From Fork to Brain: The Role of AGE–RAGE Signaling and the Western Diet in Neurodegenerative Disease
Abstract
1. Introduction
2. Advanced Glycation End Products (AGEs): Formation and Accumulation
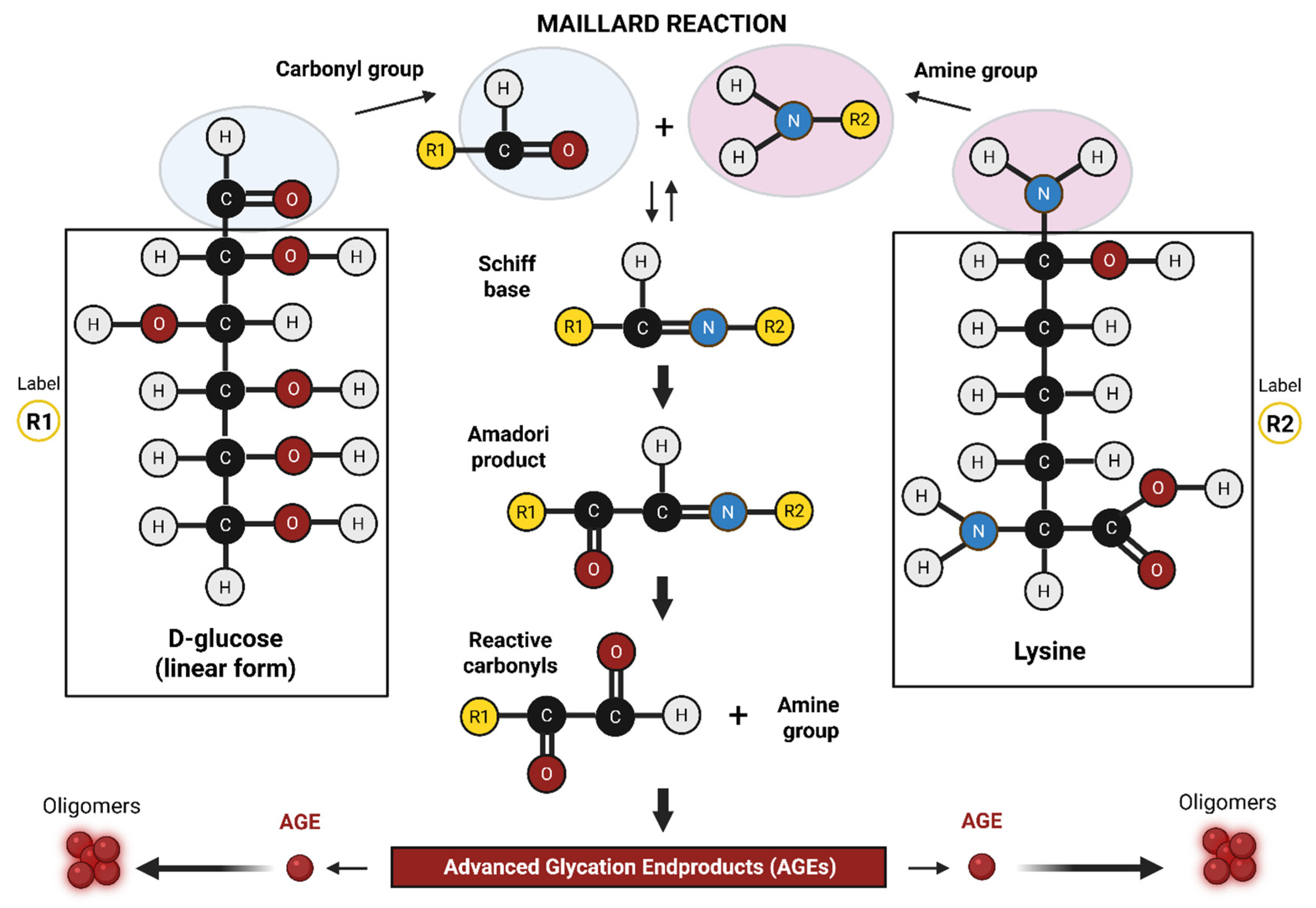
2.1. In Vitro Formation of AGEs-
2.2. In Vivo Formation of AGEs-
2.3. Tissue Storage and Accumulation of AGE
3. Exogenous AGEs: Dietary Sources and Contributions
3.1. High-AGE Foods and Cooking Practices
3.2. Gastrointestinal Absorption and Organ Distribution
3.3. Renal Clearance and Implications for Neuroinflammation
