Cervical Necrotizing Fasciitis in Adults: A Life-Threatening Emergency in Oral and Maxillofacial Surgery
Abstract
1. Introduction
2. Pathogenesis and Clinical Features
2.1. Pathogenesis
2.2. Classification
| PRESENCE OF GAS IN SOFT TISSUE (ON RADIOGRAPHIC IMAGING) | ||||
|---|---|---|---|---|
| Polymicrobial 1 | Necrotizing fasciitis type I (polymicrobial). | |||
| Necrotizing cellulitis: Nonclostridial anaerobic (crepitant) cellulitis | ||||
| Gram-positive rods | Acute clinical presentation | Clostridial myonecrosis (gas gangrene) | C. perfringens—traumatic | |
| C. septicum—Spontaneous | ||||
| Indolent clinical presentation | Clostridial (anaerobic) cellulitis | C. perfringens—more common | ||
| C. septicum—less common | ||||
| ABSENCE OF GAS IN SOFT TISSUE (ON RADIOGRAPHIC IMAGING) | ||||
| Gram-positive cocci (increasing [26]) | Necrotizing fasciitis type II (monomicrobial) | Group A Streptococcus or other beta-hemolytic streptococci (Group C–G streptococci). Increasing. | ||
| Staphylococcus aureus (methicillin-sensitive (MSSA) or methicillin-resistant (MRSA) less common but increasing (up to 16%) 2 | ||||
| Necrotizing myositis due to group A Streptococcus or other beta-hemolytic streptococci | ||||
| Enterococcus species | ||||
| Gram-negative rods | Aeromonas species—freshwater exposure | |||
| Vibrio species—Saltwater exposure, chronic hepatopathy, diabetes mellitus | ||||
| Enterobacteriaceae and non-fermenters, immunodepressed patients [27,28,29] | ||||
| Rare etiologies | Mycobacterium tuberculosis [30] | |||
| Fungal infections | ||||
2.3. Clinical Features
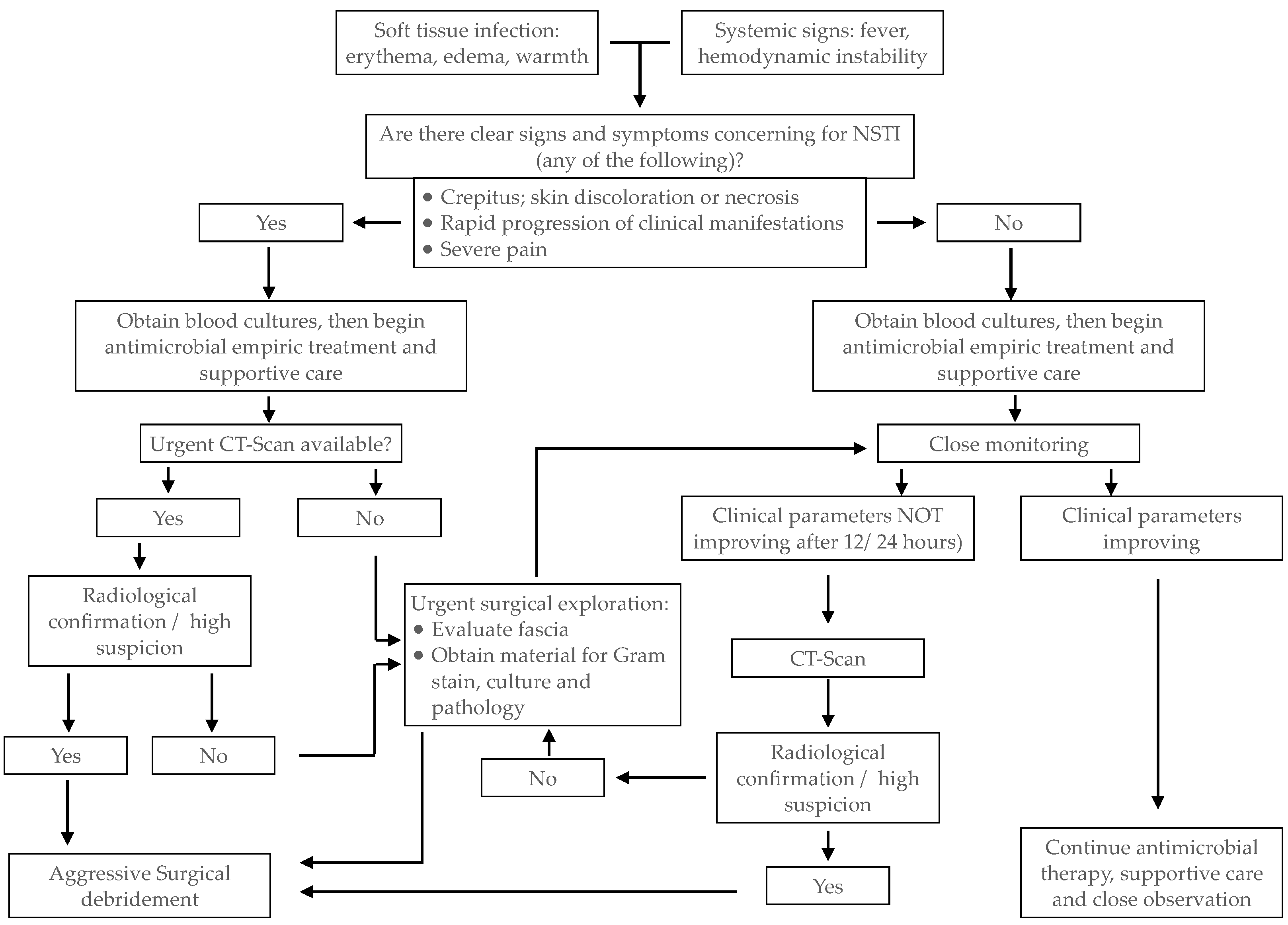
