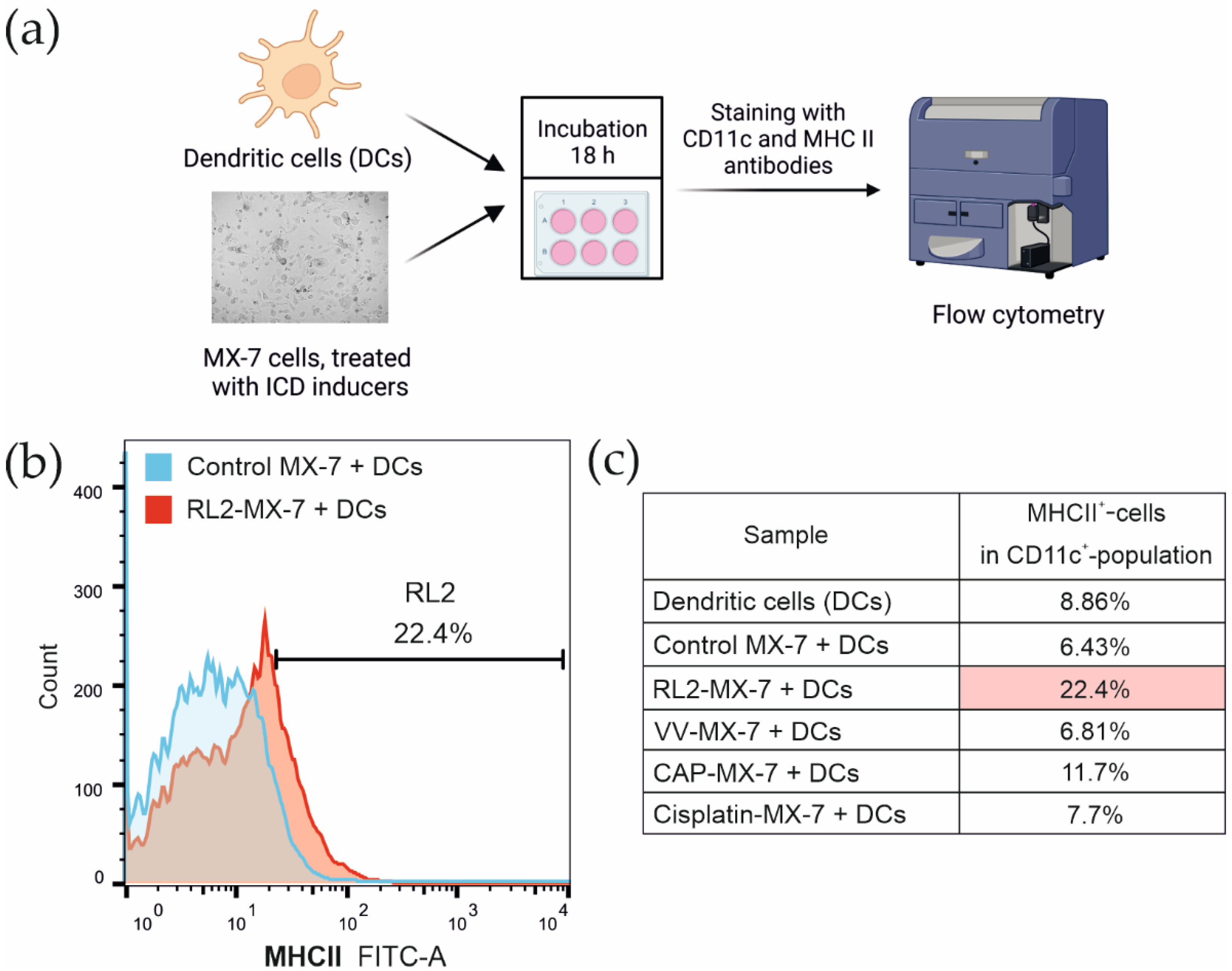
2.6. Flow Cytometry Analysis
2.7. Phagocytic Activity of Dendritic Cells
2.8. Dendritic Cell Maturation
2.9. In Vivo Assay of MX-7 Tumor Cell Uptake by Spleen CD11c+ Cells
2.10. Ethic Statement
2.11. Statistics
3. Results
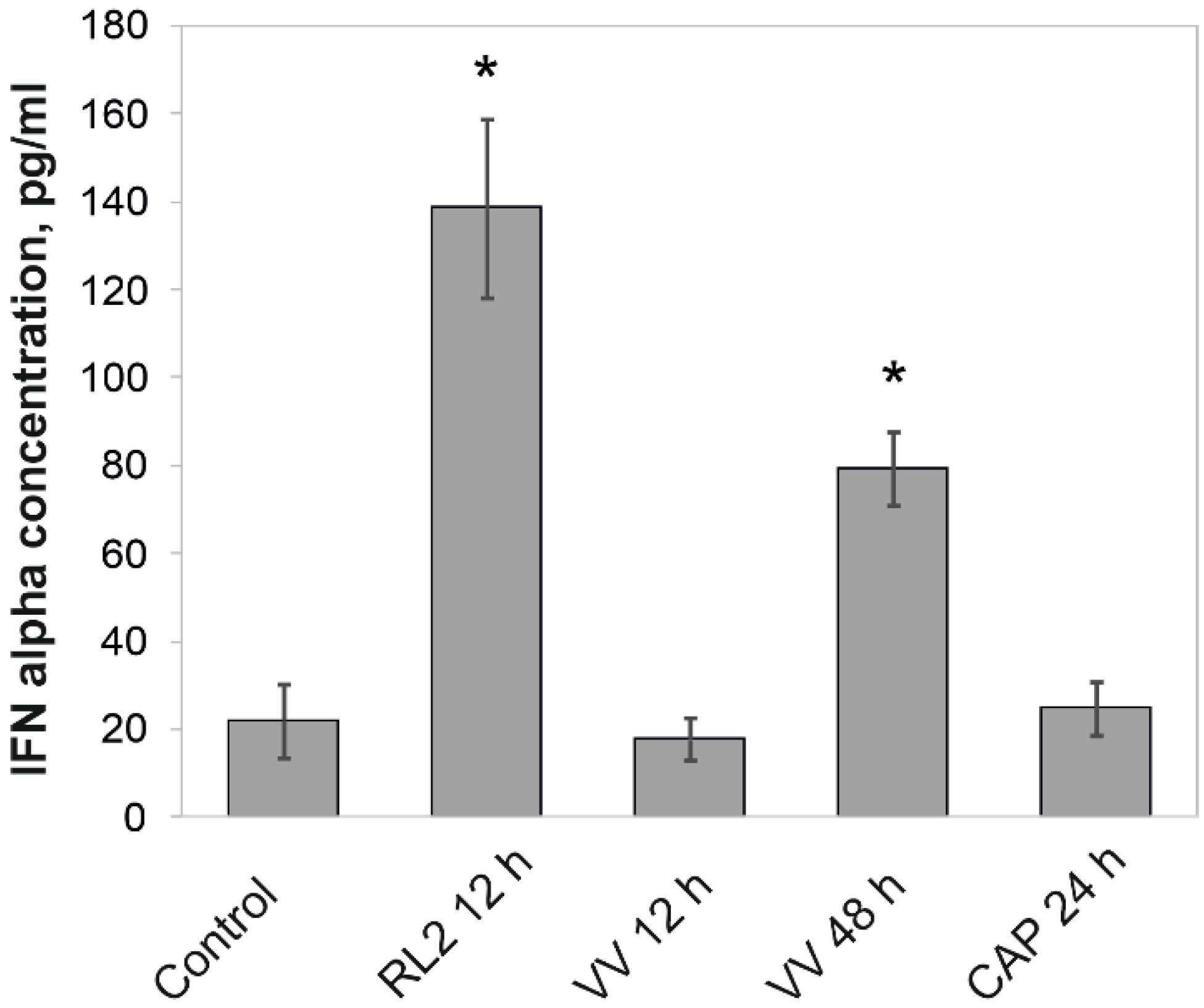
3.1. Release of IFN-α into the Intercellular Space
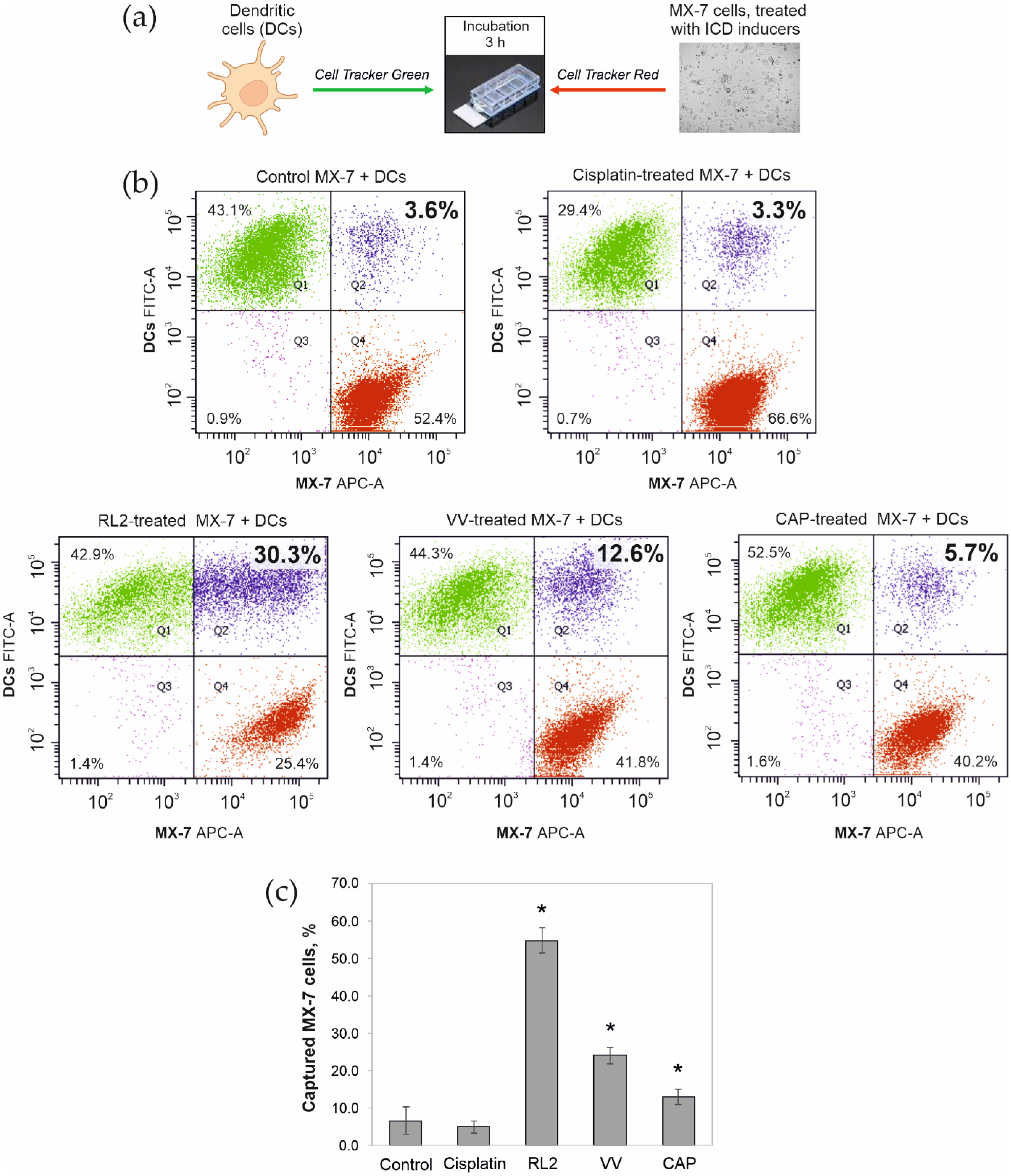
3.2. Phagocytosis of Tumor Cells Treated with ICD Inducers by Bone Marrow Dendritic Cells
3.3. Maturation of Bone Marrow Dendritic Cells after the Phagocytosis of Tumor Cells Treated with ICD Inducers
3.4. Capture of Tumor Cells by CD11c+ Mouse Spleen Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive Species in Non-Equilibrium Atmospheric-Pressure Plasmas: Generation, Transport, and Biological Effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Warren, S.; Adjemian, S.; Agostinis, P.; Martinez, A.B.; Chan, T.A.; Coukos, G.; Demaria, S.; Deutsch, E.; et al. Consensus Guidelines for the Definition, Detection and Interpretation of Immunogenic Cell Death. J. Immunother. Cancer 2020, 8, e000337. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Apetoh, L.; Ghiringhelli, F.; Kroemer, G. Immunological Aspects of Cancer Chemotherapy. Nat. Rev. Immunol. 2008, 8, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Troitskaya, O.S.; Novak, D.D.; Richter, V.A.; Koval, O.A. Immunogenic Cell Death in Cancer Therapy. Acta Nat. 2022, 14, 40–53. [Google Scholar] [CrossRef]
- Vacchelli, E.; Sistigu, A.; Yamazaki, T.; Vitale, I.; Zitvogel, L.; Kroemer, G. Autocrine Signaling of Type 1 Interferons in Successful Anticancer Chemotherapy. Oncoimmunology 2015, 4, e988042. [Google Scholar] [CrossRef]
