Abstract
Glioblastoma is the most frequent form of adult-type diffuse gliomas, and it carries a very poor prognosis. Magnetic resonance imaging (MRI) is an indispensable tool for diagnosing and monitoring brain tumors, potentially influencing choices like repeat surgery, biopsy, or alternative management strategies. However, interpreting post-surgical MRI for gliomas can be particularly demanding, especially in differentiating between tumor progression and treatment effects. Recently, a novel score specifically designed for classifying and reporting post-treatment brain tumors on MRI was proposed by a team of neuroradiologists, neurosurgeons, and neuro-oncologists: the Brain Tumor Reporting and Data System (BT-RADS). This review examines the current body of evidence regarding the use of BT-RADS in monitoring adult-type diffuse gliomas following surgery. This classification has demonstrated a number of benefits in terms of prognostic value, treatment decisions, reliability, and the quality of radiology reports. On the other hand, despite the promising diagnostic value in identifying recurrent glioblastoma, there is still some uncertainty in defining the presence or absence of tumor recurrence in the intermediate category 3. In such a category, incorporating advanced techniques such as perfusion-weighted imaging and diffusion-weighted imaging may improve the stratification of patients, avoiding repeat surgery on false positive findings.
1. Introduction
Adult-type diffuse gliomas are the most common type of primary brain tumor. Among these, glioblastoma (isocitrate dehydrogenase wild type) is the most frequent form affecting adults, and it carries a very poor prognosis [1]. For patients with newly diagnosed glioblastoma, the median survival is around 18 months, and less than 10% of patients survive beyond 5 years [2,3]. Malignant brain tumors require careful treatment planning by a team of specialists. This multidisciplinary team (MDT) typically includes a neuro-oncologist, a radiation oncologist, a radiologist, and a neurosurgeon. Together, they will discuss and recommend the most appropriate course of action, which may involve surgery (ranging from gross total to near total resection) in combination with chemotherapy, radiotherapy, or even all three [4]. Beyond the core treatments, other therapies may be employed to address specific needs. These can include newer approaches like anti-angiogenic drugs and immunotherapy. Additionally, medications like steroids, anticoagulants, and anticonvulsants can be used to manage symptoms and improve quality of life. Given the challenging nature of malignant brain tumors, with their poor prognosis, low survival rates, high recurrence, and resistance to treatment, close and regular follow-up is essential [5]. Magnetic resonance imaging (MRI) is an indispensable tool for diagnosing and monitoring brain tumors [6,7,8]. MRI scans play a key role in treatment decisions, potentially influencing choices like re-operation, biopsy, or alternative management strategies. However, interpreting post-surgical MRI for gliomas can be particularly demanding. This complexity arises from several factors, including the unpredictable course of the disease, variable tumor presentations, the difficulty in differentiating between tumor progression and treatment effects, and the inherent variability of each patient [9]. Over time, various systems have been developed to assess brain tumor treatment response. Two prominent examples include the Macdonald criteria and the Response Assessment in Neuro-Oncology guidelines [10,11]. These guidelines are constantly undergoing revision to incorporate new information and address the challenges of differentiating true tumor response from treatment-related effects (pseudo-progression) or medication influences (pseudo-response) [12]. While these criteria serve as valuable tools in clinical trials, their application in routine radiology reports and clinical practice can be hindered by several factors. These limitations include the inherent complexity of the systems, potential inconsistencies in interpretation between radiologists (high interobserver variability), the need for multiple measurements, and even a lack of a full understanding of the criteria among physicians [3]. The field of radiology has witnessed a growing trend towards standardized reporting through the implementation of Reporting and Data Systems (RADS) [13,14]. These systems aim to minimize variations and ambiguities in imaging reports by establishing common terminology and structures. In line with this cultural trend, a novel system specifically designed for classifying and reporting post-treatment brain tumors on MRI scans emerged in 2018: the Brain Tumor Reporting and Data System (BT-RADS) [3]. Initial evaluations suggest that BT-RADS criteria hold promise for enhancing the quality of MRI reports and consequently improving clinical decision-making for patients with brain tumors.
This review examines the current body of evidence regarding the use of BT-RADS in monitoring adult-type diffuse gliomas following surgery.