3. Diagnosis
3.1. Clinical Diagnosis
3.2. Laboratory Tests
3.3. Imaging Studies
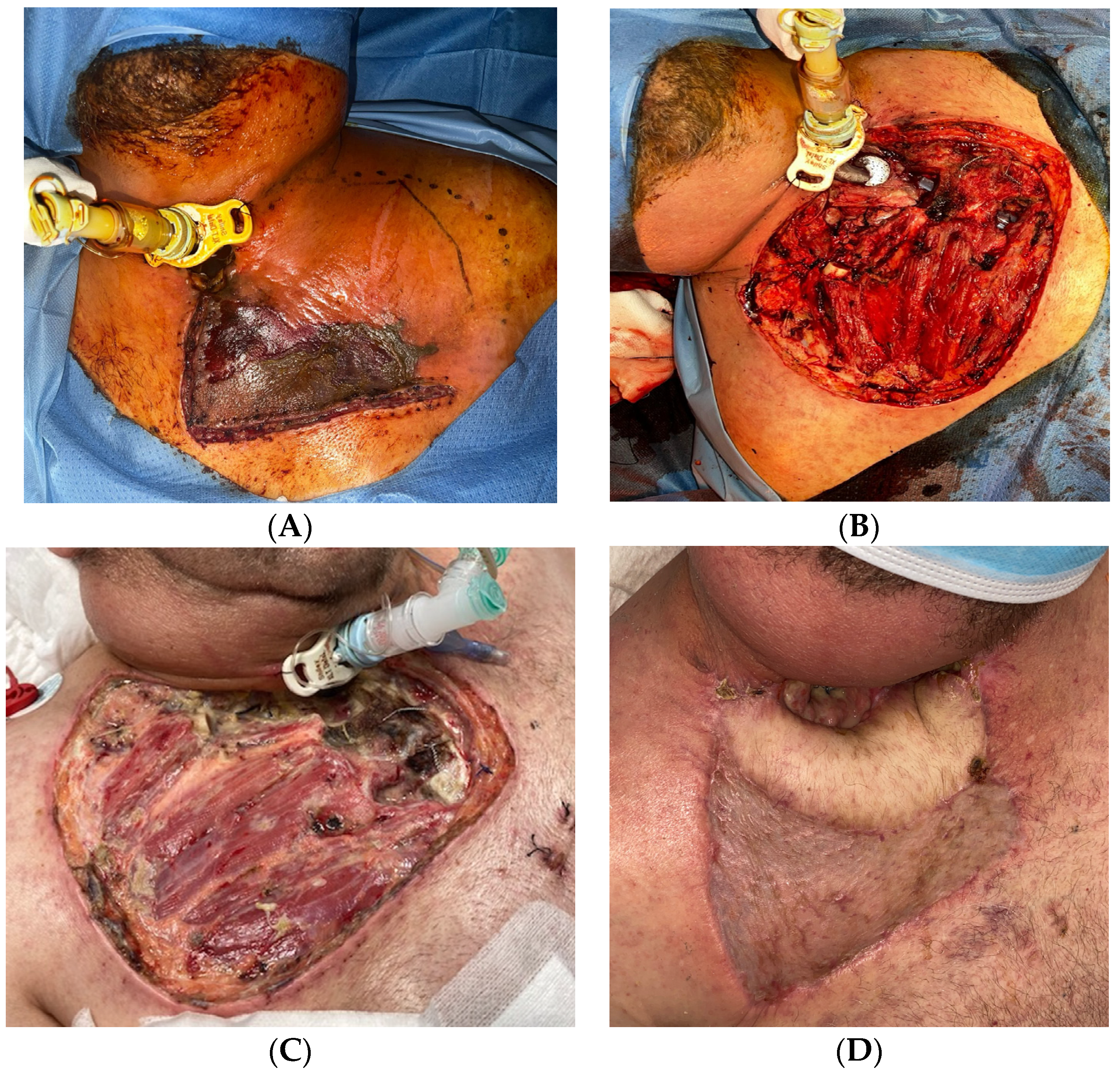
4. Management
- -
- A carbapenem: Imipenem 1 gr every 6 to 8 h or Meropenem 2 g IV every 8 h (extended infusion) or Piperacillin-tazobactam 4.5 g every 6 h PLUS an agent with activity against methicillin-resistant Staphylococcus aureus: Vancomycin (20 mg/kg initially and monitor levels) or Daptomycin (10 mg/kg every 24 h) PLUS Clindamycin 600 to 900 mg IV every 8 h or Linezolid 600 mg IV every 8 h initially—and monitor levels—if there is resistance to clindamycin (for its antitoxin effects against toxin-producing strains of beta-hemolytic streptococci and S. aureus).
- -
- Diagnosis should be made promptly. If sufficient data supports the diagnosis of NF, surgical exploration is preferred to other laboratory or imaging tests that may delay surgery;
- -
- Surgical debridement without delay (not waiting for microbiological results), removing all necrotic tissues (skin, fascia, muscle, and fat);
- -
- Tracheotomy is routinely performed to secure the airway and when a prolonged stay is anticipated;
- -
- Aggressive resuscitation measures by intensive care physicians are also key in the management of NF, together with broad-spectrum antibiotics covering the most frequent pathogens until culture results and Gram’s stain are available;
- -
- Re-interventions are usually necessary. Wounds should be closely monitored looking for signs of progression, and laboratory results and vital signs continuously assessed. When in doubt, repeat the CT scan and look for new collections/progression to descending mediastinitis.
