Slipped Capital Femoral Epiphysis Pathogenesis and Its Relation to Obesity—Where Do We Stand? A Narrative Review
Abstract
1. Introduction
2. Epidemiology
3. Slipped Capital Femoral Epiphysis and Obesity
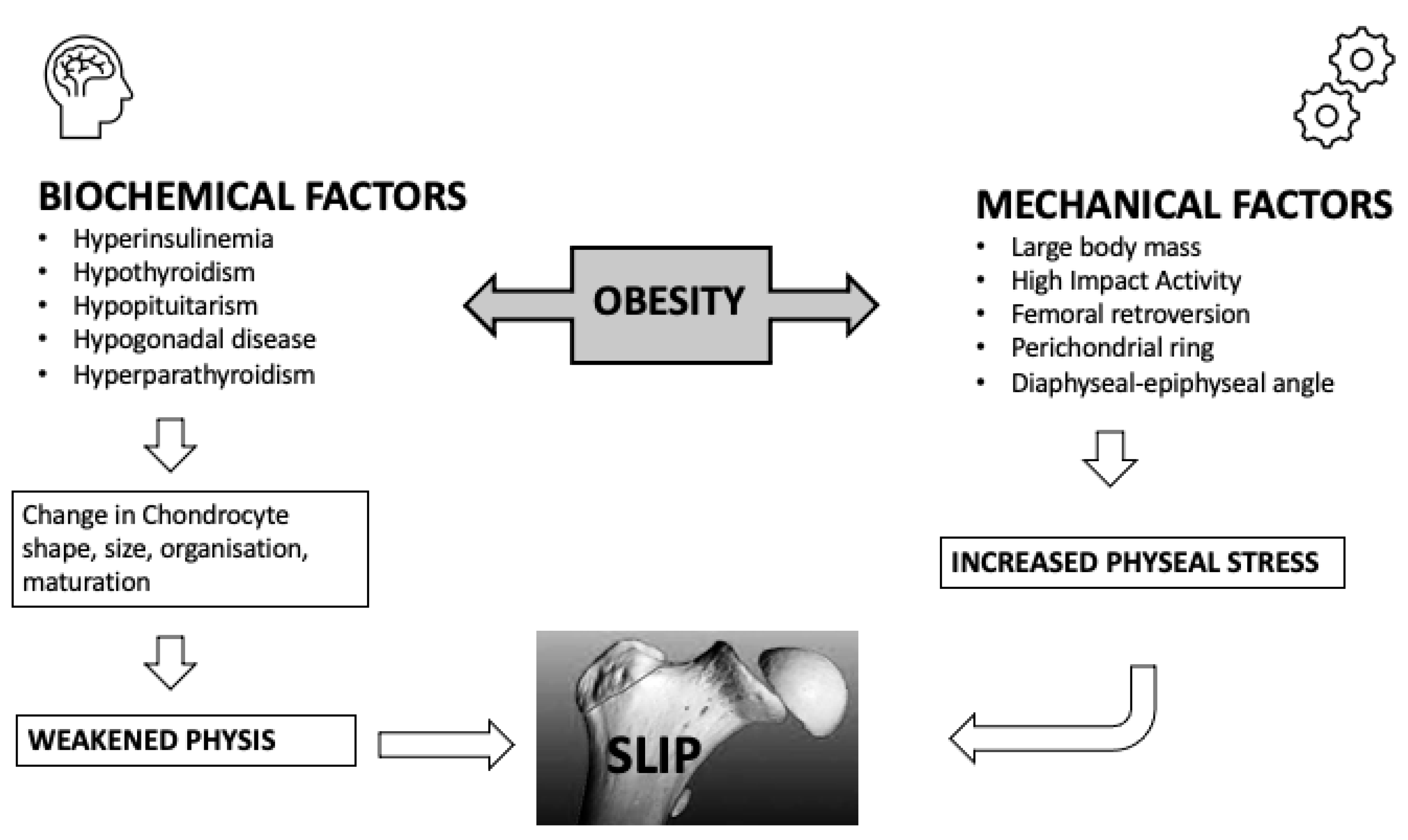
4. Pathogenesis
4.1. Endocrine Factors
4.2. Immunilogical/Biochemical Factors
4.3. Mechanical Factors
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herngren, B.; Stenmarker, M.; Vavruch, L.; Hagglund, G. Slipped Capital Femoral Epiphysis: A Population-Based Study. BMC Musculoskelet. Disord. 2017, 18, 304. [Google Scholar] [CrossRef]
- Lehmann, C.L.; Arons, R.R.; Loder, R.T.; Vitale, M.G. The Epidemiology of Slipped Capital Femoral Epiphysis: An Update. J. Pediatr. Orthop. 2006, 26, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.C.; Metcalfe, D.; Costa, M.L.; Van Staa, T. A Nationwide Cohort Study of Slipped Capital Femoral Epiphysis. Arch. Dis. Child. 2017, 102, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Kocher, M.S.; Bishop, J.A.; Weed, B.; Hresko, M.T.; Millis, M.B.; Kim, Y.J.; Kasser, J.R. Delay in Diagnosis of Slipped Capital Femoral Epiphysis. Pediatrics 2004, 113, e322–e325. [Google Scholar] [CrossRef] [PubMed]
- Loder, R.T.; Richards, B.S.; Shapiro, P.S.; Reznick, L.R.; Aronson, D.D. Acute Slipped Capital Femoral Epiphysis: The Importance of Physeal Stability. J. Bone Jt. Surg. Am. 1993, 75, 1134–1140. [Google Scholar] [CrossRef]
- Millis, M.B. SCFE: Clinical Aspects, Diagnosis, and Classification. J. Child. Orthop. 2017, 11, 93–98. [Google Scholar] [CrossRef]
- Ordeberg, G.; Hansson, L.I.; Sandström, S. Slipped Capital Femoral Epiphysis in Southern Sweden. Long-Term Result with No Treatment or Symptomatic Primary Treatment. Clin. Orthop. Relat. Res. 1984, 191, 95–104. [Google Scholar] [CrossRef]
- Carney, B.T.; Weinstein, S.L. Natural History of Untreated Chronic Slipped Capital Femoral Epiphysis. Clin. Orthop. Relat. Res. 1996, 322, 43–47. [Google Scholar] [CrossRef]
- Mathew, S.E.; Larson, A.N. Natural History of Slipped Capital Femoral Epiphysis. J. Pediatr. Orthop. 2019, 39, S23–S27. [Google Scholar] [CrossRef]
- Porter, M.; Borroff, M.; Gregg, P.; Howard, P.; MacGregor, A.; Tucker, K. National Joint Registry for England and Wales. 9th Annual Report; National Joint Registry for England and Wales: Hemel Hempstead, UK, 2012. [Google Scholar]
- Loder, R.T.; Skopelja, E.N. The Epidemiology and Demographics of Slipped Capital Femoral Epiphysis. ISRN Orthop. 2011, 2011, 486512. [Google Scholar] [CrossRef]