2. Methods
We performed a thorough literature search using the Scopus, MEDLINE, and PubMed Central databases. Our search covered all articles indexed in these databases, and the search was current as of 12 August 2024. To identify relevant studies, we used the following search terms: (“BT-RADS”) OR (“Brain Tumor Reporting and Data System”). We limited our search to articles published in English to ensure clarity. To focus on the most recent and robust research, we only included original articles. Reviews, letters to the editor, and case reports were excluded. After initial screening of titles and abstracts, we identified 12 articles out of an initial pool of 22 that appeared relevant to our research goals. We then reviewed the full text of these articles and conducted additional cross-referencing as needed to gain a comprehensive understanding of the existing literature.
3. BT-RADS Score
The BT-RADS was created to address the need for a standardized and structured assessment of treatment response in primary brain tumor MRIs. The BT-RADS was not designed to differentiate between various tumor subtypes based on initial imaging studies, nor does it encompass non-glioma tumors like metastases or meningiomas. The system was developed with a focus on practicality and user-friendliness rather than exhaustive inclusivity. The BT-RADS emerged from a collaborative effort by an MDT of neuroradiologists, neurosurgeons, and neuro-oncologists. Their development process was informed by feedback from an MDT of clinicians at an academic institution, including radiologists, neurosurgeons, pathologists, neuro-oncologists, and radiation oncologists [3].
Accurate BT-RADS scoring requires a comprehensive understanding of the patient’s clinical background. This includes details about surgical history, the timeframe since radiotherapy (particularly if it falls within the first 90 days since MRI), and specific elements of the applied therapeutic regimen, with a focus on medications like bevacizumab and steroids. Additionally, BT-RADS prioritizes specific MRI sequences, particularly fluid-attenuated inversion recovery (FLAIR) and contrast-enhanced T1-weighted images.
The BT-RADS assigns a standardized numerical score ranging from 0 to 4. Some categories, like scores of 1 and 3, have subcategories (1a/1b and 3a/3b/3c) for a more nuanced assessment. Each score directly translates to clear management recommendations, as outlined in Table 1. To facilitate widespread adoption, an educational website (www.btrads.com, accessed on 5 July 2024) was launched in May 2018, providing public access to the scoring system’s diagnostic flowchart (Figure 1) [15]. This website also offers an interactive scoring page to assist healthcare professionals in assigning the appropriate BT-RADS score to their patients. Notably, the online educational platform aided the widespread adoption of the BT-RADS within a large neuroradiology department [16].
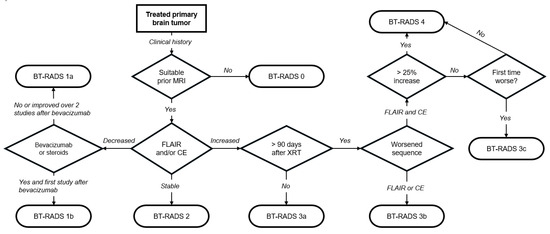
Figure 1.
BT-RADS scoring flowchart. MRI, magnetic resonance imaging; FLAIR, fluid-attenuated inversion recovery; CE, contrast enhancement; XRT, radiation therapy. Adapted from [15].
Moreover, the scoring system was incorporated into a standardized reporting format with sections covering clinical indication, technique, comparative analyses, tumor-related findings and additional findings, and overall impression [17].

Table 1.
Brain Tumor Reporting and Data System (BT-RADS) categories, imaging findings, and management [18]. FLAIR, fluid-attenuated inversion recovery.
Table 1.
Brain Tumor Reporting and Data System (BT-RADS) categories, imaging findings, and management [18]. FLAIR, fluid-attenuated inversion recovery.
| Score | Imaging Findings | Management |
|---|---|---|
| 0 | New baseline, incomplete study, or otherwise unable to categorize. | Continued follow-up; no change. |
| 1 (improvement) | Reduction in enhancing component, FLAIR component, mass effect, or resolution of lesions. 1a—Reflects decreasing tumor burden and/or improving treatment effect. 1b—Effect from medications such as increasing steroids or initiating Avastin. | |
| 2 (stable) | No appreciable change. | |
| 3 (worsening) | Increase in enhancing component, FLAIR component, and/or mass effect. 3a (<90 days since radiation therapy)—Represents treatment effects, including radiation therapy and medications. 3b (>90 days since radiation therapy)—Represents an indeterminate mix of treatment effect and tumor worsening. 3c (<25% worsening in FLAIR and enhancement and first-time worse or new indeterminate lesion outside the expected high-dose radiation field)—Represents increasing tumor burden. | Decreased time interval of follow-up. Consider change in management in 3c. |
| 4 (definitely worsening) | Increase (>25% or <25% if progressively worsened over more than 1 study) in enhancing component, FLAIR component, mass effect, and/or new definitive lesion outside the expected high-dose radiation field. Worsening of imaging findings highly suspicious for tumor progression. | Change in management. |
Figure 2 illustrates the application of the BT-RADS for the monitoring of a patient who underwent surgery for a primary brain tumor.