5. Prognosis
Complications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, B. Necrotizing fasciitis. Am. Surg. 1952, 18, 416–431. [Google Scholar] [PubMed]
- McGurk, M. Diagnosis and treatment of necrotizing fasciitis in the head and neck region. Oral Maxillofac. Surg. Clin. N. Am. 2003, 15, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Adams, F. The Genuine Works of Hippocrates, 1771st ed.; Sydenham Society: London, UK, 1849. [Google Scholar]
- Das, D.K.; Baker, M.G.; Venugopal, K. Increasing incidence of necrotizing fasciitis in New Zealand: A nationwide study over the period 1990 to 2006. J. Infect. 2011, 63, 429–433. Available online: https://pubmed.ncbi.nlm.nih.gov/21864570/ (accessed on 23 July 2023). [CrossRef] [PubMed]
- Thean, L.J.; Jenney, A.; Engelman, D.; Romani, L.; Wand, H.; Mudaliar, J.; Paka, J.; Cua, T.; Taole, S.; Sahukhan, A.; et al. Hospital admissions for skin and soft tissue infections in a population with endemic scabies: A prospective study in Fiji, 2018–2019. PLoS Neglected Trop. Dis. 2020, 14, e0008887. [Google Scholar] [CrossRef] [PubMed]
- Type, I.I. Necrotizing Fasciitis: Information for Clinicians|CDC. Available online: https://www.cdc.gov/groupastrep/diseases-hcp/necrotizing-fasciitis.html (accessed on 15 May 2023).
- Hasham, S.; Matteucci, P.; Stanley, P.R.W.; Hart, N.B. Necrotising fasciitis. BMJ 2005, 330, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, A.; Sastre, N. Necrotizing fasciitis of the face and neck. Plast. Reconstr. Surg. 1998, 102, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, S.W.; Hwang, S.H. Application of the laboratory risk indicator for necrotizing fasciitis score to the head and neck: A systematic review and meta-analysis. ANZ J. Surg. 2022, 92, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Sideris, G.; Sapountzi, M.; Malamas, V.; Papadimitriou, N.; Maragkoudakis, P.; Delides, A. Early detecting cervical necrotizing fasciitis from deep neck infections: A study of 550 patients. Eur. Arch. Otorhinolaryngol. 2021, 278, 4587–4592. [Google Scholar] [CrossRef] [PubMed]
- Salati, S.A. Necrotizing fasciitis a review. Pol. Przegl Chir. 2022, 95, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nuwayhid, Z.B.; Aronoff, D.M.; Mulla, Z.D. Blunt trauma as a risk factor for group A streptococcal necrotizing fasciitis. Ann. Epidemiol. 2007, 17, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Bryant, A.E.; Goldstein, E.J. Necrotizing Soft Tissue Infections. Infect. Dis. Clin. N. Am. 2021, 35, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Gunaratne, D.A.; Tseros, E.A.; Hasan, Z.; Kudpaje, A.S.; Suruliraj, A.; Smith, M.C.; Riffat, F.; Palme, C.E. Cervical necrotizing fasciitis: Systematic review and analysis of 1235 reported cases from the literature. Head Neck 2018, 40, 2094–2102. [Google Scholar] [CrossRef] [PubMed]
- Pitché, P.; Diata, A.-B.; Faye, O.; Tounkara, T.-M.; Niamba, P.; Mouhari-Toure, A.; Ly, F.; Soumah, M.-M.; Some-Korsaga, N.; Akakpo, A.-S.; et al. Risk factors associated with necrotizing fasciitis of the lower limbs: A multicenter case-control study. Ann. Dermatol. Venereol. 2021, 148, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.M.; Bayer, C.R.; Stevens, D.L.; Bryant, A.E. Effects of selective and nonselective nonsteroidal anti-inflammatory drugs on antibiotic efficacy of experimental group A streptococcal myonecrosis. J. Infect. Dis. 2014, 209, 1429–1435. [Google Scholar] [CrossRef]
- Weng, T.-C.; Chen, C.-C.; Toh, H.-S.; Tang, H.-J. Ibuprofen worsens Streptococcus pyogenes soft tissue infections in mice. J. Microbiol. Immunol. Infect. Wei Mian Yu Gan Ran Za Zhi 2011, 44, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Bryant, A.E. Necrotizing Soft-Tissue Infections. N. Engl. J. Med. 2017, 377, 2253–2265. [Google Scholar] [CrossRef] [PubMed]
- Anaya, D.A.; Dellinger, E.P. Necrotizing soft-tissue infection: Diagnosis and management. Clin. Infect. Dis. 2007, 44, 705–710. [Google Scholar] [PubMed]
- Lancerotto, L.; Tocco, I.; Salmaso, R.; Vindigni, V.; Bassetto, F. Necrotizing fasciitis: Classification, diagnosis, and management. J. Trauma Acute Care Surg. 2012, 72, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.G.; Perdreau-Remington, F.; Rieg, G.; Mehdi, S.; Perlroth, J.; Bayer, A.S.; Tang, A.W.; Phung, T.O.; Spellberg, B. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N. Engl. J. Med. 2005, 352, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Kaul, R.; McGeer, A.; Low, D.E.; Green, K.; Schwartz, B.; Simor, A.E. Population-based surveillance for group A streptococcal necrotizing fasciitis: Clinical features, prognostic indicators, and microbiologic analysis of seventy-seven cases. Ontario Group A Streptococcal Study. Am. J. Med. 1997, 103, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Darenberg, J.; Luca-Harari, B.; Jasir, A.; Sandgren, A.; Pettersson, H.; Schalén, C.; Norgren, M.; Romanus, V.; Norrby-Teglund, A.; Normark, B.H. Molecular and clinical characteristics of invasive group A streptococcal infection in Sweden. Clin. Infect. Dis. 2007, 45, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Bryant, A.E.; Hackett, S.P.; Chang, A.; Peer, G.; Kosanke, S.; Emerson, T.; Hinshaw, L. Group A streptococcal bacteremia: The role of tumor necrosis factor in shock and organ failure. J. Infect. Dis. 1996, 173, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Baddour, L. Necrotizing soft tissue infections. UptoDate2024. Available online: https://www.uptodate.com/contents/image?csi=3a7bd772-c906-476d-bf1c-80c663b6ddab&source=contentShare&imageKey=ID%2F116305 (accessed on 4 April 2024).