- Farrier, A.J.; Ihediwa, U.; Khan, S.; Kumar, A.; Gulati, V.; Uzoigwe, C.E.; Choudhury, M.Z. The Seasonality of Slipped Upper Femoral Epiphysis-Meta-Analysis: A Possible Association with Vitamin D. HIP Int. 2015, 25, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Loder, R.T.; Schneble, C.A. Seasonal Variation in Slipped Capital Femoral Epiphysis: New Findings Using a National Children’s Hospital Database. J. Pediatr. Orthop. 2019, 39, e44–e49. [Google Scholar] [CrossRef] [PubMed]
- Hägglund, G.; Hansson, L.I.; Ordeberg, G. Epidemiology of Slipped Capital Femoral Epiphysis in Southern Sweden. Clin. Orthop. Relat. Res. 1984, 191, 82–94. [Google Scholar] [CrossRef]
- Aprato, A.; Conti, A.; Bertolo, F.M.A. Slipped Capital Femoral Epiphysis: Current Management Strategies. Orthop. Res. Rev. 2019, 11, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.-J.; Kagda, F.; Lam, K.S.; Hui, J.H.P.; Lim, K.B.L.; Mahadev, A.; Lee, E.H. Demographics and Clinical Presentation of Slipped Capital Femoral Epiphysis in Singapore: Comparing the East with the West. J. Pediatr. Orthop. B 2008, 17, 289–292. [Google Scholar] [CrossRef]
- Larson, A.N.; Yu, E.M.; Melton, L.J., 3rd; Peterson, H.A.; Stans, A.A. Incidence of Slipped Capital Femoral Epiphysis: A Population-Based Study. J. Pediatr. Orthop. B 2010, 19, 9–12. [Google Scholar] [CrossRef]
- Witbreuk, M.M.; van Royen, B.J.; Van Kemenade, F.J.; Witte, B.I.; van der Sluijs, J.A. Incidence and Gender Differences of Slipped Capital Femoral Epiphysis in the Netherlands from 1998-2010 Combined with a Review of the Literature on the Epidemiology of SCFE. J. Child. Orthop. 2013, 7, 99–105. [Google Scholar] [CrossRef]
- Murray, A.W.; Wilson, N.I.L. Changing Incidence of Slipped Capital Femoral Epiphysis: A Relationship with Obesity? J. Bone Jt. Surg. Br. 2008, 90, 92–94. [Google Scholar] [CrossRef]
- Benson, E.C.; Miller, M.; Bosch, P.; Szalay, E.A. A New Look at the Incidence of Slipped Capital Femoral Epiphysis in New Mexico. J. Pediatr. Orthop. 2008, 28, 529–533. [Google Scholar] [CrossRef]
- Song, K.-S.; Oh, C.-W.; Lee, H.-J.; Kim, S.-D. Epidemiology and Demographics of Slipped Capital Femoral Epiphysis in Korea: A Multicenter Study by the Korean Pediatric Orthopedic Society. J. Pediatr. Orthop. 2009, 29, 683–686. [Google Scholar] [CrossRef]
- Noguchi, Y.; Sakamaki, T.; Multicenter Sutdy Commitee of the Japanese Pediatric Orthopaedic Association. Epidemiology and Demographics of Slipped Capital Femoral Epiphysis in Japan: A Multicenter Study by the Japanese Paediatric Orthopaedic Association. J. Orthop. Sci. 2002, 7, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Ravinsky, R.; Rofaiel, J.; Escott, B.G.; Lim, Z.; Ravi, B.; Howard, A. Epidemiology of Slipped Capital Femoral Epiphysis in Ontario, Canada. J. Pediatr. Orthop. 2019, 39, e165–e167. [Google Scholar] [CrossRef]
