1. Introduction
The structural build-up of cement-based materials describes the increase in rheological parameters with resting time [
1,
2,
3,
4,
5,
6,
7,
8,
9]. It originates from flocculation and the bridging effect of C-S-H between cement particles [
2,
10]. This behavior is time-dependent and reflects the structuration of cement paste at rest. If this structural build-up can be erased by shearing (structural break-down), the behavior is completely reversible. In this case, cement paste can be referred to as thixotropic material since the initial state is fully restored. This is the case for inert materials such as calcium carbonate suspension in deionized water which exhibits thixotropic behavior mainly due to flocculation [
11]. However, due to chemical changes that occur during the dormant period of cement hydration (dissolution and precipitation), the initial state cannot be fully restored. The structural build-up thus displays an irreversible part mainly due to the bridging effect (rigidification) [
2,
8].
It should be noted that controlling structural build-up is of great interest from a practical point of view. In fact, structural build-up conditions several processes for fresh cement-based materials. For instance, it allows for controlling the formwork pressure, preventing particles from settling [
12] and ensuring multi-layer casting [
13]. This is why this phenomenon is the subject of more and more attention, especially for the development of modern concretes such as printable concretes, which must meet requirements involving structural build-up [
14].
Different approaches can be used to quantify structural build-up [
1,
3,
7,
11,
15,
16,
17]. A preliminary approach consists of the measurement of the hysteresis loop [
1,
3,
7,
11,
15,
16,
17], which represents the surface between the ascending and descending flow curve. However, this method has several limitations as reported already by Banfill [
15,
18]. In fact, the hysteresis area depends not only on the resting time but also the test apparatus and shear history, including the shear rate, test conditions, and step duration. Another interesting approach consists of the determination of the evolution of storage and loss moduli with resting time, using time sweep measurements with small amplitude oscillatory shear (SAOS) [
1]. The most common approach is based on the evolution of the static yield stress with resting time. The static yield stress is the minimum shear stress required to induce flow. It originates from colloidal forces and directs contacts between particles which form a network of interacting particles able to withstand minimum stress [
19]. The slope of the yield stress–resting time curve, often noted as A
thix, characterizes the kinetics of structural build-up [
1,
8,
11] and thus the continuous formation of a network of interacting particles.
The structural build-up of cementitious materials has been the subject of a large number of studies [
1,
2,
3,
4,
6,
8,
11,
20]. However, some questions are not clearly elucidated. The contribution of physical (flocculation) and chemical effects to structural build-up remains complex. Mostafa and Yahia [
1] found that flocculation at rest can be described by a percolation time corresponding to the time needed to form a colloidal percolated network. In addition, the chemical rigidification rate of the formed network can be described by the linear increase at rest in the storage modulus within the dormant period. The authors suggested that these parameters can be used in combination to describe physical and chemical contributions to structural build-up.
Zhang et al. [
3] found that the chemical contribution is negligible until the acceleration period of hydration. Chemical structural build-up significantly increases from the onset of the acceleration period and remains constant regardless of the hydration time. The addition of a superplasticizer (SP) allows for reduction of the flocculation contribution while the chemical contribution is comparable with and without SP [
3]. For this investigation, it was considered that re-agitation before testing can erase the flocculation contribution. The chemical part is thus assumed to be irreversible.
Recently, Zhang et al. [
8] found that the evolution of static yield stress during the dormant period follows three stages. The initial stage, in the first 30 min, corresponds to a rapid linear increase in static yield stress. In the induction period (30–60 min), the static yield stress increases slowly. Finally, the acceleration stage (>60 min) is marked by an exponential increase in static yield stress. Zhang et al. [
8] indicated that the interparticle force, which is the combination of colloidal force and interaction force between C-S-H particles, is the driving force for the evolution of static yield stress. The contribution of colloidal force appears to be greater in the early period (the initial and induction period), while the C-S-H force determines the evolution trend of the static yield stress from the induction period.
Since cement hydration is a thermo-activated phenomenon, increasing temperature accelerates early-age hydration reactions, while at low temperature, early hydration can be significantly slowed down [
21,
22]. Temperature variation thus seems to be a good way to control the chemical contribution to structural build-up. In practice, the curing temperature has a significant effect on stiffening control. For prefabricated cement-based materials, increasing temperature makes it possible to accelerate the prefabrication rate, while casting in cold weather could pose problems such as a delay in structural build-up [
23], leading to stability problems which could in turn affect durability [
24].
The effect of temperature on structural build-up could be due to physicochemical processes. In fact, increasing temperature leads to an increase in the nucleation and growth rates of hydrates (such as C-S-H), which improves the bridging effect between cement particles. It is worth noting that the physical (flocculation) effect also depends on temperature. In fact, Tari et al. [
25] indicated an increase in attractive forces with temperature by using calculations based on conventional DLVO (Derjaguin, Landau, Vervey, and Overbeek) theory. According to the authors, this increase could be due to a decrease in the electrostatic repulsion induced by a decrease in the dielectric constant of the medium. De Kretser and Scales [
26] found that the assumption that the yield stress of colloidal suspensions decreases in concert with the viscosity as temperature increases is flawed. Using a theoretical framework incorporating interparticle force considerations and direct measurements of yield stress, the authors found that yield stress increases with temperature. According to the authors [
26], a change in interparticle spacing with temperature appears to be the most reasonable explanation for the observed results. For cementitious suspensions, an increase in temperature leads to an increase in the dissolution rate [
10,
22]. This leads to an increase in the number of fine cement particles and a significant increase in ionic strength, which reduces the Debye length of the diffuse layer [
10,
27,
28]. As a consequence, the flocculation of cement particles is enhanced due to the increase in the inter-particle forces dominated by van der Waals attractive forces. It can be noted that Brownian effects are also increased with temperature. The effect of temperature on structural build-up appears complex, since it involves physicochemical considerations that are difficult to separate.
Furthermore, modern concretes, such as low-carbon concretes (or green concretes [
29]) or printable concretes, contain blended cements in which a part of Portland cement is replaced by alternative powders or supplementary cementitious materials (SCMs). The effect of SCMs on structural build-up depends not only on their granular properties (particle size distribution, shape, and roughness) but also their chemical effects (filler effect and pozzolanic reaction). However, the induced effects can be different depending on the SCM and its properties. This is why the results in the scientific literature may be contradictory. When Rahman et al. [
30] found that limestone powder increases structural build-up, Zhang et al. [
31] reported a decrease in structural build-up. However, an increase was observed when increasing the limestone fineness [
31]. Moreover, the addition of silica fume, slag, or metakaolin increases structural build-up [
1,
17,
30,
32,
33], while the spherical shape of fly ash particles allows for it to be reduced [
33,
34].