- Hawkes, M.; Barton, M.; Conly, J.; Nicolle, L.; Barry, C.; Ford-Jones, E.L. Community-associated MRSA: Superbug at our doorstep. CMAJ Can. Med. Assoc. J. J. Assoc. Med. Can. 2007, 176, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Borschitz, T.; Schlicht, S.; Siegel, E.; Hanke, E.; von Stebut, E. Improvement of a Clinical Score for Necrotizing Fasciitis: ‘Pain Out of Proportion’ and High CRP Levels Aid the Diagnosis. PLoS ONE 2015, 10, e0132775. [Google Scholar] [CrossRef] [PubMed]
- Leiblein, M.; Marzi, I.; Sander, A.L.; Barker, J.H.; Ebert, F.; Frank, J. Necrotizing fasciitis: Treatment concepts and clinical results. Eur. J. Trauma Emerg. Surg. 2018, 44, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-S.; Wong, C.-H.; Tay, Y.-K. Staging of necrotizing fasciitis based on the evolving cutaneous features. Int. J. Dermatol. 2007, 46, 1036–1041. [Google Scholar] [CrossRef]
- Leyva, P.; Herrero, M.; Eslava, J.M.; Acero, J. Cervical necrotizing fasciitis and diabetic ketoacidosis: Literature review and case report. Int. J. Oral Maxillofac. Surg. 2013, 42, 1592–1595. [Google Scholar] [CrossRef] [PubMed]
- Parra Caballero, P.; Pérez Esteban, S.; Patiño Ruiz, M.E.; Sanz, S.C.; Vadillo, J.A.G. Actualización en fascitis necrotizante. Semin. Fund. Esp. Reumatol. 2012, 13, 41–48. [Google Scholar] [CrossRef]
- Hua, C.; Urbina, T.; Bosc, R.; Parks, T.; Sriskandan, S.; de Prost, N.; Chosidow, O. Necrotising soft-tissue infections. Lancet Infect. Dis. 2023, 23, e81–e94. [Google Scholar] [CrossRef] [PubMed]
- Livshits, D.; Sokup, B.; Farrell, R.; Jeong, J. Finger Test for the Diagnosis of a Critically Ill Patient with Necrotizing Fasciitis. J. Emerg. Med. 2022, 63, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.K.; Kwok, K.; Hung, Y.; Fan, C. Validation of finger test for necrotising soft tissue infection. J. Orthop. Trauma Rehabil. 2020. [Google Scholar] [CrossRef]
- Hua, J.; Friedlander, P. Cervical Necrotizing Fasciitis, Diagnosis and Treatment of a Rare Life-Threatening Infection. Ear Nose Throat J. 2023, 102, NP109–NP113. [Google Scholar] [CrossRef] [PubMed]
- Kishino, T.; Asai, N.; Ohashi, W.; Sakanashi, D.; Kato, H.; Shiota, A.; Hagihara, M.; Koizumi, Y.; Yamagishi, Y.; Suematsu, H.; et al. Usefulness of serum procalcitonin for necrotizing fasciitis as an early diagnostic tool. J. Infect. Chemother. 