- Phadnis, J.; Phillips, P.; Willoughby, R. The Epidemiologic Characteristics of Slipped Capital Femoral Epiphysis in Maori Children. J. Pediatr. Orthop. 2012, 32, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Fedorak, G.T.; Brough, A.K.; Miyamoto, R.H.; Raney, E.M. The Epidemiology of Slipped Capital Femoral Epiphysis in American Samoa. Hawaii. J. Med. Public Health 2018, 77, 215–219. [Google Scholar] [PubMed]
- Longo, U.G.; Papalia, R.; De Salvatore, S.; Ruzzini, L.; Candela, V.; Piergentili, I.; Oggiano, L.; Costici, P.F.; Denaro, V. Slipped Capital Femoral Epiphysis: An Epidemiological Nationwide Study in Italy from 2001 to 2015. BMC Musculoskelet. Disord. 2021, 22, 570. [Google Scholar] [CrossRef]
- Beharry, A.C.; Quan Soon, C.H.; Augustus, M.; Toby, D.; Thomas, D. Increasing Incidence of Slipped Capital Femoral Epiphysis in Trinidad and Tobago: A 50-Year Review. Trop. Doct. 2023, 53, 85–90. [Google Scholar] [CrossRef]
- Nguyen, A.R.; Ling, J.; Gomes, B.; Antoniou, G.; Sutherland, L.M.; Cundy, P.J. Slipped Capital Femoral Epiphysis: Rising Rates with Obesity and Aboriginality in South Australia. J. Bone Jt. Surg. Br. 2011, 93, 1416–1423. [Google Scholar] [CrossRef]
- Loder, R.T.; Greenfield, M.L. Clinical Characteristics of Children with Atypical and Idiopathic Slipped Capital Femoral Epiphysis: Description of the Age-Weight Test and Implications for Further Diagnostic Investigation. J. Pediatr. Orthop. 2001, 21, 481–487. [Google Scholar] [CrossRef]
- Obana, K.K.; Siddiqui, A.A.; Broom, A.M.; Barrett, K.; Andras, L.M.; Millis, M.B.; Goldstein, R.Y. Slipped Capital Femoral Epiphysis in Children without Obesity. J. Pediatr. 2020, 218, 192–197.e1. [Google Scholar] [CrossRef]
- Mann, D.C.; Weddington, J.; Richton, S. Hormonal Studies in Patients with Slipped Capital Femoral Epiphysis without Evidence of Endocrinopathy. J. Pediatr. Orthop. 1988, 8, 543–545. [Google Scholar] [CrossRef]
- Perry, D.C.; Metcalfe, D.; Lane, S.; Turner, S. Childhood Obesity and Slipped Capital Femoral Epiphysis. Pediatrics 2018, 142, e20181067. [Google Scholar] [CrossRef] [PubMed]
- Nittari, G.; Scuri, S.; Petrelli, F.; Pirillo, I.; di Luca, N.M.; Grappasonni, I. Fighting Obesity in Children from European World Health Organization Member States. Epidemiological Data, Medical-Social Aspects, and Prevention Programs. Clin. Ter. 2019, 170, e223–e230. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 10 January 2023).
- Escott, B.G.; De La Rocha, A.; Jo, C.-H.; Sucato, D.J.; Karol, L.A. Patient-Reported Health Outcomes After in Situ Percutaneous Fixation for Slipped Capital Femoral Epiphysis: An Average Twenty-Year Follow-up Study. J. Bone Jt. Surg. Am. 2015, 97, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Ucpunar, H.; Camurcu, I.Y.; Duman, S.; Ucpunar, E.; Sofu, H.; Bayhan, A.I. Obesity-Related Metabolic and Endocrine Disorders Diagnosed during Postoperative Follow-up of Slipped Capital Femoral Epiphysis. Acta Orthop. 2018, 89, 314–319. [Google Scholar] [CrossRef]
