Pharyngeal Stenosis and Swallowing Dysfunction Following Laryngectomy: A Scoping Review †
Abstract
1. Introduction
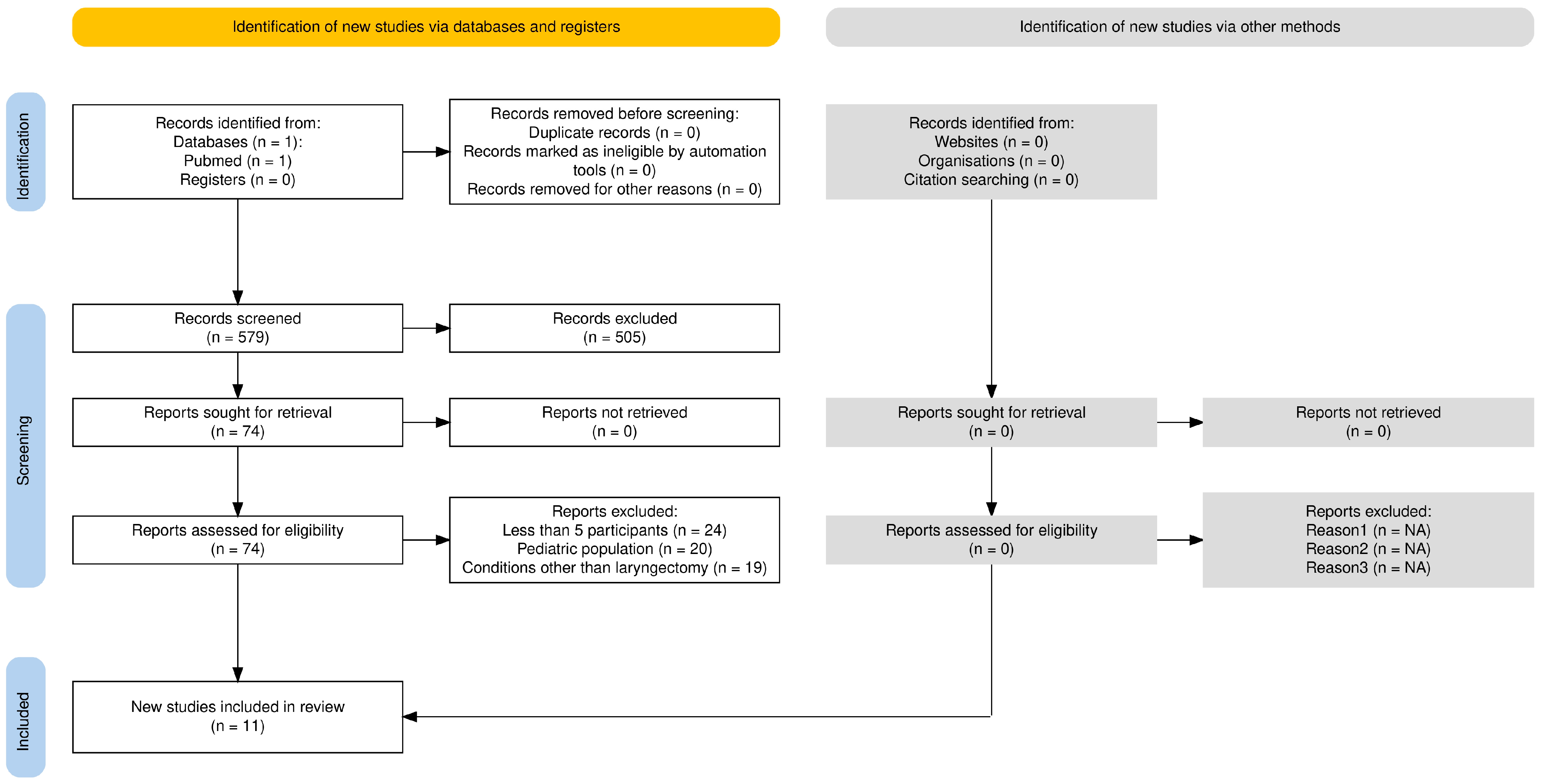
2. Methods
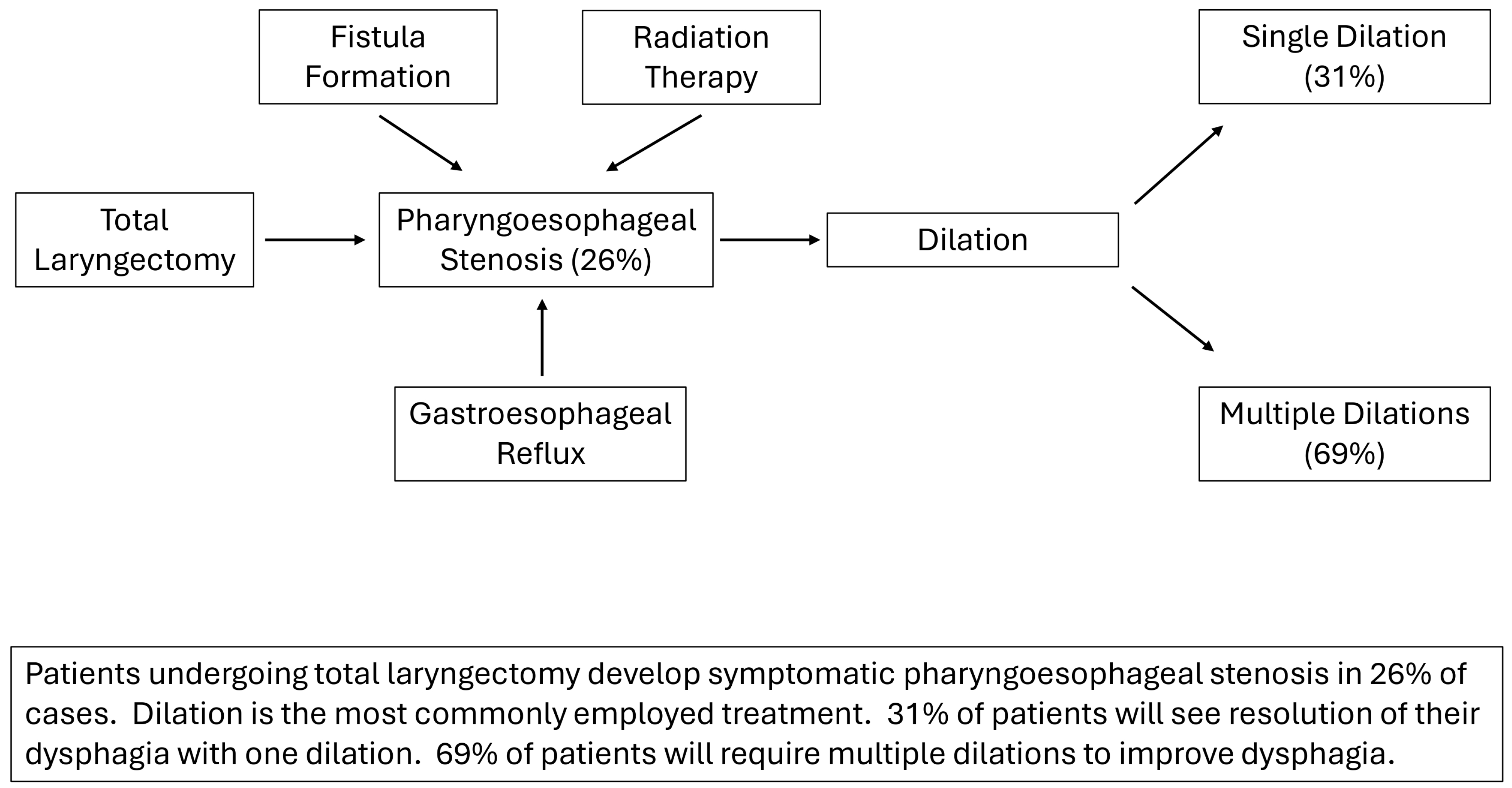
3. Results
4. Discussion
4.1. Impact of Chemoradiation on Swallowing Function
4.2. Fistula Precipitates Stricture Formation
4.3. Gastroesophageal Reflux and Stricture Formation
4.4. Outcome Measures and Efficacy of Dilation
4.5. Swallowing Function and Quality of Life
4.6. Patient-Reported Outcome Measures for Assessing Swallowing Function
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hanubal, K.S.; Chheda, N.N.; Dziegielewski, P.T. Neopharyngeal Stricture following Laryngectomy. Semin. Plast. Surg. 2022, 37, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.N.; Hoshal, S.G.; Evangelista, L.; Kuhn, M. Reconstruction technique following total laryngectomy affects swallowing outcomes. Laryngoscope Investig. Otolaryngol. 2020, 5, 703–707. [Google Scholar] [CrossRef]
- Landera, M.A.; Lundy, D.S.; Sullivan, P.A. Dysphagia After Total Laryngectomy. Perspect. Swallowing Swallowing Disord. Dysphagia 2010, 19, 39–44. [Google Scholar] [CrossRef]
- Piotet, E.; Escher, A.; Monnier, P. Esophageal and pharyngeal strictures: Report on 1,862 endoscopic dilatations using the Savary-Gilliard technique. Eur. Arch. Oto-Rhino-Laryngol. 2008, 265, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Balfe, D.M.; Koehler, R.E.; Setzen, M.; Weyman, P.J.; Baron, R.L.; Ogura, J.H. Barium examination of the esophagus after total laryngectomy. Radiology 1982, 143, 501–508. [Google Scholar] [CrossRef]
- Abu-Ghanem, S.; Sung, C.-K.; Junlapan, A.; Kearney, A.; DiRenzo, E.; Dewan, K.; Damrose, E.J. Endoscopic Management of Postradiation Dysphagia in Head and Neck Cancer Patients: A Systematic Review. Ann. Otol. Rhinol. Laryngol. 2019, 128, 767–773. [Google Scholar] [CrossRef]
- Harris, R.L.; Grundy, A.; Odutoye, T. Radiologically guided balloon dilatation of neopharyngeal strictures following total laryngectomy and pharyngolaryngectomy: 21 years’ experience. J. Laryngol. Otol. 2009, 124, 175–179. [Google Scholar] [CrossRef]
- Sweeny, L.; Golden, J.B.; White, H.N.; Magnuson, J.S.; Carroll, W.R.; Rosenthal, E.L. Incidence and Outcomes of Stricture Formation Postlaryngectomy. Otolaryngol. Neck Surg. 2011, 146, 395–402. [Google Scholar] [CrossRef]