2021, 27, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Novoa-Parra, C.D.; Wadhwani, J.; Puig-Conca, M.A.; Lizaur-Utrilla, A.; Montaner-Alonso, D.; Rodrigo-Pérez, J.L.; Morales-Suárez-Varela, M. Usefulness of a risk scale based on procalcitonin for early discrimination between necrotising fasciitis and cellulitis of the extremities. Med. Clin. 2019, 153, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-H.; Khin, L.-W.; Heng, K.-S.; Tan, K.-C.; Low, C.-O. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: A tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit. Care Med. 2004, 32, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, S.; Li, C.; Song, Z. Modified Laboratory Risk Indicator for Necrotizing Fasciitis (m-LRINEC) Score System in Diagnosing Necrotizing Fasciitis: A Nested Case-Control Study. Infect. Drug Resist. 2021, 14, 2105–2112. [Google Scholar] [CrossRef]
- Wilson, M.P.; Schneir, A.B. A case of necrotizing fasciitis with a LRINEC score of zero: Clinical suspicion should trump scoring systems. J. Emerg. Med. 2013, 44, 928–931. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.M.; Tran, A.; Cheng, W.; Rochwerg, B.; Kyeremanteng, K.; Seely, A.J.E.; Inaba, K.; Perry, J.J. Necrotizing Soft Tissue Infection: Diagnostic Accuracy of Physical Examination, Imaging, and LRINEC Score: A Systematic Review and Meta-Analysis. Ann. Surg. 2019, 269, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Carbonetti, F.; Cremona, A.; Carusi, V.; Guidi, M.; Iannicelli, E.; Di Girolamo, M.; Sergi, D.; Clarioni, A.; Baio, G.; Antonelli, G.; et al. The role of contrast enhanced computed tomography in the diagnosis of necrotizing fasciitis and comparison with the laboratory risk indicator for necrotizing fasciitis (LRINEC). Radiol. Med. 2016, 121, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Tso, D.K.; Singh, A.K. Necrotizing fasciitis of the lower extremity: Imaging pearls and pitfalls. Br. J. Radiol. 2018, 91, 20180093. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Zbären, P.; Hermans, R.; Becker, C.D.; Marchal, F.; Kurt, A.M.; Marré, S.; A Rüfenacht, D.; Terrier, F. Necrotizing fasciitis of the head and neck: Role of CT in diagnosis and management. Radiology 1997, 202, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Peponis, T.; Hage, A.; Yeh, D.D.; Kaafarani, H.M.A.; Fagenholz, P.J.; King, D.R.; de Moya, M.A.; Velmahos, G.C. The Role of Computed Tomography in the Diagnosis of Necrotizing Soft Tissue Infections. World J. Surg. 2018, 42, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-C.; Kim, S.; Cho, E.B.; Lee, G.Y.; Choi, S.-H.; Kim, S.O.; Chung, J.-W. Utility of Magnetic Resonance Imaging for Differentiating Necrotizing Fasciitis from Severe Cellulitis: A Magnetic Resonance Indicator for Necrotizing Fasciitis (MRINEC) Algorithm. J. Clin. Med. 2020, 9, 3040. [Google Scholar] [CrossRef] [PubMed]