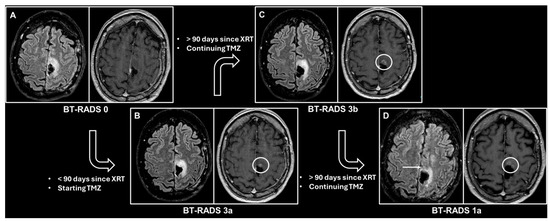
Figure 2.
Consecutive contrast-enhanced brain magnetic resonance imaging scans, each performed approximately 3 months after the previous one, in a patient who underwent surgery for an isocitrate dehydrogenase mutant anaplastic astrocytoma in the left paracentral lobule. The patient subsequently underwent radiation therapy (XRT) with concomitant temozolomide (TMZ). Every image shows the fluid-attenuated inversion recovery (FLAIR) image on the left inlet and the contrast-enhanced T1-weighted image on the right inlet. (A) The first follow-up after surgery considered as baseline, thus scored as BT-RADS 0. (B) Appearance of an area of contrast enhancement (white circle) near the surgical cavity, while the FLAIR image appears unchanged. Less than 90 days have passed since the end of XRT; thus, the assigned BT-RADS score is 3a, representing an XRT effect. (C) Slight size increase of the contrast-enhanced lesion (white circle), while the FLAIR image appears unchanged. More than 90 days have passed since the end of XRT, so the assigned BT-RADS score is 3b, representing an indeterminate mix of XRT effects and tumor worsening. (D) A decrease in the size of the contrast-enhanced area (white circle), as well as a decrease in the signal abnormality in the FLAIR image (white arrow). The assigned BT-RADS score is 1a, representing decreased tumor burden and/or improved treatment effect.
4. The BT-RADS’s Diagnostic and Prognostic Value
Distinguishing between recurrent and non-recurrent high-grade gliomas plays a critical role in optimizing patient care. While recurrent tumors may necessitate interventions like rebiopsy, repeat surgery, or additional medications alongside stricter monitoring, non-recurrent cases might benefit from discontinuing current therapies. Given the high recurrence rate of high-grade gliomas, the early recognition and treatment of recurrences is fundamental to increasing the survival probability of these patients, even if only by a few months. Therefore, accurately determining whether a new lesion represents tumor regrowth or treatment-related effects is essential for improving patient outcomes and prognosis.
A prospective study involving 81 patients with high-grade glioma demonstrated that the BT-RADS offered promising accuracy in identifying recurrent tumors. Two neuroradiologists independently evaluated the scans, and the BT-RADS achieved accuracy, specificity, and sensitivity values ranging from 83.9% to 88.9%, 76.9% to 84.6%, and 90.5% to 92.9%, respectively. Notably, BT-RADS category 3b emerged as the most effective cutoff for predicting recurrence, with accuracy, specificity, and sensitivity ranges of 83.9% to 88.9%, 76.9% to 84.6%, and 90.5% to 92.9% for the two radiologists, respectively [5].
In another prospective study, evaluating 322 MRI examinations by five neuroradiologists, the best cutoff value for predicting tumor progression was BT-RADS category 3a or higher. According to the readers, when BT-RADS 3a was used as a predictor for tumor progression, the sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of the BT-RADS were 68.6–85.7%, 84.2–92.1%, 78.1–86.3%, 80.6–89.7%, and 75.6–86.8%, respectively [19].
Kim et al. evaluated 91 patients with high-grade gliomas who underwent a total of 538 MRIs. The mortality was 53% higher with each incremental increase in BT-RADS category (hazard ratio: 1.53; 95% confidence interval: 1.07–2.19), and category 3b had the highest odds for worsening upon subsequent MRI (81%) [20].
These results are supported by those of Trivedi et al. In their study, two radiologists examined consecutive MRI scans from 100 post-operative glioma patients. The authors found that patients with BT-RADS scores of ≤3a had a significantly higher probability of surviving for 12 months (94.8%; 95% confidence interval: 89.9–99.8) compared to those with BT-RADS scores of 3b or higher (31.5%; 95% confidence interval: 4.0–59.0) (p < 0.001) [9].
