Breast Implant Illness: Surgical, Autoimmune, and Breast Reconstruction Associations
Abstract
:1. Introduction
Methodology of Literature Research
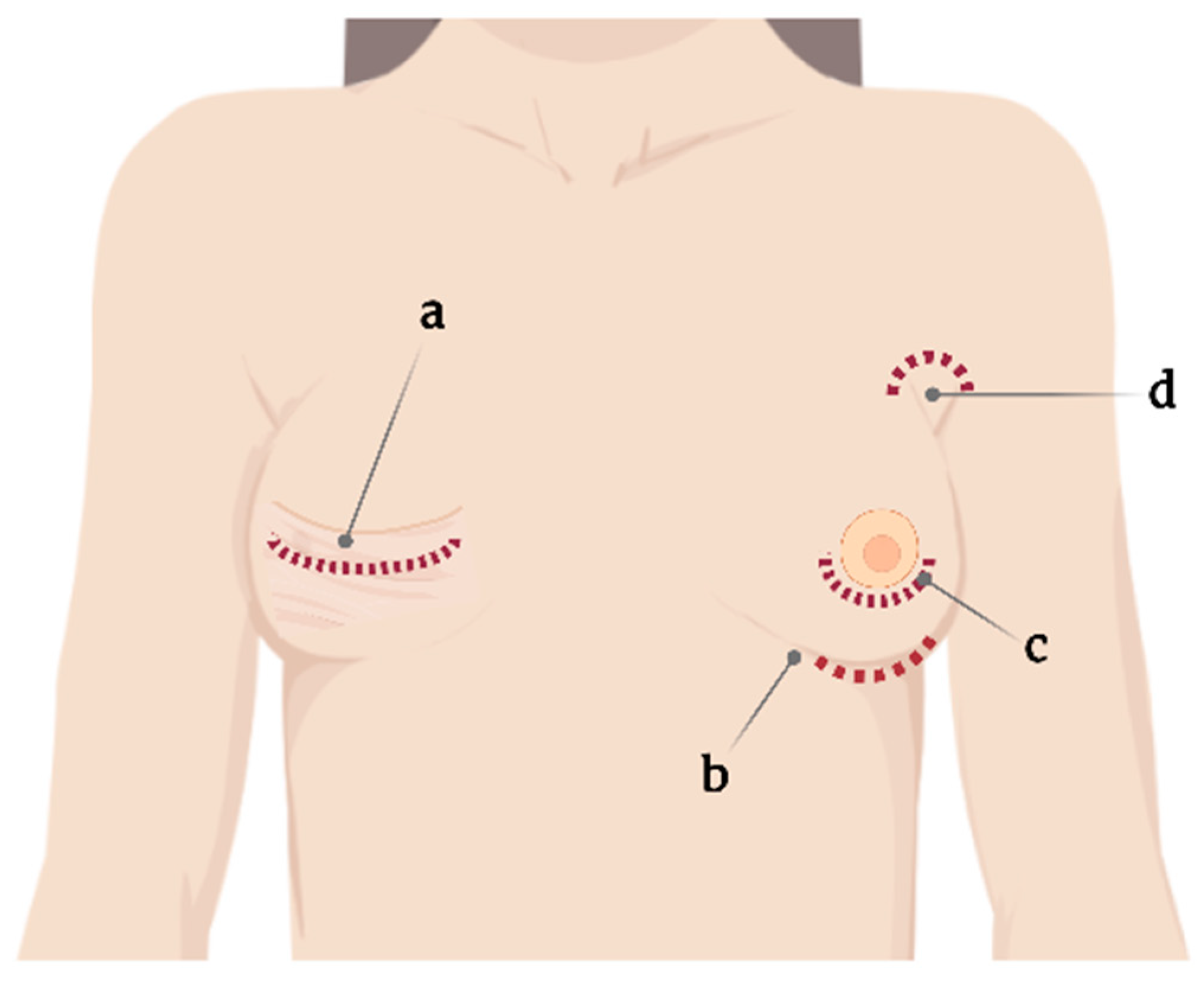
2. Surgical Implications
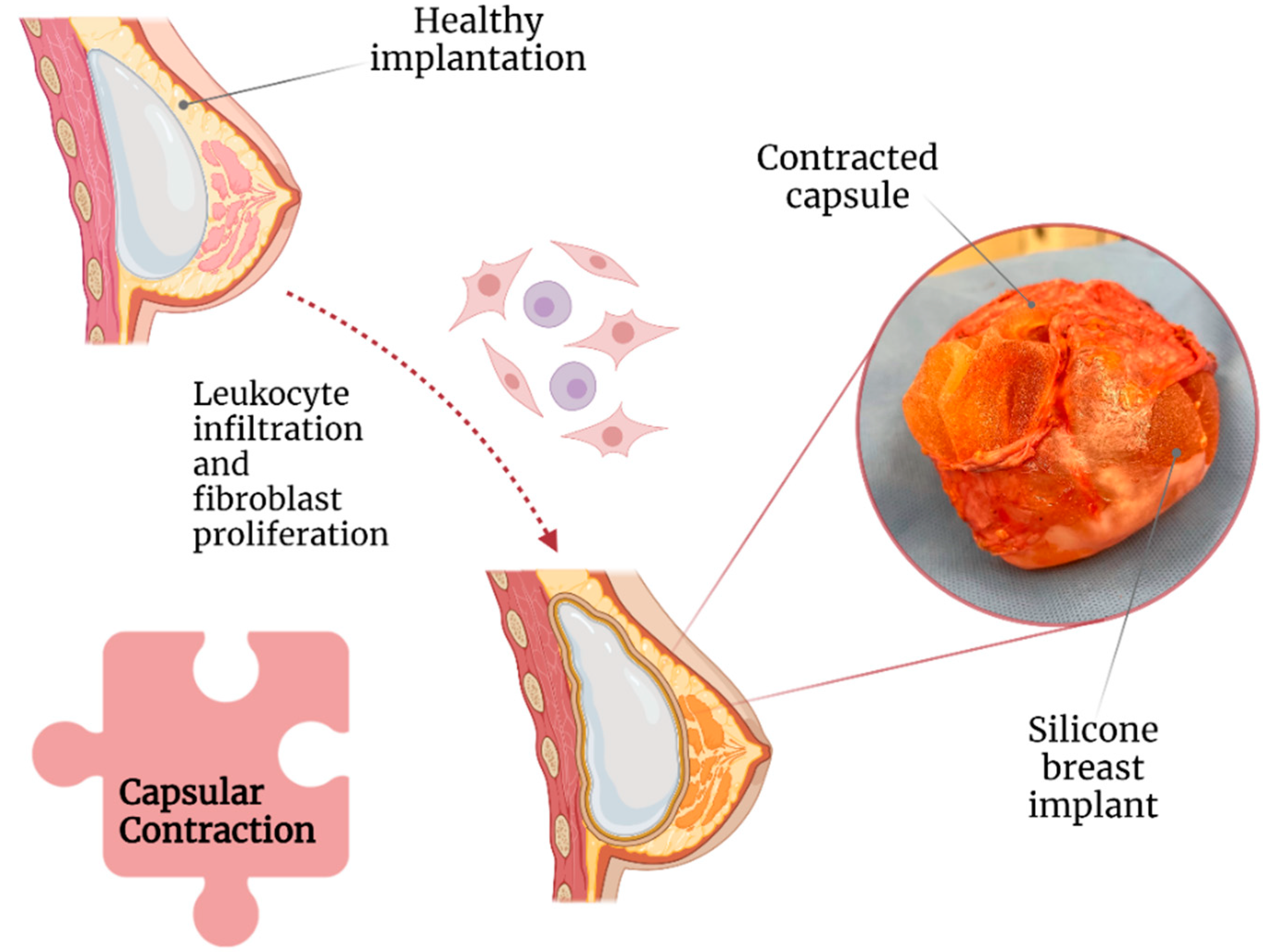
2.1. Capsular Contracture
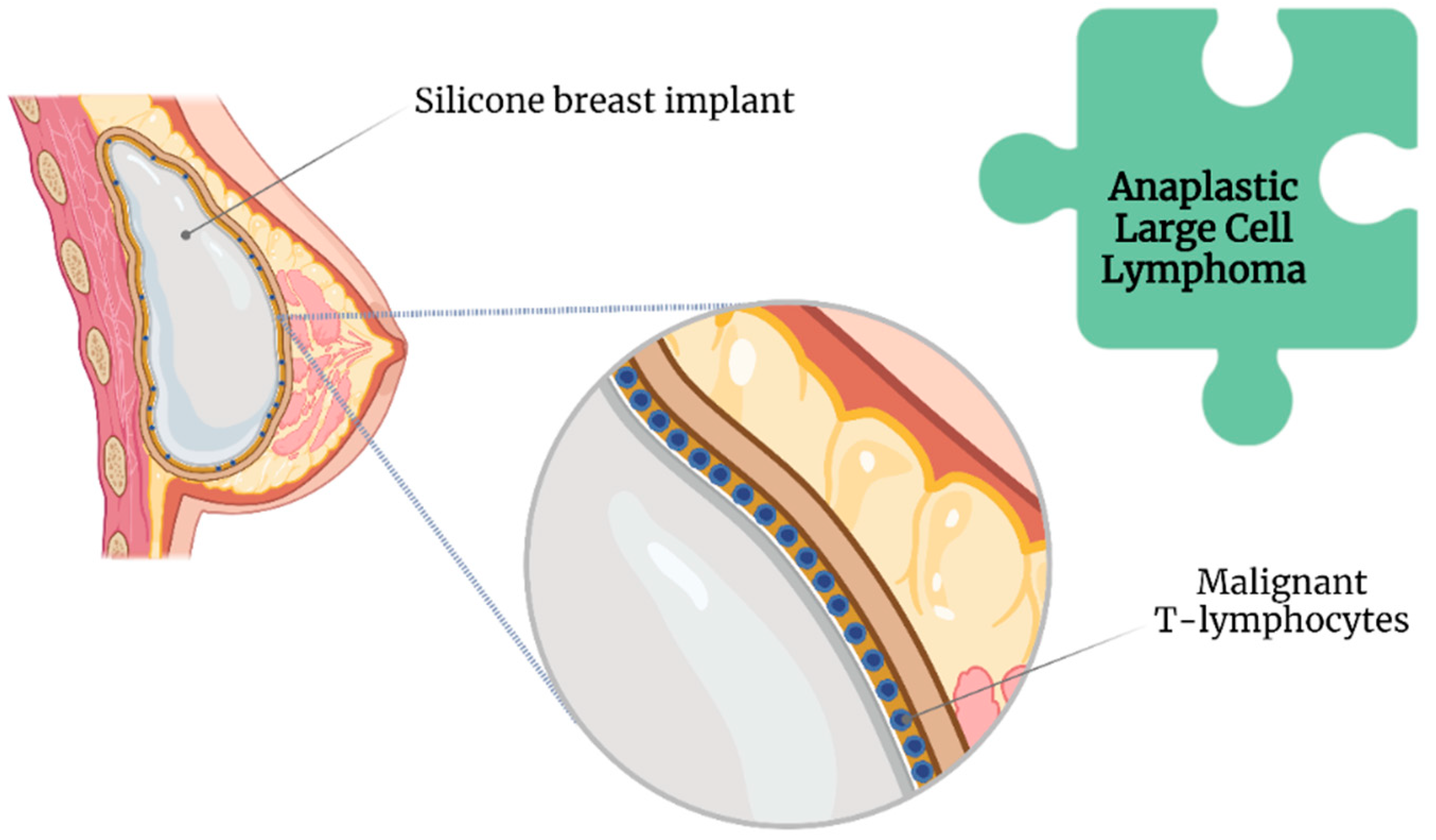
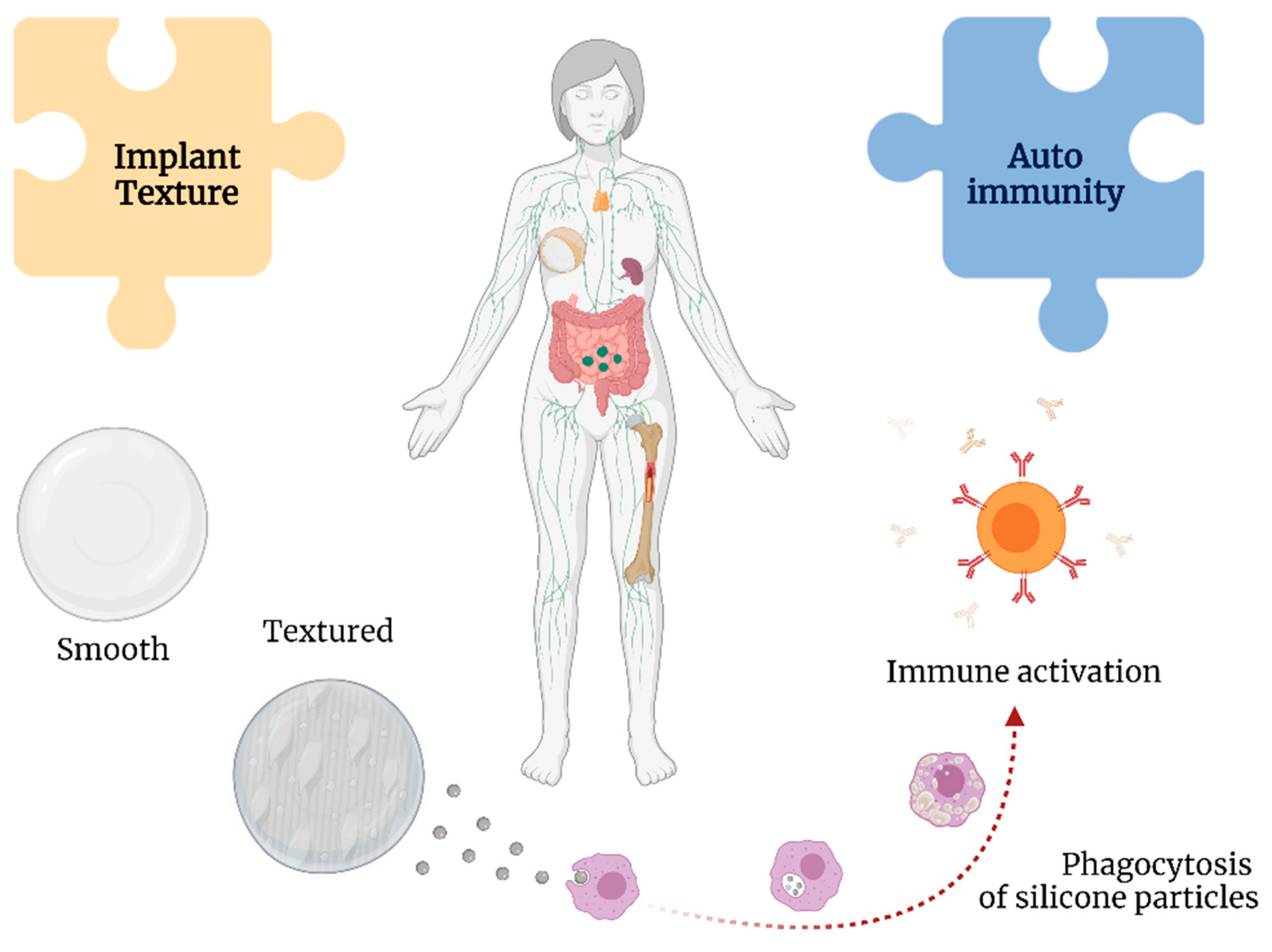
2.2. Texture of SBI and Anaplastic Large Cell Lymphoma
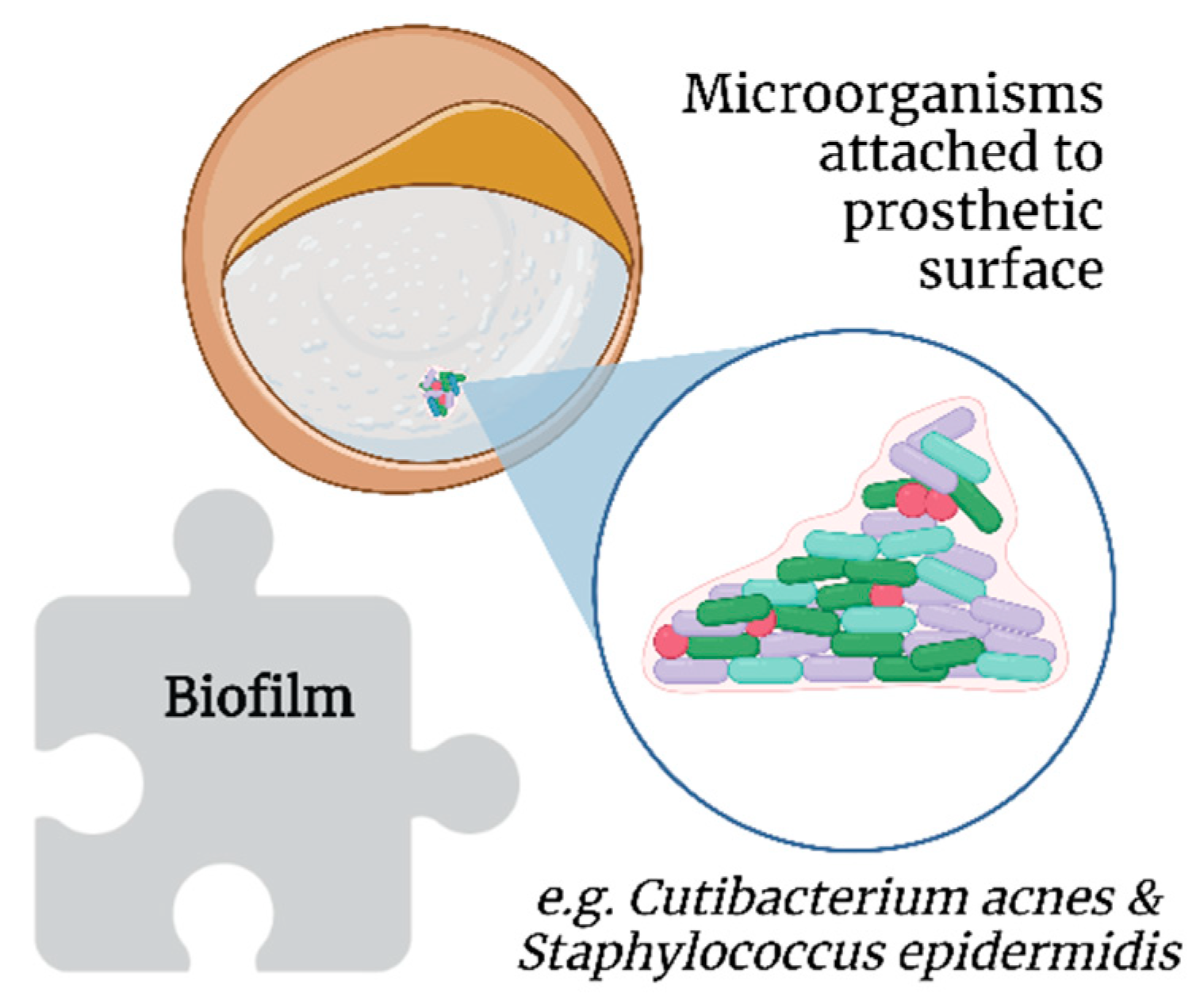
2.3. Biofilms
2.4. Infections
3. Autoimmune Responses
3.1. The Immune Response to SBI
3.2. Somatic Syndromes
3.3. Explantation and Reported Symptoms of BII
4. Breast Reconstruction
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nahabedian, M.Y. Innovations and advancements with prosthetic breast reconstruction. Breast J. 2018, 24, 586–591. [Google Scholar] [CrossRef]
- Harvey, Z.T.; Potter, B.K.; Vandersea, J.; Wolf, E. Prosthetic advances. J. Surg. Orthop. Adv. 2012, 21, 58–64. [Google Scholar] [PubMed]
- Arabian, A.; Varotsis, D.; McDonnell, C.; Meeks, E. Global social acceptance of prosthetic devices. In Proceedings of the GHTC 2016—2016 IEEE Global Humanitarian Technology Conference (GHTC) Technology for the Benefit of Humanity Conference Proceedings, Seattle, WA, USA, 13–16 October 2016; Institute of Electrical and Electronics Engineers Inc.: New York, NY, USA, 2016; pp. 563–568. [Google Scholar] [CrossRef]
- Son, W.J.; Kang, S.G.; Seo, B.F.; Choi, N.-K. A Systematic Review of the National Breast Implant Registry for Application in Korea: Can We Predict “Unpredictable” Complications? Medicina 2020, 56, 370. [Google Scholar] [CrossRef]
- American Society of Plastic Surgeons. Plastic Surgery Statistics Report 2020—ASPS National Clearinghouse of Plastic Surgery Procedural Statistics; SPS: West Valley City, UT, USA, 2021; pp. 1–26. [Google Scholar]
- U. S. Food and Drug Administration. Risks and Complications of Breast Implants Breast Implants—Certain Labeling Recommendations to Improve; FDA; Silver Spring: Montgomery County, MD, USA, 2021; pp. 1–10. [Google Scholar]
- Vasey, F.B.; Zarabadi, S.A.; Seleznick, M.; Ricca, L. Where there’s smoke there’s fire: The silicone breast implant controversy continues to flicker: A new disease that needs to be defined. J. Rheumatol. 2003, 30, 2092–2094. [Google Scholar] [PubMed]
- Bouhadana, G.; Chocron, Y.; Azzi, A.J.; Davison, P.G. Perception of implants among breast reconstruction patients in montreal. Plast. Reconstr. Surg.—Glob. Open. 2020, 8, e3116. [Google Scholar] [CrossRef]
- Siling, Y.; Klietz, M.-L.; Harren, A.K.; Wei, Q.; Hirsch, T.; Aitzetmüller, M.M. Understanding Breast Implant Illness: Etiology is the Key. Aesthet. Surg. J. 2021, 42, 370–377. [Google Scholar] [CrossRef]
- Tang, S.Y.Q.; Israel, J.S.; Afifi, A.M. Breast Implant Illness: Symptoms, Patient Concerns, and the Power of Social Media. Plast. Reconstr. Surg. 2017, 140, 765e–766e. [Google Scholar] [CrossRef]
- Miseré, R.M.L.; van der Hulst, R.R.W.J. Self-Reported Health Complaints in Women Undergoing Explantation of Breast Implants. Aesthet. Surg. J. 2022, 42, 171–180. [Google Scholar] [CrossRef]
- U. S. Food and Drug Administration. FDA Executive Summary: Breast Implant Special Topics, Prepared for the Meeting of the General and Plastic Surgery Devices Advisory Panel; FDA; Silver Spring: Montgomery County, MD, USA, 2019; pp. 1–42. [Google Scholar]
- Magnusson, M.R.; Cooter, R.D.; Rakhorst, H.; McGuire, P.A.; Adams, W.P.; Deva, A.K. Breast Implant Illness: A Way Forward. Plast. Reconstr. Surg. 2019, 143, 74S–81S. [Google Scholar] [CrossRef]
- Rohrich, R.J.; Kaplan, J.; Dayan, E. Silicone Implant Illness: Science versus Myth? Plast. Reconstr. Surg. 2019, 144, 98–109. [Google Scholar] [CrossRef]
- Magnusson, M.R.; McGuire, P. Commentary on: Understanding Breast Implant Illness: Etiology is the Key. Aesthet. Surg. J. 2022, 42, 378–380. [Google Scholar] [CrossRef]
- Bachour, Y.; Bargon, C.A.; de Blok, C.J.M.; Ket, J.C.F.; Ritt, M.J.P.F.; Niessen, F.B. Risk factors for developing capsular contracture in women after breast implant surgery: A systematic review of the literature. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, e29–e48. [Google Scholar] [CrossRef]
