The Transverse Process as a Landmark for Estimating Dural Sac Depth and Feasible Planes for Optimized Paramedian Needle Insertions †
Abstract
1. Introduction
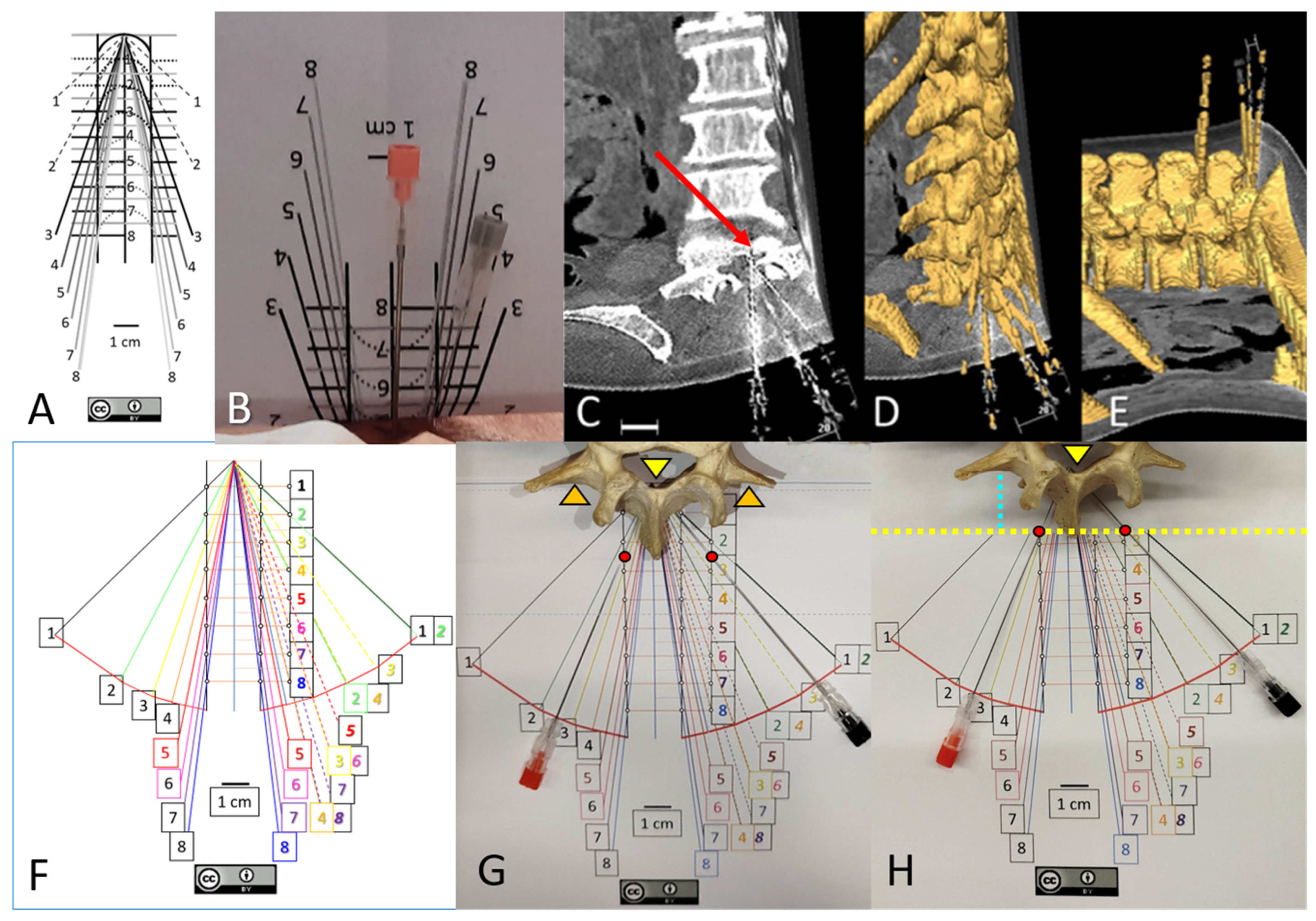
2. Methods
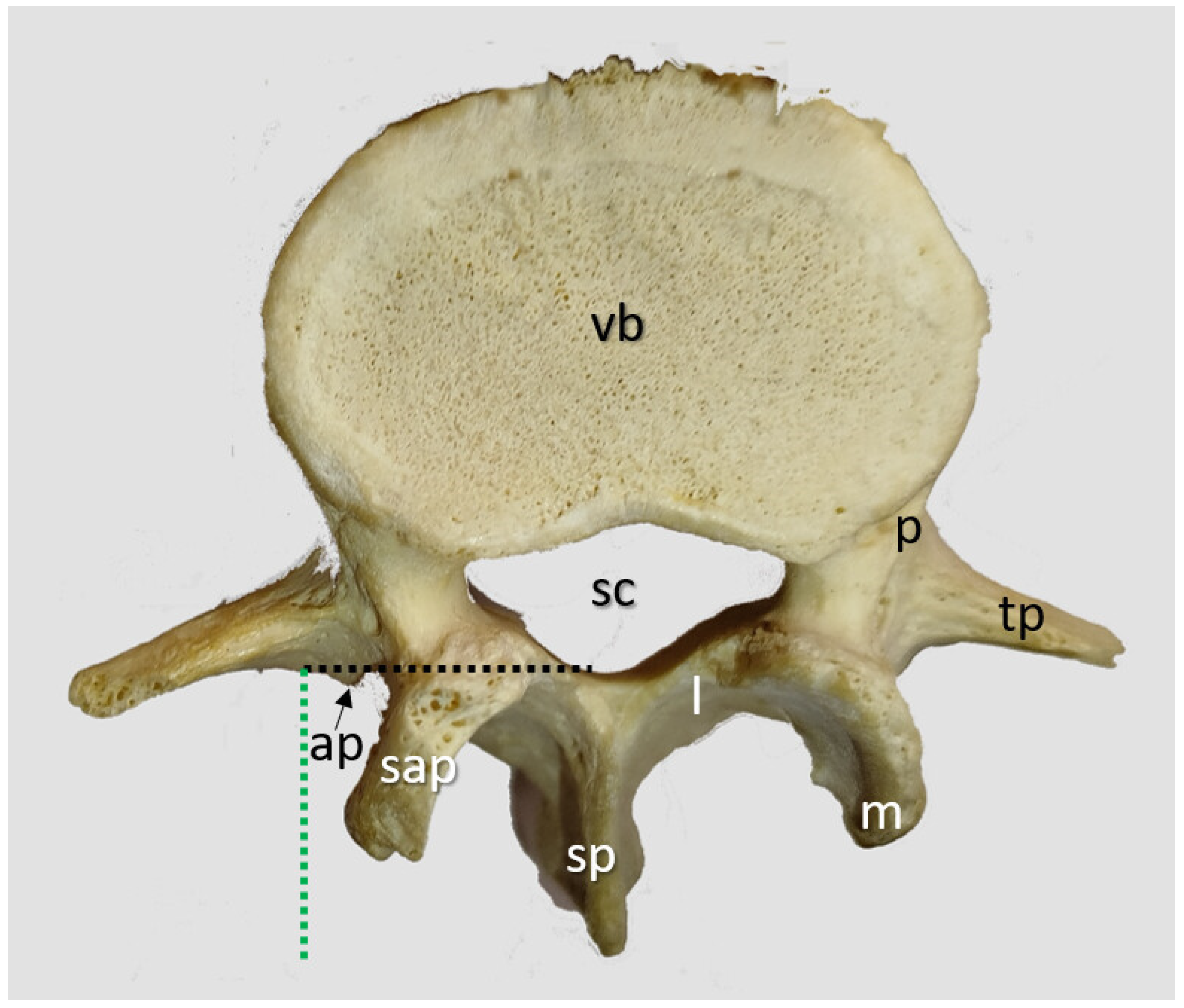
2.1. Ex Vivo Trunks, CT and 3D
2.1.1. Transverse Process and Dural Sac Alignment
2.1.2. Medial and Paramedian Paths for Perpendicular Needle Insertions
2.2. Museum Models
3. Results
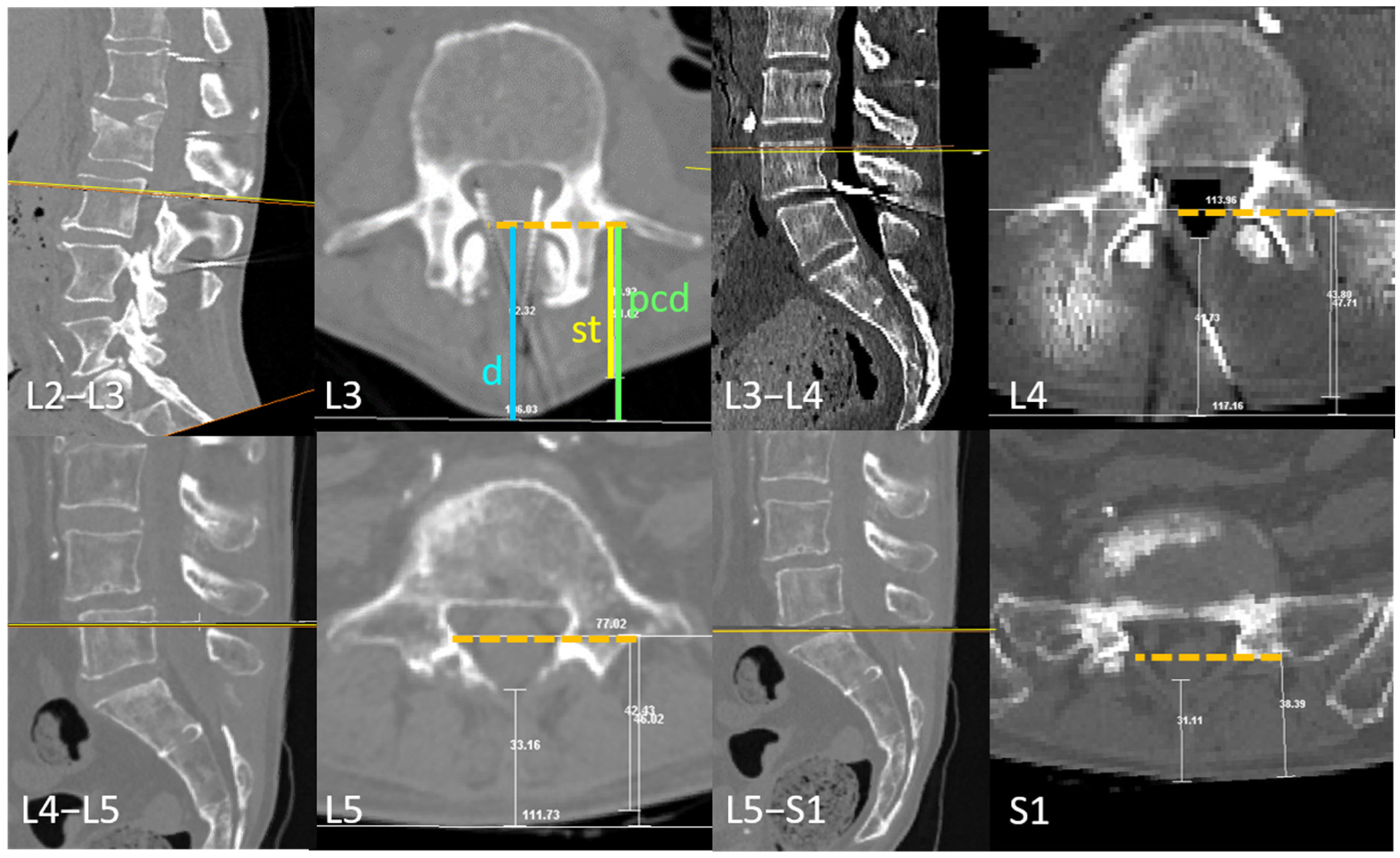
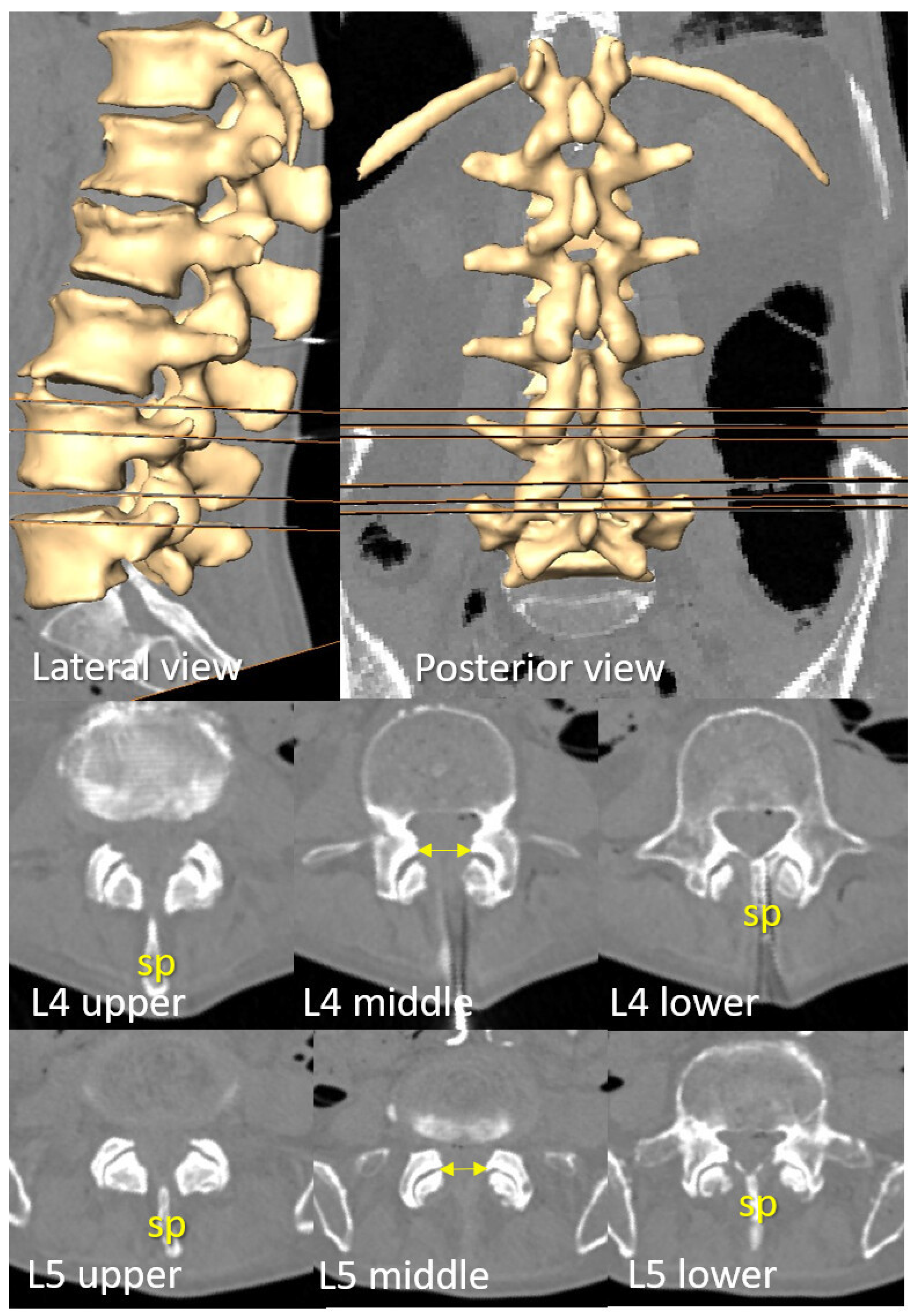
3.1. Transverse Process and Depth Level Within the Dural Sac
3.2. D Reconstruction, Transverse Process and Interlaminar Window
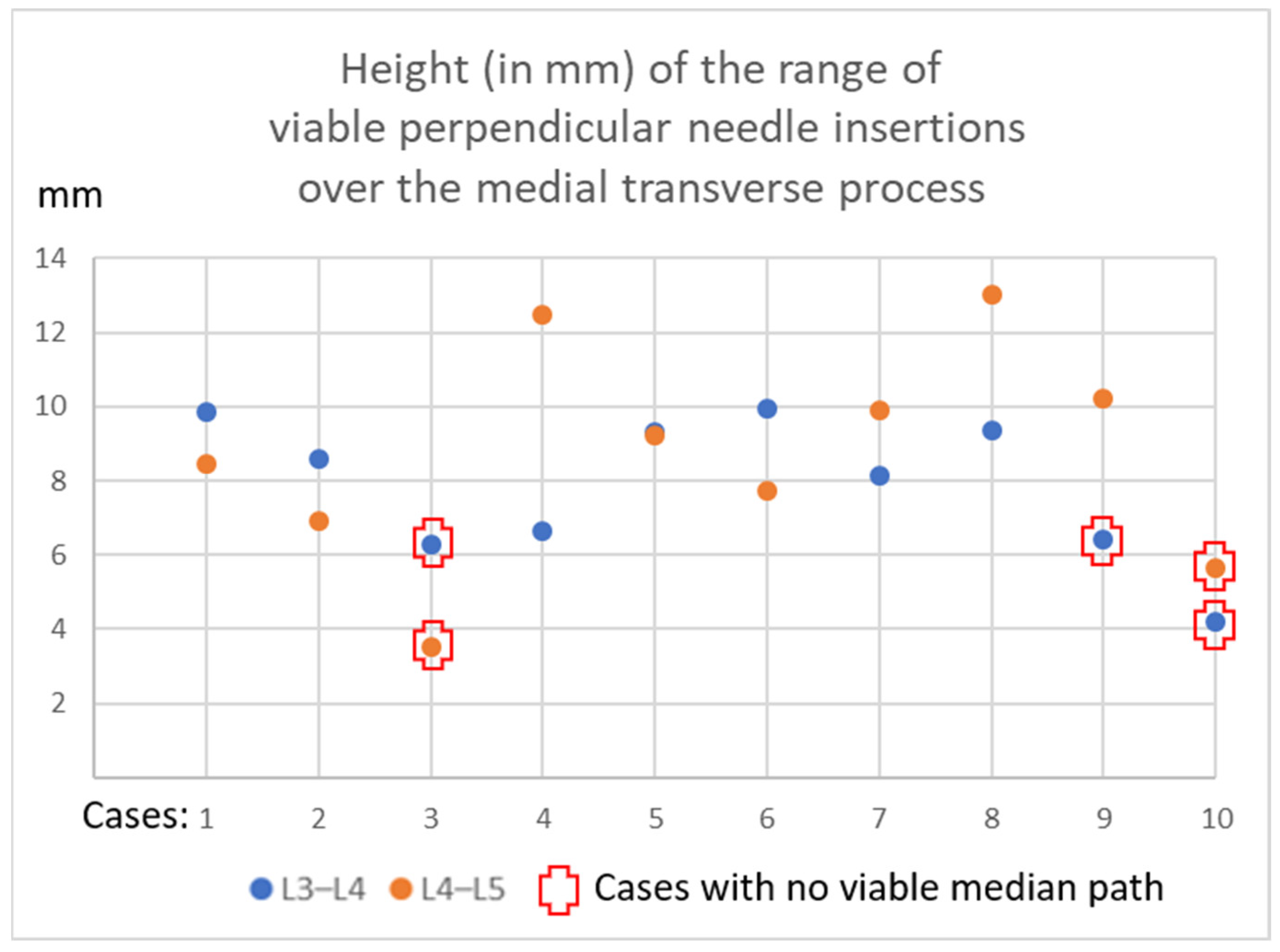
3.3. Viable or Non-Viable Medial Needle Insertions
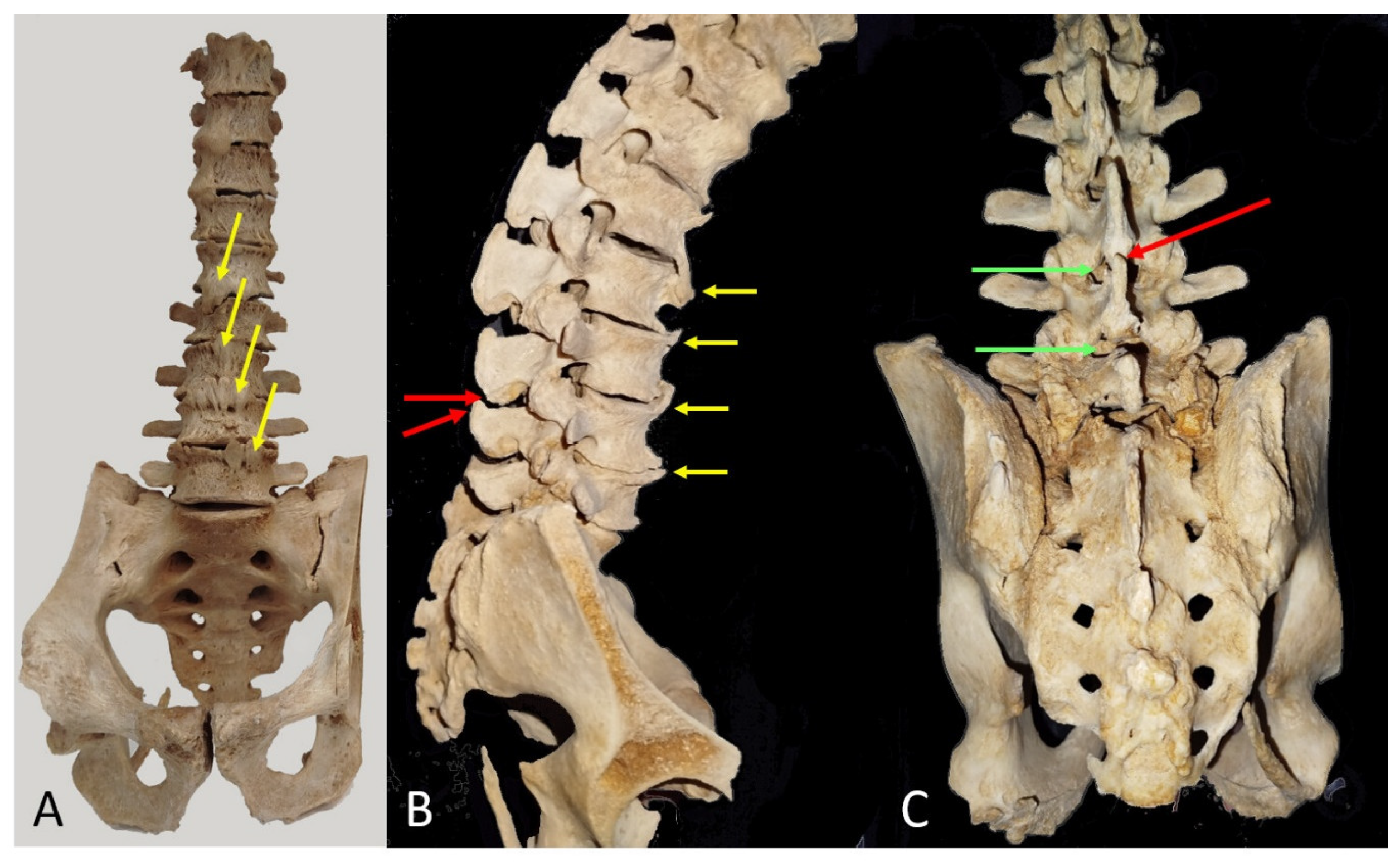
3.4. Museum Models
3.4.1. Spine with Anterior Syndesmophytes
3.4.2. Lumbar Vertebrae
4. Discussion
Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Pascarella, G.; Costa, F.; Hazboun, A.; Del Buono, R.; Strumia, A.; Longo, F.; Ruggiero, A.; Schiavoni, L.; Mattei, A.; Cataldo, R.; et al. Ultrasound predictors of difficult spinal anesthesia: A prospective single-blind observational study. Minerva Anestesiol. 2023, 89, 996–1002. [Google Scholar] [CrossRef]