- Sistigu, A.; Yamazaki, T.; Vacchelli, E.; Chaba, K.; Enot, D.P.; Adam, J.; Vitale, I.; Goubar, A.; Baracco, E.E.; Remédios, C.; et al. Cancer Cell–Autonomous Contribution of Type I Interferon Signaling to the Efficacy of Chemotherapy. Nat. Med. 2014, 20, 1301–1309. [Google Scholar] [CrossRef]
- Vacchelli, E.; Aranda, F.; Eggermont, A.; Galon, J.; Sautès-Fridman, C.; Cremer, I.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial Watch: Chemotherapy with Immunogenic Cell Death Inducers. Oncoimmunology 2014, 3, e27878. [Google Scholar] [CrossRef]
- Fond, A.M.; Ravichandran, K.S. Clearance of Dying Cells by Phagocytes: Mechanisms and Implications for Disease Pathogenesis. In Apoptosis in Cancer Pathogenesis and Anti-Cancer Therapy; Gregory, C.D., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2016; Volume 930, pp. 25–49. ISBN 978-3-319-39404-6. [Google Scholar]
- Eiz-Vesper, B.; Schmetzer, H.M. Antigen-Presenting Cells: Potential of Proven und New Players in Immune Therapies. Transfus. Med. Hemother. 2020, 47, 429–431. [Google Scholar] [CrossRef]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic Cells in Cancer Immunology and Immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef]
- Puhr, S.; Lee, J.; Zvezdova, E.; Zhou, Y.J.; Liu, K. Dendritic Cell Development—History, Advances, and Open Questions. Semin. Immunol. 2015, 27, 388–396. [Google Scholar] [CrossRef]
- Balan, S.; Saxena, M.; Bhardwaj, N. Dendritic Cell Subsets and Locations. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 348, pp. 1–68. ISBN 978-0-12-818351-9. [Google Scholar]
- Macri, C.; Fancke, B.; Radford, K.J.; O’Keeffe, M. Monitoring Dendritic Cell Activation and Maturation. In Antigen Processing; van Endert, P., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 1988, pp. 403–418. ISBN 978-1-4939-9449-6. [Google Scholar]