An additional prospective study by Abidi et al. reviewed and classified the BT-RADS scores derived from 212 MRIs of 130 patients assessed by an MDT. Their analysis revealed a clear trend: as the BT-RADS scores increased, so did the likelihood of changes in patient management (from BT-RADS 0, with a 3.1% change rate in management, to BT-RADS 4, with a 95.6% change rate in management). Interestingly, patients with an MDT BT-RADS score of 0–2 underwent repeat surgery at a lower rate (2.1%) compared to those with a score of 3–4 (10.3%, p = 0.02). Among patients who did undergo repeat surgery, 82.4% had a significant amount of tumor (>50%) detected in the pathology specimen, with the remaining cases primarily showing radiation necrosis. In addition, a high mortality rate at 12 months (30.8%) was observed in patients assigned a BT-RADS score of 3a, considerably higher compared to all other BT-RADS categories (0% to 15.6%). This result has been attributed to a possible selection bias, as the MDT setting might have preferentially included patients with a worsening prognosis for consultation [21].
In summary, these preliminary studies suggest that BT-RADS is effective in detecting tumor progression, with category 3b serving as a reliable threshold for high recurrence suspicion and worsening prognosis. Given these findings, patients scoring 3b or higher may benefit from a more aggressive management strategy, such as repeat surgery.
5. The BT-RADS’s Reliability
Similar to other established RADS, the BT-RADS score requires validation through inter-rater reliability studies. Low agreement among readers would hinder the system’s effectiveness for consistent application within and across healthcare centers.
In a single-center retrospective study, four readers with varying experience levels (two radiology residents, one general radiologist, and one neuroradiologist) independently evaluated 147 MRIs using the BT-RADS score. The overall agreement among all four readers was 82%, with a Fleiss’ kappa of 0.70, suggesting that the BT-RADS may be a reliable tool for neuroradiologists and radiologists, including those with less experience [22].
A study conducted by Essien et al. assessed the reliability of BT-RADS scoring among six readers. Four neuroradiologists and two radiology residents independently evaluated 103 MRI scans from 98 patients with brain tumors and assigned a BT-RADS score. The results showed very good agreement between the readers, with a Gwet’s index of 0.83 (95% confidence interval: 0.78–0.87). There was not a significant difference in score consistency between the neuroradiologists (Gwet’s index: 0.84) and the residents (Gwet’s index: 0.79) [23].
Almalki et al. assessed the inter-rater agreement among five neuroradiologists evaluating key features and assigning BT-RADS scores in 322 MRI examinations. The results showed strong agreement (κ = 0.89) for identifying new lesions. Agreement was good (κ between 0.67 and 0.69) for evaluating the FLAIR component (κ = 0.67) and mass effect (κ = 0.69) and determining the final BT-RADS category (κ = 0.75). However, agreement for assessing the enhancing component was moderate (κ = 0.54) [19].
Another prospective study involving 81 patients examined inter-rater agreement for BT-RADS scoring between two neuroradiologists and found substantial agreement (74%, κ = 0.71) [5].
Trivedi et al. examined the agreement between two radiologists in assigning BT-RADS scores to 100 MRIs. The overall concordance rate was 62.7% (κ = 0.67). Additionally, a high level of consistency (97.9%) was observed between BT-RADS-suggested management recommendations and the decisions made by the MDT [9].
Finally, in their study, Abidi et al. compared the BT-RADS scores assigned by an MDT to those assigned by a neuroradiologist, revealing 90.1% agreement with a linear-weighted κ of 0.95 ± 0.05 [21].
In summary, these preliminary studies highlight the strong reliability of the BT-RADS, which is essential for consistent scoring across institutions. Furthermore, utilizing the BT-RADS can serve as a guide in image interpretation for less experienced or younger physicians, assisting them in planning subsequent patient management. It is worth considering that there might be a period of adjustment when implementing new templates or structured reporting systems. Consequently, the level of agreement among users could potentially increase with the routine integration of this scoring system into the daily clinical workflow.
6. The BT-RADS’s Report Quality and Acceptance by Physicians
Brain tumor patients require frequent management decisions that are often made based on interpreting imaging in the context of MDTs, which can change the initial diagnosis, guide surgical management, and determine therapeutic plans. Thus, it is critical that the BT-RADS be approved and recognized by all physicians who are part of the MDT. By means of questionnaires submitted to various medical specialists, the impact the BT-RADS may have on the management of patients with brain tumors was evaluated.
