A New Preclinical Surgical Model for the Assessment of Dental Implant Tissue Integration
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
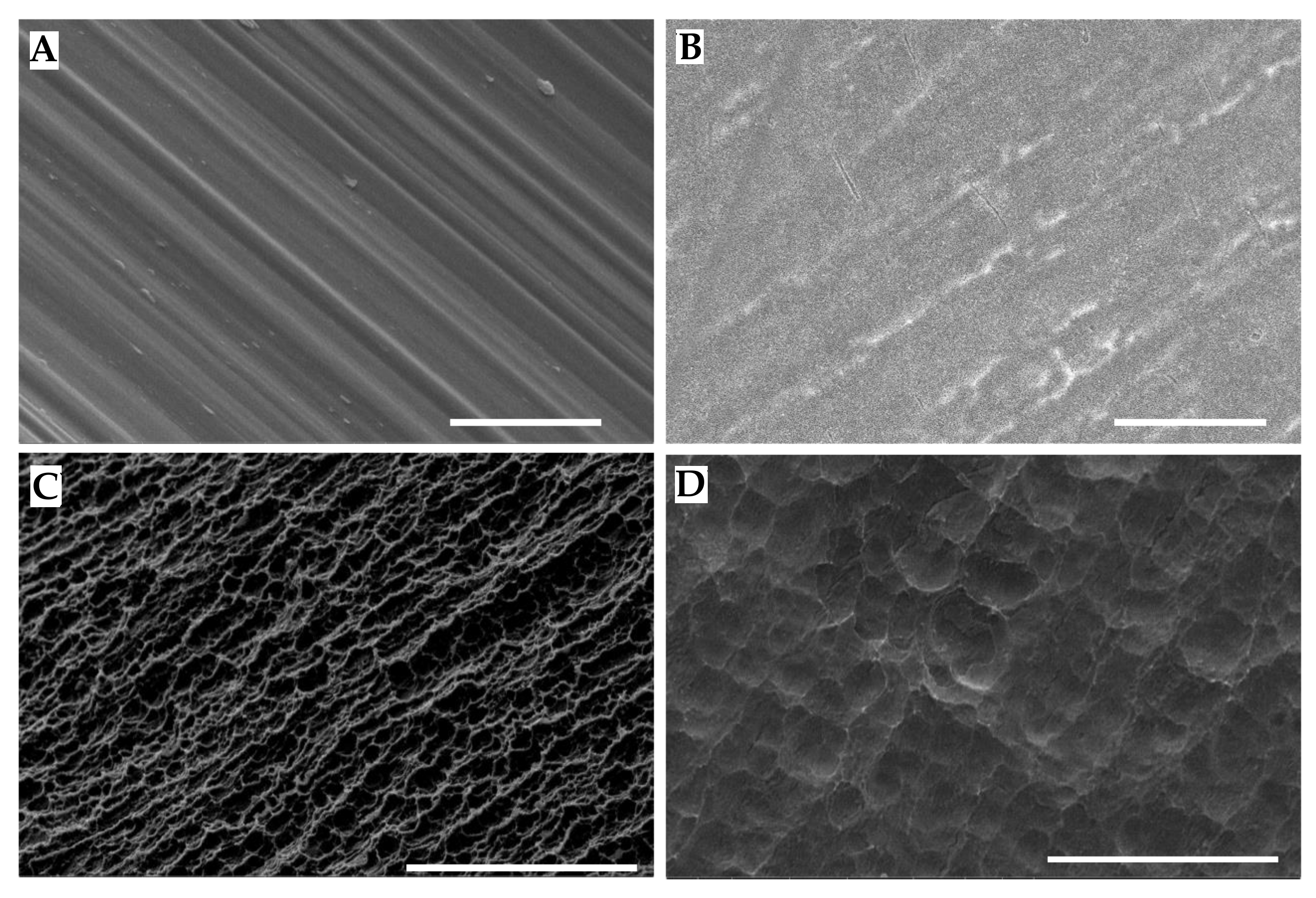
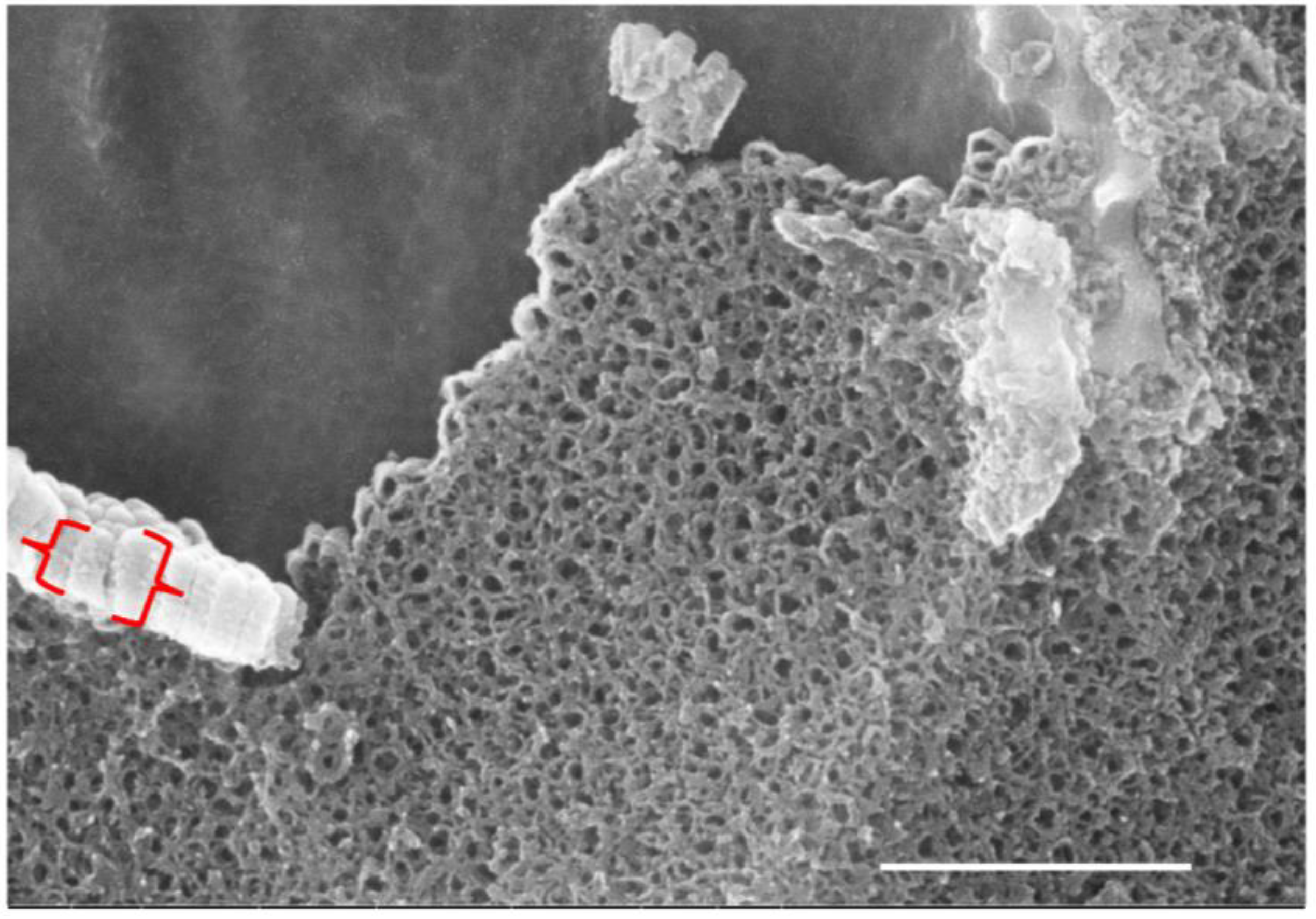
2.2. Scanning Electron Microscopy
2.3. Animal Model
2.4. Anesthesia and Pre-Surgical Preparation
2.5. Surgical Procedure
2.6. Euthanasia
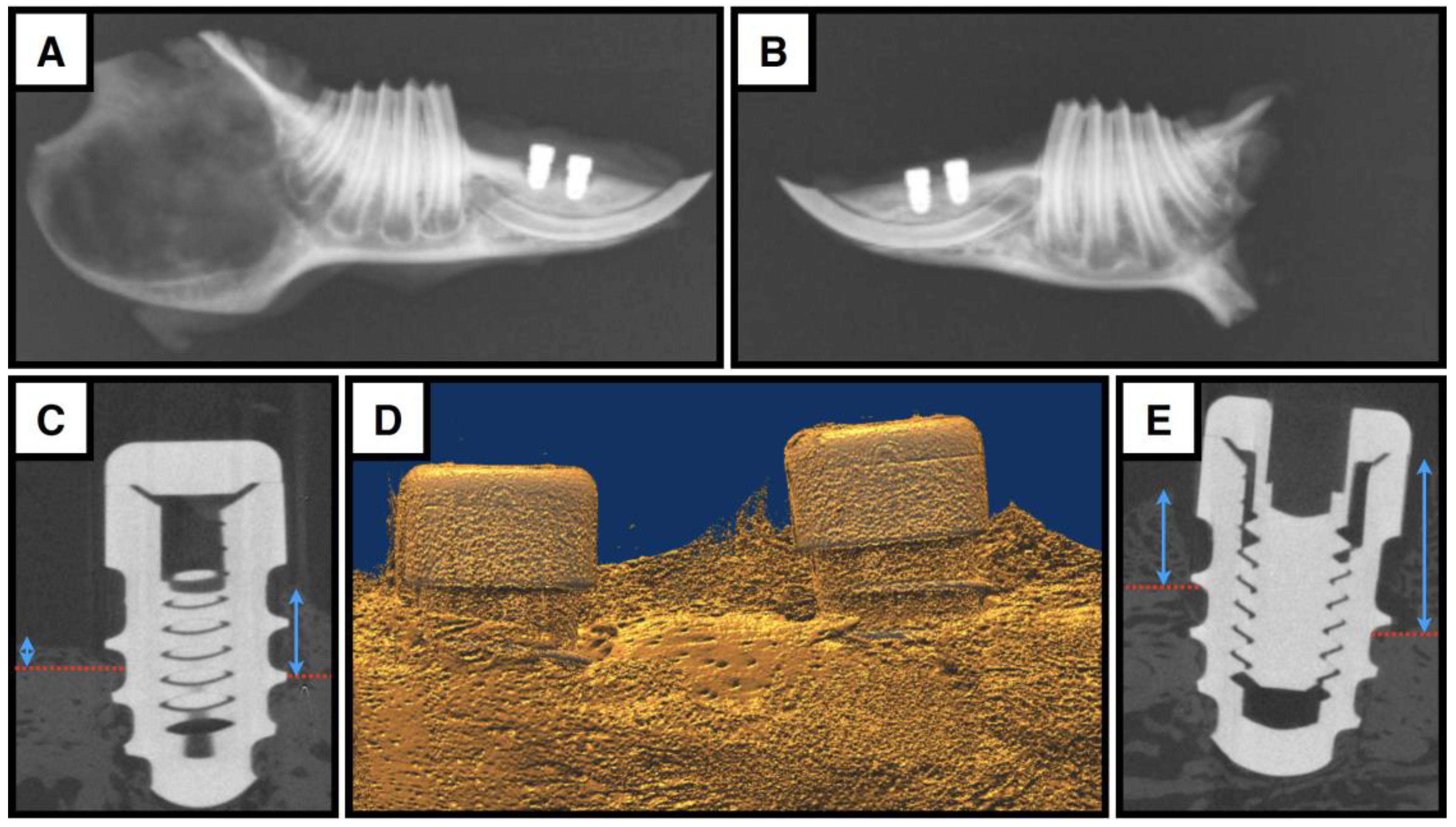
2.7. X-Ray and microCT Analysis