- Lamberti, M.J.; Nigro, A.; Mentucci, F.M.; Rumie Vittar, N.B.; Casolaro, V.; Dal Col, J. Dendritic Cells and Immunogenic Cancer Cell Death: A Combination for Improving Antitumor Immunity. Pharmaceutics 2020, 12, 256. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.D.; Romano, E.; Rufo, N.; Agostinis, P. Immunogenic versus Tolerogenic Phagocytosis during Anticancer Therapy: Mechanisms and Clinical Translation. Cell Death Differ. 2016, 23, 938–951. [Google Scholar] [CrossRef] [PubMed]
- Kepp, O.; Senovilla, L.; Vitale, I.; Vacchelli, E.; Adjemian, S.; Agostinis, P.; Apetoh, L.; Aranda, F.; Barnaba, V.; Bloy, N.; et al. Consensus Guidelines for the Detection of Immunogenic Cell Death. Oncoimmunology 2014, 3, e955691. [Google Scholar] [CrossRef] [PubMed]
- Cirone, M.; Di Renzo, L.; Lotti, L.V.; Conte, V.; Trivedi, P.; Santarelli, R.; Gonnella, R.; Frati, L.; Faggioni, A. Activation of Dendritic Cells by Tumor Cell Death. OncoImmunology 2012, 1, 1218–1219. [Google Scholar] [CrossRef]
- Di Blasio, S.; Wortel, I.M.N.; van Bladel, D.A.G.; de Vries, L.E.; Duiveman-de Boer, T.; Worah, K.; de Haas, N.; Buschow, S.I.; de Vries, I.J.M.; Figdor, C.G.; et al. Human CD1c+ DCs Are Critical Cellular Mediators of Immune Responses Induced by Immunogenic Cell Death. Oncoimmunology 2016, 5, e1192739. [Google Scholar] [CrossRef]
- Casares, N.; Pequignot, M.O.; Tesniere, A.; Ghiringhelli, F.; Roux, S.; Chaput, N.; Schmitt, E.; Hamai, A.; Hervas-Stubbs, S.; Obeid, M.; et al. Caspase-Dependent Immunogenicity of Doxorubicin-Induced Tumor Cell Death. J. Exp. Med. 2005, 202, 1691–1701. [Google Scholar] [CrossRef]
- Tesniere, A.; Schlemmer, F.; Boige, V.; Kepp, O.; Martins, I.; Ghiringhelli, F.; Aymeric, L.; Michaud, M.; Apetoh, L.; Barault, L.; et al. Immunogenic Death of Colon Cancer Cells Treated with Oxaliplatin. Oncogene 2010, 29, 482–491. [Google Scholar] [CrossRef]