- Carlos, C.; Magda, M. Slipped Capital Femoral Epiphysis: A Review for Pediatricians. Pediatr. Ann. 2018, 47, e377–e380. [Google Scholar] [CrossRef]
- Winston, T.W.; Landau, A.J.; Hosseinzadeh, P. Proximal Femoral Changes Related to Obesity: An Analysis of Slipped Capital Femoral Epiphysis Pathoanatomy. J. Pediatr. Orthop. B 2022, 31, 216–223. [Google Scholar] [CrossRef]
- Castro-Abril, H.A.; Galván, F.; Garzón-Alvarado, D.A. Geometrical and Mechanical Factors That Influence Slipped Capital Femoral Epiphysis: A Finite Element Study. J. Pediatr. Orthop. B 2015, 24, 418–424. [Google Scholar] [CrossRef]
- Witbreuk, M.; van Kemenade, F.J.; van der Sluijs, J.A.; Jansma, E.P.; Rotteveel, J.; van Royen, B.J. Slipped Capital Femoral Epiphysis and Its Association with Endocrine, Metabolic and Chronic Diseases: A Systematic Review of the Literature. J. Child. Orthop. 2013, 7, 213–223. [Google Scholar] [CrossRef]
- Montañez-Alvarez, M.; Flores-Navarro, H.H.; Cuevas-De Alba, C.; Arana-Hernández, E.I.; Ramírez-Ruiz, M. The Role of Hyperinsulinemia in Slipped Capital Femoral Epiphysis. J. Pediatr. Orthop. 2020, 40, 413–417. [Google Scholar] [CrossRef]
- Galbraith, R.T.; Gelberman, R.H.; Hajek, P.C.; Baker, L.A.; Sartoris, D.J.; Rab, G.T.; Cohen, M.S.; Griffin, P.P. Obesity and Decreased Femoral Anteversion in Adolescence. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 1987, 5, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Wabitsch, M.; Horn, M.; Esch, U.; Mayer, H.; Moss, A.; Günther, K.-P.; Nelitz, M. Silent Slipped Capital Femoral Epiphysis in Overweight and Obese Children and Adolescents. Eur. J. Pediatr. 2012, 171, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Halverson, S.J.; Warhoover, T.; Mencio, G.A.; Lovejoy, S.A.; Martus, J.E.; Schoenecker, J.G. Leptin Elevation as a Risk Factor for Slipped Capital Femoral Epiphysis Independent of Obesity Status. J. Bone Jt. Surg. 2017, 99, 865–872. [Google Scholar] [CrossRef]
- Bhatia, N.N.; Pirpiris, M.; Otsuka, N.Y. Body Mass Index in Patients with Slipped Capital Femoral Epiphysis. J. Pediatr. Orthop. 2006, 26, 197–199. [Google Scholar] [CrossRef]
- Dunger, D.B.; Ahmed, M.L.; Ong, K.K. Effects of Obesity on Growth and Puberty. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Burt Solorzano, C.M.; McCartney, C.R. Obesity and the Pubertal Transition in Girls and Boys. Reproduction 2010, 140, 399–410. [Google Scholar] [CrossRef]