2.8. Sample Harvesting
2.9. Sample Processing for Resin Embedding
2.9.1. Osteobed Method
2.9.2. Technovit Method
2.10. Histological Sectioning
2.10.1. Staining of Osteobed Sections
2.10.2. Staining of Technovit Sections
3. Results
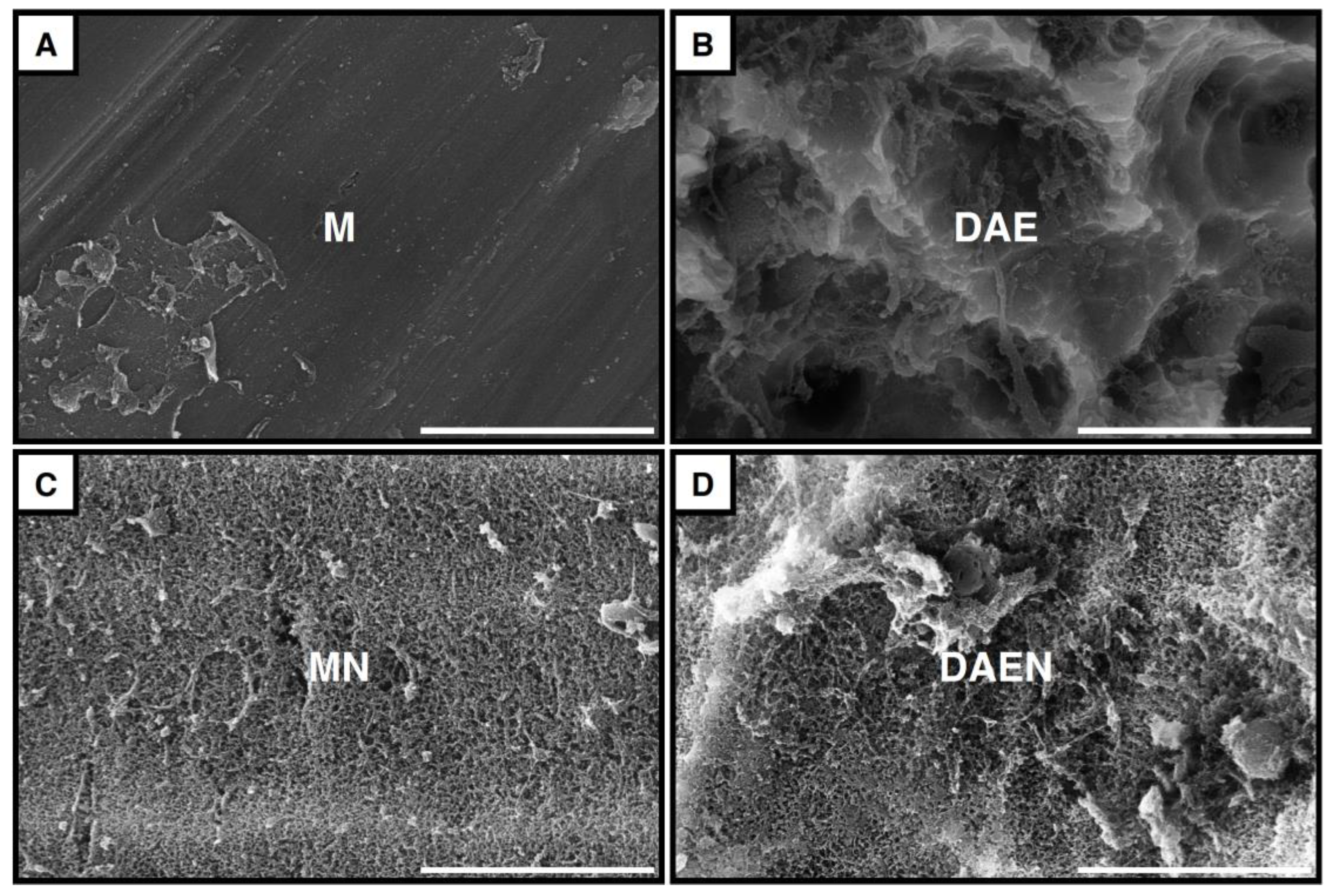
3.1. Implant Surfaces
3.2. Surgical Outcomes
3.3. The Bony Compartment
3.4. Soft Tissue Compartment
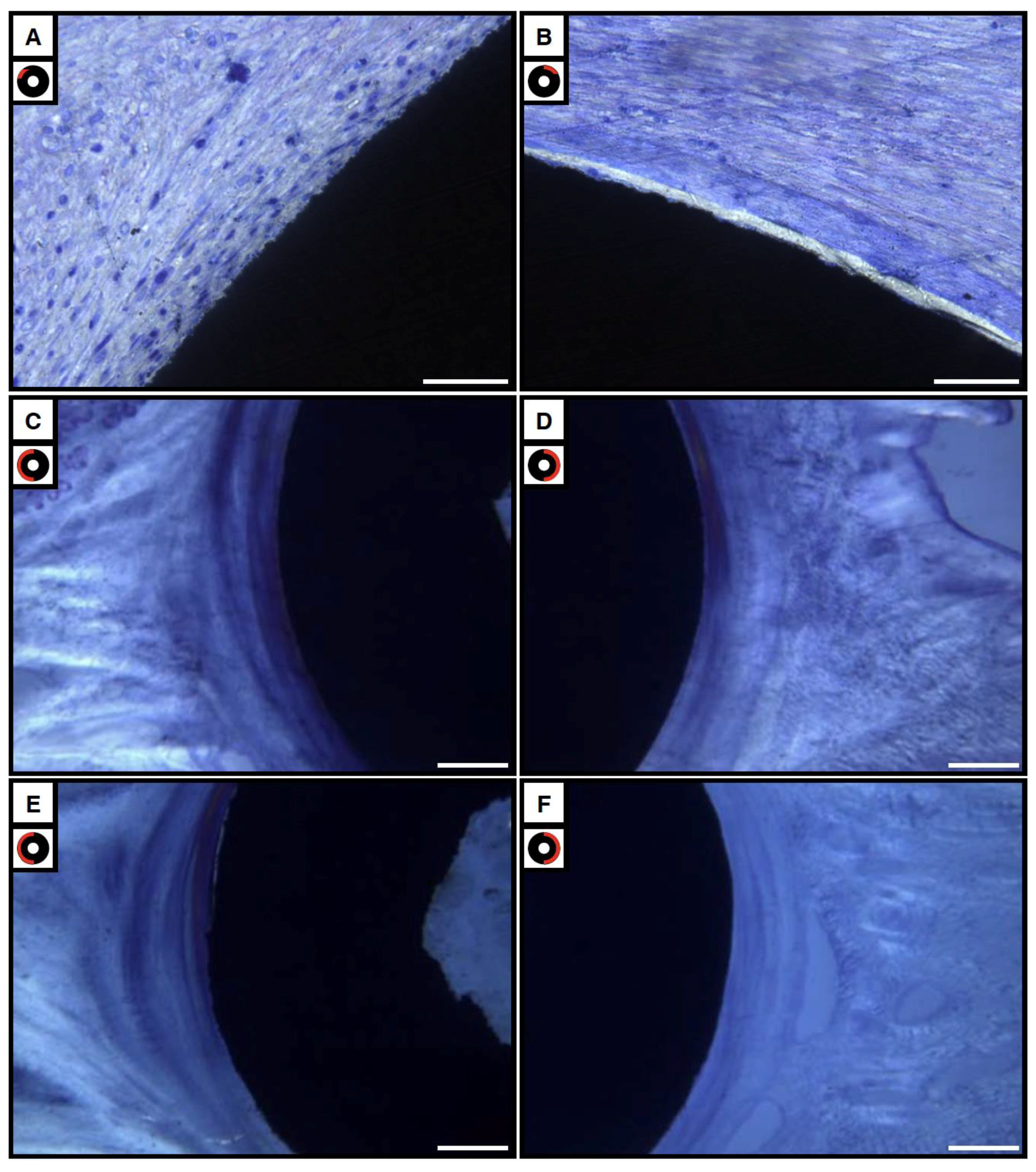
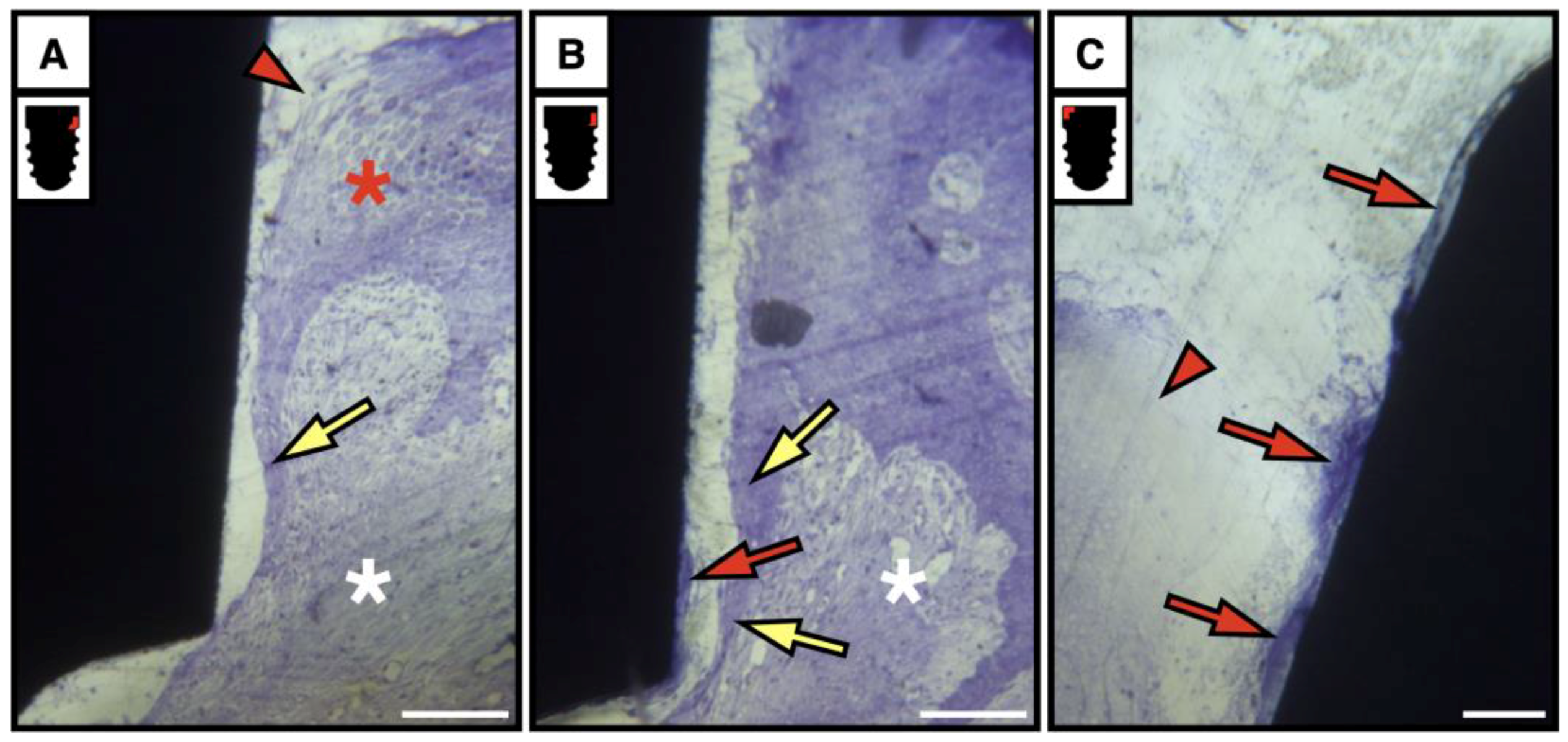