- Duewell, P.; Steger, A.; Lohr, H.; Bourhis, H.; Hoelz, H.; Kirchleitner, S.V.; Stieg, M.R.; Grassmann, S.; Kobold, S.; Siveke, J.T.; et al. RIG-I-like Helicases Induce Immunogenic Cell Death of Pancreatic Cancer Cells and Sensitize Tumors toward Killing by CD8+ T Cells. Cell Death Differ. 2014, 21, 1825–1837. [Google Scholar] [CrossRef]
- Kim, J.H.; Oh, J.Y.; Park, B.H.; Lee, D.E.; Kim, J.S.; Park, H.E.; Roh, M.S.; Je, J.E.; Yoon, J.H.; Thorne, S.H.; et al. Systemic Armed Oncolytic and Immunologic Therapy for Cancer with JX-594, a Targeted Poxvirus Expressing GM-CSF. Mol. Ther. 2006, 14, 361–370. [Google Scholar] [CrossRef]
- Turubanova, V.D.; Balalaeva, I.V.; Mishchenko, T.A.; Catanzaro, E.; Alzeibak, R.; Peskova, N.N.; Efimova, I.; Bachert, C.; Mitroshina, E.V.; Krysko, O.; et al. Immunogenic Cell Death Induced by a New Photodynamic Therapy Based on Photosens and Photodithazine. J. Immunother. Cancer 2019, 7, 350. [Google Scholar] [CrossRef]
- Koval, O.A.; Fomin, A.S.; Kaledin, V.I.; Semenov, D.V.; Potapenko, M.O.; Kuligina, E.V.; Nikolin, V.P.; Nikitenko, E.V.; Richter, V.A. A Novel Pro-Apoptotic Effector Lactaptin Inhibits Tumor Growth in Mice Models. Biochimie 2012, 94, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Kochneva, G.; Sivolobova, G.; Tkacheva, A.; Grazhdantseva, A.; Troitskaya, O.; Nushtaeva, A.; Tkachenko, A.; Kuligina, E.; Richter, V.; Koval, O. Engineering of Double Recombinant Vaccinia Virus with Enhanced Oncolytic Potential for Solid Tumor Virotherapy. Oncotarget 2016, 7, 74171–74188. [Google Scholar] [CrossRef] [PubMed]
- Schweigert, I.; Zakrevsky, D.; Gugin, P.; Yelak, E.; Golubitskaya, E.; Troitskaya, O.; Koval, O. Interaction of Cold Atmospheric Argon and Helium Plasma Jets with Bio-Target with Grounded Substrate Beneath. Appl. Sci. 2019, 9, 4528. [Google Scholar] [CrossRef]
