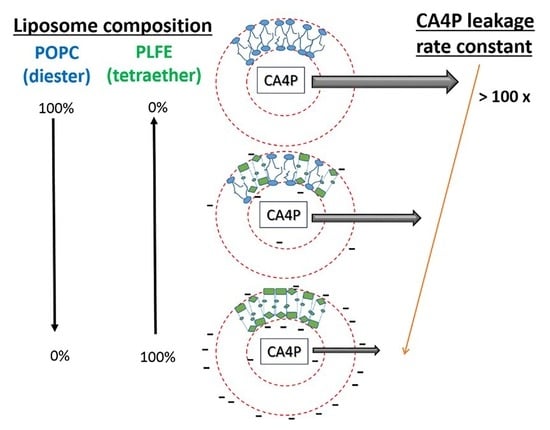
The Polar Lipid Fraction E from Sulfolobus acidocaldarius Can Be Used as Liposomal Drug Stabilizing Agents to Reduce the Leakage of the Antivascular Drug Combretastatin A4 Disodium Phosphate from Tetraether/Diester Hybrid Archaeosomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Archaea Cells and PLFE Lipids
2.2. Particle Size, Size Distribution, and Zeta Potential
2.3. Liposome Preparation
2.4. DPH Fluorescence Polarization Measurements
2.5. Entrapment of CA4P in Archaeosomes
2.6. Separation of Free CA4P from Archaeosomall CA4P
2.7. Drug Leakage Assay
2.8. MCF-7 Cell Line
2.9. Cytotoxicity Assay
2.10. Statistical Analysis
3. Results and Discussion
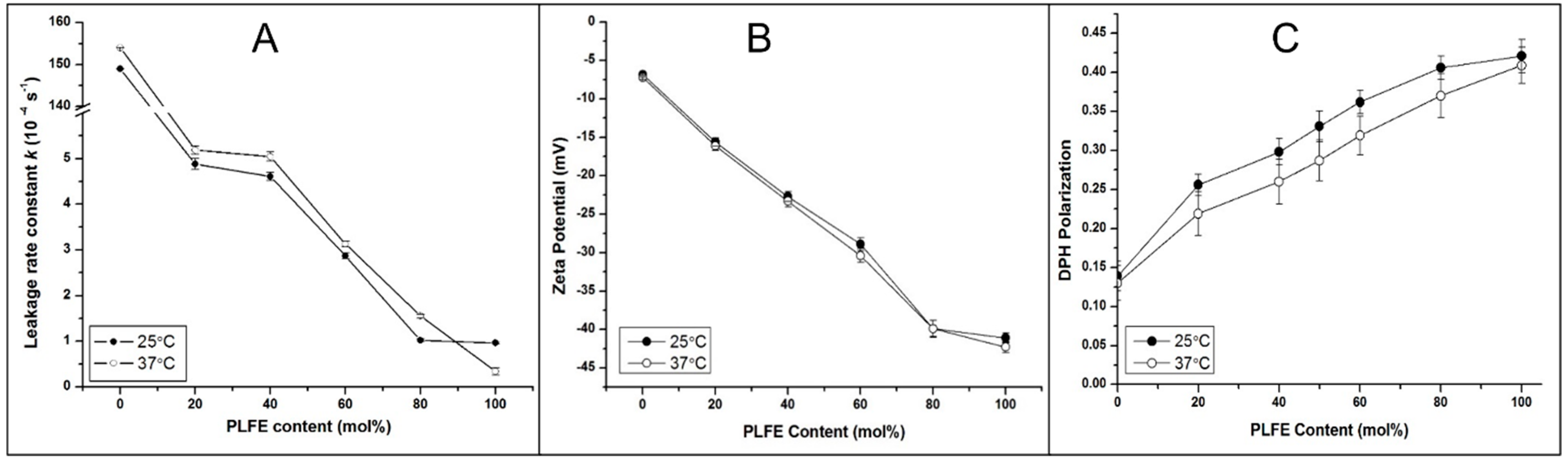
3.1. CA4P Leakage
3.2. Zeta Potential
3.3. Effect of PLFE Content on Archaeosomal Membrane Packing in the Hydrophobic Core
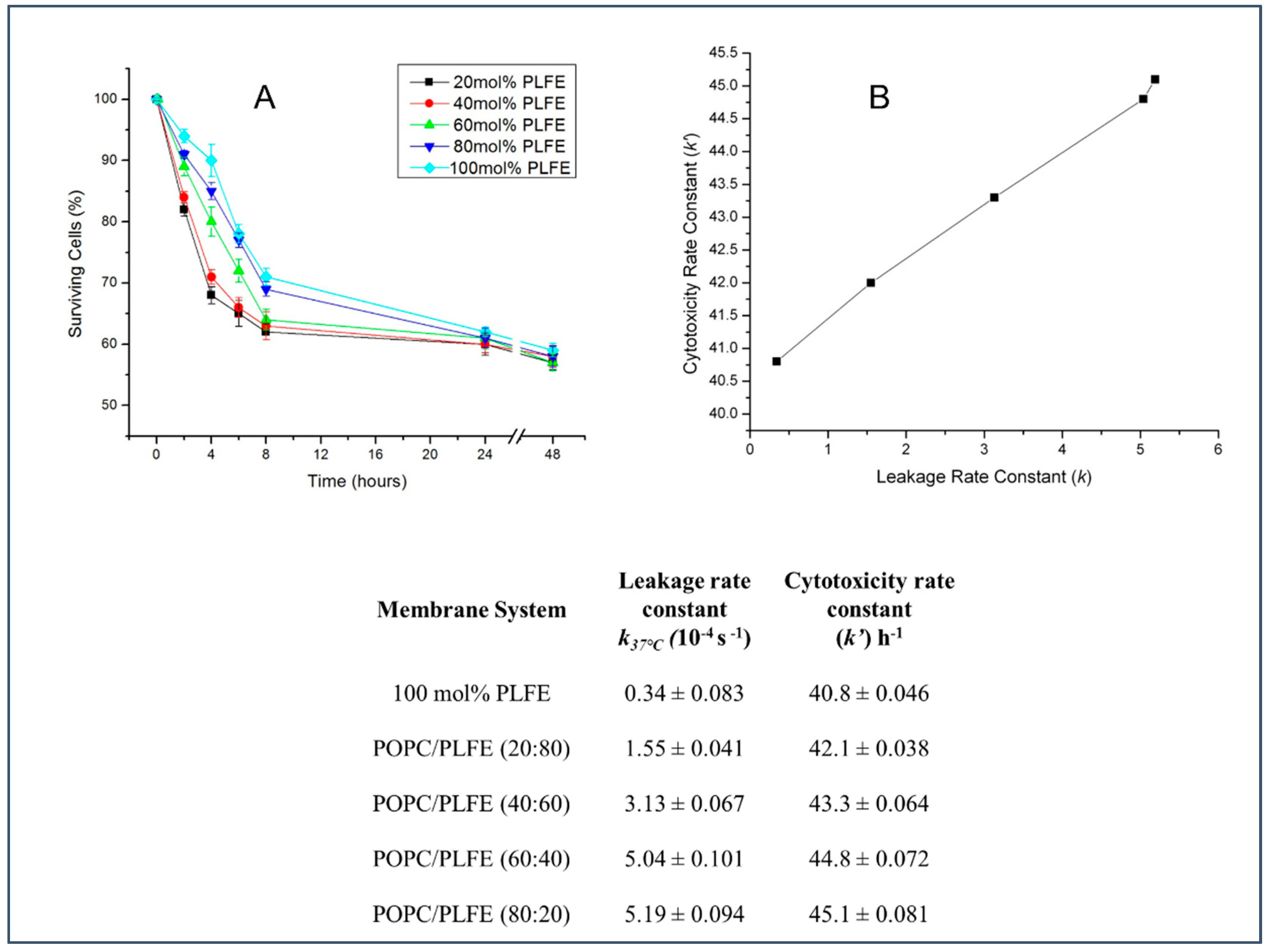
3.4. Effect of PLFE Content on Cytotoxicity of Archaeosomal CA4P against MCF-7 Breast Cancer Cells
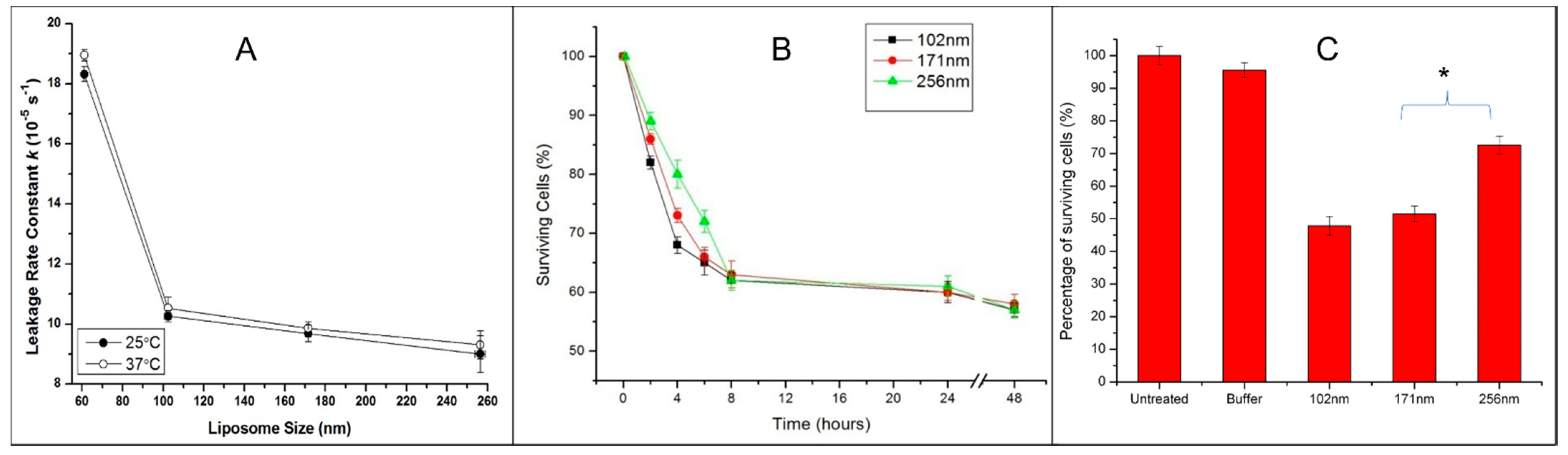
3.5. Effect of Archaeosome Size on Drug Leakage and Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, T.O.B.; Ahmad, R.R.H.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal drug delivery systems and anticancer drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Chang, H.; Yeh, M. Clinical development of liposome-based drugs: Formulation, characterization and therapeutic efficacy. Int. J. Nanomed. 2012, 7, 49–60. [Google Scholar]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update post COVID-19 vaccines. Bioeng. Transl. Med. 2021, 6, e10246. [Google Scholar] [CrossRef] [PubMed]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Venegas, B.; Zhu, W.; Haloupek, N.B.; Lee, J.; Zellhart, E.; Sugar, I.P.; Kiani, M.; Chong, P.L.-G. Cholesterol superlattice modulates combretastatin A4 disodium phosphate (CA4P) release from liposomes and CA4P cytotoxicity on mammary cancer cells. Biophys. J. 2012, 102, 2086–2094. [Google Scholar] [CrossRef]
- Young, S.L.; Chaplin, D.J. Combretastatin A4 phosphate: Background and current clinical status. Expert Opin. Investig. Drugs 2004, 13, 1171–1182. [Google Scholar] [CrossRef]