3.4.1. Bright and Polarized Light Microscopy
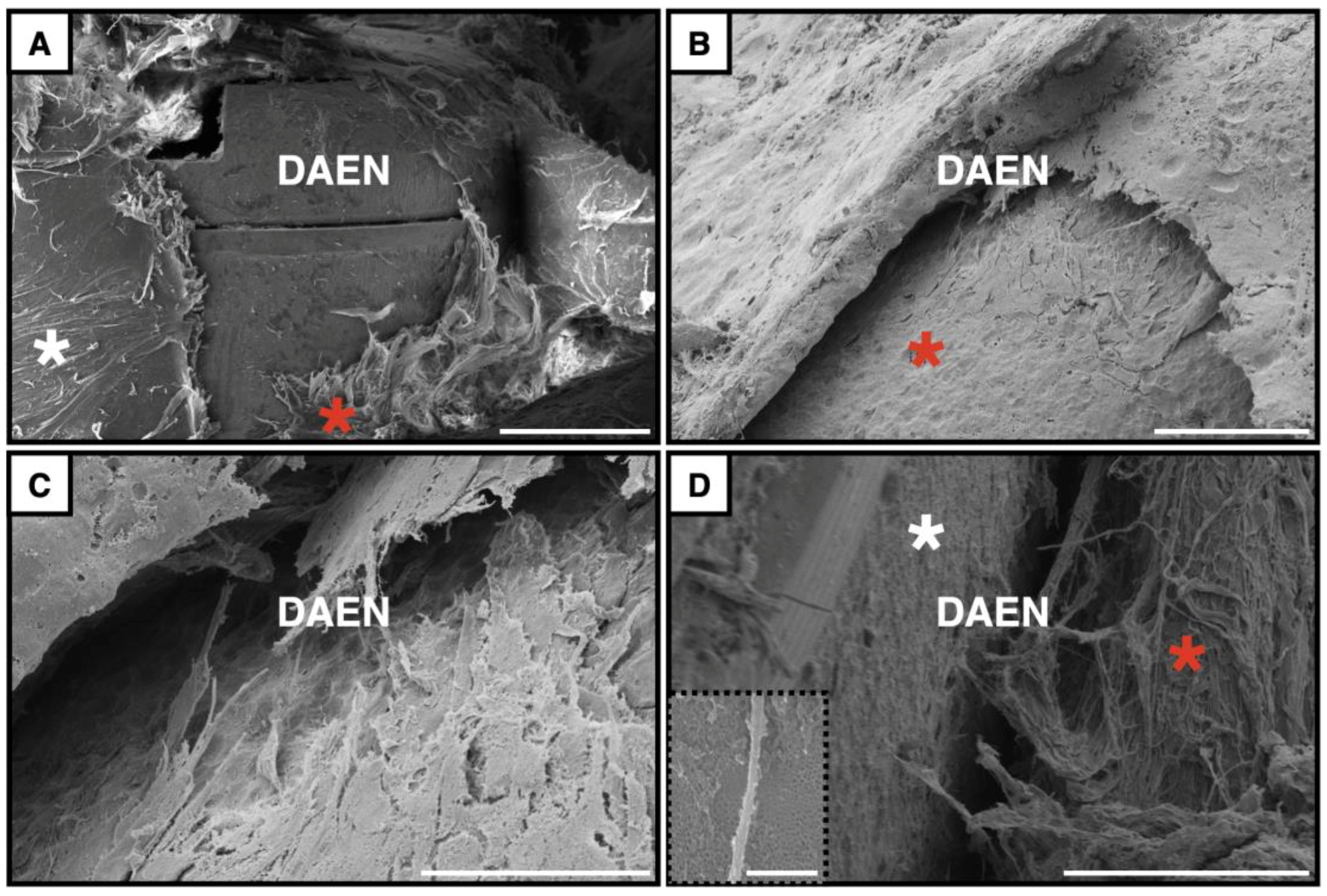
3.4.2. Scanning Electron Microscopy Observations
3.5. A Note on the Prematurely Exposed Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MNGCs | multinucleate giant cells |
| M | machined surface |
| MN | machined surface with nanotubes |
| DAE | dual-acid-etched surface |
| DAEN | dual-acid-etched surface with nanotubes |
| microCT | micro-computed tomography |
| SEM | scanning electron microscopy |
| TEM | transmission electron microscopy |
| PMMA | poly-methyl methacrylate |
| TRAP | tartrate-resistant acid phosphatase |
| JE | junctional epithelium |
| IBL | internal basal lamina |
| DAT cells | directly attached to tooth cells |
| FIB | focused ion beam |
References
- Karoussis, I.K.; Salvi, G.E.; Bürgin, W.; Lang, N.P. Effect of implant design on survival and success rates of titanium oral implants: A 10-year prospective cohort study of the ITI Dental Implant System. Clin. Oral Implants Res. 2004, 15, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, C. Development of a New Experimental Model for Investigating Osseointegration of Titanium Mini-Screws Placed Intraorally. Master’s Thesis, University of Toronto, Toronto, ON, Canada, 2020. Available online: http://hdl.handle.net/1807/101040 (accessed on 13 April 2025).