- Schweigert, I.; Alexandrov, A.; Zakrevsky, D.; Milakhina, E.; Patrakova, E.; Troitskaya, O.; Birykov, M.; Koval, O. Mismatch of Frequencies of Ac Voltage and Streamers Propagation in Cold Atmospheric Plasma Jet for Typical Regimes of Cancer Cell Treatment. J. Phys. Conf. Ser. 2021, 2100, 012020. [Google Scholar] [CrossRef]
- Schweigert, I.; Alexandrov, A.; Zakrevsky, D.; Gugin, P.; Milakhina, E.; Golubitskaya, E.; Troitskaya, O.; Biryukov, M.; Koval, O. Analysis of Grounded Substrate Effects on Cold Atmospheric Plasma Jet Irradiation of Cellular and Animal Models. J. Phys. Conf. Ser. 2020, 1698, 012010. [Google Scholar] [CrossRef]
- Golubitskaya, E.A.; Troitskaya, O.S.; Yelak, E.V.; Gugin, P.P.; Richter, V.A.; Schweigert, I.V.; Zakrevsky, D.E.; Koval, O.A. Cold Physical Plasma Decreases the Viability of Lung Adenocarcinoma Cells. Acta Nat. 2019, 11, 16–19. [Google Scholar] [CrossRef]
- Troitskaya, O.; Varlamov, M.; Nushtaeva, A.; Richter, V.; Koval, O. Recombinant Lactaptin Induces Immunogenic Cell Death and Creates an Antitumor Vaccination Effect in Vivo with Enhancement by an IDO Inhibitor. Molecules 2020, 25, 2804. [Google Scholar] [CrossRef]
- Koval, O.; Kochneva, G.; Tkachenko, A.; Troitskaya, O.; Sivolobova, G.; Grazhdantseva, A.; Nushtaeva, A.; Kuligina, E.; Richter, V. Recombinant Vaccinia Viruses Coding Transgenes of Apoptosis-Inducing Proteins Enhance Apoptosis But Not Immunogenicity of Infected Tumor Cells. BioMed Res. Int. 2017, 2017, 3620510. [Google Scholar] [CrossRef]
- Troitskaya, O.; Golubitskaya, E.; Biryukov, M.; Varlamov, M.; Gugin, P.; Milakhina, E.; Richter, V.; Schweigert, I.; Zakrevsky, D.; Koval, O. Non-Thermal Plasma Application in Tumor-Bearing Mice Induces Increase of Serum HMGB1. IJMS 2020, 21, 5128. [Google Scholar] [CrossRef]