- Maejima, R.; Iijima, K.; Koike, T.; Ara, N.; Uno, K.; Hatta, W.; Ogawa, T.; Watanabe, K.; Katori, Y.; Shimosegawa, T. Endoscopic balloon dilatation for pharyngo-upper esophageal stricture after treatment of head and neck cancer. Dig. Endosc. 2014, 27, 310–316. [Google Scholar] [CrossRef]
- Zhang, T.; Szczesniak, M.; Maclean, J.; Bertrand, P.; Wu, P.I.; Omari, T.; Cook, I.J. Biomechanics of Pharyngeal Deglutitive Function following Total Laryngectomy. Otolaryngol. Neck Surg. 2016, 155, 295–302. [Google Scholar] [CrossRef]
- Petersen, J.F.; Pézier, T.F.; van Dieren, J.M.; van der Noort, V.; van Putten, T.; Bril, S.I.; Janssen, L.; Dirven, R.; Brekel, M.W.v.D.; de Bree, R. Dilation after laryngectomy: Incidence, risk factors and complications. Oral Oncol. 2019, 91, 107–112. [Google Scholar] [CrossRef]
- Stoner, P.L.; Fullerton, A.L.; Freeman, A.M.; Chheda, N.N.; Estores, D.S. Endoscopic Dilation of Refractory Postlaryngectomy Strictures: A Case Series and Literature Review. Gastroenterol. Res. Pract. 2019, 2019, 8905615. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.I.; Szczesniak, M.M.; Maclean, J.; Graham, P.H.; Quon, H.; Choo, L.; Cook, I.J. Endoscopic dilatation improves long-term dysphagia following head and neck cancer therapies: A randomized control trial. Dis. Esophagus 2018, 32, doy087. [Google Scholar] [CrossRef] [PubMed]
- Farlow, J.L.; Birkeland, A.C.; Hardenbergh, A.; Lyden, T.; Brenner, J.C.; Shuman, A.G.; Chinn, S.B.; Stucken, C.L.; Malloy, K.M.; Moyer, J.S.; et al. Speech and swallowing outcomes after laryngectomy for the dysfunctional irradiated larynx. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 1459–1465. [Google Scholar] [CrossRef]
- Schuman, A.D.; Birkeland, A.C.; Farlow, J.L.; Lyden, T.; Blakely, A.; Spector, M.E.; Rosko, A.J. Predictors of Stricture and Swallowing Function Following Salvage Laryngectomy. Laryngoscope 2020, 131, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Schimberg, A.S.; Wellenstein, D.J.; Schutte, H.W.; Honings, J.; Marres, H.A.M.; Takes, R.P.; Broek, G.B.v.D. Feasibility and Safety of Office-Based Transnasal Balloon Dilation for Neopharyngeal and Proximal Esophageal Strictures in Patients with a History of Head and Neck Carcinoma. Dysphagia 2021, 37, 93–98. [Google Scholar] [CrossRef]