- Yazan, M.; Atil, F.; Gonen, Z.B.; Kocyigit, I.D.; Tekin, U. A Novel Experimental Model for Dental Implant Research. Int. J. Exp. Dent. Sci. 2018, 7, 43–47. [Google Scholar] [CrossRef]
- Freilich, M.; Shafer, D.; Wei, M.; Kompalli, R.; Adams, D.; Kuhn, L. Implant system for guiding a new layer of bone. Computed microtomography and histomorphometric analysis in the rabbit mandible. Clin. Oral Implants Res. 2009, 20, 201–207. [Google Scholar] [CrossRef]
- Cordioli, G.; Atiyeh, F.; Piattelli, A.; Majzoub, Z. Healing of transplanted composite bone grafts–implants: A pilot animal study. Clin. Oral Implants Res. 2003, 14, 750–758. [Google Scholar] [CrossRef]
- Munhoz, E.A.; Bodanezi, A.; Cestari, T.M.; Taga, R.; de Carvalho, P.S.; Ferreira, O., Jr. Long-term rabbits bone response to titanium implants in the presence of inorganic bovine-derived graft. J. Biomater. Appl. 2012, 27, 91–98. [Google Scholar] [CrossRef]
- Munhoz, E.A.; Bodanezi, A.; Cestari, T.M.; Taga, R.; Ferreira, O., Jr.; de Carvalho, P.S. Biomechanical and Microscopic Response of Bone to Titanium Implants in the Presence of Inorganic Grafts. J. Oral Implantol. 2011, 37, 19–25. [Google Scholar] [CrossRef]
- Munhoz, E.A.; Bodanezi, A.; Biol, T.M.C.; Graeff, M.S.Z.; Ferreira, O., Jr.; de Carvalho, P.S.; Taga, R. Impact of Inorganic Xenograft on Bone Healing and Osseointegration: An Experimental Study in Rabbits. Implant Dent. 2017, 26, 875–881. [Google Scholar] [CrossRef]
- Weber, J.B.B.; Mayer, L.; Cenci, R.A.; Baraldi, C.E.; Ponzoni, D.; Gerhardt de Oliveira, M. Effect of Three Different Protocols of Low-Level Laser Therapy on Thyroid Hormone Production After Dental Implant Placement in an Experimental Rabbit Model. Photomed. Laser Surg. 2014, 32, 612–617. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, W.Q.; Xu, L.N.; Ming, P.P.; Shao, S.Y.; Qiu, J. Osseointegration of titanium dental implant under fluoride exposure in rabbits: Micro-CT and histomorphometry study. Clin. Oral Implants Res. 2019, 30, 1038–1048. [Google Scholar] [CrossRef]
- AlOtaibi, N.M.; Dunne, M.; Ayoub, A.F.; Naudi, K.B. A novel surgical model for the preclinical assessment of the osseointegration of dental implants: A surgical protocol and pilot study results. J. Transl. Med. 2021, 19, 276. [Google Scholar] [CrossRef]
- Donnelly, T.M.; Vella, D. Anatomy, Physiology and Non-dental Disorders of the Mouth of Pet Rabbits. Veterinary Clin. N. Am. Exot. Anim. Pract. 2016, 19, 737–756. [Google Scholar] [CrossRef]
- Choe, S.; Ma, T.; Jones, D.; Shiau, H.J.; Saito, H. Peri-implant mucosal tissue attachment: Narrative review. Dent. Rev. 2024, 4, 100141. [Google Scholar] [CrossRef]
- Renvert, S.; Polyzois, I. Risk indicators for peri-implant mucositis: A systematic literature review. J. Clin. Periodontol. 2015, 42, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Kroger, A.; Hülsmann, C.; Fickl, S.; Spinell, T.; Hüttig, F.; Kaufmann, F.; Heimbach, A.; Hoffmann, P.; Enkling, N.; Renvert, S.; et al. The severity of human peri-implantitis lesions correlates with the level of submucosal microbial dysbiosis. J. Clin. Periodontol. 2018, 45, 1498–1509. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S286–S291. [Google Scholar] [CrossRef]
- Jepsen, S.; Berglundh, T.; Genco, R.; Aass, A.M.; Demirel, K.; Derks, J.; Figuero, E.; Giovannoli, J.L.; Goldstein, M.; Lambert, F.; et al. Primary prevention of peri-implantitis: Managing peri-implant mucositis. J. Clin. Periodontol. 2015, 42, 152–157. [Google Scholar] [CrossRef]
- Masoud, I.; Shapiro, F.; Moses, A. Longitudinal roentgencephalometric study of the growth of the New Zealand white rabbit: Cumulative and biweekly incremental growth rates for skull and mandible. J. Craniofacial Genet. Dev. Biol. 1986, 6, 259–287. [Google Scholar] [CrossRef]
- Stübinger, S.; Dard, M. The Rabbit as Experimental Model for Research in Implant Dentistry and Related Tissue Regeneration. J. Investig. Surg. 2013, 26, 266–282. [Google Scholar] [CrossRef]
- Liddell, R.S.; Ajami, E.; Li, Y.; Bajenova, E.; Yang, Y.; Davies, J.E. The influence of implant design on the kinetics of osseointegration and bone anchorage homeostasis. Acta Biomater. 2021, 121, 514–526. [Google Scholar] [CrossRef]
- Warda, N.; Noh, R.; Davies, J.E. TRAP-Positive Cells: Potential Mediators of Peri-Implant Bone Healing. Master’s Thesis, University of Toronto, Toronto, ON, Canada. manuscript in preparation.