- Dong, D.; Ko, B.; Baumeister, P.; Swenson, S.; Costa, F.; Markland, F.; Stiles, C.; Patterson, J.B.; Bates, S.E.; Lee, A.S. Vascular targeting and antiangiogenesis agents induce drug resistance effector GRP78 within the tumor microenvironment. Cancer Res. 2005, 65, 5785–5791. [Google Scholar] [CrossRef]
- Dark, G.G.; Hill, S.A.; Prise, V.E.; Tozer, G.M.; Pettit, G.R.; Chaplin, D.J. Combretastatin A-4, an agent that displays potent and selective toxicity toward tumor vasculature. Cancer Res. 1997, 57, 1829–1834. [Google Scholar]
- Pattillo, C.B.; Venegas, B.; Donelson, F.J.; Del Valle, L.; Knight, L.; Chong, P.L.-G.; Kiani, M.F. Radiation-guided targeting of combretastatin encapsulated immunoliposomes to mammary tumors. Pharm. Res. 2009, 26, 1093–1100. [Google Scholar] [CrossRef]
- Daswani, V.P.; Ayesa, U.; Venegas, B.; Chong, P.L.-G. Concentration-induced J-aggregate formation causes a biphasic change in the release of trans-combretastatin A4 disodium phosphate from archaeosomes and the subsequent cytotoxicity on mammary cancer cells. Mol. Pharm. 2015, 12, 3724–3734. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.L.; Montague, C.E.; Chang, E.L. Purification of glycerol dialkyl nonitol tetraether from Sulfolobus acidocaldarius. J. Lipid Res. 1989, 30, 944–949. [Google Scholar] [CrossRef]
- Chang, E.L.; Lo, S.L. Extraction and purification of tetraether lipids from Sulfolobus acidocaldarius. In Protocols for Archaebacterial Research; Fleischmann, E.M., Place, A.R., Robb, R.T., Schreier, H.J., Eds.; Maryland Biotechnology Institute: Baltimore, MD, USA, 1991; pp. 2.3.1–2.3.14. [Google Scholar]
- De Rosa, M.; Gambacorta, A.; Nicolaus, B.; Chappe, B.; Albrecht, P. Isoprenoid ethers: Backbone of complex lipids of the archaebacterium Sulfolobus solfataricus. Biochim. Biophys. Acta 1983, 753, 249–256. [Google Scholar] [CrossRef]
- De Rosa, M.; Esposito, E.; Gambacorta, A.; Nicholaus, B.; Bu’lock, J.D. Effects of temperature on ether lipid composition of Caldariella acidophila. Phytochemistry 1980, 19, 827–831. [Google Scholar] [CrossRef]
- Yang, L.L.; Haug, A. Structure of membrane lipids and physico-biochemical properties of the plasma membrane from Thermoplasma acidophilum, adapted to growth at 37 °C. Biochim. Biophys. Acta 1979, 573, 308–320. [Google Scholar] [CrossRef]