The effect of temperature on structural build-up was investigated by Huan et al. [
35]. They found that an increase in temperature leads to the improvement of structural-build-up due to the increase in the number and the growth rate of C-S-H bridges. In addition, Huan et al. [
35] found that supplementary cementitious materials (SCMs) change the temperature dependence of structural build-up. Therefore, the addition of fly ash and slag decreases the structural build-up of cement paste at low temperature (10 °C), while at high temperature (40 °C), structural build-up increases. The addition of Nano-SiO
2 and Nano-CaCO
3 allows to increase the formation of C-S-H and thus causes a faster build-up rate.
Although there are a large number of studies, the reversibility of structural build-up, especially with temperature variation, has not been addressed in the literature. In fact, it is often assumed that the chemical changes have a permanent effect on structural build-up, and strong shearing does not allow the chemical contribution to be erased [
2,
3]. Only the flocculation effect is assumed to be reversible. It would have been interesting to evaluate the reversibility of structural build-up, in which the chemical contribution can be reduced at low temperature or increased at high temperature. It has to be kept in mind that the effect of temperature, as discussed above, involves physicochemical considerations, and chemical changes also affect flocculation. The addition of SCMs would induce other physicochemical effects. Therefore, this paper aims to examine the reversibility of structural build-up with temperature. The effect of limestone filler, metakaolin, fly ash, and slag is also investigated.
2. Materials and Methods
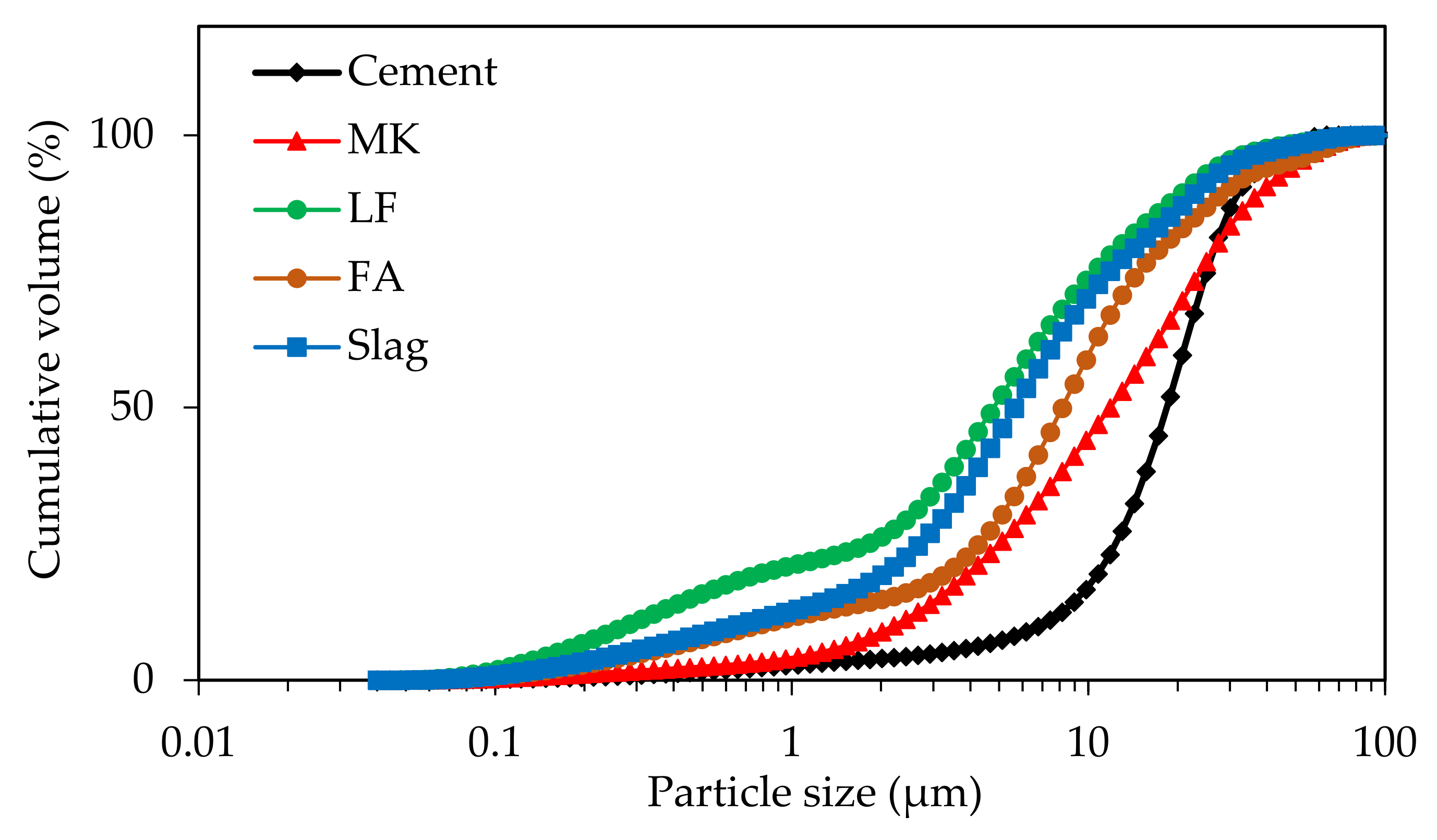
Portland cement (CEM I from Lafarge Holcim) with different mineral additions (fly ash (FA from Cemex), slag (S from Cemex), limestone filler (LF from Omya BL), and metakaolin (MK from Argeco) was used to prepare binary blends of 70CEMI/30 SCM (wt%). The granular properties of the raw materials are summarized in
Table 1. The particle size distributions of the raw materials were determined in water by using a laser granulometer (LS 13,320 Beckman Coulter) with an adapted optical model (
Figure 1). The chemical composition of the raw materials is presented in
Appendix A.
Pastes were produced with a water-to-binder ratio (w/b) of 0.5 and were mixed with deionized water in a planetary agitator according to the following sequence: 1 min mixing at 500 rpm, scraping the mixer walls to homogenize the mix (30 s), and 30 s mixing at 1000 rpm. Sample preparation was carried out at ambient temperature (20 ± 2 °C).