- Green, H.; Morikawa, M.; Nixon, T. A Dual Effector Theory of Growth-Hormone Action. Differentiation 1985, 29, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, Y.; De Luca, F. The Effect of a High-Calorie Diet on Bone Growth Is Mediated by the Insulin Receptor. Bone 2019, 122, 166–175. [Google Scholar] [CrossRef]
- Zhang, F.; He, Q.; Tsang, W.P.; Garvey, W.T.; Chan, W.Y.; Wan, C. Insulin Exerts Direct, IGF-1 Independent Actions in Growth Plate Chondrocytes. Bone Res. 2014, 2, 14012. [Google Scholar] [CrossRef]
- Wu, S.; Aguilar, A.L.; Ostrow, V.; De Luca, F. Insulin Resistance Secondary to a High-Fat Diet Stimulates Longitudinal Bone Growth and Growth Plate Chondrogenesis in Mice. Endocrinology 2011, 152, 468–475. [Google Scholar] [CrossRef]
- Torres, E.S.; Andrade, C.V.; Fonseca, E.C.; Mello, M.A.; Duarte, M.E.L. Insulin Impairs the Maturation of Chondrocytes In Vitro. Braz. J. Med. Biol. Res. 2003, 36, 1185–1192. [Google Scholar] [CrossRef]
- Tresoldi, I.; Modesti, A.; Dragoni, M.; Potenza, V.; Ippolito, E. Histological, Histochemical and Ultrastructural Study of Slipped Capital Femoral Epiphysis. J. Child. Orthop. 2017, 11, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Ko, K.R.; Kim, J.H.; Shim, J.S. Clinical and Radiographic Characteristics of Atypical Slipped Capital Femoral Epiphysis. J. Pediatr. Orthop. 2019, 39, e742–e749. [Google Scholar] [CrossRef] [PubMed]
- Zelaya, R.; Zarka, A.; Byerly, D. Slipped Capital Femoral Epiphysis as a Presentation of Underlying Metabolic Disorders: Pseudohypoparathyroidism and Juvenile Hypothyroidism. Cureus 2021, 13, e13775. [Google Scholar] [CrossRef] [PubMed]
- Gutch, M.; Philip, R.; Philip, R.; Toms, A.; Saran, S.; Gupta, K.K. Skeletal Manifestations of Juvenile Hypothyroidism and the Impact of Treatment on Skeletal System. Indian J. Endocrinol. Metab. 2013, 17 (Suppl. 1), S181–S183. [Google Scholar] [CrossRef]
- Tank, J.C.; Weiner, D.S.; Jacquet, R.; Childs, D.; Ritzman, T.F.; Horne, W.I.; Steiner, R.; Morscher, M.A.; Landis, W.J. The Effects of Hypothyroidism on the Proximal Femoral Physis in Miniature Swine. J. Orthop. Res. 2013, 31, 1986–1991. [Google Scholar] [CrossRef]
- Moyer, J.; Jacks, L.; Hunter, J.D.; Chan, G. Slipped Capital Femoral Epiphysis and Associated Hypothyroidism. A Review of the Literature with Two Classic Case Examples. J. Pediatr. Endocrinol. Metab. 2016, 29, 427–434. [Google Scholar] [CrossRef]
- Kadowaki, S.; Hori, T.; Matsumoto, H.; Kanda, K.; Ozeki, M.; Shirakami, Y.; Kawamoto, N.; Ohnishi, H.; Fukao, T. Prepubertal Onset of Slipped Capital Femoral Epiphysis Associated with Hypothyroidism: A Case Report and Literature Review. BMC Endocr. Disord. 2017, 17, 59. [Google Scholar] [CrossRef]
- Agarwal, C.; Seigle, R.; Agarwal, S.; Bilezikian, J.P.; Hyman, J.E.; Oberfield, S.E. Pseudohypoparathyroidism: A Rare Cause of Bilateral Slipped Capital Femoral Epiphysis. J. Pediatr. 2006, 149, 406–408. [Google Scholar] [CrossRef]