- Uda, I.; Sugai, A.; Itoh, Y.H.; Itoh, T. Variation in molecular species of polar lipids from Thermoplasma acidophilum depends on growth temperature. Lipids 2001, 36, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Nemoto, N.; Shida, Y.; Oshima, T.; Yamagishi, A. Effects of pH and temperature on the composition of polar lipids in Thermoplasma acidophilum HO-62. J. Bacteriol. 2008, 190, 5404–5411. [Google Scholar] [CrossRef] [PubMed]
- Bagatolli, L.A.; Gratton, E.; Khan, T.K.; Chong, P.L.-G. Two-photon fluorescence microscopy studies of bipolar tetraether giant liposomes from thermoacidophilic archaebacteria Sulfolobus acidocaldarius. Biophys. J. 2000, 79, 416–425. [Google Scholar] [CrossRef]
- Ren, X.; Liu, K.; Zhang, Q.; Noh, H.M.; Kumbur, E.C.; Yuan, W.W.; Zhou, J.G.; Chong, P.L.-G. Design, fabrication and characterization of archaeal tetraether free-standing planar membranes in a PDMS- and PCB-based fluidic platform. ACS Appl. Mater. Interfaces 2014, 6, 12618–12628. [Google Scholar] [CrossRef]
- Ren, X.; Kumbur, E.C.; Zhou, J.G.; Noh, H.M.; Chong, P.L.-G. Stability of free-standing tetraether planar membranes in microchips. J. Membr. Sci. 2017, 540, 27–34. [Google Scholar] [CrossRef]
- Jeworrek, C.; Evers, F.; Erlkamp, M.; Grobelny, S.; Tolan, M.; Chong, P.L.-G.; Winter, R. Structure and phase behavior of archaeal lipid monolayers. Langmuir 2011, 27, 13113–13121. [Google Scholar] [CrossRef] [PubMed]
- Elferink, M.G.; de Wit, J.G.; Demel, R.; Driessen, A.J.; Konings, W.N. Functional reconstitution of membrane proteins in monolayer liposomes from bipolar lipids of Sulfolobus acidocaldarius. J. Biol. Chem. 1992, 267, 1375–1381. [Google Scholar] [CrossRef]
- Chong, P.L.-G. Archaebacterial bipolar tetraether lipids: Physico-chemical and membrane properties. Chem. Phys. Lipids 2010, 163, 253–265. [Google Scholar] [CrossRef]
- Chong, P.L.-G.; Ayesa, U.; Daswani, V.P.; Hur, E.C. On physical properties of tetraether lipid membranes: Effects of cyclopentane rings. Archaea 2012, 2012, 138439. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.L.-G.; Bonanno, A.; Ayesa, U. Dynamics and organization of archaeal tetraether lipid membranes. In Membrane Organization and Dynamics, Springer Series in Biophysics; Chattopadhyay, A., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 11–30. [Google Scholar]
- Freisleben, H.-J.; Bormann, J.; Litzinger, D.C.; Lehr, F.; Rudolph, P.; Schatton, M.; Huang, L. Toxicity and biodistribution of liposomes of the main phospholipid from the archaebacterium Thermoplasma acidophilum. J. Liposome Res. 1995, 5, 215–223. [Google Scholar] [CrossRef]