- Cortina, L.E.; Wu, M.P.; Meyer, C.D.; Feng, A.L.; Varvares, M.A.; Richmon, J.D.; Deschler, D.G.; Lin, D.T. Predictors of multiple dilations and functional outcomes after total laryngectomy and laryngopharyngectomy. Head Neck 2023, 46, 138–144. [Google Scholar] [CrossRef]
- Forastiere, A.A.; Zhang, Q.; Weber, R.S.; Maor, M.H.; Goepfert, H.; Pajak, T.F.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; et al. Long-Term Results of RTOG 91-11: A Comparison of Three Nonsurgical Treatment Strategies to Preserve the Larynx in Patients With Locally Advanced Larynx Cancer. J. Clin. Oncol. 2013, 31, 845–852. [Google Scholar] [CrossRef]
- Lazarus, C.L. Effects of chemoradiotherapy on voice and swallowing. Curr. Opin. Otolaryngol. Head Neck Surg. 2009, 17, 172–178. [Google Scholar] [CrossRef]
- Mendelson, A.H.; Small, A.J.; Agarwalla, A.; Scott, F.I.; Kochman, M.L. Esophageal Anastomotic Strictures: Outcomes of Endoscopic Dilation, Risk of Recurrence and Refractory Stenosis, and Effect of Foreign Body Removal. Clin. Gastroenterol. Hepatol. 2014, 13, 263–271. [Google Scholar] [CrossRef]
- Lew, R.J.; Kochman, M.L. A Review of Endoscopic Methods of Esophageal Dilation. J. Clin. Gastroenterol. 2002, 35, 117–126. [Google Scholar] [CrossRef]
- Urken, M.L.; Jacobson, A.S.; Lazarus, C.L. Comprehensive approach to restoration of function in patients with radiation-induced pharyngoesophageal stenosis. Head Neck 2012, 34, 1317–1328. [Google Scholar] [CrossRef]
- Paknezhad, H.; Borchard, N.A.; Lee, G.K.; Damrose, E.J. The Sternocleidomastoid Myocutaneous Flap: A Laryngeal Preservation Option for Total Hypopharyngoesophageal Stenosis. Otolaryngol. Neck Surg. 2019, 161, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Penêda, J.F.; Fernandes, J.; Monteiro, E. Risk Factors for Pharyngocutaneous Fistula Following Total Laryngectomy. Indian J. Otolaryngol. Head Neck Surg. 2022, 75, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Lebo, N.L.; Caulley, L.; Alsaffar, H.; Corsten, M.J.; Johnson-Obaseki, S. Peri-operative factors predisposing to pharyngocutaneous fistula after total laryngectomy: Analysis of a large multi-institutional patient cohort. J. Otolaryngol. Head Neck Surg. 2017, 46, 1–8. [Google Scholar] [CrossRef]