- Jacobson, J.M.; Gatti, M.E.; Schaffner, A.D.; Hill, L.M.; Spear, S.L. Effect of incision choice on outcomes in primary breast augmentation. Aesthet. Surg. J. 2012, 32, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Chen, L.; Liu, W.; Mu, D.; Luan, J. Capsular Contracture Rate After Breast Augmentation with Periareolar Versus Other Two (Inframammary and Transaxillary) Incisions: A Meta-Analysis. Aesthet. Plast. Surg. 2018, 42, 32–37. [Google Scholar] [CrossRef]
- Blount, A.L.; Martin, M.D.; Lineberry, K.D.; Kettaneh, N.; Alfonso, D.R. Capsular contracture rate in a low-risk population after primary augmentation mammaplasty. Aesthet. Surg. J. 2013, 33, 516–521. [Google Scholar] [CrossRef]
- Wiener, T.C. Relationship of incision choice to capsular contracture. Aesthet. Plast. Surg. 2008, 32, 303–306. [Google Scholar] [CrossRef]
- Galdiero, M.; Larocca, F.; Iovene, M.R.; Francesca, M.; Pieretti, G.; D’Oriano, V.; Franci, G.; Ferraro, G.; D’Andrea, F.; Nicoletti, G.F. Microbial Evaluation in Capsular Contracture of Breast Implants. Plast. Reconstr. Surg. 2018, 141, 23–30. [Google Scholar] [CrossRef]
- Prantl, L.; Angele, P.; Schreml, S.; Ulrich, D.; Pöppl, N.; Eisenmann-Klein, M. Determination of serum fibrosis indexes in patients with capsular contracture after augmentation with smooth silicone gel implants. Plast. Reconstr. Surg. 2006, 118, 224–229. [Google Scholar] [CrossRef]
- Sood, A.; Xue, E.Y.; Sangiovanni, C.; Therattil, P.J.; Lee, E.S. Breast Massage, Implant Displacement, and Prevention of Capsular Contracture After Breast Augmentation With Implants: A Review of the Literature. Eplasty 2017, 17, e41. [Google Scholar]
- Wee, C.E.; Younis, J.; Isbester, K.; Smith, A.; Wangler, B.; Sarode, A.L.; Patil, N.; Grunzweig, K.; Boas, S.; Harvey, D.J.; et al. Understanding Breast Implant Illness, Before and After Explantation: A Patient-Reported Outcomes Study. Ann. Plast. Surg. 2020, 85, S82–S86. [Google Scholar] [CrossRef]
- Calobrace, M.B.; Schwartz, M.R.; Zeidler, K.R.; Pittman, T.A.; Cohen, R.; Stevens, W.G. Long-term safety of textured and smooth breast implants. Aesthet. Surg. J. 2018, 38, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Hammond, J.B.; Kosiorek, H.E.; Cronin, P.A.; Rebecca, A.M.; Casey, W.J.; Wong, W.W.; Vargas, C.E.; Vern-Gross, T.Z.; McGee, L.A.; Pockaj, B.A. Capsular contracture in the modern era: A multidisciplinary look at the incidence and risk factors after mastectomy and implant-based breast reconstruction. Am. J. Surg. 2021, 221, 1005–1010. [Google Scholar] [CrossRef]
- Stevens, W.G.; Nahabedian, M.Y.; Calobrace, M.B.; Harrington, J.L.; Capizzi, P.J.; Cohen, R.; D’incelli, R.C.; Beckstrand, M. Risk factor analysis for capsular contracture: A 5-year sientra study analysis using round, smooth, and textured implants for breast augmentation. Plast. Reconstr. Surg. 2013, 132, 1115–1123. [Google Scholar] [CrossRef]
- Jones, P.; Mempin, M.; Hu, H.; Chowdhury, D.; Foley, M.; Cooter, R.; Adams, W.P.; Vickery, K.; Deva, A.K. The functional influence of breast implant outer shell morphology on bacterial attachment and growth. Plast. Reconstr. Surg. 2018, 142, 837–849. [Google Scholar] [CrossRef]
- Munhoz, A.M.; Clemens, M.W.; Nahabedian, M.Y. Breast Implant Surfaces and Their Impact on Current Practices. Plast. Reconstr. Surg.—Glob. Open 2019, 7, e2466. [Google Scholar] [CrossRef] [Green Version]
- Luvsannyam, E.; Patel, D.; Hassan, Z.; Nukala, S.; Somagutta, M.R.; Hamid, P. Overview of Risk Factors and Prevention of Capsular Contracture Following Implant-Based Breast Reconstruction and Cosmetic Surgery: A Systematic Review. Cureus 2020, 12, e10341. [Google Scholar] [CrossRef] [PubMed]
- Mallucci, P.; Bistoni, G. The Use of Anatomic Implants in Aesthetic Breast Surgery. Clin. Plast. Surg. 2021, 48, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Vinci, V.; Domenico, C.; Luca, M.; Silvia, G.; Alessandra, V.; Barbara, C.; Valeria, B.; Andrea, B.; Mattia, S.; Federico, B.; et al. The evolution of breast prostheses. Breast J. 2020, 26, 1801–1804. [Google Scholar] [CrossRef]
