Exploring the Impact of Diabetes Mellitus on Clinical Outcomes in Patients Following Severe Traumatic Brain Injury Using the TriNetX Database
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
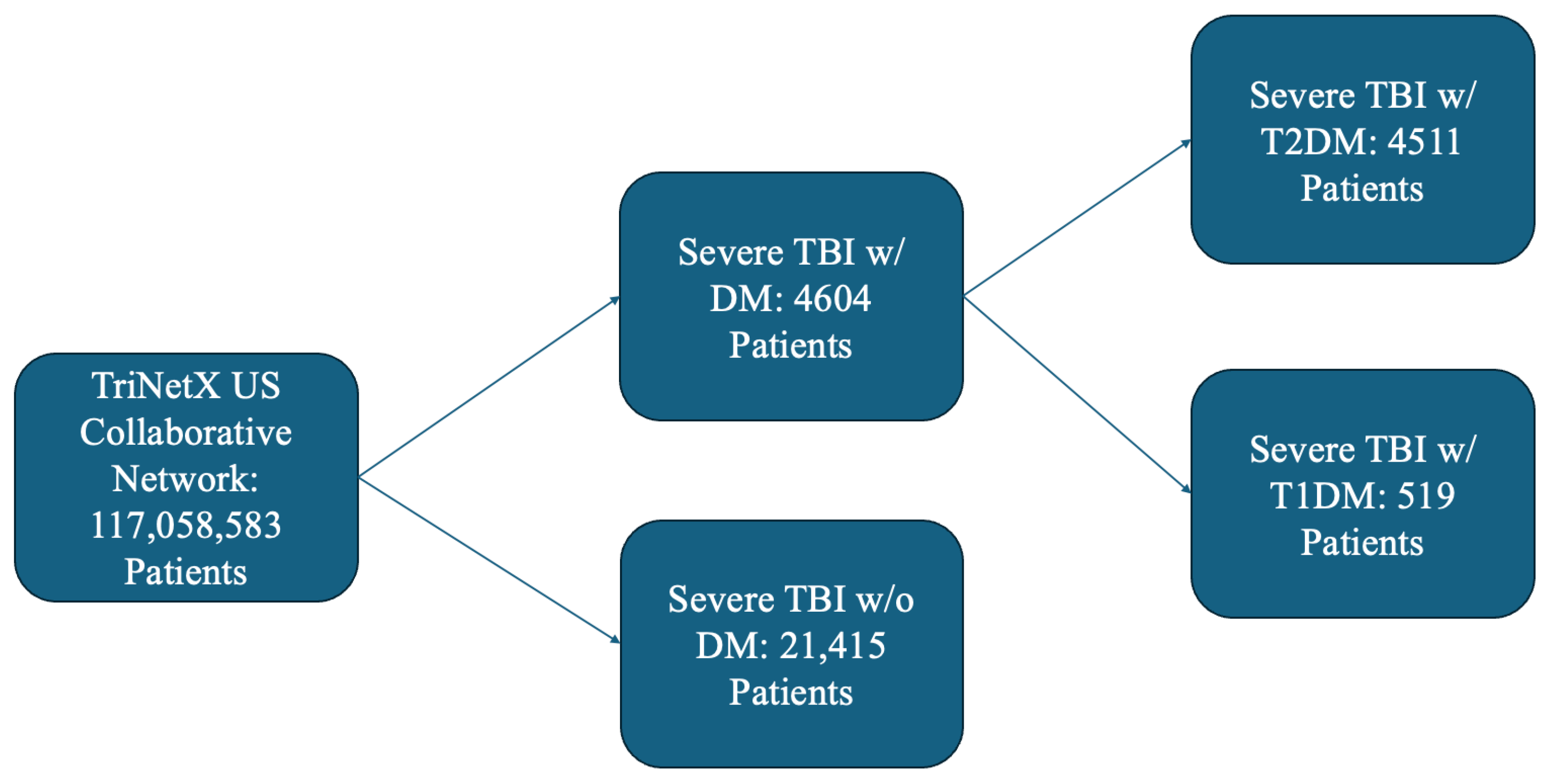
2.2. Cohort Selection
2.3. Variables
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. Diabetic vs. Non-Diabetic Cohorts
3.2. Insulin-Dependent Diabetes vs. Insulin-Independent Diabetes Cohorts
4. Discussion
4.1. Impact of DM on Outcomes
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| LOINC | Logical Observation Identifiers Names and Codes |
| DM | Diabetes mellitus |
| DVT | Deep vein thrombosis |
| ICD-10-CM | International Classification of Diseases, Tenth Revision, Clinical Modification |
| IDDM | Insulin-dependent diabetes mellitus |
| MI | Myocardial infarction |
| NIDDM | Non-insulin dependent diabetes mellitus |
| PE | Pulmonary embolism |
| PEG | Percutaneous endoscopic gastrostomy |
| RR | Risk ratio |
| SMD | Standardized mean difference |
| TBI | Traumatic brain injury |
References
- Wong, V.S.; Langley, B. Epigenetic changes following traumatic brain injury and their implications for outcome, recovery and therapy. Neurosci. Lett. 2016, 625, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Rovlias, A.; Kotsou, S. The influence of hyperglycemia on neurological outcome in patients with severe head injury. Neurosurgery 2000, 46, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Cochran, A.; Scaife, E.R.; Hansen, K.W.; Downey, E.C. Hyperglycemia and outcomes from pediatric traumatic brain injury. J. Trauma-Inj. Infect. Crit. Care 2003, 55, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Bürki, A.; Chesnut, R.M.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef]