- Patel, G.B.; Ponce, A.; Zhou, H.; Chen, W. Safety of intranasally administered archaeal lipid mucosal vaccine adjuvant and delivery (AMVAD) vaccine in mice. Int. J. Toxicol. 2008, 27, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.B.; Sprott, G.D. Archaeobacterial ether lipid liposomes (archaeosomes) as novel vaccine and drug delivery systems. Crit. Rev. Biotechnol. 1999, 19, 317–357. [Google Scholar] [CrossRef]
- Klasczyk, B.; Knecht, V.; Lipowsky, R.; Dimova, R. Interactions of alkali metal chlorides with phosphatidylcholine vesicles. Langmuir 2010, 26, 18951–18958. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.; Chen, M.; Patty, P.J.; Code, C.; Cheng, J.; Frisken, B.J.; Zuckermann, M.; Thewalt, J. Ergosterol in POPC membranes: Physical properties and comparison with structurally similar sterols. Biophys. J. 2007, 92, 1606–1615. [Google Scholar] [CrossRef]
- Drummond, D.C.; Meyer, O.; Hong, K.; Kirpotin, D.B.; Papahadjopoulos, D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol. Rev. 1999, 51, 691–743. [Google Scholar] [PubMed]
- Bartlett, G.R. Phosphorus assay in column chromatography. J. Biol. Chem. 1959, 234, 466–468. [Google Scholar] [CrossRef]
- Kanichay, R.; Boni, L.T.; Cooke, P.H.; Khan, T.K.; Chong, P.L.-G. Calcium-induced aggregation of archaeal bipolar tetraether liposomes derived from thermoacidophilic archaeon Sulfolobus acidocaldarius. Archaea 2003, 1, 175–183. [Google Scholar] [CrossRef]
- Dobro, M.J.; Samson, R.Y.; Yu, Z.; McCullough, J.; Ding, H.J.; Chong, P.L.-G.; Bell, S.D.; Jensen, G.J. Electron cryotomography of ESCRT assemblies and dividing Sulfolobus cells suggests that spiraling filaments are involved in membrane scission. Mol. Biol. Cell 2013, 24, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Jameson, D.M. Introduction to Fluorescence; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Pattillo, C.B.; Sari-Sarraf, F.; Nallamothu, R.; Moore, B.M.; Wood, G.C.; Kiani, M.F. Targeting of the antivascular drug combretastatin to irradiated tumors results in tumor growth delay. Pharm. Res. 2005, 22, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Gorner, H.; Kuhn, H.J. Cis-trans Photoisomerisation of Stilbenes and Stilbene-Like Molecules. Adv. Photochem. 2005, 19, 1–117. [Google Scholar]