- Mowlds, D.S.; Salibian, A.A.; Scholz, T.; Paydar, K.Z.; Wirth, G.A. Capsular contracture in implant-based breast reconstruction: Examining the role of acellular dermal matrix fenestrations. Plast. Reconstr. Surg. 2015, 136, 629–635. [Google Scholar] [CrossRef]
- Ajdic, D.; Zoghbi, Y.; Gerth, D.; Panthaki, Z.J.; Thaller, S. The relationship of bacterial biofilms and capsular contracture in breast implants. Aesthet. Surg. J. 2016, 36, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Shauly, O.; Gould, D.J.; Patel, K.M. Microtexture and the cell/biomaterial interface: A systematic review and meta-analysis of capsular contracture and prosthetic breast implants. Aesthet. Surg. J. 2019, 39, 603–614. [Google Scholar] [CrossRef]
- Turner, S.D.; Inghirami, G.; Miranda, R.N.; Kadin, M.E. Cell of Origin and Immunologic Events in the Pathogenesis of Breast Implant–Associated Anaplastic Large-Cell Lymphoma. Am. J. Pathol. 2020, 190, 2–10. [Google Scholar] [CrossRef] [Green Version]
- Swanson, E. The Food and Drug Administration Bans Biocell Textured Breast Implants: Lessons for Plastic Surgeons. Ann. Plast. Surg. 2020, 84, 343–345. [Google Scholar] [CrossRef]
- McKernan, C.D.; Vorstenbosch, J.; Chu, J.J.; Nelson, J.A. Breast Implant Safety: An Overview of Current Regulations and Screening Guidelines. J. Gen. Intern. Med. 2022, 37, 212–216. [Google Scholar] [CrossRef]
- Johal, K.S.; Floyd, D. To bloc or not to bloc: Challenges in the management of patients requesting “En-Bloc capsulectomy”. Aesthet. Surg. J. 2020, 40, NP561–NP563. [Google Scholar] [CrossRef]
- Keane, G.; Chi, D.; Ha, A.Y.; Myckatyn, T.M. En Bloc Capsulectomy for Breast Implant Illness: A Social Media Phenomenon? Aesthet. Surg. J. 2021, 41, 448–459. [Google Scholar] [CrossRef]
- Swanson, E. Evaluating the necessity of capsulectomy in cases of textured breast implant replacement. Ann. Plast. Surg. 2020, 85, 691–698. [Google Scholar] [CrossRef]
- Abi-Rafeh, J.; Safran, T.; Winocour, S.; Dionisopoulos, T.; Davison, P.; Vorstenbosch, J. Lack of Evidence on Complication Profile of Breast Implant Capsulectomy: A Call to Action for Plastic Surgeons. Plast. Reconstr. Surg. 2021, 148, 157e–158e. [Google Scholar] [CrossRef]
- Venkatesan, N.; Perumal, G.; Doble, M. Bacterial resistance in biofilm-associated bacteria. Future Microbiol. 2015, 10, 1743–1750. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Park, M.D.; Otto, M. Host response to Staphylococcus epidermidis colonization and infections. Front. Cell. Infect. Microbiol. 2017, 7, 90. [Google Scholar] [CrossRef] [Green Version]
- Beam, E.; Osmon, D. Prosthetic Joint Infection Update. Infect. Dis. Clin. N. Am. 2018, 32, 843–859. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, C.; Bellingeri, A.; Carville, K.; Garten, A.; Woo, K. A route to more effective infection management: The Infection Management Pathway. Wounds Int. 2020, 11, 50–57. [Google Scholar]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial adherence and biofilm formation on medical implants: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2014, 228, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Moure, J.S.; Mydlowska, A.; Shin, C.; Vella, M.; Kaplan, L.J. Nanometric Considerations in Biofilm Formation. Surg. Infect. 2019, 20, 167–173. [Google Scholar] [CrossRef]
- Achermann, Y.; Goldstein, E.J.C.; Coenye, T.; Shirtliffa, M.E. Propionibacterium acnes: From Commensal to opportunistic biofilm-associated implant pathogen. Clin. Microbiol. Rev. 2014, 27, 419–440. [Google Scholar] [CrossRef] [Green Version]
- James, G.A.; Boegli, L.; Hancock, J.; Bowersock, L.; Parker, A.; Kinney, B.M. Bacterial Adhesion and Biofilm Formation on Textured Breast Implant Shell Materials. Aesthet. Plast. Surg. 2019, 43, 490–497. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, P.H.; Teitler, N.A.; Hon, H.H.; Miller, J.J. Breast Implant Illness and Cutibacterium acnes: A Case Report. Plast. Reconstr. Surg.—Glob. Open 2022, 10, E4146. [Google Scholar] [CrossRef]