- Jeremitsky, E.; Omert, L.A.; Dunham, C.M.; Wilberger, J.; Rodriguez, A. The impact of hyperglycemia on patients with severe brain injury. J. Trauma-Inj. Infect. Crit. Care 2005, 58, 47–50. [Google Scholar] [CrossRef]
- Prisco, L.; Iscra, F.; Ganau, M.; Berlot, G. Early predictive factors on mortality in head injured patients: A retrospective analysis of 112 traumatic brain injured patients. J. Neurosurg. Sci. 2012, 56, 131–136. [Google Scholar]
- Chong, S.L.; Harjanto, S.; Testoni, D.; Ng, Z.M.; Low, C.Y.D.; Lee, K.P.; Lee, J.H. Early Hyperglycemia in Pediatric Traumatic Brain Injury Predicts for Mortality, Prolonged Duration of Mechanical Ventilation, and Intensive Care Stay. Int. J. Endocrinol. 2015, 2015, 719476. [Google Scholar] [CrossRef]
- Shi, J.; Dong, B.; Mao, Y.M.; Guan, W.; Cao, J.C.; Zhu, R.X.; Wang, S.N. Review: Traumatic brain injury and hyperglycemia, a potentially modifiable risk factor. Oncotarget 2016, 7, 71052–71061. [Google Scholar] [CrossRef]
- Van den Berghe, G.; Wouters, P.; Weekers, F.; Verwaest, C.; Bruyninckx, F.; Schetz, M.; Vlasselaers, D.; Ferdinande, P.; Lauwers, P.; Bouillon, R. Intensive insulin therapy in critically ill patients. N. Engl. J. Med. 2001, 345, 1359–1367. [Google Scholar] [CrossRef]
- Type 2 Diabetes. Available online: https://www.cdc.gov/diabetes/about/about-type-2-diabetes.html (accessed on 18 April 2023).