- Huang, J.; Buboltz, J.T.; Feigenson, G.W. Maximum solubility of cholesterol in phosphatidylcholine and phosphatidylethanolamine bilayers. Biochim. Biophys. Acta 1999, 1417, 89–100. [Google Scholar] [CrossRef]
- Elferink, M.G.; De Wit, J.G.; Driessen, A.J.; Konings, W.N. Energy-transducing properties of primary proton pumps reconstituted into archaeal bipolar lipid vesicles. Eur. J. Biochem. 1993, 214, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Vilalta, I.; Gliozzi, A.; Prats, M. Interfacial air/water proton conduction from long distances by Sulfolobus solfataricus archaeal bolaform lipids. Eur. J. Biochem. 1996, 240, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, J.L.; Chong, P.L.-G. Molecular modeling of archaebacterial bipolar tetraether lipid membranes. Chem. Phys. Lipids 2000, 105, 193–200. [Google Scholar] [CrossRef]
- Eisenberg, M.; Gresalfi, T.; Riccio, T.; McLaughlin, S. Adsorption of monovalent cations to bilayer membranes containing negative phospholipids. Biochemistry 1979, 18, 5213–5223. [Google Scholar] [CrossRef]
- Matos, C.; de Castro, B.; Gameiro, P.; Lima, J.L.F.; Reis, S. Zeta-potential measurements as a tool to quantify the effect of charged drugs on the surface potential of egg phosphatidylcholine liposomes. Langmuir 2004, 20, 369–377. [Google Scholar] [CrossRef]
- Komatsu, H.; Chong, P.L.-G. Low permeability of liposomal membranes composed of bipolar tetraether lipids from Thermoacidophilic archaebacterium Sulfolobus acidocaldarius. Biochemistry 1998, 37, 107–115. [Google Scholar] [CrossRef]
- Herbig, M.E.; Fromm, U.; Leuenberger, J.; Krauss, U.; Beck-Sickinger, A.G.; Merkle, H.P. Bilayer interaction and localization of cell penetrating peptides with model membranes: A comparative study of a human calcitonin (hCT)-derived peptide with pVEC and pAntp(43–58). Biochim. Biophys. Acta 2005, 1712, 197–211. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ayesa, U.; Chong, P.L.-G. Polar lipid fraction E from Sulfolobus acidocaldarius and dipalmitoylphosphatidylcholine can form stable yet thermo-sensitive tetraether/diester hybrid archaeosomes with controlled release capability. Int. J. Mol. Sci. 2020, 21, 8388. [Google Scholar] [CrossRef]