Nine months after implementing the BT-RADS, a study at a single institution surveyed faculty, residents, and other healthcare providers directly involved in brain tumor patient care. The results showed a significant improvement in provider perception across several areas (p-values < 0.003), including consistency, communication between radiologist and physician, report ambiguity, confidence in reports, and facilitation of patient management. Overall, these findings suggested a strong preference for the structured reporting format provided by the BT-RADS compared with the free-text reporting format [24].
A separate survey targeting oncologists, neurosurgeons, radiation oncologists, and radiologists revealed a notable improvement in BT-RADS reports in terms of consistency, ambiguity, and confidence in imaging interpretation. The implementation of the BT-RADS led to a statistically significant increase in both physician satisfaction and their perception of patient satisfaction [16].
A study investigating the use of the BT-RADS in clinical practice found strong support from neuroradiologists. Five participants endorsed both encouraging the application of the BT-RADS and using a structured format for reporting post-treatment glioma imaging. However, the survey also identified a need for further refinement of the BT-RADS [19].
Another study employed objective metrics to assess the benefits of structured brain tumor reporting via the BT-RADS. The researchers compared pre- and post-BT-RADS implementation time periods by analyzing post-treatment glioma reports. The findings showed that the use of the BT-RADS reporting template led to increased inclusion of historical information (terms like “Avastin” and “methylguanine-DNA methyltransferase” were reported significantly more frequently), reduced use of uncertain language (hedge words like “possibly” and “likely” were used significantly less often), increased conciseness (overall report and impression section length decreased significantly), and decreased use of addenda to clarify information [25].
In summary, these preliminary studies suggest that the BT-RADS structured reporting format enhances the quality of communication within MDTs managing patients with brain tumors. By providing a standardized format, the BT-RADS ensures that all relevant information are clearly and concisely conveyed among specialists.
7. Future Perspectives
The BT-RADS primarily relies on the analysis of FLAIR and contrast-enhanced T1-weighted images. While this facilitates the multicentric application of the scoring system, as these sequences are routinely included in MRI protocols for brain tumors, it may limit diagnostic accuracy in more complex cases. For such cases, advanced imaging techniques like perfusion-weighted imaging (PWI) and diffusion-weighted imaging (DWI) could prove valuable in identifying post-surgical tumor recurrence. Notably, a study retrospectively evaluated the value of DWI and dynamic susceptibility contrast-enhanced PWI for differentiating recurrent and non-recurrent high-grade gliomas in 91 post-operative patients with BT-RADS category 3 lesions. The rate of recurrence progressively increased with higher BT-RADS categories: 21.4% for category 3a, 61.5% for category 3b, and 78.4% for category 3c. Those with recurrent gliomas had significantly higher relative cerebral blood volume values than those with non-recurrent gliomas (2.3 vs. 1.3, p < 0.001). The apparent diffusion coefficient mean was significantly lower in the recurrent gliomas than in the non-recurrent gliomas (0.8 vs. 1.3 × 10−3 mm2/s, p < 0.001). When PWI was combined with the BT-RADS, the accuracy in distinguishing recurrence from non-recurrence increased significantly, as measured by the area under the curve rising from 0.76 to 0.90. Similarly, adding DWI to the BT-RADS resulted in an area under the curve increase from 0.76 to 0.88. The strongest diagnostic performance was achieved by combining all three variables (BT-RADS, PWI, and DWI), achieving an area under the curve of 0.95 (with a 95% confidence interval of 0.88–0.98) [26]. A separate study focusing on BT-RADS category 3 tumors also reported comparable findings. The mean apparent diffusion coefficient value was significantly lower in the recurrent group (0.9 × 10−3 mm2/s) compared to the non-recurrent group (1.15 × 10−3 mm2/s; p < 0.001). Incorporating DWI into the BT-RADS assessment yielded a specificity of 71.4%, a sensitivity of 84.6%, an accuracy of 77.8%, and an area under the curve of 0.78 (95% CI: 0.58–0.92) [27]. Given that DWI is now included in all standard brain MRI protocols and that PWI is becoming increasingly widespread, it seems reasonable to integrate these techniques in the evaluation of brain tumors, especially in more uncertain cases (e.g., indeterminate cases in category 3). The authors of the BT-RADS could potentially implement these techniques into the BT-RADS flowchart, for example, by upgrading a lesion classified as 3b to 3c when it shows restricted diffusion or increased perfusion indices.