- Noh, R. Investigation of Soft Tissue Healing around Osseointegrated Titanium Mini-Implants Placed in the Mandibular Diastema of Rabbits. Master’s Thesis, University of Toronto, Toronto, ON, Canada, 2022. Available online: http://hdl.handle.net/1807/125654 (accessed on 13 April 2025).
- Velasco-Ortega, E.; Ortiz-Garcia, I.; Jiménez-Guerra, A.; Núñez-Márquez, E.; Moreno-Muñoz, J.; Rondón-Romero, J.L.; Cabanillas-Balsera, D.; Gil, J.; Muñoz-Guzón, F.; Monsalve-Guil, L. Osseointegration of Sandblasted and Acid-Etched Implant Surfaces. A Histological and Histomorphometric Study in the Rabbit. Int. J. Mol. Sci. 2021, 22, 8507. [Google Scholar] [CrossRef]
- de Barros e Lima Bueno, R.; Ponce, K.J.; Dias, A.P.; Guadarrama Bello, D.; Brunski, J.B.; Nanci, A. Influence of 528 nanotopography on early bone healing during controlled implant loading. Nanomaterials 2020, 10, 2191. [Google Scholar] [CrossRef] [PubMed]
- Kligman, S.; Ren, Z.; Chung, C.H.; Perillo, M.A.; Chang, Y.C.; Koo, H.; Zheng, Z.; Li, C. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef] [PubMed]
- Mattheck, C. Design in Nature—Learning from Trees; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Warda, N. TRAP+ Cells: Potential Mediators of Peri-Implant Bone Healing. Master’s Thesis, University of Toronto, Toronto, ON, Canada, 2022. Available online: http://hdl.handle.net/1807/125683 (accessed on 13 April 2025).
- Reinedahl, D.; Chrcanovic, B.; Albrektsson, T.; Tengvall, P.; Wennerberg, A. Ligature-Induced Experimental Peri-Implantitis-A Systematic Review. J. Clin. Med. 2018, 7, 492. [Google Scholar] [CrossRef]
- de Araújo Silva, D.N.; Casarin, M.; Monajemzadeh, S.; Menezes da Silveira, T.; Lubben, J.; Bezerra, B.; Pirih, F.Q. Experimental Model of Ligature-Induced Peri-Implantitis in Mice. J. Vis. Exp. 2024, 207, e66316. [Google Scholar] [CrossRef]
- Shah, F.A.; Ruscsák, K.; Palmquist, A. 50 years of scanning electron microscopy of bone—A comprehensive overview of the important discoveries made and insights gained into bone material properties in health, disease, and taphonomy. Bone Res. 2019, 7, 15. [Google Scholar] [CrossRef]
- Davies, J.; Hosseini, M. Histodynamics of Endosseous Wound Healing. In Bone Engineering; Davies, J.E., Ed.; EM Squared: Toronto, ON, Canada, 2000; pp. 1–14. [Google Scholar]
- Polak, J.M.; Priestley, J.V. Electron Microscopic Immunocytochemistry, Principles and Practices; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Graham, L.; Orenstein, J. Processing tissue and cells for transmission electron microscopy in diagnostic pathology and research. Nat. Protoc. 2007, 2, 2439–2450. [Google Scholar] [CrossRef]
- Kuroiwa, M.; Chihara, K.; Higashi, S. Electron microscopic studies on Sharpey’s fibers in the alveolar bone of rat molars. Kaibogaku Zasshi 1994, 69, 776–782. [Google Scholar] [PubMed]
- Schroeder, H.E.; Listgarten, M.A. The junctional epithelium: From strength to defense. J. Dent. Res. 2003, 82, 158–161. [Google Scholar] [CrossRef]
- Schupbach, P.; Glauser, R. The defense architecture of the human periimplant mucosa: A histological study. J Prosthet. Dent. 2007, 97 (Suppl. S6), S15–S25. [Google Scholar] [CrossRef]
- Vignoletti, F.; de Sanctis, M.; Berglundh, T.; Abrahamsson, I.; Sanz, M. Early healing of implants placed into fresh extraction sockets: An experimental study in the beagle dog. III: Soft tissue findings. J. Clin. Periodontol. 2009, 36, 1059–1066. [Google Scholar] [CrossRef]
- Glauser, R.; Schupbach, P. Early bone formation around immediately placed two-piece tissue-level zirconia implants with a modified surface: An experimental study in the miniature pig mandible. Int. J. Implant Dent. 2022, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Al Rezk, F.; Trimpou, G.; Lauer, H.C.; Weigl, P.; Krockow, N. Response of soft tissue to different abutment materials with different surface topographies: A review of the literature. Gen. Dent. 2018, 66, 18–25. [Google Scholar] [PubMed]
- Deporter, D.A.; Watson, P.A.; Pilliar, R.M.; Howley, T.P.; Winslow, J. A histological evaluation of a functional endosseous, porous-surfaced, titanium alloy dental implant system in the dog. J. Dent. Res. 1988, 67, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Pilliar, R.M.; Davies, J.E.; Smith, D.C. The bone-biomaterial interface for load-bearing implants. MRS Bull. 1991, 9, 55–61. [Google Scholar] [CrossRef]
- Nevins, M.; Nevins, M.L.; Carnelo, M.; Boyesen, J.L.; Kim, D.M. Human histologic evidence of a connective tissue attachment to a dental implant. Int. J. Periodontics Restor. Dent. 2008, 28, 111–121. [Google Scholar] [PubMed]
- Liñares, A.; Domken, O.; Dard, M.; Blanco, J. Peri-implant soft tissues around implants with a modified neck surface. Part 1. Clinical and histometric outcomes: A pilot study in minipigs. J. Clin. Periodontol. 2013, 40, 412–420. [Google Scholar] [CrossRef]
