[18F]-FDG PET/CT of the Pulmonary Benign Metastasizing Leiomyoma in a Breast Cancer Patient: A Case Report
Abstract
1. Introduction
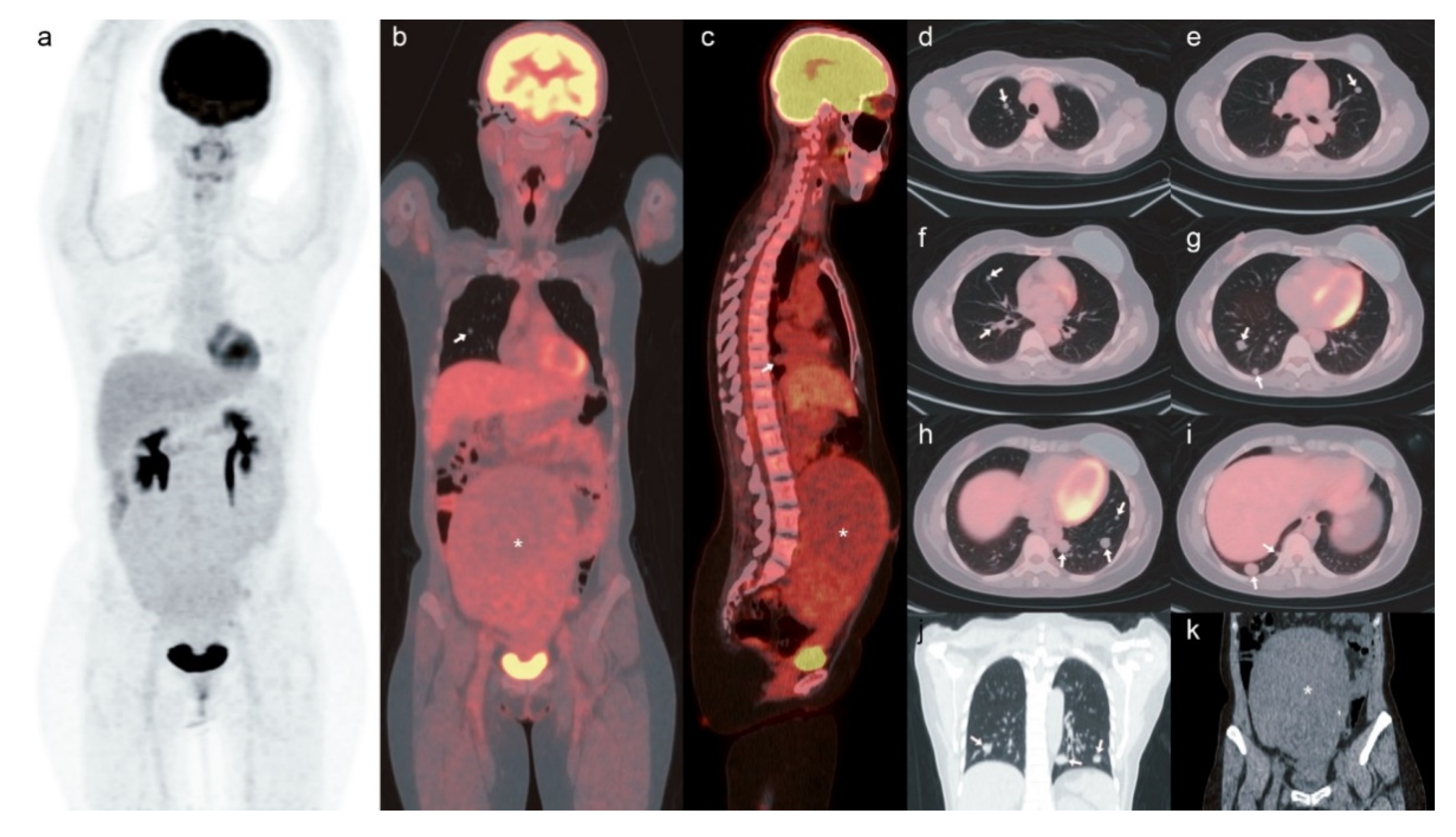
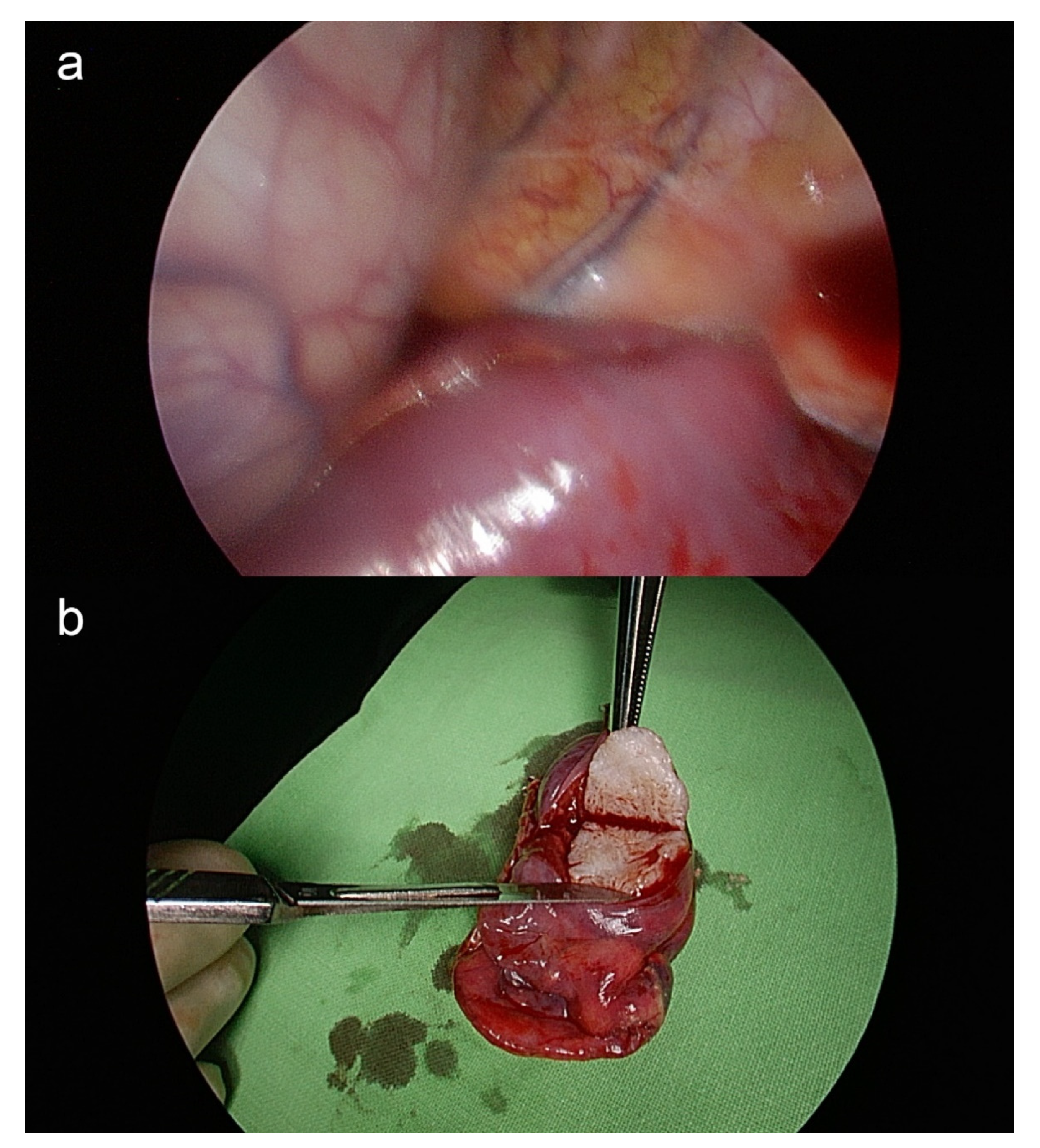
2. Case
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pacheco-Rodriguez, G.; Taveira-DaSilva, A.M.; Moss, J. Benign Metastasizing Leiomyoma. Clin. Chest Med. 2016, 37, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, B.; Allan, A.L. Molecular Mechanisms of Breast Cancer Metastasis to the Lung: Clinical and Experimental Perspectives. Int. J. Mol. Sci. 2019, 20, 2272. [Google Scholar] [CrossRef] [PubMed]
- Abbott, G.F.; Vlahos, I. CT Diagnosis and Management of Focal Lung Disease. In Diseases of the Chest, Breast, Heart and Vessels 2019–2022: Diagnostic and Interventional Imaging; Hodler, J., Kubik-Huch, R.A., von Schulthess, G.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 47–55. [Google Scholar]
- Asumu, H.; Estrin, Y.; Mohammed, T.L.; Verma, N. Benign Metastasizing Leiomyoma. Curr. Probl. Diagn. Radiol. 2017, 46, 257–259. [Google Scholar] [CrossRef]
- Nucci, M.R.; Drapkin, R.; Dal Cin, P.; Fletcher, C.D.; Fletcher, J.A. Distinctive cytogenetic profile in benign metastasizing leiomyoma: Pathogenetic implications. Am. J. Surg. Pathol. 2007, 31, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Sun, Y.; Jin, Y.; Xu, L.; Dai, H.; Wang, J.; Zhang, Z.; Chen, X. Multiple organ benign metastasizing leiomyoma: A case report and literature review. J. Obstet. Gynaecol. Res. 2019, 45, 2132–2136. [Google Scholar] [CrossRef]