Today, technology plays a fundamental role in radiological innovation, and it could further promote the dissemination and use of BT-RADS in clinical practice in the future. For instance, a cloud-based platform called the Brain Imaging Collaborative Suite’s Longitudinal Imaging Tracker (BrICS-LIT) has been developed to aid in accurate and objective patient monitoring during follow-up. This platform utilizes semi-automated tumor segmentation algorithms for both contrast-enhanced T1-weighted and FLAIR MRI images, assisting clinicians in quantitatively assessing brain tumors. Thus, the BrICS-LIT could enhance objectivity when measuring the effectiveness of new treatments for brain tumor patients [28]. Researchers have also developed a fully automated system to classify brain tumors using BT-RADS categories. This system analyzes both structured (BT-RADS-formatted) and unstructured MRI reports, learning from BT-RADS-formatted reports and then using this knowledge to estimate the BT-RADS category for both structured and unstructured reports. These automatically generated categories could then be used to support the clinical interpretation of MRI findings and investigate large-scale outcomes, such as overall survival and progression-free rates, for different BT-RADS categories [29].
Furthermore, a collaborative effort is underway between the BT-RADS’s authors and the American College of Radiology to integrate the BT-RADS into the existing RADS framework [30]. This process involves incorporating insights from published research, probably leading to score updates in the near future.
Table 2 lists the main articles that assessed the role of the BT-RADS for the surveillance of adult-type diffuse gliomas after surgery.

Table 2.
Main articles that have evaluated the role of the Brain Tumor Reporting and Data System (BT-RADS) in clinical practice, listed in order of citation in the text. MRI, magnetic resonance imaging; MDT, multidisciplinary team; PWI, perfusion-weighted imaging; DWI, diffusion-weighted imaging; FLAIR, fluid-attenuated inversion recovery.
8. A Summary of the BT-RADS’s Pros and Cons
In summary, preliminary studies have shown a range of positive effects associated with the use of the BT-RADS for the surveillance of adult-type diffuse gliomas after surgery [2], such as the BT-RADS’s promising prognostic value in predicting mortality; its excellent ability to influence treatment decisions, including surgical interventions, made by the MDT; its good inter-observer reliability, even among physicians with different experience levels and those from non-radiological backgrounds; and its facilitation of improvements in the quality of radiology reports, yielding better acceptance by MDT members.
On the other hand, despite the good diagnostic value in distinguishing recurrent glioblastoma from non-recurrent tumors, there is still room for improvement [31,32], particularly in defining the presence or absence of tumor recurrence in intermediate category 3. In this category, incorporating advanced techniques such as DWI and PWI may improve the stratification of patients, avoiding repeat surgery on false positive findings.
For optimal BT-RADS utilization, access to complete patient clinical data is crucial. Moreover, the BT-RADS must be widely known and used among all MDT members involved in primary brain tumor follow-up; including a score within an MRI report could lead to more confusion than benefits if it is not agreed with by other physicians. However, even if it is decided not to indicate the final BT-RADS category, this system has proven to be a good guide in the interpretation of images of brain tumor patients under surveillance, and therefore, it deserves to be known. Although not without limitations, the current BT-RADS appears to be a good starting point for updates in the near future based on the strengths and weaknesses that have emerged from the studies analyzed in this overview.
Author Contributions