- Marseglia, A.; Fratiglioni, L.; Kalpouzos, G.; Wang, R.; Bäckman, L.; Xu, W.L. Prediabetes and diabetes accelerate cognitive decline and predict microvascular lesions: A population-based cohort study. Alzheimers Dement. 2019, 15, 25–33. [Google Scholar] [CrossRef]
- Chaudhry, R.; Kukreja, N.; Tse, A.; Pednekar, G.; Mouchli, A.; Young, L.; Didyuk, O.; Wegner, R.C.; Grewal, N.; Williams, G.W. Trends and Outcomes of Early Versus Late Percutaneous Endoscopic Gastrostomy Placement in Patients with Traumatic Brain Injury: Nationwide Population-based Study. J. Neurosurg. Anesthesiol. 2018, 30, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Galimberti, S.; Graziano, F.; Wiegers, E.J.A.; Lingsma, H.F.; Iaquaniello, C.; Stocchetti, N.; Menon, D.; Citerio, G.; Participants, C.-T.I. Tracheostomy practice and timing in traumatic brain-injured patients: A CENTER-TBI study. Intensive Care Med. 2020, 46, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Harhangi, B.S.; Kompanje, E.J.O.; Leebeek, F.W.G.; Maas, A.I.R. Coagulation disorders after traumatic brain injury. Acta Neurochir. 2008, 150, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Lustenberger, T.; Talving, P.; Lam, L.; Inaba, K.; Bass, M.; Plurad, D.; Demetriades, D. Effect of diabetes mellitus on outcome in patients with traumatic brain injury: A national trauma databank analysis. Brain Inj. 2013, 27, 281–285. [Google Scholar] [CrossRef]
- Tseng, C.C.; Huang, Y.C.; Tu, P.H.; Yip, P.K.; Chang, T.W.; Lee, C.C.; Chen, C.C.; Chen, N.Y.; Liu, Z.H. Impact of Diabetic Hyperglycemia on Clinical Outcomes in Patients with Diabetes Mellitus Following Traumatic Brain Injury. Turk. Neurosurg. 2023, 33, 548–555. [Google Scholar] [CrossRef]
- Schaffer, S.W.; Croft, C.B.; Solodushko, V. Cardioprotective effect of chronic hyperglycemia: Effect on hypoxia-induced apoptosis and necrosis. Am. J. Physiol.-Heart Circ. Physiol. 2000, 278, H1948–H1954. [Google Scholar] [CrossRef]
- Rojas, D.R.; Tegeder, I.; Kuner, R.; Agarwal, N. Hypoxia-inducible factor 1 protects peripheral sensory neurons from diabetic peripheral neuropathy by suppressing accumulation of reactive oxygen species. J. Mol. Med. 2018, 96, 1395–1405. [Google Scholar] [CrossRef]
- Pasquel, F.J.; Lansang, M.C.; Dhatariya, K.; Umpierrez, G.E. Management of diabetes and hyperglycaemia in the hospital. Lancet Diabetes Endocrinol. 2021, 9, 174–188. [Google Scholar] [CrossRef]
- Patil, S.P.; Jain, P.D.; Ghumatkar, P.J.; Tambe, R.; Sathaye, S. Neuroprotective effect of metformin in mptp-induced parkinson’s disease in mice. Neuroscience 2014, 277, 747–754. [Google Scholar] [CrossRef]
- Cai, X.S.; She, M.Q.; Xu, M.Y.; Chen, H.Y.; Li, J.J.; Chen, X.L.; Zheng, D.P.; Liu, J.; Chen, S.L.; Zhu, J.B.; et al. GLP-1 treatment protects endothelial cells from oxidative stress-induced autophagy and endothelial dysfunction. Int. J. Biol. Sci. 2018, 14, 1696–1708. [Google Scholar] [CrossRef]