- Dedivitis, R.A.; Aires, F.T.; Cernea, C.R.; Brandão, L.G. Pharyngocutaneous fistula after total laryngectomy: Systematic review of risk factors. Head Neck 2015, 37, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Cecatto, S.B.; Soares, M.M.; Henriques, T.; Monteiro, E.; Moura, C.I.F.P. Predictive factors for the postlaryngectomy pharyngocutaneous fistula development: Systematic review. Braz. J. Otorhinolaryngol. 2014, 80, 167–177. [Google Scholar] [CrossRef]
- Timmermans, A.J.; Lansaat, L.; Theunissen, E.A.R.; Hamming-Vrieze, O.; Hilgers, F.J.M.; Brekel, M.W.M.v.D. Predictive Factors for Pharyngocutaneous Fistulization After Total Laryngectomy. Ann. Otol. Rhinol. Laryngol. 2014, 123, 153–161. [Google Scholar] [CrossRef]
- Casasayas, M.; Sansa, A.; García-Lorenzo, J.; López, M.; Orús, C.; Peláez, X.; Quer, M.; León, X. Pharyngocutaneous fistula after total laryngectomy: Multivariate analysis of risk factors and a severity-based classification proposal. Eur. Arch. Oto-Rhino-Laryngol. 2018, 276, 143–151. [Google Scholar] [CrossRef]
- Morton, R.P.; Mehanna, H.; Hall, F.T.; McIvor, N.P. Prediction of pharyngocutaneous fistulas after laryngectomy. Otolaryngol. Neck Surg. 2007, 136, 46–49. [Google Scholar] [CrossRef]
- Benson, E.M.; Hirata, R.M.; Thompson, C.B.; Ha, P.K.; Fakhry, C.; Saunders, J.R.; Califano, J.A.; Arnaoutakis, D.; Levine, M.; Tang, M.; et al. Pharyngocutaneous fistula after total laryngectomy: A single-institution experience, 2001–2012. Am. J. Otolaryngol. 2015, 36, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Roh, J.L.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Prediction of pharyngocutaneous fistula and survival after salvage laryngectomy for laryngohypopharyngeal carcinoma. Head Neck 2019, 41, 3002–3008. [Google Scholar] [CrossRef]
- Kiliç, C.; Tuncel, U.; Cömert, E. Pharyngocutaneous fistulae after total laryngectomy: Analysis of the risk factors and treatment approaches. B-ENT 2015, 11, 95–100. [Google Scholar]
- Basheeth, N.; O’Leary, G.; Sheahan, P. Pharyngocutaneous fistula after salvage laryngectomy: Impact of interval between radiotherapy and surgery, and performance of bilateral neck dissection. Head Neck 2014, 36, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Formeister, E.J.; Alemi, A.S.; El-Sayed, I.; George, J.R.; Ha, P.; Knott, P.D.; Ryan, W.R.; Seth, R.; Tamplen, M.L.; Heaton, C.M. Shorter interval between radiation therapy and salvage laryngopharyngeal surgery increases complication rates following microvascular free tissue transfer. Am. J. Otolaryngol. 