- Mempin, M.; Hu, H.; Chowdhury, D.; Deva, A.; Vickery, K. The A, B and C’s of silicone breast implants: Anaplastic large cell lymphoma, biofilm and capsular contracture. Materials 2018, 11, 2393. [Google Scholar] [CrossRef] [Green Version]
- Giordano, S.; Peltoniemi, H.; Lilius, P.; Salmi, A. Povidone-iodine combined with antibiotic topical irrigation to reduce capsular contracture in cosmetic breast augmentation: A comparative study. Aesthet. Surg. J. 2013, 33, 675–680. [Google Scholar] [CrossRef] [Green Version]
- Carvajal, J.; Carvajal, M.; Hernández, G. Back to Basics: Could the Preoperative Skin Antiseptic Agent Help Prevent Biofilm-Related Capsular Contracture? Aesthet. Surg. J. 2019, 39, 848–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yalanis, G.C.; Liu, E.W.; Cheng, H.T. Efficacy and safety of povidone-iodine irrigation in reducing the risk of capsular contracture in aesthetic breast augmentation: A systematic review and meta-analysis. Plast. Reconstr. Surg. 2015, 136, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Ponraja, G.; McLeod, K.; Chong, S. Breast Implant Illness: A Biofilm Hypothesis. Plast. Reconstr. Surg.—Glob. Open 2020, 8, e2755. [Google Scholar] [CrossRef] [PubMed]
- Loch-Wilkinson, A.; Beath, K.J.; Knight, R.J.W.; Wessels, W.L.F.; Magnusson, M.; Papadopoulos, T.; Connell, T.; Lofts, J.; Locke, M.; Hopper, I.; et al. Breast implant-associated anaplastic large cell lymphoma in Australia and New Zealand: High-surface-area textured implants are associated with increased risk. Plast. Reconstr. Surg. 2017, 140, 645–654. [Google Scholar] [CrossRef]
- Rieger, U.M.; Pierer, G.; Lüscher, N.J.; Trampuz, A. Sonication of removed breast implants for improved detection of subclinical infection. Aesthet. Plast. Surg. 2009, 33, 404–408. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, P.; Fink, B.; Sandow, D.; Margull, A.; Berger, I.; Frommelt, L. Prolonged bacterial culture to identify late periprosthetic joint infection: A promising strategy. Clin. Infect. Dis. 2008, 47, 1403–1409. [Google Scholar] [CrossRef] [Green Version]
- Portillo, M.E.; Salvadó, M.; Alier, A.; Martínez, S.; Sorli, L.; Horcajada, J.P.; Puig, L. Advantages of sonication fluid culture for the diagnosis of prosthetic joint infection. J. Infect. 2014, 69, 35–41. [Google Scholar] [CrossRef]
- Miseré, R.; Maartje, C.; van der Hulst, R.R.W.J. The Prevalence of Self-Reported Health Complaints and Health-Related Quality of Life in Women With Breast Implants. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2021, 41, 661–668. [Google Scholar] [CrossRef]
- Shoenfeld, Y.; Agmon-Levin, N. “ASIA”—Autoimmune/inflammatory syndrome induced by adjuvants. J. Autoimmun. 2011, 36, 4–8. [Google Scholar] [CrossRef]
- Watad, A.; Rosenberg, V.; Tiosano, S.; Tervaert, J.W.C.; Yavne, Y.; Shoenfeld, Y.; Shalev, V.; Chodick, G.; Amital, H. Silicone breast implants and the risk of autoimmune/rheumatic disorders: A real-world analysis. Int. J. Epidemiol. 2018, 47, 1846–1854. [Google Scholar] [CrossRef]
- Moraitis, S.D.; Agrafiotis, A.C.; Kapranou, A.; Kanakakis, K. Mediastinal silicone lymphadenopathy revealed after thymectomy for autoimmune myasthenia gravis. Monaldi Arch. Chest Dis. 2018, 88, 83–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barsky, A.J.; Borus, J.F. Functional somatic syndromes. Ann. Intern. Med. 1999, 130, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Vera-Lastra, O.; Medina, G.; Cruz-Dominguez, M.D.P.; Jara, L.J.; Shoenfeld, Y. Autoimmune/inflammatory syndrome induced by adjuvants (Shoenfeld’s syndrome): Clinical and immunological spectrum. Expert Rev. Clin. Immunol. 2013, 9, 361–373. [Google Scholar] [CrossRef]
- Maijers, M.C.; de Blok, C.J.M.; Niessen, F.B.; van der Veldt, A.A.M.; Ritt, M.J.P.F.; Winters, H.A.H.; Kramer, M.H.H.; Nanayakkara, P.W.B. Women with silicone breast implants and unexplained systemic symptoms: A descriptive cohort study. Neth. J. Med. 2014, 71, 534–540. [Google Scholar]
- Gadarowski, M.B.; Pukhalskaya, T.; Farah, R.; Smoller, B.R. Acquired anhidrosis in a patient with Sjogren syndrome and silicone breast implants. JAAD Case Rep. 2020, 6, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Akyol, L.; Onem, S.; Ozgen, M.; Sayarlioglu, M. Sjögren’s syndrome after silicone breast implantation. Eur. J. Rheumatol. 2015, 2, 165–166. [Google Scholar] [CrossRef]