Conceptualization, M.P.; methodology, M.P.; software, M.P.; validation, C.C.Q.; formal analysis, C.C.Q.; investigation, M.P.; resources, C.C.Q.; data curation, M.P.; writing—original draft preparation, M.P.; writing—review and editing, C.C.Q.; visualization, C.C.Q.; supervision, C.C.Q.; project administration, M.P.; funding acquisition, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.E.; Waite, K.A.; Jemal, A.; Siegel, R.L.; et al. Brain and Other Central Nervous System Tumor Statistics, 2021. CA. Cancer J. Clin. 2021, 71, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Hilario, A. Standardized Brain Tumor Reporting in the Multidisciplinary Spotlight: Pros of the BT-RADS. Eur. Radiol. 2024; Epub ahead of print. [Google Scholar] [CrossRef]
- Weinberg, B.D.; Gore, A.; Shu, H.-K.G.; Olson, J.J.; Duszak, R.; Voloschin, A.D.; Hoch, M.J. Management-Based Structured Reporting of Posttreatment Glioma Response with the Brain Tumor Reporting and Data System. J. Am. Coll. Radiol. JACR 2018, 15, 767–771. [Google Scholar] [CrossRef]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO Guidelines on the Diagnosis and Treatment of Diffuse Gliomas of Adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Ebaid, N.Y.; Ahmed, R.N.; Assy, M.M.; Amin, M.I.; Alaa Eldin, A.M.; Alsowey, A.M.; Abdelhay, R.M. Diagnostic Validity and Reliability of BT-RADS in the Management of Recurrent High-Grade Glioma. J. Neuroradiol. 2024, 51, 101190. [Google Scholar] [CrossRef]
- Parillo, M.; Vertulli, D.; Vaccarino, F.; Mallio, C.A.; Beomonte Zobel, B.; Quattrocchi, C.C. The Sensitivity of MIPs of 3D Contrast-Enhanced VIBE T1-Weighted Imaging for the Detection of Small Brain Metastases (≤5 Mm) on 1.5 Tesla MRI. Neuroradiol. J. 2024, 19714009241260802, Epub ahead of print. [Google Scholar] [CrossRef]
- Parillo, M.; Vertulli, D.; Mallio, C.A.; Quattrocchi, C.C. Imaging Findings in Carcinomatous Encephalitis Secondary to Malignant Melanoma. Egypt. J. Neurol. Psychiatry Neurosurg. 2023, 59, 76. [Google Scholar] [CrossRef]
- Parillo, M.; Vaccarino, F.; Quattrocchi, C.C. Imaging Findings in a Case of Leptomeningeal Myelomatosis, a Rare but Critical Central Nervous System Complication of Multiple Myeloma. Neuroradiol. J. 2023, 36, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, K.H.; Guha, A.; Thakur, M.; Mahajan, A.; Bhole, P.; Gupta, T. External Validation of the Brain Tumour Reporting and Data System (BT-RADS) in the Multidisciplinary Managementof Post-Treatment Gliomas. Pol. J. Radiol. 2024, 89, e148–e155. [Google Scholar] [CrossRef]
- Macdonald, D.R.; Cascino, T.L.; Schold, S.C.; Cairncross, J.G. Response Criteria for Phase II Studies of Supratentorial Malignant Glioma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1990, 8, 1277–1280. [Google Scholar] [CrossRef]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; Degroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated Response Assessment Criteria for High-Grade Gliomas: Response Assessment in Neuro-Oncology Working Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef]
- Ramakrishnan, D.; von Reppert, M.; Krycia, M.; Sala, M.; Mueller, S.; Aneja, S.; Nabavizadeh, A.; Galldiks, N.; Lohmann, P.; Raji, C.; et al. Evolution and Implementation of Radiographic Response Criteria in Neuro-Oncology. Neuro-Oncol. Adv. 2023, 5, vdad118. [Google Scholar] [CrossRef]
- Parillo, M.; Mallio, C.A.; Van der Molen, A.J.; Rovira, À.; Dekkers, I.A.; Karst, U.; Stroomberg, G.; Clement, O.; Gianolio, E.; Nederveen, A.J.; et al. The Role of Gadolinium-Based Contrast Agents in Magnetic Resonance Imaging Structured Reporting and Data Systems (RADS). Magn. Reson. Mater. Phys. Biol. Med. 2024, 37, 15–25. [Google Scholar] [CrossRef]
- Parillo, M.; van der Molen, A.J.; Asbach, P.; Elsholtz, F.H.J.; Laghi, A.; Ronot, M.; Wu, J.S.; Mallio, C.A.; Quattrocchi, C.C. The Role of Iodinated Contrast Media in Computed Tomography Structured Reporting and Data Systems (RADS): A Narrative Review. Quant. Imaging Med. Surg. 2023, 13, 7621–7631. [Google Scholar] [CrossRef]
- BT-RADS Flowchart. Available online: https://btrads.com/wp-content/uploads/2018/05/BT-RADS-flow-chart-2018_02_01.pdf (accessed on 5 July 2024).
- Kim, S.; Hoch, M.J.; Cooper, M.E.; Gore, A.; Weinberg, B.D. Using a Website to Teach a Structured Reporting System, the Brain Tumor Reporting and Data System. Curr. Probl. Diagn. Radiol. 2021, 50, 356–361. [Google Scholar] [CrossRef]
- BT-RADS Template. Available online: https://btrads.com/wp-content/uploads/2019/11/BT-RADS-followup-template-v1_03_short-sample.pdf (accessed on 29 August 2024).
- Brain Tumor Reporting and Data System (BT-RADS). Available online: https://btrads.com/ (accessed on 5 July 2024).