- Khalili, M.; Daniels, L.; Lin, A.; Krebs, F.C.; Snook, A.E.; Bekeschus, S.; Bowne, W.B.; Miller, V. Non-Thermal Plasma-Induced Immunogenic Cell Death in Cancer. J. Phys. D Appl. Phys. 2019, 52, 423001. [Google Scholar] [CrossRef]
- Lin, A.; Truong, B.; Patel, S.; Kaushik, N.; Choi, E.; Fridman, G.; Fridman, A.; Miller, V. Nanosecond-Pulsed DBD Plasma-Generated Reactive Oxygen Species Trigger Immunogenic Cell Death in A549 Lung Carcinoma Cells through Intracellular Oxidative Stress. IJMS 2017, 18, 966. [Google Scholar] [CrossRef] [PubMed]
- Van Loenhout, J.; Freire Boullosa, L.; Quatannens, D.; De Waele, J.; Merlin, C.; Lambrechts, H.; Lau, H.W.; Hermans, C.; Lin, A.; Lardon, F.; et al. Auranofin and Cold Atmospheric Plasma Synergize to Trigger Distinct Cell Death Mechanisms and Immunogenic Responses in Glioblastoma. Cells 2021, 10, 2936. [Google Scholar] [CrossRef] [PubMed]
- Semenov, D.V.; Fomin, A.S.; Kuligina, E.V.; Koval, O.A.; Matveeva, V.A.; Babkina, I.N.; Tikunova, N.V.; Richter, V.A. Recombinant Analogs of a Novel Milk Pro-Apoptotic Peptide, Lactaptin, and Their Effect on Cultured Human Cells. Protein J. 2010, 29, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Schweigert, I.V.; Alexandrov, A.L.; Zakrevsky, D.E. Self-Organization of Touching-Target Current with Ac Voltage in Atmospheric Pressure Plasma Jet for Medical Application Parameters. Plasma Sources Sci. Technol. 2020, 29, 12LT02. [Google Scholar] [CrossRef]
- Markov, O.V.; Mironova, N.L.; Sennikov, S.V.; Vlassov, V.V.; Zenkova, M.A. Prophylactic Dendritic Cell-Based Vaccines Efficiently Inhibit Metastases in Murine Metastatic Melanoma. PLoS ONE 2015, 10, e0136911. [Google Scholar] [CrossRef]
- Lorenzi, S.; Mattei, F.; Sistigu, A.; Bracci, L.; Spadaro, F.; Sanchez, M.; Spada, M.; Belardelli, F.; Gabriele, L.; Schiavoni, G. Type I IFNs Control Antigen Retention and Survival of CD8α+ Dendritic Cells after Uptake of Tumor Apoptotic Cells Leading to Cross-Priming. J. Immunol. 2011, 186, 5142–5150. [Google Scholar] [CrossRef]