2018, 39, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Lansaat, L.; van der Noort, V.; Bernard, S.E.; Eerenstein, S.E.J.; Plaat, B.E.C.; Langeveld, T.A.P.M.; Lacko, M.; Hilgers, F.J.M.; de Bree, R.; Takes, R.P.; et al. Predictive factors for pharyngocutaneous fistulization after total laryngectomy: A Dutch Head and Neck Society audit. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 783–794. [Google Scholar] [CrossRef]
- Ismi, O.; Unal, M.; Vayisoglu, Y.; Yesilova, M.; Helvaci, I.; Gorur, K.; Ozcan, C. Stapler Esophageal Closure During Total Laryngectomy. J. Craniofacial Surg. 2017, 28, e35–e40. [Google Scholar] [CrossRef]
- Guimarães, A.V.; Aires, F.T.; Dedivitis, R.A.; Kulcsar, M.A.V.; Ramos, D.M.; Cernea, C.R.; Brandão, L.G. Efficacy of pectoralis major muscle flap for pharyngocutaneous fistula prevention in salvage total laryngectomy: A systematic review. Head Neck 2015, 38, e2317–e2321. [Google Scholar] [CrossRef]
- Šifrer, R.; Aničin, A.; Pohar, M.P.; Žargi, M.; Pukl, P.; Soklič, T.; Strojan, P. Pharyngocutaneous fistula: The incidence and the risk factors. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 3393–3399. [Google Scholar] [CrossRef]
- Cardoso, A.J.; Sandy, N.S.; Gomez, G.S.; Servidoni, M.d.F.; Lomazi, E.A.; Bellomo-Brandao, M.A. Factors associated with a higher number of esophageal dilations in children with a history of alkaline ingestion. Arch. Gastroenterol. 2024, 61, e23061. [Google Scholar] [CrossRef]
- Koufman, J.A. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): A clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope 1991, 101, 1–78. [Google Scholar] [PubMed]
- Cocuzza, S.; Bonfiglio, M.; Chiaramonte, R.; Aprile, G.; Mistretta, A.; Grosso, G.; Serra, A. Gastroesophageal reflux disease and postlaryngectomy tracheoesophageal fistula. Eur. Arch. Oto-Rhino-Laryngol. 2012, 269, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Smit, C.F.; Tan, J.; Mathus-Vliegen, L.M.H.; Devriese, P.P.; Brandsen, M.; Grolman, W.; Schouwenburg, P.F. High incidence of gastropharyngeal and gastroesophageal reflux after total laryngectomy. Head Neck 1998, 20, 619–622. [Google Scholar] [CrossRef]
- Gilmore, K.R.; Hutcheson, K.A.; Chapman, P.H.; Bi, W.; Goepfert, R.; Pytynia, K.B.; Rodriguez, M.A. Symptom profile of head and neck patients initiating care in a survivorship clinic. J. Clin. Oncol. 2022, 40, e24047. [Google Scholar] [CrossRef]