The static yield stress measurements were performed using a rotational rheometer equipped with a four-blade vane geometry. This geometry has an internal diameter of 28 mm, and the outer cup diameter is 30 mm. The resulting gap is 1 mm. The geometry constants were calibrated using the Couette analogy as suggested by Aït-Kadi et al. [
36].
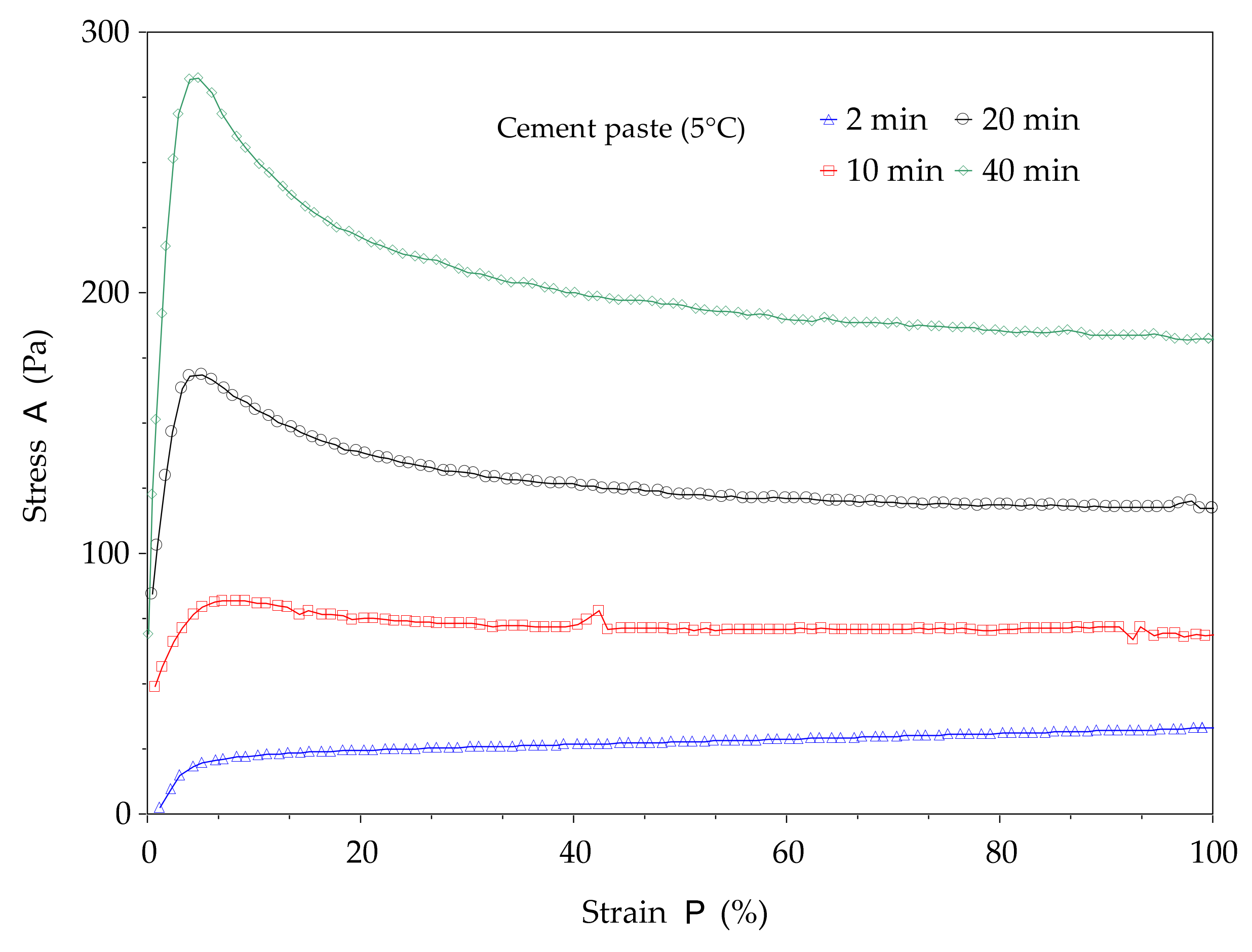
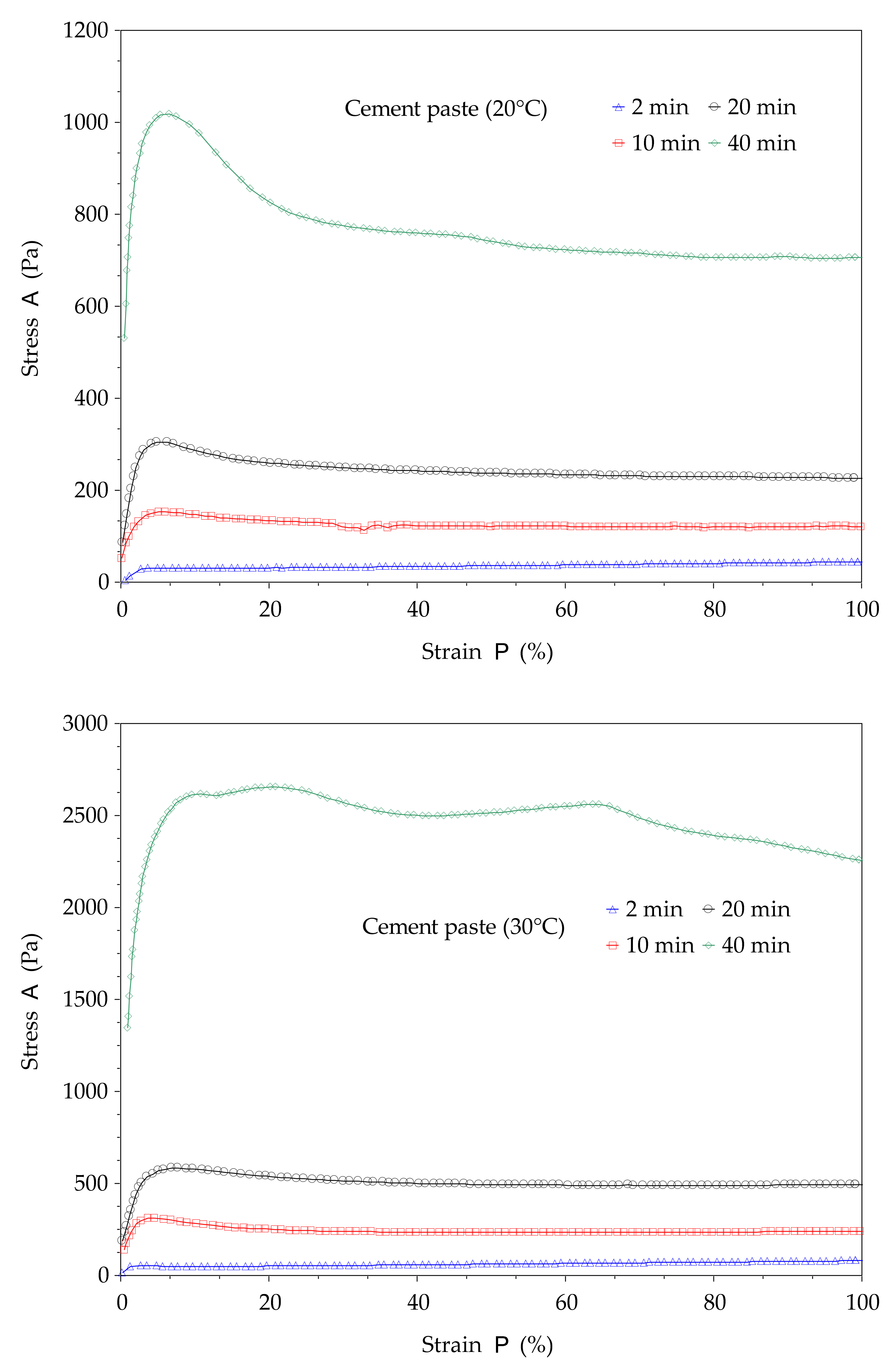
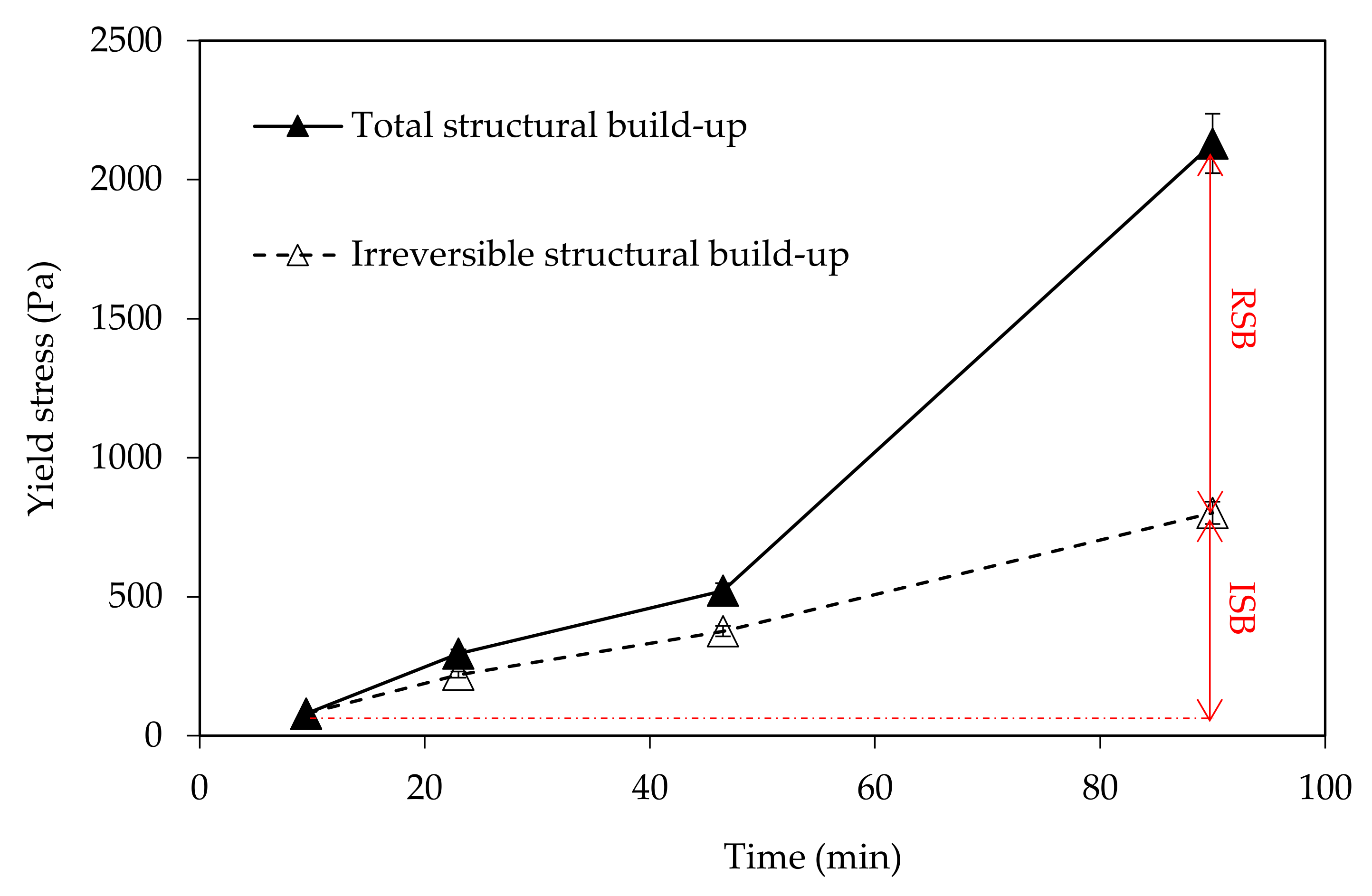
The structural build-up of cement pastes can be described by the evolution of static yield stress with resting time (or time). In this study, the method proposed in [