- Sugár, I.P. A generalization of the shell theorem. Electric potential of charged spheres and charged vesicles surrounded by electrolyte. AIMS Biophys. 2020, 7, 76–89. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta potential measurements. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Humana Press: New York, NY, USA, 2011; pp. 63–70. [Google Scholar]
- Arora, A.; Raghuraman, H.; Chattopadhyay, A. Influence of cholesterol and ergosterol on membrane dynamics: A fluorescence approach. Biochem. Biophys. Res. Commun. 2004, 318, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Pottel, H.; van der Meer, W.; Herreman, W. Correlation between the order parameter and the steady-state fluorescence anisotropy of 1,6-diphenyl-1,3,5-hexatriene and an evaluation of membrane fluidity. Biochim. Biophys. Acta 1983, 730, 181–186. [Google Scholar] [CrossRef]
- Lian, B.; Wei, H.; Pan, R.; Sun, J.; Zhang, B.; Wu, J.; Li, X.; Tian, G. Galactose modified liposomes for effective co-delivery of doxorubicin and combretastatin A4. Int. J. Nanomed. 2021, 16, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Sprott, G.D.; Dicaire, C.J.; Cote, J.; Whitfield, D.M. Adjuvant potential of archaeal synthetic glycolipid mimetics critically depends on the glyco head group structure. Glycobiology 2008, 18, 559–565. [Google Scholar] [CrossRef]
- Šuštar, V.; Zelko, J.; Lopalco, P.; Lobasso, S.; Ota, A.; Ulrih, N.P.; Corcelli, A.; Kralj-Iglič, V. Morphology, biophysical properties and protein-mediated fusion of archaeosomes. PLoS ONE 2012, 7, e39401. [Google Scholar] [CrossRef]
- Sprott, G.D.; Dicaire, C.J.; Gurnani, K.; Deschatelets, L.A.; Krishnan, L. Liposome adjuvants prepared from the total polar lipids of Haloferax volcanii, Planococcus spp. and Bacillus firmus differ in ability to elicit and sustain immune responses. Vaccine 2004, 22, 2154–2162. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Gruhle, K.; Meister, A.; Hause, G.; Drescher, S. Bolalipid-doped liposomes: Can bolalipids increase the integrity of liposomes exposed to gastrointestinal fluids? Pharmaceutics 2019, 11, 646. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, J.; Hofhaus, G.; Thomas, S.; Cuesta, L.C.; Gropp, F.; Schroder, R.; Hartmann, K.; Fricker, G. Improved oral bioavailability of human growth hormone by a combination of liposomes containing bio-enhancers and tetraether lipids and omeprazole. J. Pharm. Sci. 2014, 103, 3985–3993. [Google Scholar] [CrossRef] [PubMed]