- Jara, L.J.; Medina, G.; Gómez-Bañuelos, E.; Saavedra, M.A.; Vera-Lastra, O. Still’s disease, lupus-like syndrome, and silicone breast implants. A case of “ASIA” (Shoenfeld’s syndrome). Lupus 2012, 21, 140–145. [Google Scholar] [CrossRef]
- Lappe, M.A. Silicone-reactive disorder: A new autoimmune disease caused by immunostimulation and superantigens. Med. Hypotheses 1993, 41, 348–352. [Google Scholar] [CrossRef]
- Tervaert, J.W.C.; Kappel, R.M. Silicone implant incompatibility syndrome (SIIS): A frequent cause of ASIA (Shoenfeld’s syndrome). Immunol. Res. 2013, 56, 293–298. [Google Scholar] [CrossRef]
- Fuzzard, S.K.; Teixeira, R.; Zinn, R. A Review of the Literature on the Management of Silicone Implant Incompatibility Syndrome. Aesthet. Plast. Surg. 2019, 43, 1145–1149. [Google Scholar] [CrossRef]
- Khoo, T.; Proudman, S.; Limaye, V. Silicone breast implants and depression, fibromyalgia and chronic fatigue syndrome in a rheumatology clinic population. Clin. Rheumatol. 2019, 38, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Colaris, M.J.L.; de Boer, M.; van der Hulst, R.R.; Tervaert, J.W.C. Two hundreds cases of ASIA syndrome following silicone implants: A comparative study of 30 years and a review of current literature. Immunol. Res. 2017, 65, 120–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felger, J.C.; Miller, A.H. Cytokine effects on the basal ganglia and dopamine function: The subcortical source of inflammatory malaise. Front. Neuroendocrinol. 2012, 33, 315–327. [Google Scholar] [CrossRef] [Green Version]
- Tervaert, J.W.C.; Colaris, M.J.; van der Hulst, R.R. Silicone breast implants and autoimmune rheumatic diseases: Myth or reality. Curr. Opin. Rheumatol. 2017, 29, 348–354. [Google Scholar] [CrossRef] [PubMed]
- de Faria Castro Fleury, E.; Rêgo, M.M.; Ramalho, L.C.; Ayres, V.J.; Seleti, R.O.; Ferreira, C.A.P.; Roveda, D., Jr. Silicone-induced granuloma of breast implant capsule (SIGBIC): Similarities and differences with anaplastic large cell lymphoma (ALCL) and their differential diagnosis. Breast Cancer Targets Ther. 2017, 9, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Faria Castro Fleury, E.; D’Alessandro, G.S.; Wludarski, S.C.L. Silicone-induced granuloma of breast implant capsule (SIGBIC): Histopathology and radiological correlation. J. Immunol. Res. 2018, 2018, 6784971. [Google Scholar] [CrossRef] [Green Version]
- Caravantes-Cortes, M.I.; Roldan-Valadez, E.; Zwojewski-Martinez, R.D.; Salazar-Ruiz, S.Y.; Carballo-Zarate, A.A. Breast Prosthesis Syndrome: Pathophysiology and Management Algorithm. Aesthet. Plast. Surg. 2020, 44, 1423–1437. [Google Scholar] [CrossRef]
- Fleury, E.d.C.; Gianini, A.C.; Ayres, V.; Ramalho, L.C.; Seleti, R.O.; Roveda, D. Breast magnetic resonance imaging: Tips for the diagnosis of silicone-induced granuloma of a breast implant capsule (SIGBIC). Insights Imaging 2017, 8, 439–446. [Google Scholar] [CrossRef]
- Favi, P.M.; Valencia, M.M.; Elliott, P.R.; Restrepo, A.; Gao, M.; Huang, H.; Pavon, J.J.; Webster, T.J. Shape and surface chemistry effects on the cytotoxicity and cellular uptake of metallic nanorods and nanospheres. J. Biomed. Mater. Res.—Part A 2015, 103, 3940–3955. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; de Haan, L.H.J.; Evers, N.M.; Jiang, X.; Marcelis, A.T.M.; Zuilhof, H.; Rietjens, I.M.C.M.; Alink, G.M. Role of surface charge and oxidative stress in cytotoxicity of organic monolayer-coated silicon nanoparticles towards macrophage NR8383 cells. Part. Fibre Toxicol. 2010, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Tervaert, J.W.C.; Mohazab, N.; Redmond, D.; van Eeden, C.; Osman, M. Breast implant illness: Scientific evidence of its existence. Expert Rev. Clin. Immunol. 2022, 18, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Katzin, W.E.; Centeno, J.A.; Feng, L.J.; Kiley, M.; Mullick, F.G. Pathology of lymph nodes from patients with breast implants: A histologic and spectroscopic evaluation. Am. J. Surg. Pathol. 2005, 29, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Ganau, S.; Tortajada, L.; Rodríguez, X.; González, G.; Sentís, M. Silicone lymphadenopathy: An unusual cause of internal mammary lymph node enlargement. Breast J. 2008, 14, 502–503. [Google Scholar] [CrossRef] [PubMed]