11] is used. This method consists of a succession of static yield stress measurements with different resting times, as shown in
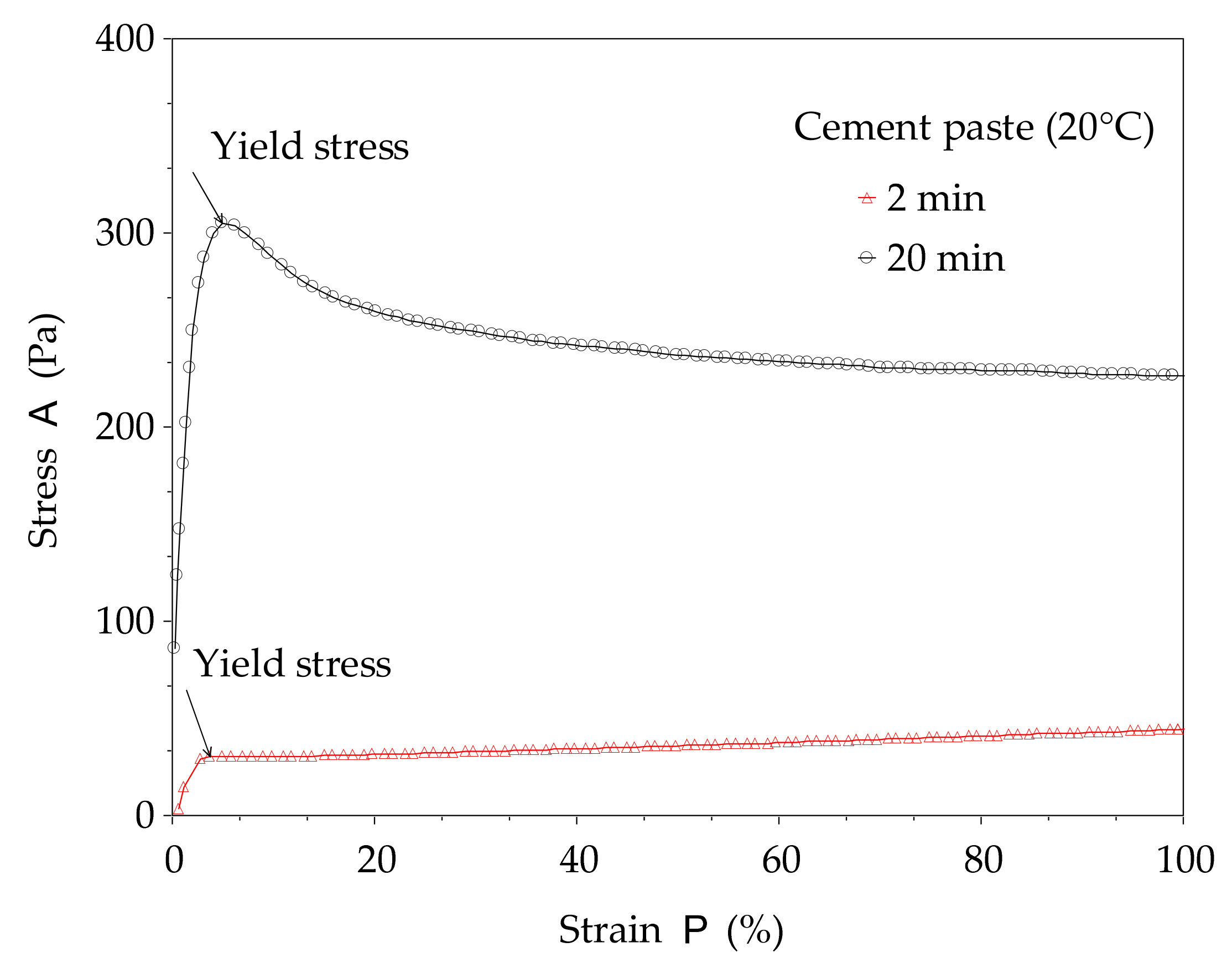
Figure 2. The testing procedures begin with a strong pre-shear process (150 s
−1 during 30 s) to homogenize the paste in the rheometer cup followed by a resting time of 2 min, 10 min, 20 min, and 40 min. After each resting time, a stress growth procedure with a constant shear rate of 0.005 s
−1 is applied over 3 min (
Figure 2a). The typical curve given by the stress growth procedure is presented in