- Bellon, B.; Pippenger, B.; Stähli, A.; Degen, M.; Parisi, L. Cementum and enamel surface mimicry influences soft tissue cell behavior. J. Periodontal Res. 2025, 60, 64–76. [Google Scholar] [CrossRef]
- Schroeder, H.E.; Listgarten, M. The gingival tissues: The architecture of periodontal protection. Periodontology 2000 1997, 13, 91–120. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Lang, N.P. The junctional epithelium: From health top disease. J. Dent. Res. 2005, 84, 9–20. [Google Scholar] [CrossRef]
- Ten Cate, A.R. Oral Histology Development, Structure, and Function; C.V. Mosby: St. Louis, MO, USA, 1980; pp. 234–238. [Google Scholar]
- Hormia, M.C.; Sahlberg, C.I.; Thesleff, I.; Airenne, T. The epithelium-tooth interface−−A basal lamina rich in Laminin-5 and lacking other known laminin isoforms. J. Dent. Res. 1998, 77, 1479–1485. [Google Scholar] [CrossRef]
- James, R.A.; Schulz, R.L. Hemidesmosomes and the adhesion of junctional epithelial cells to metal implants—A preliminary report. Oral. Implantol. 1974, 4, 294–302. [Google Scholar] [PubMed]
- Listgarten, M.A.; Lai, C.H. Ultrastructure of the intact interface between an endosseous epoxy resin dental implant and the host tissues. J. Biol. Buccale 1975, 3, 13–28. [Google Scholar] [PubMed]
- Hansson, H.A.; Albrektsson, T.; Brånemark, P.-I. Structural aspects of the interface between tissue and titanium implants. J. Prosthet. Dent. 1983, 50, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Gould, T.R.; Westbury, L.; Brunette, D.M. Ultrastructural study of the attachment of human gingiva to titanium in vivo. J. Prosteth Dent. 1984, 52, 418–420. [Google Scholar] [CrossRef]
- Listgarten, M.A. Soft and hard tissue response to endosseous dental implants. Anat. Rec. 1996, 245, 410–425. [Google Scholar] [CrossRef]
- Lilienberg, B.; Gualin, F.; Berglundh, T.; Tonetti, M.; Lindhe, J. Some characteristics of the ridge mucosa before and after implant installation. A prospective study in humans. J. Clin. Periodontol. 1996, 23, 1008–1013. [Google Scholar] [CrossRef]
- Jarmar, T.; Palmquist, A.; Brånemark, R.; Hermansson, L.; Engqvist, H.; Thomsen, P. Technique for preparation and characterization in cross-section of oral titanium implant surfaces using focused ion beam and transmission electron microscopy. J. Biomed. Mater. Res. Part A 2008, 87, 1003–1009. [Google Scholar] [CrossRef]
- Grandfield, K.; Engqvist, H. Focused Ion Beam in the Study of Biomaterials and Biological Matter. Adv. Mater. Sci. Eng. 2012, 2012, 841961. [Google Scholar] [CrossRef]
- Wang, X.; Liddell, R.S.; Wen, H.B.; Davies, J.E.; Ajami, E. The role of implant coronal surface properties on early adhesion of streptococcus oralis—An in vitro comparative study. J. Biomed. Mater. Res. A 2025, 113, e37866. [Google Scholar] [CrossRef]





| Basis | Powder | Hardener 1 | Hardener 2 | Regulator | |
|---|---|---|---|---|---|
| Component Number | 1 | 2 | 3 | 4 | 5 |
| Pre-Infiltration | 200 mL | 1 g | |||
| Infiltration | 250 mL | 20 g | 1 g | ||
| Stock Solution A | 500 mL | 80 g | 3 g | ||
| Stock Solution B | 500 mL | 4 mL | 2 mL | ||
| Polymerization Mixture | 9 parts (v/v) 1 part (v/v) | plus | Stock Solution A Stock Solution B | ||
| Chemical Product | Product Number | Vendor |
|---|---|---|
| TRAP Basic Incubation Medium | ||
| Sodium Acetate Anhydrous | S-2889 | Sigma-Aldrich |
| L-(+) Tartaric Acid | T-6521 | |
| Glacial Acetic Acid | A-6283 | |
| Napthol AS-MX Phosphate Substrate Mix | ||
| Napthol AS-MX Phosphate | N-4875 | Sigma-Aldrich |
| Ethylene Glycol Monoethyl Ether | E-2632 | |
| TRAP Staining Solution Mix | ||
| TRAP Basic Incubation Medium | - | Sigma-Aldrich |
| Fast Red Violet LB Salt | F-3381 | |
| Napthol AS-MX Phosphate Substrate Mix | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, R.; Warda, N.; Tremblay, C.; Davies, J.E. A New Preclinical Surgical Model for the Assessment of Dental Implant Tissue Integration. Surgeries 2025, 6, 36. https://doi.org/10.3390/surgeries6020036
Noh R, Warda N, Tremblay C, Davies JE. A New Preclinical Surgical Model for the Assessment of Dental Implant Tissue Integration. Surgeries. 2025; 6(2):36. https://doi.org/10.3390/surgeries6020036
Chicago/Turabian StyleNoh, Ryan, Nahrain Warda, Charles Tremblay, and John E. Davies. 2025. "A New Preclinical Surgical Model for the Assessment of Dental Implant Tissue Integration" Surgeries 6, no. 2: 36. https://doi.org/10.3390/surgeries6020036
APA StyleNoh, R., Warda, N., Tremblay, C., & Davies, J. E. (2025). A New Preclinical Surgical Model for the Assessment of Dental Implant Tissue Integration. Surgeries, 6(2), 36. https://doi.org/10.3390/surgeries6020036