- Bae, J.; Park, S.K.; Yoo, S.; Lim, Y.J.; Kim, J.T. Influence of age, laterality, patient position, and spinal level on the interlamina space for spinal puncture. Reg. Anesth. Pain Med. 2019, 45, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Labandeyra, H.; Sala-Blanch, X.; Prats-Galino, A.; Puigdellívol-Sánchez, A. Neuraxial Anesthesia and Risk of Root Damage: A 3D Ex Vivo Study. NeuroSci 2024, 5, 623–634. [Google Scholar] [CrossRef]
- Hagenaars, M.; van den Dobbelsteen, J.J.; van Gerwen, D.J. Changes in needle maneuver space and optimal insertion site for midline neuraxial puncture with progressive age: An analysis in computed tomography scans. Reg. Anesth. Pain Med. 2023, 49, 853–860. [Google Scholar] [CrossRef]
- Hameed, F.; Hunter, D.J.; Rainville, J.; Li, L.; Suri, P. Prevalence of anatomic impediments to interlaminar lumbar epidural steroid injection. Arch. Phys. Med. Rehabil. 2012, 93, 339–343. [Google Scholar] [CrossRef]
- Blomberg, R.G.; Jaanivald, A.; Walther, S. Advantages of the paramedian approach for lumbar epidural analgesia with catheter technique. Anaesthesia 1989, 44, 742–746. [Google Scholar] [CrossRef]
- Marcucci, C.; Ahmed, M.M. Consider the paramedian approach for spinal anesthetic placement if the patient is in the lateral position. In Avoiding Common Anesthesia Errors; Marcucci, L., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; pp. 439–440. [Google Scholar]
- Kallidaikurichi-Srinivasan, K.; Iohom, G.; Loughnane, F.; Lee, P.J. Conventional landmark-guided midline versus preprocedure ultrasound guided paramedian techniques in spinal anesthesia. Anesth. Analg. 2015, 121, 1089–1096. [Google Scholar] [CrossRef]
- Puigdellívol-Sánchez, A.; Reina, M.A.; Sala-Blanch, X.; Pomés-Talló, J.; Prats-Galino, A. Pythagoras and cosines: The skin-dural sac distance and optimal angles in paramedian spinal anesthesia. Clin. Anat. 2016, 29, 1046–1052. [Google Scholar] [CrossRef]