Figure 3. It can be observed that the shear stress–strain curve can display a peak or plateau. This peak or value at the plateau defines the static yield stress. This procedure allows for determination of the total structural build-up. Other illustrations of yield stress measurements are presented in
Appendix A (
Figure A1).
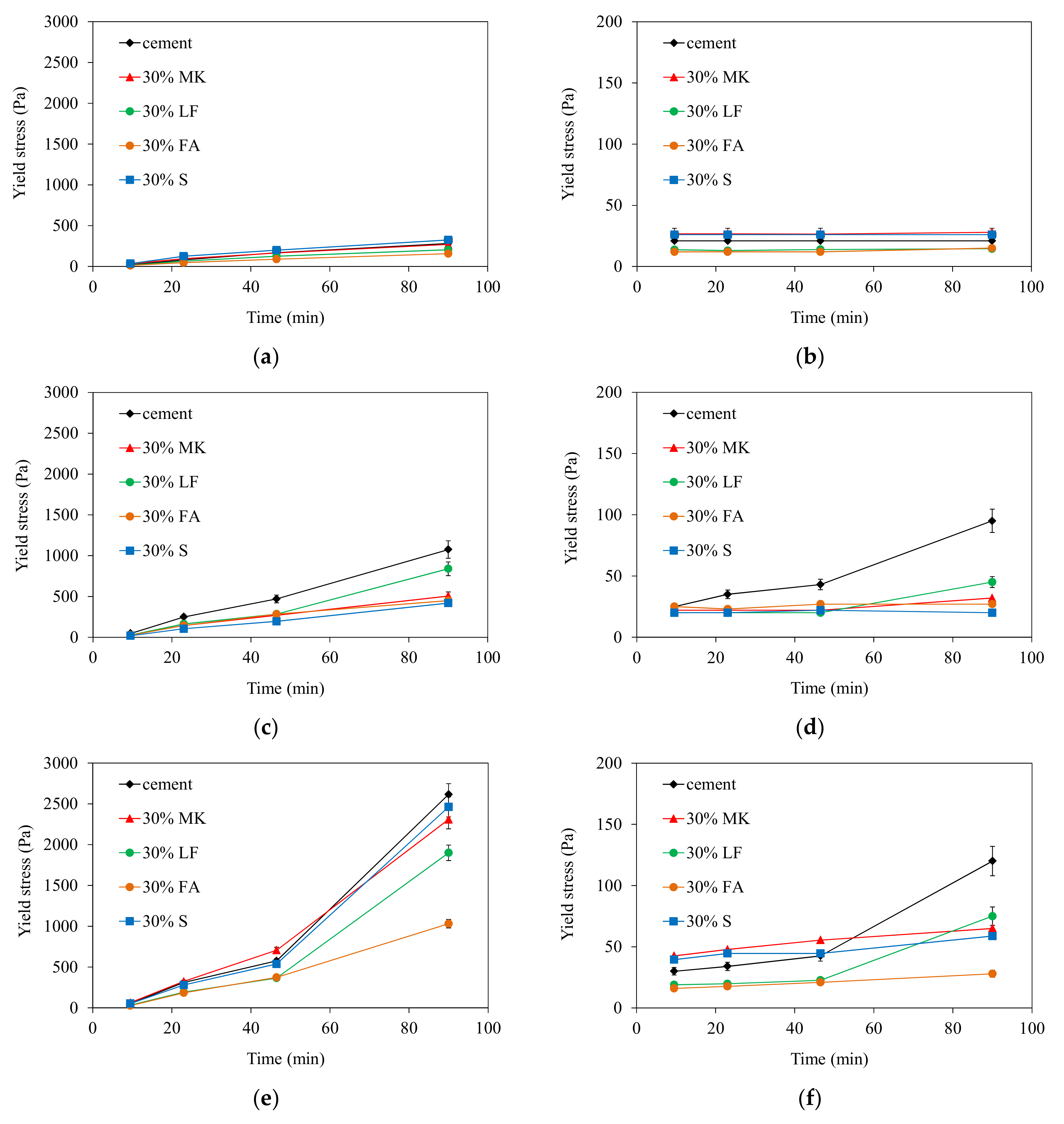
Furthermore, the same method was applied to determine the evolution of static yield stress with time, but the pastes were re-sheared (at 150 s
−1) prior to testing (
Figure 2b). This allows for erasure of the reversible part of structural build-up [
11] and for determination of the irreversible part.