- Malghem, J.; Lecouvet, F.E.; Omoumi, P.; Maldague, B.E.; Berg, B.C.V. Necrotizing fasciitis: Contribution and limitations of diagnostic imaging. Jt. Bone Spine 2013, 80, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Kwee, R.M.; Kwee, T.C. Diagnostic performance of MRI and CT in diagnosing necrotizing soft tissue infection: A systematic review. Skelet. Radiol. 2022, 51, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.C.; Gorbach, S.L.; Hirschmann, J.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C.; et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin. Infect. Dis. 2014, 59, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, T.J.; Green, S.D.; Childers, B.J. Massive infectious soft-tissue injury: Diagnosis and management of necrotizing fasciitis and purpura fulminans. Plast. Reconstr. Surg. 2001, 107, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.; Allen, S.M.; Stocks, R.M.S.; Thompson, J.W. Deep neck infection. Otolaryngol. Clin. N. Am. 2008, 41, 459–483. [Google Scholar] [CrossRef] [PubMed]
- Mladenov, A.; Diehl, K.; Müller, O.; von Heymann, C.; Kopp, S.; Peitsch, W.K. Outcome of necrotizing fasciitis and Fournier’s gangrene with and without hyperbaric oxygen therapy: A retrospective analysis over 10 years. World J. Emerg. Surg. WJES 2022, 17, 43. [Google Scholar] [CrossRef] [PubMed]
- Parks, T.; Wilson, C.; Curtis, N.; Norrby-Teglund, A.; Sriskandan, S. Polyspecific Intravenous Immunoglobulin in Clindamycin-treated Patients With Streptococcal Toxic Shock Syndrome: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2018, 67, 1434–1436. [Google Scholar] [CrossRef]
- Kadri, S.S.; Swihart, B.J.; Bonne, S.L.; Hohmann, S.F.; Hennessy, L.V.; Louras, P.; Evans, H.L.; Rhee, C.; Suffredini, A.F.; Hooper, D.C.; et al. Impact of Intravenous Immunoglobulin on Survival in Necrotizing Fasciitis With Vasopressor-Dependent Shock: A Propensity Score-Matched Analysis From 130 US Hospitals. Clin. Infect. Dis. 2017, 64, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Sarna, T.; Sengupta, T.; Miloro, M.; Kolokythas, A. Cervical Necrotizing Fasciitis With Descending Mediastinitis: Literature Review and Case Report. J. Oral Maxillofac. Surg. 2012, 70, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-H.; Chang, H.-C.; Pasupathy, S.; Khin, L.-W.; Tan, J.-L.; Low, C.-O. Necrotizing fasciitis: Clinical presentation, microbiology, and determinants of mortality. J. Bone Jt. Surg. Am. 2003, 85, 1454–1460. [Google Scholar] [CrossRef]