- Siddeeque, N.; Hussein, M.H.; Abdelmaksoud, A.; Bishop, J.; Attia, A.S.; Elshazli, R.M.; Fawzy, M.S.; Toraih, E.A. Neuroprotective effects of GLP-1 receptor agonists in neurodegenerative Disorders: A Large-Scale Propensity-Matched cohort study. Int. Immunopharmacol. 2024, 143, 113537. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Oelze, M.; Hanf, A.; Kröller-Schön, S.; Kashani, F.; Roohani, S.; Welschof, P.; Kopp, M.; Gödtel-Armbrust, U.; Xia, N.; et al. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol. 2017, 13, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gallego, C.G.; Requena-Ibanez, J.A.; Antonio, R.S.; Ishikawa, K.; Watanabe, S.; Picatoste, B.; Flores, E.; Garcia-Ropero, A.; Sanz, J.; Hajjar, R.J.; et al. Empagliflozin Ameliorates Adverse Left Ventricular Remodeling in Nondiabetic Heart Failure by Enhancing Myocardial Energetics. J. Am. Coll. Cardiol. 2019, 73, 1931–1944. [Google Scholar] [CrossRef] [PubMed]
- Nojima, I.; Eikawa, S.; Tomonobu, N.; Hada, Y.; Kajitani, N.; Teshigawara, S.; Miyamoto, S.; Tone, A.; Uchida, H.A.; Nakatsuka, A.; et al. Dysfunction of CD8+PD-1+T cells in type 2 diabetes caused by the impairment of metabolism-immune axis. Sci. Rep. 2020, 10, 14928. [Google Scholar] [CrossRef]
- Zhao, J.L.; Yang, S.; Shu, B.; Chen, L.; Yang, R.H.; Xu, Y.B.; Xie, J.L.; Liu, X.S.; Qi, S.H. Transient High Glucose Causes Persistent Vascular Dysfunction and Delayed Wound Healing by the DNMT1-Mediated Ang-1/NF-κB Pathway. J. Investig. Dermatol. 2021, 141, 1573–1584. [Google Scholar] [CrossRef]
- Kopf, S.; Groener, J.B.; Kender, Z.; Fleming, T.; Brune, M.; Riedinger, C.; Volk, N.; Herpel, E.; Pesta, D.; Szendrödi, J.; et al. Breathlessness and Restrictive Lung Disease: An Important Diabetes-Related Feature in Patients with Type 2 Diabetes. Respiration 2018, 96, 29–40. [Google Scholar] [CrossRef]
- Cheng, S.J.; Hou, G.J.; Liu, Z.P.; Lu, Y.; Liang, S.C.; Cang, L.; Zhang, X.Y.; Zou, C.L.; Kang, J.; Chen, Y. Risk prediction of in-hospital mortality among patients with type 2 diabetes mellitus and concomitant community-acquired pneumonia. Ann. Pallliat. Med. 2020, 9, 3313–3325. [Google Scholar] [CrossRef]
- Langmore, S.E.; Krisciunas, G.P.; Warner, H.; White, S.D.; Dvorkin, D.; Fink, D.; McNally, E.; Scheel, R.; Higgins, C.; Levitt, J.E.; et al. Abnormalities of Aspiration and Swallowing Function in Survivors of Acute Respiratory Failure. Dysphagia 2021, 36, 831–841. [Google Scholar] [CrossRef]
- Tang, F.; Lane, S.; Korsak, A.; Paton, J.F.R.; Gourine, A.V.; Kasparov, S.; Teschemacher, A.G. Lactate-mediated glia-neuronal signalling in the mammalian brain. Nat. Commun. 2014, 5, 3284. [Google Scholar] [CrossRef]
- Xing, G.Q.; Ren, M.; O’Neill, J.T.; Verma, A.; Watson, W.D. Controlled cortical impact injury and craniotomy result in divergent alterations of pyruvate metabolizing enzymes in rat brain. Exp. Neurol. 2012, 234, 31–38. [Google Scholar] [CrossRef]
- Reno, C.M.; Skinner, A.; Bayles, J.; Chen, Y.S.; Daphna-Iken, D.; Fisher, S.J. Severe hypoglycemia-induced sudden death is mediated by both cardiac arrhythmias and seizures. Am. J. Physiol.-Endocrinol. Metab. 2018, 315, E240–E249. [Google Scholar] [CrossRef] [PubMed]
- Hermanides, J.; Plummer, M.P.; Finnis, M.; Deane, A.M.; Coles, J.P.; Menon, D.K. Glycaemic control targets after traumatic brain injury: A systematic review and meta-analysis. Crit. Care 2018, 22, 11. [Google Scholar] [CrossRef]