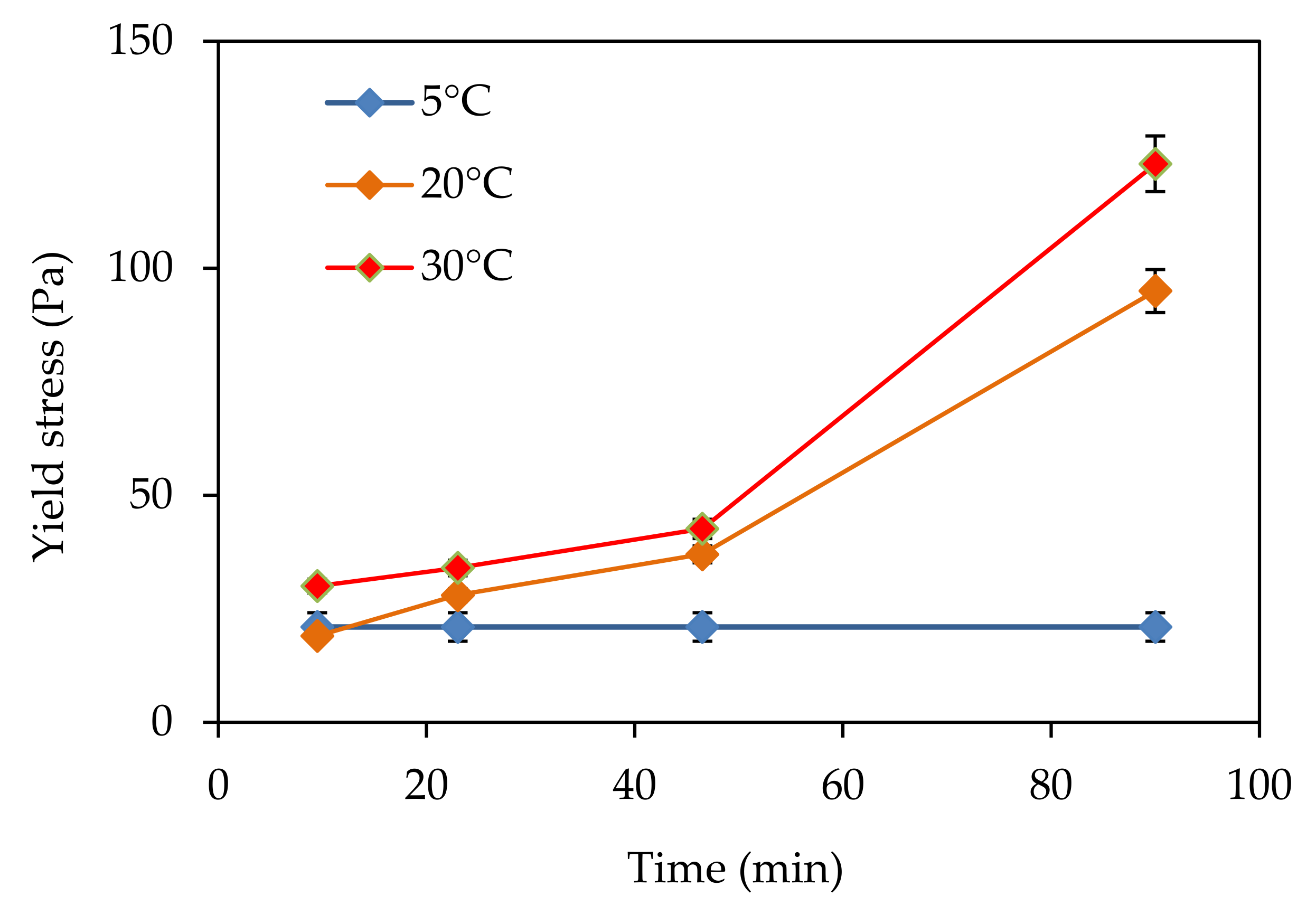
After the end of mixing carried out at room temperature (20 ± 2 °C), the cement paste is introduced into a concentric Peltier cylinder capable of controlling the test temperature in a range of −20 °C to 150 °C. Yield stress measurement is then performed at controlled temperatures (5 °C, 20 °C, or 30 °C). The rheometer cup was covered with a solvent trap during testing. The first yield stress measurement was performed 7.5 min after water/cement contact.
Tests were carried out in triplicate. A total of 90 yield stress measurements were performed.