- Nilsen, M.L.; Mady, L.J.; Hodges, J.; Wasserman-Wincko, T.; Johnson, J.T. Burden of treatment: Reported outcomes in a head and neck cancer survivorship clinic. Laryngoscope 2019, 129, E437–E444. [Google Scholar] [CrossRef]
- Sellstrom, D.; Haighton, C.; Finch, T.; O’Hara, J.; Patterson, J.M. Assessment and management of late radiation-associated dysphagia after treatment for head and neck cancer: A scoping review and survey of UK speech and language therapists. Int. J. Lang. Commun. Disord. 2025, 60, e13154. [Google Scholar] [CrossRef]
- Stradling, E.J.; Barnhart, M.K.; Robinson, R.A.; Mogg, P.J.; Ward, E.C.; Smee, R.I. Implementing flexible endoscopic evaluationof swallow screening within annual cancer surveillance appoint-ments to monitor for late-stage radiation-induced dysphagia: A feasibility study. Head Neck 2024, 46, 615–662. [Google Scholar] [CrossRef]
- Ebersole, B.; McCarroll, L.; Ridge, J.A.; Liu, J.C.; Bauman, J.; Donnelly, S.; Galloway, T.J. Identification and management of late dysfunction in survivors of head and neck cancer: Implementation and outcomes of an interdisciplinary quality of life (IQOL) clinic. Head Neck 2021, 43, 2124–2135. [Google Scholar] [CrossRef]
- Langmore, S.E.; McCulloch, T.M.; Krisciunas, G.P.; Lazarus, C.L.; Van Daele, D.J.; Pauloski, B.R.; Rybin, D.; Doros, G. Efficacy of electrical stimulation and exercise for dysphagia in patients with head and neck cancer: A randomized clinical trial. Head Neck 2015, 38, E1221–E1231. [Google Scholar] [CrossRef]
- Massonet, H.; Goeleven, A.; Steen, L.V.D.; Vergauwen, A.; Baudelet, M.; Van Haesendonck, G.; Vanderveken, O.; Bollen, H.; van der Molen, L.; Duprez, F.; et al. Home-based intensive treatment of chronic radiation-associated dysphagia in head and neck cancer survivors (HIT-CRAD trial). Trials 2022, 23, 893. [Google Scholar] [CrossRef]
- Van Daele, D.J.; Langmore, S.E.; Krisciunas, G.P.; Lazarus, C.L.; Pauloski, B.R.; McCulloch, T.M.; Gramigna, G.D.; Ma, B.P.M.; Wagner, C.W.; Mott, S.L. The impact of time after radiation treatment on dysphagia in patients with head and neck cancer enrolled in a swallowing therapy program. Head Neck 2019, 41, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.T.; Govender, R.; Roy, P.J.; Vaz, F.; Hilari, K. Factors affecting swallowing outcomes after total laryngectomy: Participant self-report using the swallowing outcomes after laryngectomy questionnaire. Head Neck 2020, 42, 1963–1969. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, A.C.; King, S.; Renslo, B.; Sawaf, T.; Karadaghy, O.; Kraft, S. Functional Oral Intake in Primary Versus Salvage Laryngectomy. Otolaryngol. Head Neck Surg. 2024, 171, 756–763. [Google Scholar] [CrossRef] [PubMed]
| Paper Author/Year | Study Design (Timeframe) | Literature Quality | Patients | Male (%) | Mean Age | Primary (P) vs. Salvage (S) Laryngectomy | Number of Patients Undergoing Dilation (%) |
|---|---|---|---|---|---|---|---|
| Harris et al., 2010 [7] | Retrospective case series (1989–2006) | Fair | 112 | 17 (85%) | 64.5 | 9 (P); 6 (S) | 15 (13.4%) |
| Sweeny et al., 2012 [8] | Retrospective cohort (2003–2009) | Fair | 263 | 214 (81%) | 59 | 118 (P); 145 (S) | 45 (17%) |
| Maejima et al., 2015 [9] | Retrospective cohort (2010–2013) | Fair | 19 | 18 (95%) | 66.3 | 17 (P); 2 (S) | 17 (89%) |
| Zhang et al., 2016 [10] | Cross-sectional study (2014–2015) | Good | 31 | 23 (74%) | 68 | NS | 5 (16.1%) |
| Petersen et al., 2019 [11] | Retrospective cohort (2000–2016) | Fair | 477 | 385 (81%) | 64 | 193 (P); 211 (S) | 111 (23%) |
| Stoner et al., 2019 [12] | Retrospective case series (2013–2017) | Fair | 7 | 4 (57%) | 60 | 6 (P); 1 (S) | 7 (100%) |
| Wu et al., 2019 [13] | Randomized controlled trial (2013–2017) | Good | 21 | 14 (66%) | 65.1 | NS | 11 (100%) |
| Farlow et al., 2020 [14] | Retrospective case series (2000–2018) | Fair | 32 | 30 (70%) | 61 | 32 (S) | 32 (100%) |
| Schuman et al., 2021 [15] | Retrospective cohort (1997–2016) | Fair | 233 | 185 (80%) | 59.8 | 233 (S) | 68 (39.2%) |
| Schimberg et al., 2022 [16] | Prospective case series (2018–2019) | Fair | 10 | 10 (83%) | 72.2 | 5 (P); 5 (S) | 10 (100%) |
| Cortina et al., 2024 [17] | Retrospective cohort (2013–2022) | Fair | 217 | 39 (80%) | 65 | 19 (P); 30 (S) | 49 (23%) |
| Total Number of Patients | 1421 |
|---|---|
| Male:Female | 3.4:1 |
| Mean age at TL, years | 64.1 |
| Resection, n (%) | |
| Sole primary treatment | 286 (20) |
| Salvage | 659 (46) |
| Primary followed by adjuvant treatment | 153 (11) |
| Reconstruction, n (%) | |
| Primary | 1166 (82) |
| Locoregional | 86 (6) |
| Free flap | 169 (12) |