- Jingushi, S.; Suenaga, E. Slipped Capital Femoral Epiphysis: Etiology and Treatment. J. Orthop. Sci. 2004, 9, 214–219. [Google Scholar] [CrossRef]
- Eisenstein, A.; Rothschild, S. Biochemical Abnormalities in Patients with Slipped Capital Femoral Epiphysis and Chondrolysis. J. Bone Jt. Surg. Am. 1976, 58, 459–467. [Google Scholar] [CrossRef]
- Liu, R.W.; Armstrong, D.G.; Levine, A.D.; Gilmore, A.; Thompson, G.H.; Cooperman, D.R. An Anatomic Study of the Epiphyseal Tubercle and Its Importance in the Pathogenesis of Slipped Capital Femoral Epiphysis. J. Bone Jt. Surg. Am. 2013, 95, e341–e348. [Google Scholar] [CrossRef] [PubMed]
- Kiapour, A.M.; Kiapour, A.; Maranho, D.A.; Kim, Y.-J.; Novais, E.N. Relative Contribution of Epiphyseal Tubercle and Peripheral Cupping to Capital Femoral Epiphysis Stability during Daily Activities. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2019, 37, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.M.; Batterman, S.C.; Brighton, C.T. Shear Strength of the Human Femoral Capital Epiphyseal Plate. J. Bone Jt. Surg. Am. 1976, 58, 94–103. [Google Scholar] [CrossRef]
- Loder, R.T.; Gunderson, Z.J.; Sun, S.; Liu, R.W.; Novais, E.V. Slipped Capital Femoral Epiphysis Associated with Athletic Activity. Sport. Health 2022, 19417381221093044. [Google Scholar] [CrossRef]
- Manoff, E.M.; Banffy, M.B.; Winell, J.J. Relationship Between Body Mass Index and Slipped Capital Femoral Epiphysis. J. Pediatr. Orthop. 2005, 25, 744–746. [Google Scholar] [CrossRef] [PubMed]
- Poussa, M.; Schlenzka, D.; Yrjönen, T. Body Mass Index and Slipped Capital Femoral Epiphysis. J. Pediatr. Orthop. B 2003, 12, 369–371. [Google Scholar] [CrossRef]
- Azzopardi, T.; Sharma, S.; Bennet, G.C. Slipped Capital Femoral Epiphysis in Children Aged Less than 10 Years. J. Pediatr. Orthop. B 2010, 19, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Chatziravdeli, V.; Stefanou, M.; Pilichou, A.; Krallis, P.; Anastasopoulos, J. Early Onset Slipped Capital Femoral Epiphysis in Children under 10 Years Old. Surgical Treatment with Two Different Methods and Results. Hippokratia 2019, 23, 165–168. [Google Scholar]
- Fishkin, Z.; Armstrong, D.G.; Shah, H.; Patra, A.; Mihalko, W.M. Proximal Femoral Physis Shear in Slipped Capital Femoral Epiphysis—A Finite Element Study. J. Pediatr. Orthop. 2006, 26, 291–294. [Google Scholar] [CrossRef]
- Farzaneh, S.; Paseta, O.; Gómez-Benito, M.J. Multi-Scale Finite Element Model of Growth Plate Damage during the Development of Slipped Capital Femoral Epiphysis. Biomech. Model. Mechanobiol. 2015, 14, 371–385. [Google Scholar] [CrossRef]