- Arif, N.; Yousfi, S.; Vinnard, C. Deaths from necrotizing fasciitis in the United States, 2003–2013. Epidemiol. Infect. 2016, 144, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.; Gottlieb, M.; Long, B.; Perkins, J.C. Necrotizing Soft Tissue Infections (NSTI): Pearls and Pitfalls for the Emergency Clinician. J. Emerg. Med. 2022, 62, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-F.; Hung, M.-H.; Lin, Y.-S.; Lu, C.-L.; Liu, C.; Chen, C.-C.; Lee, Y.-H. Independent predictors of mortality for necrotizing fasciitis: A retrospective analysis in a single institution. J. Trauma 2011, 71, 467–473, discussion 473. [Google Scholar] [CrossRef] [PubMed]
- Prado-Calleros, H.M.; Jiménez-Fuentes, E.; Jiménez-Escobar, I. Descending necrotizing mediastinitis: Systematic review on its treatment in the last 6 years, 75 years after its description. Head. Neck 2016, 38 (Suppl. 1), E2275–E2283. [Google Scholar] [CrossRef] [PubMed]
- Sumi, Y. Descending necrotizing mediastinitis: 5 years of published data in Japan. Acute Med. Surg. 2015, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
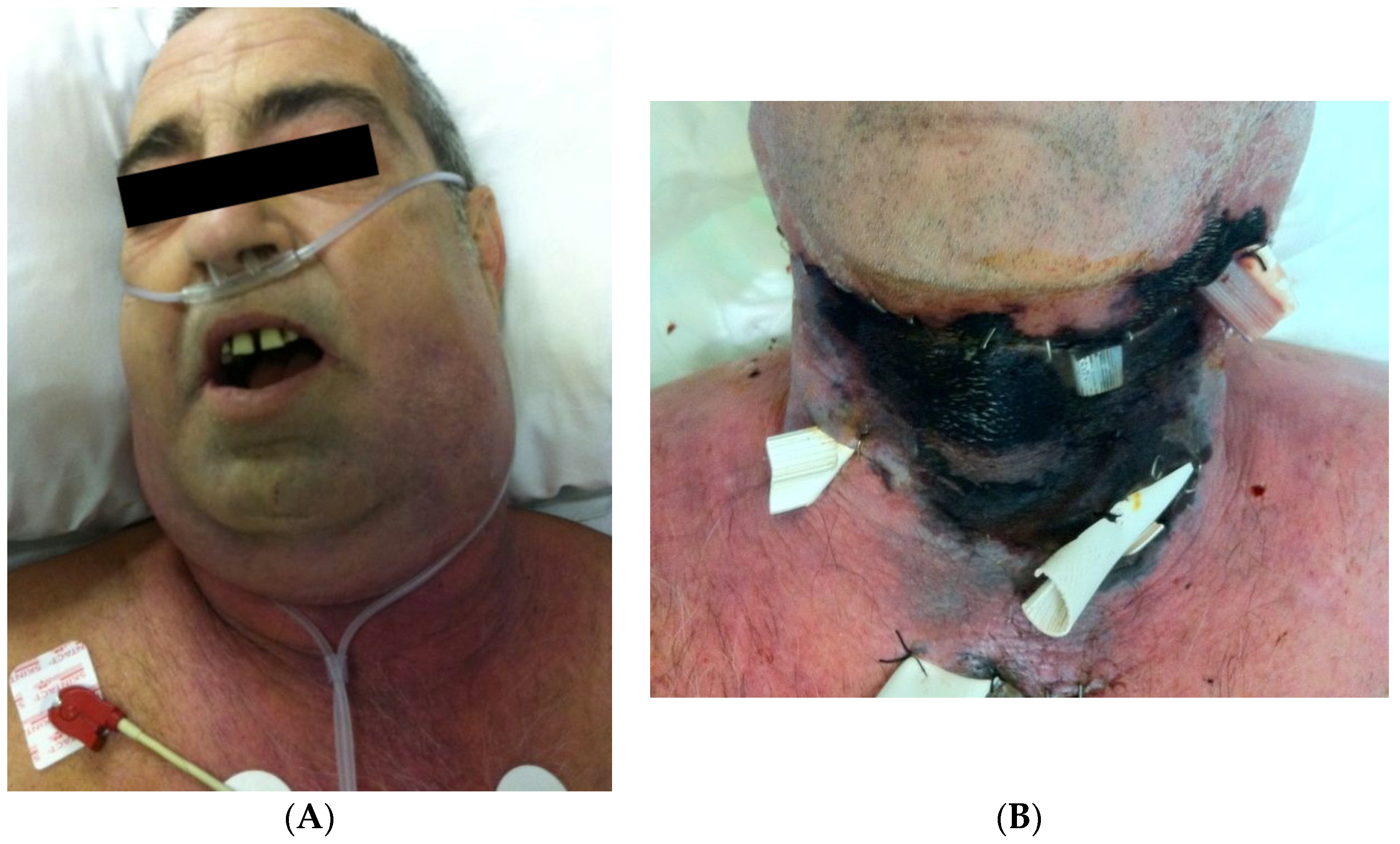


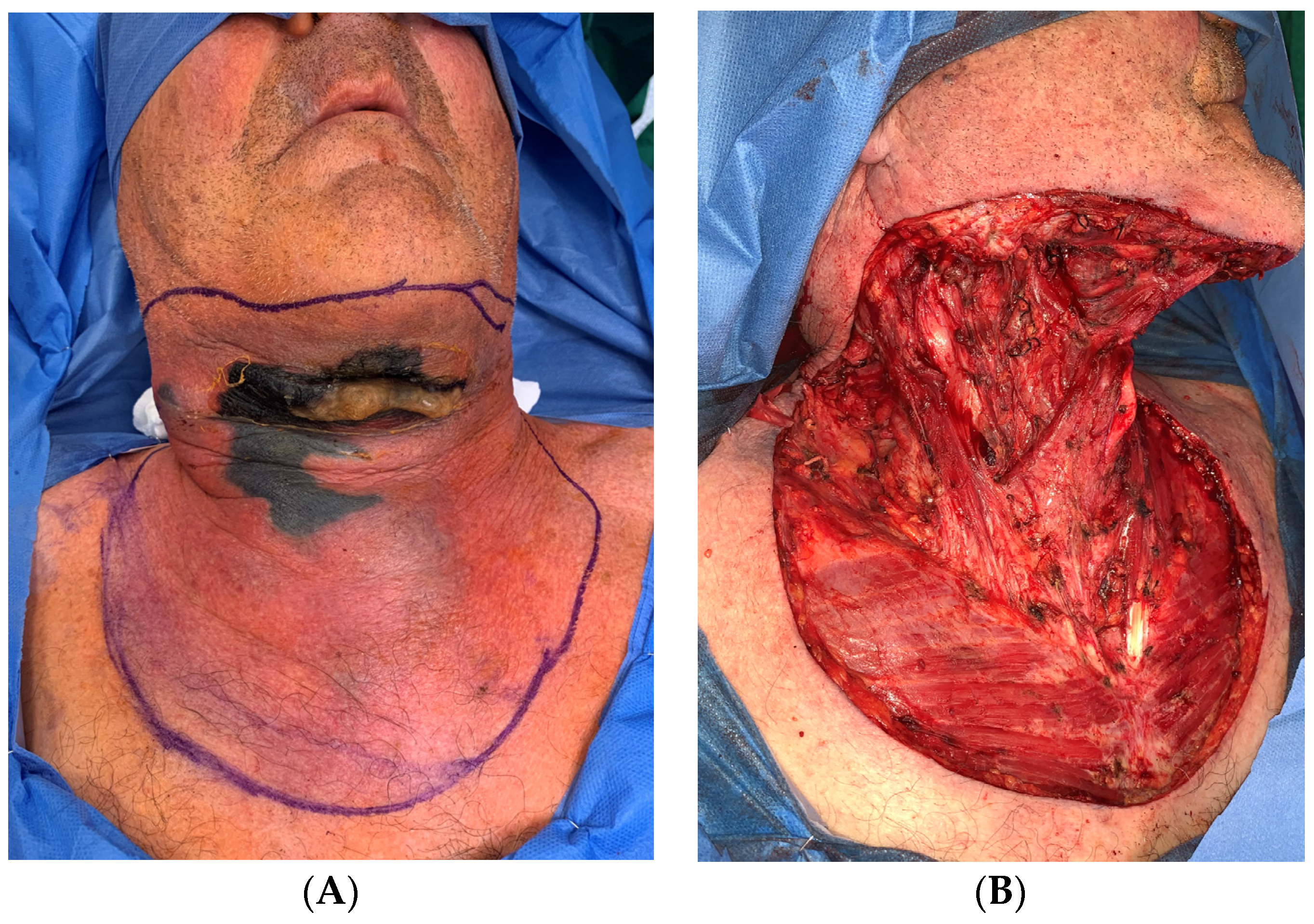
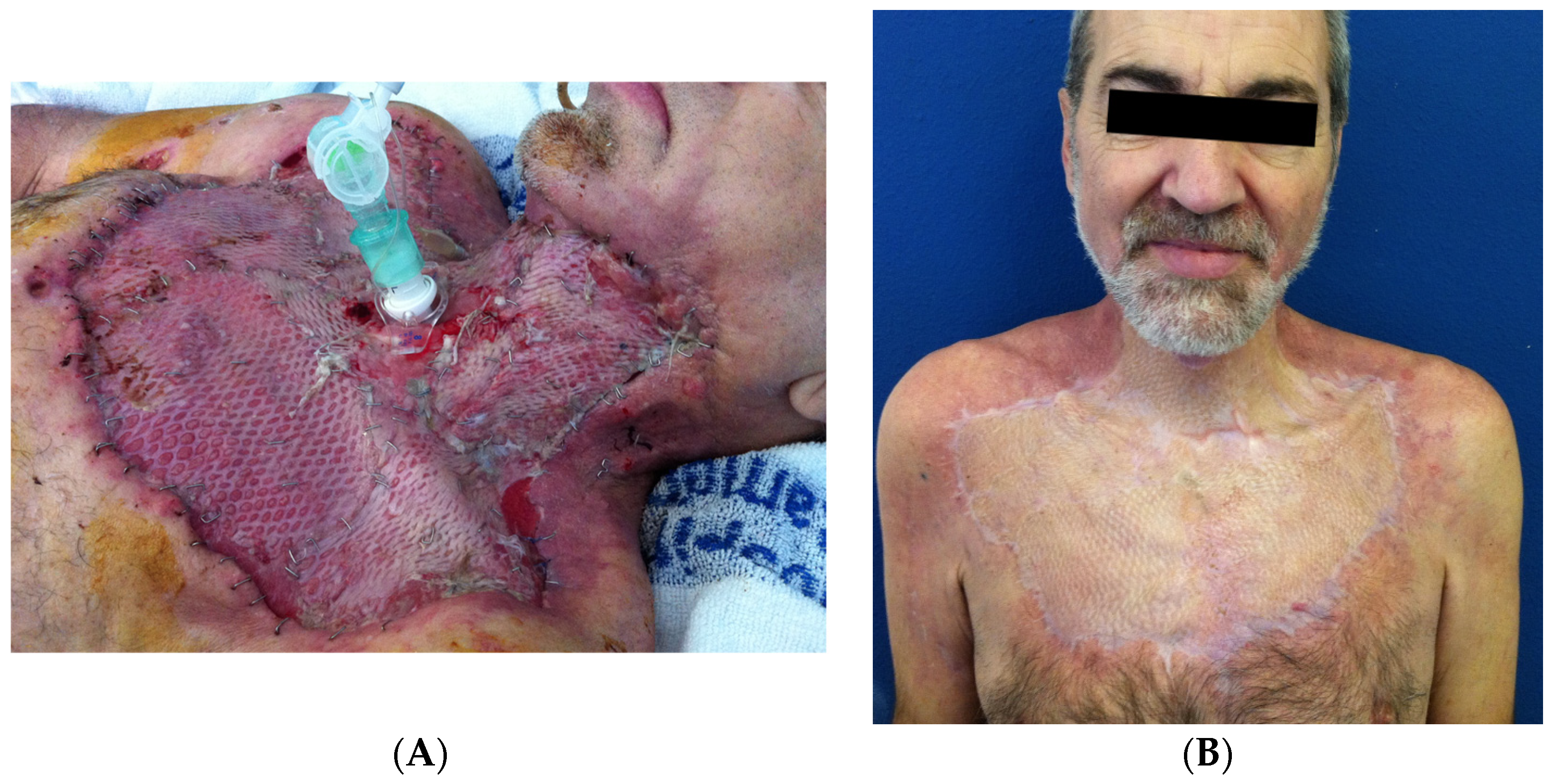
| Causes of CNF | Frequency |
|---|---|
| Odontogenic | 47% |
| Pharyngeal | 28% |
| Tonsillar/Peritonsillar | 6% |
| Major salivary glands | 2.5% |
| Skin disruption: | 1.7% |
| Surgical wounds | |
| Animal bites | |
| Lacerations and scratches | |
| Injection (i.e., iv drug use) | |
| No source identified | 10% |
| Others: | 4.8% |
| Otitis media and mastoiditis | |
| Blunt trauma without laceration | |
| Radiotherapy |
| Clinical Features (Combination of Local + Systemic Signs and Rapid Progression) | Laboratory Findings (Generally Nonspecific) | Radiological Findings |
|---|---|---|
| Local Signs | Leukocytosis (left shift) | Gas in soft tissues |
| Facial/cervical swelling/edema/erythema/warmth | Elevated C-reactive protein and/or erythrocyte sedimentation rate | Absence or heterogeneity of tissue enhancement with IV contrast |
| Crepitus +/− skin necrosis | Coagulopathy | Fluid collections |
| Severe pain | Hyponatremia | Inflammatory changes beneath the fascia |