- Zhou, H.; Forveille, S.; Sauvat, A.; Yamazaki, T.; Senovilla, L.; Ma, Y.; Liu, P.; Yang, H.; Bezu, L.; Müller, K.; et al. The Oncolytic Peptide LTX-315 Triggers Immunogenic Cell Death. Cell Death Dis. 2016, 7, e2134. [Google Scholar] [CrossRef]
- Huang, B.; Sikorski, R.; Kirn, D.H.; Thorne, S.H. Synergistic Anti-Tumor Effects between Oncolytic Vaccinia Virus and Paclitaxel Are Mediated by the IFN Response and HMGB1. Gene Ther. 2011, 18, 164–172. [Google Scholar] [CrossRef]
- Schiavoni, G.; Mattei, F.; Gabriele, L. Type I Interferons as Stimulators of DC-Mediated Cross-Priming: Impact on Anti-Tumor Response. Front. Immunol. 2013, 4, 483. [Google Scholar] [CrossRef]
- Lebon, A.; Tough, D. Type I Interferon as a Stimulus for Cross-Priming. Cytokine Growth Factor Rev. 2008, 19, 33–40. [Google Scholar] [CrossRef]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Álvaro-Benito, M.; Stolzenberg, S.; Noé, F.; Freund, C. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front. Immunol. 2017, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.; Toomre, D.; Garrett, W.; Mellman, I. Dendritic Cell Maturation Triggers Retrograde MHC Class II Transport from Lysosomes to the Plasma Membrane. Nature 2002, 418, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Veyer, D.L.; Carrara, G.; Maluquer de Motes, C.; Smith, G.L. Vaccinia Virus Evasion of Regulated Cell Death. Immunol. Lett. 2017, 186, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Kleinpeter, P.; Remy-Ziller, C.; Winter, E.; Gantzer, M.; Nourtier, V.; Kempf, J.; Hortelano, J.; Schmitt, D.; Schultz, H.; Geist, M.; et al. By Binding CD80 and CD86, the Vaccinia Virus M2 Protein Blocks Their Interactions with Both CD28 and CTLA4 and Potentiates CD80 Binding to PD-L1. J. Virol. 2019, 93, e00207-19. [Google Scholar] [CrossRef]