- Soudack, M.; Yelin, A.; Simansky, D.; Ben-Nun, A. Fluorodeoxyglucose-positive internal mammary lymph node in breast cancer patients with silicone implants: Is it always metastatic cancer? Eur. J. Cardio-Thoracic Surg. 2013, 44, 79–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grubstein, A.; Cohen, M.; Steinmetz, A.; Cohen, D. Siliconomas mimicking cancer. Clin. Imaging 2011, 35, 228–231. [Google Scholar] [CrossRef]
- Cohen Tervaert, J.W. Autoinflammatory/autoimmunity syndrome induced by adjuvants (ASIA; Shoenfeld’s syndrome): A new flame. Autoimmun. Rev. 2018, 17, 1259–1264. [Google Scholar] [CrossRef]
- Coroneos, C.J.; Selber, J.C.; Offodile, A.C.; Butler, C.E.; Clemens, M.W. US FDA Breast Implant Postapproval Studies: Long-term Outcomes in 99,993 Patients. Ann. Surg. 2019, 269, 30–36. [Google Scholar] [CrossRef]
- Hortolam, J.G.; de Carvalho, J.F.; Appenzeller, S. Connective tissue diseases following silicone breast implantation: Where do we stand? Clinics 2013, 68, 281. [Google Scholar] [CrossRef]
- Dijkman, H.B.P.M.; Slaats, I.; Bult, P. Assessment of Silicone Particle Migration among Women Undergoing Removal or Revision of Silicone Breast Implants in the Netherlands. JAMA Netw. Open 2021, 4, e2125381. [Google Scholar] [CrossRef]
- Karlson, E.W.; Lee, I.M.; Cook, N.R.; Manson, J.A.E.; Buring, J.E.; Hennekens, C.H. Comparison of self-reported diagnosis of connective tissue disease with medical records in female health professionals. The women’s health cohort study. Am. J. Epidemiol. 1999, 150, 652–660. [Google Scholar] [CrossRef] [Green Version]
- Valente, D.S.; Zanella, R.K.; Mulazzani, C.M.; Valente, S.S. Risk Factors for Explantation of Breast Implants: A Cross-Sectional Study. Aesthet. Surg. J. 2021, 41, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Virevialle, C.; Labbé, A.; Dupont-Monod, S.; Parent, F.; Baudouin, C. Can breast implants be responsible for dry eye? Eye 2014, 28, 633–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teuber, S.S.; Howell, L.P.; Yoshida, S.H.; Gershwin, M.E. Remission of sarcoidosis following removal of silicone gel breast implants. Int. Arch. Allergy Immunol. 1994, 105, 404–407. [Google Scholar] [CrossRef] [PubMed]
- de Boer, M.; Colaris, M.; van der Hulst, R.R.W.J.; Tervaert, J.W.C. Is explantation of silicone breast implants useful in patients with complaints? Immunol. Res. 2017, 65, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Rohrich, R.J.; Kenkel, J.M.; Adams, W.P.; Beran, S.; Conner, W.C.H. A prospective analysis of patients undergoing silicone breast implant explantation. Plast. Reconstr. Surg. 2000, 105, 2529–2537. [Google Scholar] [CrossRef]
- Vasey, F.B.; Havice, D.L.; Bocanegra, T.S.; Seleznick, M.J.; Bridgeford, P.H.; Martinez-Osuna, P.; Espinoza, L.R. Clinical findings in symptomatic women with silicone breast implants. Semin. Arthritis Rheum. 1994, 24, 22–28. [Google Scholar] [CrossRef]
- Aziz, N.; Vasey, F.; Leaverton, P. Comparison of clinical status among women retaining or removing gel breast implants. Am. J. Epidemiol. 1997, 145, 191. [Google Scholar]
- Thomas, W., 3rd; Harper, L.; Wong, S.; Michalski, J.; Harris, C.; Moore, J.; Rodning, C. Explantation of silicone breast implants. Am. Surg. 1997, 63, 421–429. [Google Scholar]
- Kappel, R.M.; Pruijn, G.J.M. The monobloc hydrogel breast implant, experiences and ideas. Eur. J. Plast. Surg. 2012, 35, 229–233. [Google Scholar] [CrossRef] [Green Version]
- Svahn, J.; Vastine, V.; Landon, B.; Dobke, M. Outcome of mammary prostheses explantation: A patient perspective. Ann. Plast. Surg. 1996, 36, 594–600. [Google Scholar] [CrossRef]