- Garcia-Ballestas, E.; Villafañe, J.; Nuñez-Baez, K.; Perdomo, W.A.F.; Duran, M.A.; Janjua, T.; Moscote-Salazar, L.R.; Agrawal, A. A systematic review and meta-analysis on glycemic control in traumatic brain injury. Clin. Neurol. Neurosurg. 2024, 245, 108504. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, S.; Xiong, C.; Chan, V.; Sutton, M.; Escobar, M.; Colantonio, A.; Mollayeva, T. Comorbidity in traumatic brain injury and functional outcomes: A systematic review. Eur. J. Phys. Rehabil. Med. 2021, 57, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Stoudt, K.; Chawla, S. Don’t Sugar Coat It: Glycemic Control in the Intensive Care Unit. J. Intensive Care Med. 2019, 34, 889–896. [Google Scholar] [CrossRef]
- Finfer, S.; Blair, D.; Bellomo, R.; McArthur, C.; Mitchell, I.; Myburgh, J.; Norton, R.; Potter, J.; Chittock, D.; Dhingra, V.; et al. Intensive versus Conventional Glucose Control in Critically Ill Patients. N. Engl. J. Med. 2009, 360, 1283–1297. [Google Scholar] [CrossRef]
- Zhu, C.R.; Chen, J.J.; Pan, J.C.; Qiu, Z.C.; Xu, T. Therapeutic effect of intensive glycemic control therapy in patients with traumatic brain injury: A systematic review and meta-analysis of randomized controlled trials. Medicine 2018, 97, e11671. [Google Scholar] [CrossRef]
- Vespa, P.; McArthur, D.L.; Stein, N.; Huang, S.C.; Shao, W.; Filippou, M.; Etchepare, M.; Glenn, T.; Hovda, D.A. Tight glycemic control increases metabolic distress in traumatic brain injury: A randomized controlled within-subjects trial. Crit. Care Med. 2012, 40, 1923–1929. [Google Scholar] [CrossRef]
- Laing, J.; Gabbe, B.; Chen, Z.B.; Perucca, P.; Kwan, P.; O’Brien, T.J. Risk Factors and Prognosis of Early Posttraumatic Seizures in Moderate to Severe Traumatic Brain Injury. JAMA Neurol. 2022, 79, 334–341. [Google Scholar] [CrossRef]
- Gupta, M.; Pandey, S.; Rumman, M.; Singh, B.; Mahdi, A.A. Molecular mechanisms underlying hyperglycemia associated cognitive decline. IBRO Neurosci. Rep. 2023, 14, 57–63. [Google Scholar] [CrossRef]
- Lai, J.Q.; Shi, Y.C.; Lin, S.; Chen, X.R. Metabolic disorders on cognitive dysfunction after traumatic brain injury. Trends Endocrinol. Metab. 2022, 33, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.L.; Siu, J.J.; Huang, W.; Askwith, C.; Cao, L. Insulin Modulates Excitatory Synaptic Transmission and Synaptic Plasticity in the Mouse Hippocampus. Neuroscience 2019, 411, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Perazzo, P.; Bottinelli, E.; Possenti, F.; Banfi, G. Managing a tertiary orthopedic hospital during the covid-19 epidemic, main challenges and solutions adopted. Int. J. Environ. Res. Public Health 2020, 17, 4818. [Google Scholar] [CrossRef] [PubMed]
- Mackersie, R.C.; Dicker, R.A. Pitfalls in the Evaluation and Management of the Trauma Patient. Curr. Probl. Surg. 2007, 44, 778–833. [Google Scholar] [CrossRef]
- Eriksson, E.A.; Christianson, D.A.; Vanderkolk, W.E.; Bonnell, B.W.; Hoogeboom, J.E.; Ott, M.M. Tight blood glucose control in trauma patients: Who really benefits. J. Emerg. Trauma Shock 2011, 4, 359–364. [Google Scholar] [CrossRef]
- Kurtz, P.; Rocha, E.E.M. Nutrition Therapy, Glucose Control, and Brain Metabolism in Traumatic Brain Injury: A Multimodal Monitoring Approach. Front. Neurosci. 2020, 14, 190. [Google Scholar] [CrossRef]
- Späni, C.B.; Braun, D.J.; Van Eldik, L.J. Sex-related responses after traumatic brain injury: Considerations for preclinical modeling. Front. Neuroendocrinol. 2018, 50, 52–66. [Google Scholar] [CrossRef]