| Blistering and bullae | Acidosis | |
| Systemic manifestations | Elevation in serum creatinine, lactate, CK, and AST | |
| Fever | ||
| Hemodynamic instability |
| Differential Diagnosis | |
|---|---|
| Cellulitis | Generally not associated with hemodynamic instability (fever may be present) |
| Pyoderma gangrenosum | Slower progression, unlikely to develop sepsis, strong link with inflammatory bowel disease, does not resemble cellulitis, violaceous ulcer edge is typical. Fascial planes have normal resistance to dissection. Worsens with surgery. No response to antibiotics. Usually negative blood and tissue cultures. |
| Gas gangrene (clostridial myonecrosis) | Spontaneous or after traumatic injury. Gram-positive rods are typical. May require amputation (instead of debridement). |
| Pyomyositis | Abscess formation in skeletal muscle. S. aureus usually. Less systemic toxicity. |
| Deep venous thrombosis | Previous manipulations of the neck (operations, punctures, drug use, trauma). Sore throat, impression of swelling, restricted movement of the neck with tilting of the head |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Leyva, P.; Dios-Díez, P.; Cárdenas-Serres, C.; Bueno-de Vicente, Á.; Ranz-Colio, Á.; Sánchez-Jáuregui, E.; Almeida-Parra, F.; Acero-Sanz, J. Cervical Necrotizing Fasciitis in Adults: A Life-Threatening Emergency in Oral and Maxillofacial Surgery. Surgeries 2024, 5, 517-531. https://doi.org/10.3390/surgeries5030042
de Leyva P, Dios-Díez P, Cárdenas-Serres C, Bueno-de Vicente Á, Ranz-Colio Á, Sánchez-Jáuregui E, Almeida-Parra F, Acero-Sanz J. Cervical Necrotizing Fasciitis in Adults: A Life-Threatening Emergency in Oral and Maxillofacial Surgery. Surgeries. 2024; 5(3):517-531. https://doi.org/10.3390/surgeries5030042
Chicago/Turabian Stylede Leyva, Patricia, Paula Dios-Díez, Cristina Cárdenas-Serres, Ángela Bueno-de Vicente, Álvaro Ranz-Colio, Eduardo Sánchez-Jáuregui, Fernando Almeida-Parra, and Julio Acero-Sanz. 2024. "Cervical Necrotizing Fasciitis in Adults: A Life-Threatening Emergency in Oral and Maxillofacial Surgery" Surgeries 5, no. 3: 517-531. https://doi.org/10.3390/surgeries5030042
APA Stylede Leyva, P., Dios-Díez, P., Cárdenas-Serres, C., Bueno-de Vicente, Á., Ranz-Colio, Á., Sánchez-Jáuregui, E., Almeida-Parra, F., & Acero-Sanz, J. (2024). Cervical Necrotizing Fasciitis in Adults: A Life-Threatening Emergency in Oral and Maxillofacial Surgery. Surgeries, 5(3), 517-531. https://doi.org/10.3390/surgeries5030042