- Barnaś, E.; Książek, M.; Raś, R.; Skręt, A.; Skręt-Magierło, J.; Dmoch-Gajzlerska, E. Benign metastasizing leiomyoma: A review of current literature in respect to the time and type of previous gynecological surgery. PLoS ONE 2017, 12, e0175875. [Google Scholar] [CrossRef] [PubMed]
- Awonuga, A.O.; Shavell, V.I.; Imudia, A.N.; Rotas, M.; Diamond, M.P.; Puscheck, E.E. Pathogenesis of benign metastasizing leiomyoma: A review. Obstet. Gynecol. Surv. 2010, 65, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Yoon, G.; Lee, J.-S.; Han, J.; Song, S.Y.; Kim, B.-G.; Bae, D.-S.; Lee, J.-W. Pulmonary benign metastasizing leiomyoma: Clinical and therapeutic analyses of 11 patients treated at a single institution. Int. J. Clin. Exp. Med. 2016, 9, 19654–19663. [Google Scholar]
- Sawai, Y.; Shimizu, T.; Yamanaka, Y.; Niki, M.; Nomura, S. Benign metastasizing leiomyoma and 18-FDG-PET/CT: A case report and literature review. Oncol. Lett. 2017, 14, 3641–3646. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wei, W.T.; Chen, P.C. Benign metastasizing leiomyoma of the lung: A case report and literature review. Oncol. Lett. 2015, 10, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Zong, D.; He, W.; Li, J.; Peng, H.; Chen, P.; Ouyang, R. Concurrent benign metastasizing leiomyoma in the lung and lumbar spine with elevated standardized uptake value level in positron-emission tomography computed tomography: A case report and literature review. Medicine 2018, 97, e11334. [Google Scholar] [CrossRef] [PubMed]