- Godfrey, P.; Godfrey, N. Response of locoregional and systemic symptoms to breast implant replacement with autologous tissues: Experience in 37 consecutive patients. Plast. Reconstr. Surg. 1996, 97, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Peters, W.; Smith, D.; Fornasier, V.; Lugowski, S.; Ibanez, D. An outcome analysis of 100 women after explantation of silicone gel breast implants. Ann. Plast. Surg. 1997, 39, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Picha, G.J.; Hardas, B.; Schumacher, A.; Murphy, D.K. Five-year safety data for more than 55,000 subjects following breast implantation: Comparison of rare adverse event rates with silicone implants versus national norms and saline implants. Plast. Reconstr. Surg. 2017, 140, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Santiago, E.A.; de Paula, I.B. Autoimmune/Inflammatory Syndrome Induced by Adjuvants (Asia Syndrome) Associated with Silicone Breast Implant Rupture. Arch. Breast Cancer 2021, 8, 156–161. [Google Scholar] [CrossRef]
- Jara, L.J.; García-Collinot, G.; Medina, G.; Cruz-Dominguez, M.d.; Vera-Lastra, O.; Carranza-Muleiro, R.A.; Saavedra, M.A. Severe manifestations of autoimmune syndrome induced by adjuvants (Shoenfeld’s syndrome). Immunol. Res. 2017, 65, 8–16. [Google Scholar] [CrossRef]
- Barbosa, M.R.; Makris, U.E.; Mansi, I.A. Association of Breast Implants with Nonspecific Symptoms, Connective Tissue Diseases, and Allergic Reactions: A Retrospective Cohort Analysis. Plast. Reconstr. Surg. 2021, 147, 42E–49E. [Google Scholar] [CrossRef]
- Fleury, E.d.C.; Bernal, K.J.H.; Madeiro, A.L.M.; Ocana, W.L.C.; Fleury, J.C.V.; Caobianco, L. Side effects in breast implants related to radiotherapy in breast cancer reconstructive surgery. Tech. Innov. Patient Support Radiat. Oncol. 2021, 18, 8–11. [Google Scholar] [CrossRef]
- Roy, P.G.; Yan, Z.; Nigam, S.; Maheshwari, K. Aesthetic breast surgery: Putting in context—a narrative review. Gland Surg. 2021, 10, 2832–2846. [Google Scholar] [CrossRef]
- Miseré, R.M.; van Kuijk, S.M.; Claassens, E.L.; Heuts, E.M.; Piatkowski, A.A.; van der Hulst, R.R. Breast-related and body-related quality of life following autologous breast reconstruction is superior to implant-based breast reconstruction—A long-term follow-up study. Breast 2021, 59, 176–182. [Google Scholar] [CrossRef]
- Susarla, S.M.; Ganske, I.; Helliwell, L.; Morris, D.; Eriksson, E.; Chun, Y.S. Comparison of clinical outcomes and patient satisfaction in immediate single-stage versus two-stage implant-based breast reconstruction. Plast. Reconstr. Surg. 2015, 135, 1e–8e. [Google Scholar] [CrossRef]
- Rodriguez-Feliz, J.; Codner, M.A. Embrace the change: Incorporating single-stage implant breast reconstruction into your practice. Plast. Reconstr. Surg. 2015, 136, 221–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.K.; Sheckter, C.C. Breast Reconstruction Following Breast Cancer Treatment—2018. JAMA 2018, 320, 1277. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.L.; Bovill, E.S.; Macadam, S.A.; Tyldesley, S.; Giang, J.; Lennox, P.A. Postmastectomy Radiation Therapy after Immediate Two-Stage Tissue Expander/Implant Breast Reconstruction. Plast. Reconstr. Surg. 2014, 134, 1e–10e. [Google Scholar] [CrossRef] [PubMed]
- Magill, L.J.; Ricketts, K.; Keshtgar, M.; Mosahebi, A.; Jell, G. Impact of post mastectomy radiotherapy on the silicone breast implant. Mater. Sci. Eng. C 2019, 98, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Ricci, J.A.; Epstein, S.; Momoh, A.O.; Lin, S.J.; Singhal, D.; Lee, B.T. A meta-analysis of implant-based breast reconstruction and timing of adjuvant radiation therapy. J. Surg. Res. 2017, 218, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Fleury, E.D.F.C. Silicone Induced Granuloma of Breast Implant Capsule (SIGBIC) diagnosis: Breast Magnetic Resonance (BMR) sensitivity to detect silicone bleeding. PLoS ONE 2020, 15, e0235050. [Google Scholar] [CrossRef]
- Kaplan, J.; Rohrich, R. Breast implant illness: A topic in review. Gland Surg. 2021, 10, 430–443. [Google Scholar] [CrossRef]
- Fleury, E.; Nimir, C.; D’alessandro, G.S. The breast tumor microenvironment: Could silicone breast implant elicit breast carcinoma? Breast Cancer Targets Ther. 2021, 13, 45–58. [Google Scholar] [CrossRef]