- Puigdellívol-Sánchez, A.; Sala-Blanch, X.; Reina, M.A.; Prats-Galino, A. Anatomical Variability and Biomedical Imaging for Spinal Anesthesia Individualization: How 3D Tools Enhance Understanding. In Technological Adoption and Trends in Health Sciences Teaching, Learning, and Practice; Marcos-Pablos, S., Juanes-Méndez, J.A., Eds.; IGI Global: Hershey, PA, USA, 2022; pp. 126–146. [Google Scholar]
- Ranjini, S.S.; Malarvizhi, A.C. Ultrasonographic Guidance in the Prediction of difficult Spinal Anaesthesia in Older Patients. J. Evol. Med. Dent. Sci. 2014, 3, 8340–8354. [Google Scholar]
- Kampitak, W.; Werawatganon, T.; Uerpairojkit, K.; Songthamwat, B. Paramedian Spinal Anesthesia: Landmark vs. Ultrasound-Guid. Approaches J. Anesth. Clin. Res. 2018, 9, 6. [Google Scholar]
- Uyel, Y.; Kilicaslan, A. Preprocedural Ultrasonography Versus Landmark-Guided Spinal Anesthesia in Geriatric Patients with Difficult Anatomy: A Prospective Randomized Trial. Eurasian J. Med. 2021, 53, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Kasai, Y.; Kawakita, E.; Sakakibara, T.; Akeda, K.; Uchida, A. Direction of the formation of anterior lumbar vertebral osteophytes. BMC Musculoskelet. Disord. 2009, 10, 4. [Google Scholar] [CrossRef]