- Engelmayer, J.; Larsson, M.; Subklewe, M.; Chahroudi, A.; Cox, W.I.; Steinman, R.M.; Bhardwaj, N. Vaccinia Virus Inhibits the Maturation of Human Dendritic Cells: A Novel Mechanism of Immune Evasion. J. Immunol. 1999, 163, 6762–6768. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troitskaya, O.; Novak, D.; Varlamov, M.; Biryukov, M.; Nushtaeva, A.; Kochneva, G.; Zakrevsky, D.; Schweigert, I.; Richter, V.; Koval, O. Immunological Effects of Cold Atmospheric Plasma-Treated Cells in Comparison with Those of Cells Treated with Lactaptin-Based Anticancer Drugs. Biophysica 2022, 2, 266-280. https://doi.org/10.3390/biophysica2030025
Troitskaya O, Novak D, Varlamov M, Biryukov M, Nushtaeva A, Kochneva G, Zakrevsky D, Schweigert I, Richter V, Koval O. Immunological Effects of Cold Atmospheric Plasma-Treated Cells in Comparison with Those of Cells Treated with Lactaptin-Based Anticancer Drugs. Biophysica. 2022; 2(3):266-280. https://doi.org/10.3390/biophysica2030025
Chicago/Turabian StyleTroitskaya, Olga, Diana Novak, Mikhail Varlamov, Mikhail Biryukov, Anna Nushtaeva, Galina Kochneva, Dmitriy Zakrevsky, Irina Schweigert, Vladimir Richter, and Olga Koval. 2022. "Immunological Effects of Cold Atmospheric Plasma-Treated Cells in Comparison with Those of Cells Treated with Lactaptin-Based Anticancer Drugs" Biophysica 2, no. 3: 266-280. https://doi.org/10.3390/biophysica2030025
APA StyleTroitskaya, O., Novak, D., Varlamov, M., Biryukov, M., Nushtaeva, A., Kochneva, G., Zakrevsky, D., Schweigert, I., Richter, V., & Koval, O. (2022). Immunological Effects of Cold Atmospheric Plasma-Treated Cells in Comparison with Those of Cells Treated with Lactaptin-Based Anticancer Drugs. Biophysica, 2(3), 266-280. https://doi.org/10.3390/biophysica2030025