- Almalki, Y.E.; Basha, M.A.A.; Metwally, M.I.; Zeed, N.A.; Nada, M.G.; Alduraibi, S.K.; Morsy, A.A.; Balata, R.; Al Attar, A.Z.; Amer, M.M.; et al. Validating Brain Tumor Reporting and Data System (BT-RADS) as a Diagnostic Tool for Glioma Follow-Up after Surgery. Biomedicines 2024, 12, 887. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hoch, M.J.; Peng, L.; Somasundaram, A.; Chen, Z.; Weinberg, B.D. A Brain Tumor Reporting and Data System to Optimize Imaging Surveillance and Prognostication in High-Grade Gliomas. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2022, 32, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Abidi, S.A.; Hoch, M.J.; Hu, R.; Sadigh, G.; Voloschin, A.; Olson, J.J.; Shu, H.-K.G.; Neill, S.G.; Weinberg, B.D. Using Brain Tumor MRI Structured Reporting to Quantify the Impact of Imaging on Brain Tumor Boards. Tomography 2023, 9, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Parillo, M.; Mallio, C.A.; Pileri, M.; Dirawe, D.; Romano, A.; Bozzao, A.; Weinberg, B.; Quattrocchi, C.C. Interrater Reliability of Brain Tumor Reporting and Data System (BT-RADS) in the Follow up of Adult Primary Brain Tumors: A Single Institution Experience in Italy. Quant. Imaging Med. Surg. 2023, 13, 7423–7431. [Google Scholar] [CrossRef]
- Essien, M.; Cooper, M.E.; Gore, A.; Min, T.L.; Risk, B.B.; Sadigh, G.; Hu, R.; Hoch, M.J.; Weinberg, B.D. Interrater Agreement of BT-RADS for Evaluation of Follow-Up MRI in Treated Primary Brain Tumor Patients. AJNR Am. J. Neuroradiol. 2024; Epub ahead of print. [Google Scholar] [CrossRef]
- Gore, A.; Hoch, M.J.; Shu, H.-K.G.; Olson, J.J.; Voloschin, A.D.; Weinberg, B.D. Institutional Implementation of a Structured Reporting System: Our Experience with the Brain Tumor Reporting and Data System. Acad. Radiol. 2019, 26, 974–980. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Weinberg, B.D.; Hu, R.; Saindane, A.; Mullins, M.; Allen, J.; Hoch, M.J. Quantitative Improvement in Brain Tumor MRI Through Structured Reporting (BT-RADS). Acad. Radiol. 2020, 27, 780–784. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Wu, X.; Pan, Y.; Zhou, D.; Zhang, H.; Chen, Y.; Zhao, J.; Mo, Z.; Huang, B. Adding DSC PWI and DWI to BT-RADS Can Help Identify Postoperative Recurrence in Patients with High-Grade Gliomas. J. Neurooncol. 2020, 146, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Metwally, M.I.; Hafez, F.F.M.; Ibrahim, S.A.; Morsy, A.A.; Zeed, N.A. The Value of Adding DWI and FLAIR Signal Changes in the Resection Cavity on the Diagnostic Performance of BT-RADS Category 3 for Tumor Progression Prediction in Post-Treated Glioma Patients: A Prospective Pilot Study. Egypt. J. Radiol. Nucl. Med. 2023, 54, 52. [Google Scholar] [CrossRef]
- Ramesh, K.; Gurbani, S.S.; Mellon, E.A.; Huang, V.; Goryawala, M.; Barker, P.B.; Kleinberg, L.; Shu, H.-K.G.; Shim, H.; Weinberg, B.D. The Longitudinal Imaging Tracker (BrICS-LIT):A Cloud Platform for Monitoring Treatment Response in Glioblastoma Patients. Tomography 2020, 6, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Weinberg, B.D.; Gore, A.; Banerjee, I. A Scalable Natural Language Processing for Inferring BT-RADS Categorization from Unstructured Brain Magnetic Resonance Reports. J. Digit. Imaging 2020, 33, 1393–1400. [Google Scholar] [CrossRef]
- Weinberg, B.D.; Hoch, M.J. Brain Tumor-Radiology and Data System (BT-RADS)-an Imperfect System but a Worthwhile Start. Eur. Radiol. 2024; Epub ahead of print. [Google Scholar] [CrossRef]
- Martín-Noguerol, T.; Cabrera-Zubizarreta, A.; Luna, A. Standardized Reporting Systems for (Which?) Brain Tumors from in the Dark: Cons of the BT-RADS. Eur. Radiol. 2024; Epub ahead of print. [Google Scholar] [CrossRef]
- Rao, B.; Ikuta, I.; Mahajan, A.; Karam, A.A.; Zohrabian, V.M. Brain Tumor Reporting and Data System: A Pictorial Review. Neurographics 2021, 11, 175–185. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).