- González-Paredes, A.; Clarés-Naverosb, B.; Ruiz-Martínezb, M.A.; Durbán-Fornielesa, J.J.; Ramos-Cormenzanaa, A.; Monteoliva-Sáncheza, M. Delivery systems for natural antioxidant compounds: Archaeosomes and archaeosomal hydrogels characterization and release study. Int. J. Pharm. 2011, 421, 321–331. [Google Scholar] [CrossRef]
- Jacobsen, A.; Jensen, S.M.; Fricker, G.; Brandl, M.; Treusch, A.H. Archaeal lipids in oral delivery of therapeutic peptides. Eur. J. Pharm. Sci. 2017, 108, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Duse, L.; Pinnapireddy, S.R.; Strehlow, B.; Jedelska, J.; Bakowsky, U. Low level LED photodynamic therapy using curcumin loaded tetraether liposomes. Eur. J. Pharm. Biopharm. 2018, 126, 233–241. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daswani, V.P.; Ayesa, U.; Chong, P.L.-G. The Polar Lipid Fraction E from Sulfolobus acidocaldarius Can Be Used as Liposomal Drug Stabilizing Agents to Reduce the Leakage of the Antivascular Drug Combretastatin A4 Disodium Phosphate from Tetraether/Diester Hybrid Archaeosomes. Biophysica 2021, 1, 474-486. https://doi.org/10.3390/biophysica1040034
Daswani VP, Ayesa U, Chong PL-G. The Polar Lipid Fraction E from Sulfolobus acidocaldarius Can Be Used as Liposomal Drug Stabilizing Agents to Reduce the Leakage of the Antivascular Drug Combretastatin A4 Disodium Phosphate from Tetraether/Diester Hybrid Archaeosomes. Biophysica. 2021; 1(4):474-486. https://doi.org/10.3390/biophysica1040034
Chicago/Turabian StyleDaswani, Varsha P., Umme Ayesa, and Parkson Lee-Gau Chong. 2021. "The Polar Lipid Fraction E from Sulfolobus acidocaldarius Can Be Used as Liposomal Drug Stabilizing Agents to Reduce the Leakage of the Antivascular Drug Combretastatin A4 Disodium Phosphate from Tetraether/Diester Hybrid Archaeosomes" Biophysica 1, no. 4: 474-486. https://doi.org/10.3390/biophysica1040034
APA StyleDaswani, V. P., Ayesa, U., & Chong, P. L.-G. (2021). The Polar Lipid Fraction E from Sulfolobus acidocaldarius Can Be Used as Liposomal Drug Stabilizing Agents to Reduce the Leakage of the Antivascular Drug Combretastatin A4 Disodium Phosphate from Tetraether/Diester Hybrid Archaeosomes. Biophysica, 1(4), 474-486. https://doi.org/10.3390/biophysica1040034