- Takahashi, T.; Yoshii, T.; Mori, K.; Kobayashi, S.; Inoue, H.; Tada, K.; Tamura, N.; Hirai, T.; Sugimura, N.; Nagoshi, N.; et al. Comparison of radiological characteristics between diffuse idiopathic skeletal hyperostosis and ankylosing spondylitis: A multicenter study. Sci. Rep. 2023, 13, 1849. [Google Scholar] [CrossRef] [PubMed]
- Furness, G.; Reilly, M.P.; Kuchi, S. An evaluation of ultrasound imaging for identification of lumbar intervertebral level. Anaesthesia 2002, 57, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Ilfeld, B.M.; Loland, V.J.; Mariano, E.R. Prepuncture ultrasound imaging to predict transverse process and lumbar plexus depth for psoas compartment block and perineural catheter insertion: A prospective, observational study. Anesth. Analg. 2010, 110, 1725–1728. [Google Scholar] [CrossRef]
- Vogt, M.; van Gerwen, D.J.; Lubbers, W.; van den Dobbelsteen, J.J.; Hagenaars, M. Optimal Point of Insertion and Needle Angle in Neuraxial Blockade Using a Midline Approach: A Study in Computed Tomography Scans of Adult Patients. Reg. Anesth. Pain Med. 2017, 42, 600–608. [Google Scholar] [CrossRef]