- Abu Saadeh, F.; Riain, C.O.; Cormack, C.M.; Gleeson, N. Lung metastases from benign uterine leiomyoma: Does 18-FDG-PET/CT have a role to play? Ir. J. Med. Sci. 2019, 188, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Kiyokawa, H.; Omiya, H.; Takami, K.; Sekimoto, M.; Mori, K. Metastasizing Leiomyoma of the Lung Detected on Chest X-ray after Surgery for Breast Cancer; Report of a Case. Kyobu Geka 2015, 68, 1103–1106. [Google Scholar] [PubMed]
- Ishibashi, H.; Ohta, S.; Hirose, M.; Nakada, C.; Arai, K.; Etou, H. Pulmonary benign metastasizing leiomyoma from uterine myoma; report of a case. Kyobu Geka 2006, 59, 951–954. [Google Scholar] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kao, C.-H.; Gao, H.-W.; Ko, K.-H.; Liao, G.-S.; Tsai, C.-J. [18F]-FDG PET/CT of the Pulmonary Benign Metastasizing Leiomyoma in a Breast Cancer Patient: A Case Report. Surgeries 2021, 2, 231-236. https://doi.org/10.3390/surgeries2030023
Kao C-H, Gao H-W, Ko K-H, Liao G-S, Tsai C-J. [18F]-FDG PET/CT of the Pulmonary Benign Metastasizing Leiomyoma in a Breast Cancer Patient: A Case Report. Surgeries. 2021; 2(3):231-236. https://doi.org/10.3390/surgeries2030023
Chicago/Turabian StyleKao, Chun-Hao, Hong-Wei Gao, Kai-Hsiung Ko, Guo-Shiou Liao, and Chi-Jung Tsai. 2021. "[18F]-FDG PET/CT of the Pulmonary Benign Metastasizing Leiomyoma in a Breast Cancer Patient: A Case Report" Surgeries 2, no. 3: 231-236. https://doi.org/10.3390/surgeries2030023
APA StyleKao, C.-H., Gao, H.-W., Ko, K.-H., Liao, G.-S., & Tsai, C.-J. (2021). [18F]-FDG PET/CT of the Pulmonary Benign Metastasizing Leiomyoma in a Breast Cancer Patient: A Case Report. Surgeries, 2(3), 231-236. https://doi.org/10.3390/surgeries2030023