- Lakshmipathy, D.; Rangarajan, S.; Barreau, A.; Lu, J.; Kleinberg, G.; Lucke-Wold, B. Genetic Contributions to Recovery following Brain Trauma: A Narrative Review. Front. Biosci. 2024, 29, 103. [Google Scholar] [CrossRef]
- Dasic, D.; Morgan, L.; Panezai, A.; Syrmos, N.; Ligarotti, G.K.I.; Zaed, I.; Chibbaro, S.; Khan, T.; Prisco, L.; Ganau, M. A scoping review on the challenges, improvement programs, and relevant output metrics for neurotrauma services in major trauma centers. Surg. Neurol. Int. 2022, 13, 171. [Google Scholar] [CrossRef]
- Hiroyuki, H.; Shigeto, O.; Masataka, N. Blood glucose control in patients with severe sepsis and septic shock. World J. Gastroenterol. 2009, 15, 4132. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Diao, L.; Stanojcic, M.; Xiu, F.; Eckardt, K. Stress Hyperglycemia, Insulin Treatment, and Innate Immune Cells. Int. J. Endocrinol. 2014, 2014, 486403. [Google Scholar] [CrossRef]
| Presence of Diabetes Cohort Demographics | |||
|---|---|---|---|
| Characteristics | Propensity-Score Matched Sample No. (%) | ||
| With DM | Without DM | SMD | |
| N = 3986 | N = 3986 | ||
| Age at Index (years) | 63 | 64 | 0.0878 |
| Gender | |||
| Female | 1286 (32.26) | 1289 (32.24) | 0.00016 |
| Male | 2552 (64.02) | 2553 (64.05) | 0.0005 |
| Race | |||
| White | 1927 (48.34) | 1968 (49.37) | 0.0206 |
| Black | 606 (15.20) | 581 (14.58) | 0.0176 |
| Asian | 193 (4.842) | 174 (4.365) | 0.0227 |
| Unknown Race | 1012 (25.39) | 1046 (26.24) | 0.0195 |
| Chronic Medical Conditions | |||
| Hypertension | 3163 (79.35) | 3180 (79.78) | 0.0106 |
| Dyslipidemia | 1950 (48.92) | 1875 (47.04) | 0.0377 |
| Heart Failure | 802 (20.12) | 766 (19.22) | 0.0227 |
| Liver Disease | 194 (4.867) | 188 (4.717) | 0.007 |
| Obesity | 800 (20.07) | 749 (18.79) | 0.0323 |
| Alcohol Abuse | 619 (15.53) | 613 (15.38) | 0.0042 |
| Alcohol Dependence | 442 (11.09) | 455 (11.42) | 0.0103 |
| Nicotine Dependence | 876 (21.98) | 869 (21.80) | 0.0042 |
| Chronic Lower Respiratory Disease | 929 (23.31) | 889 (22.30) | 0.0239 |
| Ischemic Heart Disease | 1363 (34.20) | 1328 (33.32) | 0.0186 |
| Atrial Fibrillation and Flutter | 896 (22.48) | 913 (22.91) | 0.0102 |
| Acute Kidney Failure and Chronic Kidney Disease | 1642 (41.19) | 1611 (40.42) | 0.0158 |
| Other Peripheral Vascular Diseases | 297 (7.451) | 272 (6.824) | 0.0244 |
| Medications | |||
| Cardiovascular Medications | 2384 (59.81) | 2363 (59.28) | 0.0107 |
| Blood Products/Modifiers/Volume Expanders | 1845 (46.29) | 1817 (45.59) | 0.0141 |
| Complications Risk | ||||
|---|---|---|---|---|
| Outcomes | Risk Ratio | Lower Bound | Upper Bound | p-Value |
| Mortality | 0.815 | 0.771 | 0.861 | <0.0001 |
| Sepsis | 1.321 | 1.163 | 1.5 | <0.0001 |
| Tracheostomy | 1.278 | 1.137 | 1.436 | <0.0001 |
| PEG | 1.265 | 1.122 | 1.428 | 0.0001 |
| Pneumonia | 1.2 | 1.088 | 1.322 | 0.0002 |
| Seizure | 1.174 | 1.069 | 1.289 | 0.0007 |
| Craniotomy | 1.052 | 0.907 | 1.221 | 0.5004 |
| Surgical Site Infection | 1.001 | 0.417 | 2.402 | 0.9987 |
| Ventilator Dependence | 0.902 | 0.844 | 0.963 | 0.0021 |
| MI | 1.08 | 0.889 | 1.311 | 0.4367 |
| Cerebral Infarction | 1.309 | 1.142 | 1.5 | <0.0001 |
| DVT | 1.11 | 0.845 | 1.457 | 0.4543 |
| PE | 0.986 | 0.765 | 1.271 | 0.9146 |
| Diabetes Type Cohort Demographics | |||
|---|---|---|---|
| Characteristics | Propensity-Score Matched Sample No. (%) | ||