- Poots, C.; Chin, K.J. Strategies for successful lumbar neuraxial anaesthesia and analgesia in patients with challenging anatomy. BJA Educ. 2024, 24, 46–56. [Google Scholar] [CrossRef]
- Bae, J.; Kim, Y.; Yoo, S.; Kim, J.T.; Park, S.K. Handheld ultrasound-assisted versus palpation-guided combined spinal-epidural for labor analgesia: A randomized controlled trial. Sci. Rep. 2023, 13, 23009. [Google Scholar] [CrossRef]
- Young, B.; Onwochei, D.; Desai, N. Conventional landmark palpation vs. preprocedural ultrasound for neuraxial analgesia and anaesthesia in obstetrics—A systematic review and meta-analysis with trial sequential analyses. Anaesthesia 2021, 76, 818–831. [Google Scholar]
- Kim, Y.; Yoo, S.; Park, S.K.; Bae, H.; Lim, Y.J.; Tae, J. Optimal angle of needle insertion for spinal anesthesia in patients with spondylolisthesis: An ultrasonographic study. BMC Anesthesiol. 2021, 21, 221. [Google Scholar] [CrossRef]
- Özdemir, H.H.; Demir, C.F.; Varol, S.; Arslan, D.; Yıldız, M.; Akil, E. The effects of needle deformation during lumbar puncture. J. Neurosci. Rural. Pract. 2015, 6, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Ultrasound-assisted Spinal Anesthesia in Midline Versus Paramedian Approach in Adult Orthopedic Surgery. Available online: https://clinicaltrials.gov/study/NCT07047469 (accessed on 3 November 2025).
- Paramedian Incidence Angles in Spinal Anaesthesia. Available online: https://clinicaltrials.gov/study/NCT07210892 (accessed on 3 November 2025).

| Case | L4–L5 mm Sup-Inf | Viable Median Path | L3–L4 mm Sup-Inf | Viable Median Path |
|---|---|---|---|---|
| 1 | 8.5 | yes | 9.9 | yes |
| 2 | 6.9 | yes | 8.6 | yes |
| 3 | 3.5 | no | 6.3 | no |
| 4 | 12.5 | yes | 6.7 | yes |
| 5 | 9.2 | yes | 9.3 | yes |
| 6 | 7.7 | yes | 10.0 | yes |
| 7 | 9.9 | yes | 8.1 | yes |
| 8 | 13.0 | yes | 9.4 | yes |
| 9 | 10.2 | yes | 6.4 | no |
| 10 | 5.6 | no | 4.2 | no |
| Mean | 8.7 | 80% | 7.9 | 70% |