4. Conclusions
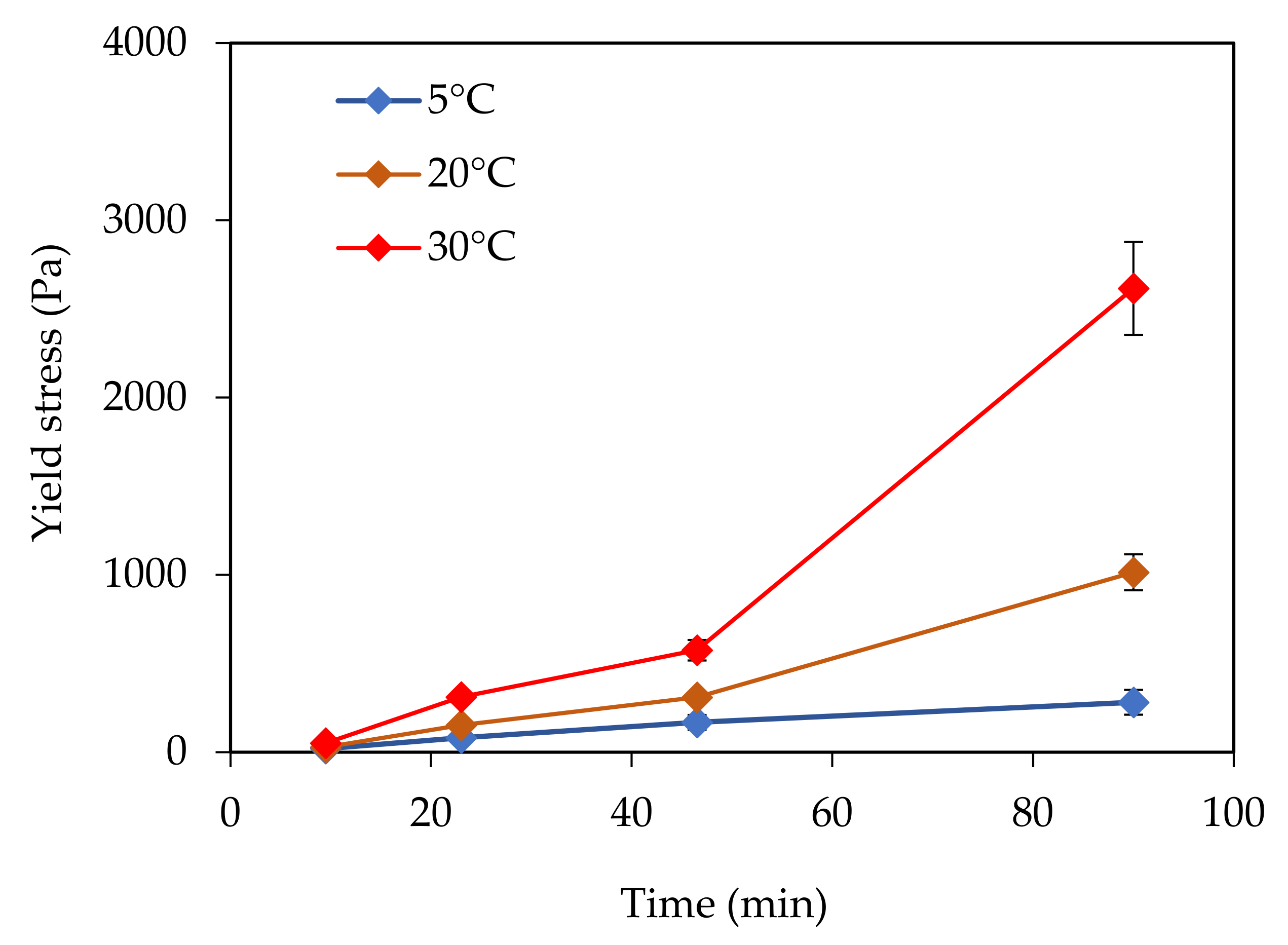
In this paper, the effect of temperature on structural build-up was investigated. It was shown that structural build-up follows at least three periods. The first period is marked by the rapid growth of yield stress, followed by a period of slow increase. Approximately 50 min after water/cement contact, the yield stress increases exponentially. This evolution was not observed at 5 °C. It should be noted that the yield stress 90 min after water/cement contact is multiplied by 10 when the temperature increases from 5 to 30 °C.
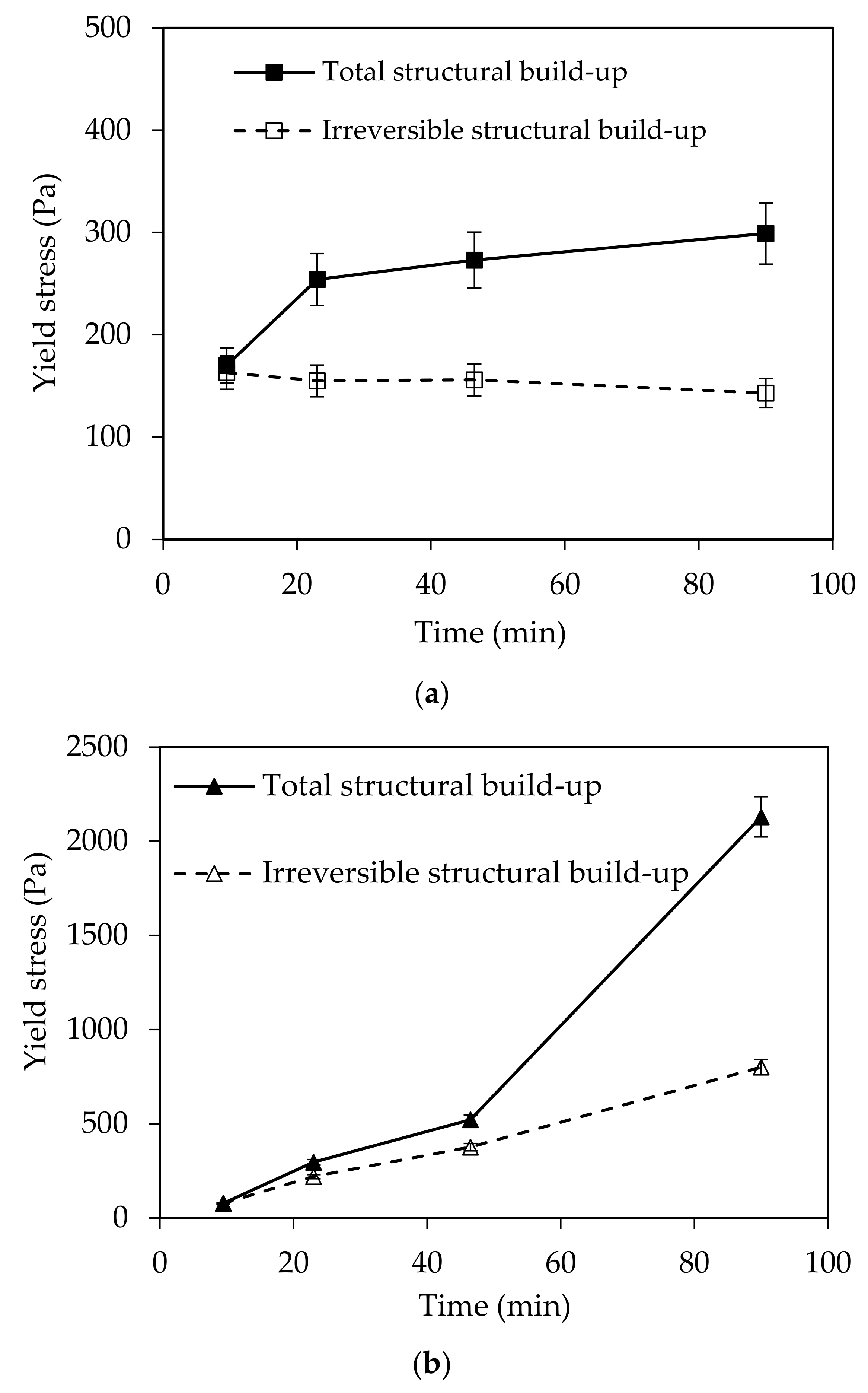
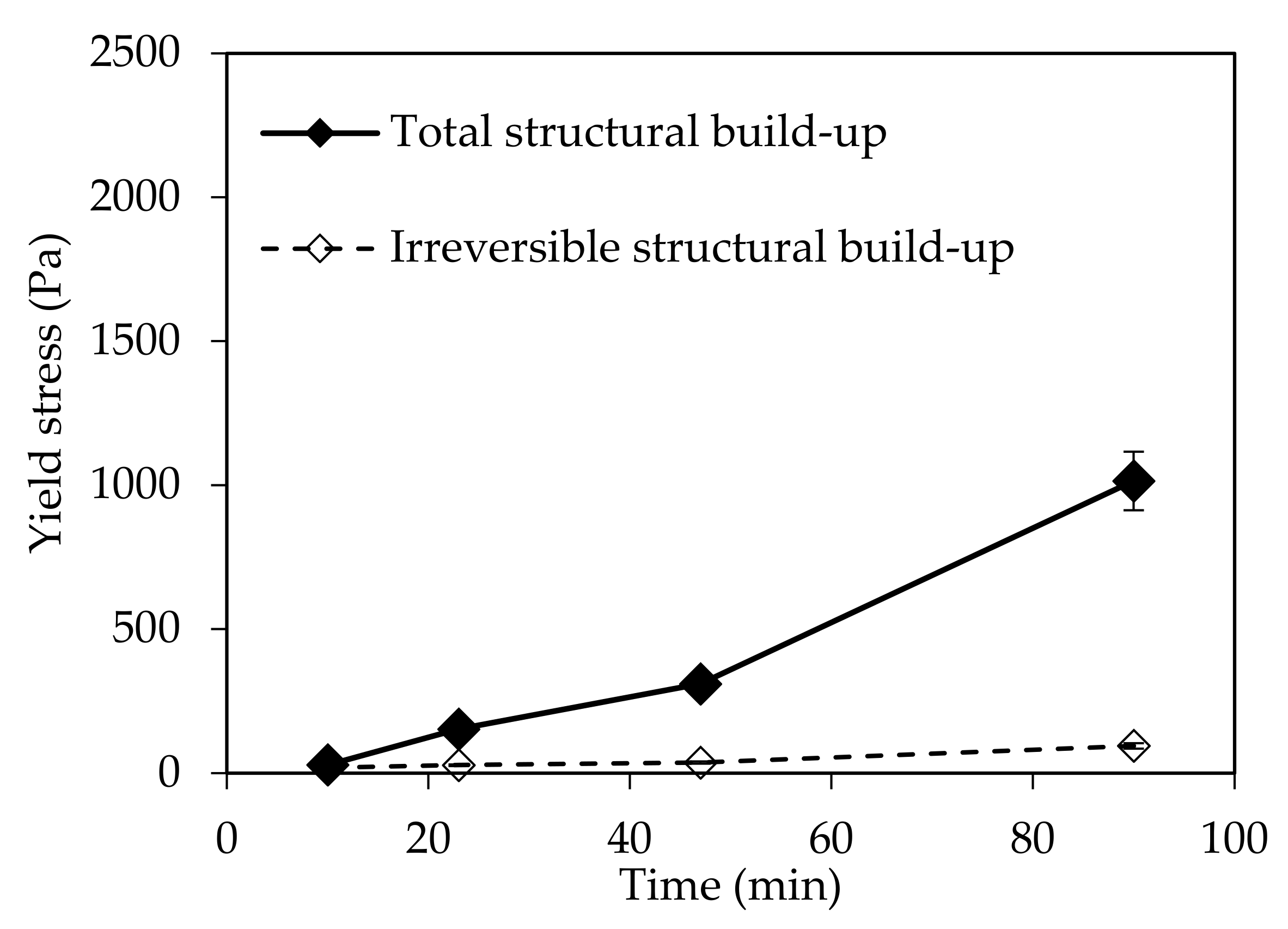
Furthermore, the reversibility of the structural build-up of cementitious materials was also examined. It appears that structural build-up can display an irreversible part originating from chemical changes, which depends on several factors. The results show that an increase in temperature leads to an increase in irreversible structural build-up. The increase of the structural build-up with temperature rise can be explained in part by the acceleration of hydration kinetics and the bridging effect due to the formation of early hydrates at the surface of particles. In fact, increasing temperature leads to an increase in the dissolution rate, which induces an increase in the number of fine particles likely to agglomerate. This agglomeration could be reversible. However, the bridging effect induced by early hydrates could be the cause of the exponential increase in yield stress observed after 50 min. This bridging effect is expected to be irreversible.
Furthermore, the observations suggest that the interparticle distance plays a major role in the reversibility of structural build-up, since it conditions not only the magnitude of attractive forces and agglomeration but also C-S-H forces (bridges between particles).
The addition of metakaolin (MK), fly ash (FA), or slag (S) allows for the irreversible structural build-up to be decreased significantly, while cement paste with limestone filler (LF) exhibits the same trend as neat cement paste, but with lower yield stresses.