| With IDDM N = 519 | With NIDDM N = 519 | SMD | |
| Age at Index (years) | 60 | 61 | 0.0445 |
| Gender | |||
| Female | 174 (33.53) | 168 (32.37) | 0.0246 |
| Male | 317 (61.08) | 323 (62.24) | 0.0238 |
| Race | |||
| White | 254 (48.94) | 247 (47.59) | 0.027 |
| Black | 114 (21.97) | 115 (22.16) | 0.0046 |
| Asian | 14 (2.697) | 15 (2.89) | 0.0117 |
| Unknown Race | 103 (19.85) | 108 (20.81) | 0.0239 |
| Chronic Medical Conditions | |||
| Hypertension | 453 (87.28) | 457 (88.05) | 0.0234 |
| Dyslipidemia | 355 (68.40) | 372 (71.68) | 0.0715 |
| Heart Failure | 200 (38.54) | 203 (39.11) | 0.0119 |
| Liver Disease | 52 (10.02) | 56 (10.79) | 0.0252 |
| Obesity | 189 (36.42) | 200 (38.54) | 0.0438 |
| Alcohol Abuse | 97 (18.69) | 105 (20.23) | 0.0389 |
| Alcohol Dependence | 68 (13.10) | 69 (13.30) | 0.0057 |
| Nicotine Dependence | 154 (29.67) | 158 (30.44) | 0.01068 |
| Chronic Lower Respiratory Disease | 174 (33.53) | 178 (34.30) | 0.0163 |
| Ischemic Heart Disease | 262 (50.48) | 269 (51.83) | 0.027 |
| Atrial Fibrillation and Flutter | 136 (26.20) | 137 (26.40) | 0.044 |
| Acute Kidney Failure and Chronic Kidney Disease | 337 (64.93) | 339 (65.32) | 0.0081 |
| Other Peripheral Vascular Diseases | 130 (25.05) | 133 (25.63) | 0.0133 |
| Medications | |||
| Cardiovascular Medications | 320 (61.66) | 323 (62.24) | 0.0119 |
| Blood Products/Modifiers/Volume Expanders | 289 (55.68) | 300 (57.80) | 0.0428 |
| Complications Risk | ||||
|---|---|---|---|---|
| Outcomes | Risk Ratio | Lower Bound | Upper Bound | p-Value |
| Mortality | 0.987 | 0.838 | 1.163 | 0.8783 |
| Sepsis | 1.105 | 0.752 | 1.623 | 0.6103 |
| Tracheostomy | 0.952 | 0.672 | 1.348 | 0.7816 |
| PEG | 0.923 | 0.636 | 1.34 | 0.6736 |
| Pneumonia | 0.99 | 0.701 | 1.399 | 0.9549 |
| Seizure | 0.918 | 0.717 | 1.175 | 0.4951 |
| Craniotomy | 0.842 | 0.512 | 1.384 | 0.4972 |
| Surgical Site Infection | 1 | 0.42 | 2.382 | 1 |
| Ventilator Dependence | 0.928 | 0.742 | 1.16 | 0.5094 |
| MI | 0.972 | 0.557 | 1.695 | 0.9197 |
| Cerebral Infarction | 0.943 | 0.598 | 1.485 | 0.7997 |
| DVT | 0.844 | 0.382 | 1.867 | 0.6757 |
| PE | 1.002 | 0.421 | 2.386 | 0.9963 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaik, K.; Rasmussen, S.; Rahme, R.; Karsy, M. Exploring the Impact of Diabetes Mellitus on Clinical Outcomes in Patients Following Severe Traumatic Brain Injury Using the TriNetX Database. Surgeries 2025, 6, 38. https://doi.org/10.3390/surgeries6020038
Shaik K, Rasmussen S, Rahme R, Karsy M. Exploring the Impact of Diabetes Mellitus on Clinical Outcomes in Patients Following Severe Traumatic Brain Injury Using the TriNetX Database. Surgeries. 2025; 6(2):38. https://doi.org/10.3390/surgeries6020038
Chicago/Turabian StyleShaik, Kamal, Spencer Rasmussen, Rudy Rahme, and Michael Karsy. 2025. "Exploring the Impact of Diabetes Mellitus on Clinical Outcomes in Patients Following Severe Traumatic Brain Injury Using the TriNetX Database" Surgeries 6, no. 2: 38. https://doi.org/10.3390/surgeries6020038
APA StyleShaik, K., Rasmussen, S., Rahme, R., & Karsy, M. (2025). Exploring the Impact of Diabetes Mellitus on Clinical Outcomes in Patients Following Severe Traumatic Brain Injury Using the TriNetX Database. Surgeries, 6(2), 38. https://doi.org/10.3390/surgeries6020038