| ±SD | 2.9 | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puigdellívol-Sánchez, A.; Labandeyra, H.; Prats-Galino, A.; Sala-Blanch, X. The Transverse Process as a Landmark for Estimating Dural Sac Depth and Feasible Planes for Optimized Paramedian Needle Insertions. NeuroSci 2025, 6, 119. https://doi.org/10.3390/neurosci6040119
Puigdellívol-Sánchez A, Labandeyra H, Prats-Galino A, Sala-Blanch X. The Transverse Process as a Landmark for Estimating Dural Sac Depth and Feasible Planes for Optimized Paramedian Needle Insertions. NeuroSci. 2025; 6(4):119. https://doi.org/10.3390/neurosci6040119
Chicago/Turabian StylePuigdellívol-Sánchez, Anna, Hipólito Labandeyra, Alberto Prats-Galino, and Xavier Sala-Blanch. 2025. "The Transverse Process as a Landmark for Estimating Dural Sac Depth and Feasible Planes for Optimized Paramedian Needle Insertions" NeuroSci 6, no. 4: 119. https://doi.org/10.3390/neurosci6040119
APA StylePuigdellívol-Sánchez, A., Labandeyra, H., Prats-Galino, A., & Sala-Blanch, X. (2025). The Transverse Process as a Landmark for Estimating Dural Sac Depth and Feasible Planes for Optimized Paramedian Needle Insertions. NeuroSci, 6(4), 119. https://doi.org/10.3390/neurosci6040119