- Adidharma, W.; Latack, K.R.; Colohan, S.M.; Morrison, S.D.; Cederna, P.S. Breast Implant Illness: Are Social Media and the Internet Worrying Patients Sick? Plast. Reconstr. Surg. 2020, 145, 225e–227e. [Google Scholar] [CrossRef]
- Tian, W.M.; Rames, J.D.; Blau, J.A.; Taskindoust, M.; Hollenbeck, S.T. Contextualizing Breast Implant Removal Patterns with Google Trends: Big Data Applications in Surgical Demand. Plast. Reconstr. Surg.—Glob. Open 2022, 10, e4005. [Google Scholar] [CrossRef]
- Cole, N.M. Consequences of the U.S. Food and Drug Administration-Directed Moratorium on Silicone Gel Breast Implants: 1992 to 2006. Plast. Reconstr. Surg. 2018, 141, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Kricheldorff, J.; Fallenberg, E.M.; Solbach, C.; Gerber-Schäfer, C.; Rancsó, C.; von Fritschen, U. Breast Implant-Associated Lymphoma The Diagnosis and Treatment of a New Disease Entity. Dtsch. Arztebl. Int. 2018, 115, 625–635. [Google Scholar] [CrossRef]
- Kaderbhai, A.; Broomfield, A.; Cuss, A.; Shaw, K.; Deva, A.K. Breast implants: A guide for general practice. Aust. J. Gen. Pract. 2021, 50, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, P.; Tay, V. Transitioning from Conventional Textured to Nanotextured Breast Implants: Our Early Experience and Modifications for Optimal Breast Augmentation Outcomes. Aesthet. Surg. J. 2021, 41, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Asaad, M.; Offodile, A.C.; di Pompeo, F.S.; Bevers, T.B.; Stelly, S.; Carew, L.A.; Barnea, Y.; Miranda, R.N.; Butler, C.E.; Clemens, M.W. Management of Symptomatic Patients with Textured Implants. Plast. Reconstr. Surg. 2021, 147, 58S–68S. [Google Scholar] [CrossRef] [PubMed]
- Groth, A.K.; Graf, R. Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) and the Textured Breast Implant Crisis. Aesthet. Plast. Surg. 2020, 44, 1–12. [Google Scholar] [CrossRef]
- Limberger, G.M.; Esteves, K.P.; Halal, L.M.; Nery, L.E.M.; da Fonseca, D.B. Chronic immune challenge is detrimental to female survival, feeding behavior, and reproduction in the field cricket Gryllus assimilis (Fabricius, 1775). J. Comp. Physiol. B 2022, 2022, 1–12. [Google Scholar] [CrossRef]
- Atiyeh, B.; Enisieh, S. Breast Implant Illness (BII): Real Syndrome or a Social MediaPhenomenon? A Narrative Review of the Literature. Aesthet. Plast. Surg. 2021, 46, 43–57. [Google Scholar] [CrossRef]
- Mcguire, P.A.; Haws, M.J.; Nahai, F. Breast Implant Illness: How Can We Help? Aesthet. Surg. J. 2019, 39, 1260–1263. [Google Scholar] [CrossRef]
- Trinidad-Calderón, P.A.; López-Castillo, L.M.; Gallegos-Martínez, S.; Trujillo-de Santiago, G.; García-Lara, S.; Álvarez, M.M. nurP28, a New-to-Nature Zein-Derived Peptide, Enhances the Therapeutic Effect of Docetaxel in Breast Cancer Monolayers and Spheroids. Molecules 2022, 27, 2824. [Google Scholar] [CrossRef]
- Logothetis, M.L. Women’s Reports of Breast Implant Problems and Silicone-Related Illness. J. Obstet. Gynecol. Neonatal Nurs. 1995, 24, 609–616. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varela-Chinchilla, C.D.; Salinas-McQuary, G.; Segura-Azuara, N.d.l.Á.; Trinidad-Calderón, P.A. Breast Implant Illness: Surgical, Autoimmune, and Breast Reconstruction Associations. Surgeries 2022, 3, 111-125. https://doi.org/10.3390/surgeries3020013
Varela-Chinchilla CD, Salinas-McQuary G, Segura-Azuara NdlÁ, Trinidad-Calderón PA. Breast Implant Illness: Surgical, Autoimmune, and Breast Reconstruction Associations. Surgeries. 2022; 3(2):111-125. https://doi.org/10.3390/surgeries3020013
Chicago/Turabian StyleVarela-Chinchilla, Carlos Daniel, Gabriel Salinas-McQuary, Nancy de los Ángeles Segura-Azuara, and Plinio A. Trinidad-Calderón. 2022. "Breast Implant Illness: Surgical, Autoimmune, and Breast Reconstruction Associations" Surgeries 3, no. 2: 111-125. https://doi.org/10.3390/surgeries3020013
APA StyleVarela-Chinchilla, C. D., Salinas-McQuary, G., Segura-Azuara, N. d. l. Á., & Trinidad-Calderón, P. A. (2022). Breast Implant Illness: Surgical, Autoimmune, and Breast Reconstruction Associations. Surgeries, 3(2), 111-125. https://doi.org/10.3390/surgeries3020013