- Mirkopulos, N.; Weiner, D.S.; Askew, M. The Evolving Slope of the Proximal Femoral Growth Plate Relationship to Slipped Capital Femoral Epiphysis. J. Pediatr. Orthop. 1988, 8, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, S.; Novais, E.N.; Maranho, D.A.; Emami, S.A.; Portilla, G.; Kim, Y.-J.; Kiapour, A.M. Age- and Sex-Specific Morphologic Changes in the Metaphyseal Fossa Adjacent to Epiphyseal Tubercle in Children and Adolescents without Hip Disorders. J. Orthop. Res. 2020, 38, 2213–2219. [Google Scholar] [CrossRef] [PubMed]

| Author | Study Years | Region | Incidence per 100.000 | Age Range (in Years) |
|---|---|---|---|---|
| Larson et al. | 1965–2005 | Midwest USA | 8.3 | 9–16 |
| Herngren et al. | 2007–2013 | Sweden | 44 females 57 males | 11.7 * females 13 * males |
| Witbreuk et al. | 1998–2010 | Netherlands | 11.6 | 5–19 |
| Murray and Wilson | 1981–2000 | Scotland | 9.66 | 11.6 * females 12.6 * males |
| Benson et al. | 1995–2006 | New Mexico | 5.99 | 8–17 |
| Lehmann et al. | 1997, 2000 | USA | 10.8 | 9–16 |
| Kwang-Soon Song et al. | 1989- 2003 | Korea | 0.499 males 0.142 females | 10–14 |
| Perry et al. | 2016–2017 | Great Britain | 3.34 | 6–18 |
| Noguchi et al. | 1997–1999 | Japan | 2.22 males 0.76 females | 10–14 |
| Ravinsky et al. | 2002–2011 | Ontario, Canada | 5.68 | 9–16 |
| Phadnis et al. | 2000–2010 | Maori/ New Zealand | 81 Maori 11.3 New Zealand | 5–14 |
| Fedorak et al. | 2005–2014 | American Samoa | 53.1 | 5–14 |
| Longo et al. | 2001–2015 | Italy | 2.9 | 10–14 |
| Beharry et al. | 2008–2018 | North Trinidad | 2.2 | |
| Nguyen et al. | 1988–2007 | South Australia | 8.2 | 10–19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatziravdeli, V.; Psaroulaki, E.; Rodiftsis, G.; Katsaras, G. Slipped Capital Femoral Epiphysis Pathogenesis and Its Relation to Obesity—Where Do We Stand? A Narrative Review. Surgeries 2023, 4, 152-163. https://doi.org/10.3390/surgeries4020017
Chatziravdeli V, Psaroulaki E, Rodiftsis G, Katsaras G. Slipped Capital Femoral Epiphysis Pathogenesis and Its Relation to Obesity—Where Do We Stand? A Narrative Review. Surgeries. 2023; 4(2):152-163. https://doi.org/10.3390/surgeries4020017
Chicago/Turabian StyleChatziravdeli, Vasiliki, Evdokia Psaroulaki, Grigoriοs Rodiftsis, and Georgios Katsaras. 2023. "Slipped Capital Femoral Epiphysis Pathogenesis and Its Relation to Obesity—Where Do We Stand? A Narrative Review" Surgeries 4, no. 2: 152-163. https://doi.org/10.3390/surgeries4020017
APA StyleChatziravdeli, V., Psaroulaki, E., Rodiftsis, G., & Katsaras, G. (2023). Slipped Capital Femoral Epiphysis Pathogenesis and Its Relation to Obesity—Where Do We Stand? A Narrative Review. Surgeries, 4(2), 152-163. https://doi.org/10.3390/surgeries4020017