| Paper Author/Year | Number of Patients Undergoing Dilation (%) | Technique | Setting (Office vs. OR) | Mean Dilations Per Patient (Range) | Patients Requiring ≥1 Dilation (%) | Median Time from Laryngectomy to Dilation (Months) | Complications (%) | Overall Rate of Post-Dilation Complications |
|---|---|---|---|---|---|---|---|---|
| Harris et al., 2010 [7] | 15 (13.4%) | Balloon | OR | 2.25 (1–6) | 10 (75%) | 18 | None | 0 (0%) |
| Sweeny et al., 2012 [8] | 45 (17%) | NS | NS | NS | 22 (45%) | NS | None | 0 (0%) |
| Maejima et al., 2015 [9] | 17 (100%) | Balloon | OR | 6.6 (1–30) | 15 (88%) | 23.1 | Bleeding (6%) Pharyngeal edema (6%) | 2 (12%) |
| Zhang et al., 2016 [10] | 5 (16.1%) | Bougie dilator | OR | 1 | 0 | NS | NS | NA |
| Petersen et al., 2019 [11] | 111 (23%) | Bougie dilator | OR | 3 (1–113) | 84 (76%) | 9 | Perforation (5%) Loss of voice prosthesis (5%) | 12 (11%) |
| Stoner et al., 2019 [12] | 7 (100%) | Bougie dilator | OR | 12 (7–48) | 7 (100%) | 14.5 | None | 0 (0%) |
| Wu et al., 2019 [13] | 11 (100%) | Bougie dilator | OR | 3.5 (1–13) | 27 (66%) | NS | Throat pain (4.76%) | 1 (9%) |
| Farlow et al., 2020 [14] | 32 (74%) | NS | NS | NS | 19 (60%) | 24 | NS | 0 (0%) |
| Schuman et al., 2021 [15] | 68 (29.2%) | NS | Both | NS | 41 (60.3%) | 10–15 | NS | 0 (0%) |
| Schimberg et al., 2022 [16] | 10 (100%) | Transnasal balloon | Office | 2.2 | NS | NS | Local infection (10%) | 1 (10%) |
| Cortina et al., 2024 [17] | 49 (22.5%) | Bougie dilator | OR | 4 | 35 (71%) | 8.6 | None | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halagur, A.; Sheth, A.; Wu, S.; Belsky, M.; Damrose, E.J. Pharyngeal Stenosis and Swallowing Dysfunction Following Laryngectomy: A Scoping Review. Surgeries 2025, 6, 41. https://doi.org/10.3390/surgeries6020041
Halagur A, Sheth A, Wu S, Belsky M, Damrose EJ. Pharyngeal Stenosis and Swallowing Dysfunction Following Laryngectomy: A Scoping Review. Surgeries. 2025; 6(2):41. https://doi.org/10.3390/surgeries6020041
Chicago/Turabian StyleHalagur, Akash, Amar Sheth, Shannon Wu, Michael Belsky, and Edward J. Damrose. 2025. "Pharyngeal Stenosis and Swallowing Dysfunction Following Laryngectomy: A Scoping Review" Surgeries 6, no. 2: 41. https://doi.org/10.3390/surgeries6020041
APA StyleHalagur, A., Sheth, A., Wu, S., Belsky, M., & Damrose, E. J. (2025). Pharyngeal Stenosis and Swallowing Dysfunction Following Laryngectomy: A Scoping Review. Surgeries, 6(2), 41. https://doi.org/10.3390/surgeries6020041