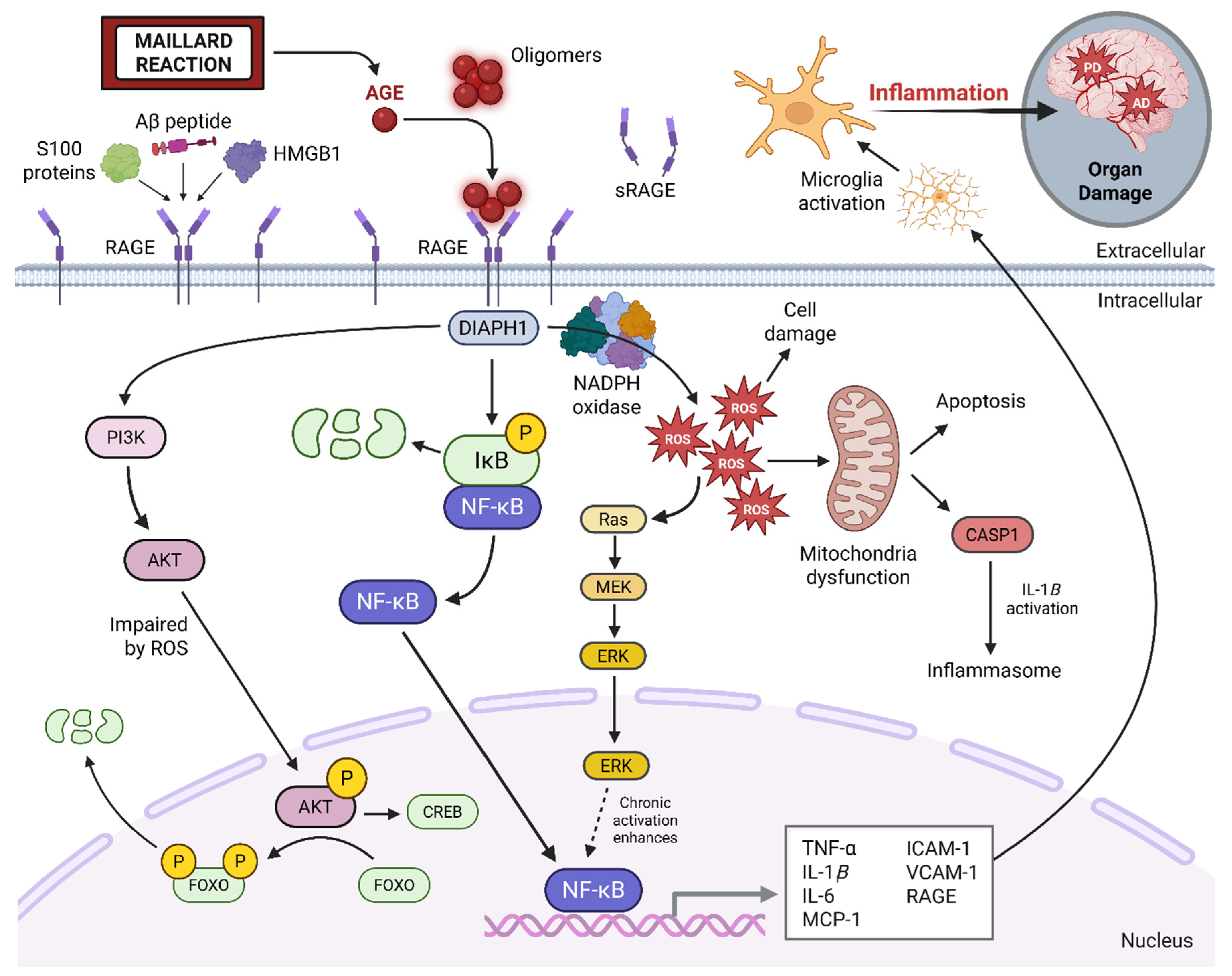
4. AGE–RAGE Signaling and Neuroinflammation
5. Post-Viral Syndromes and the AGE–RAGE Axis
5.1. AGE–RAGE Signaling in Post-Viral Disease: A Mechanistic Proposal
5.2. SARS-CoV-2 and AGE–RAGE Signaling
6. The Western Diet, AGE Formation, and Neurodegeneration
6.1. Western Diet as a Source of Exogenous and Endogenous AGEs
6.2. Environmental Contributors
7. Pharmacological and Lifestyle Interventions
7.1. RAGE-Targeting Therapies: Current Limitations
7.2. Dietary and Lifestyle Strategies to Reduce AGE Burden
8. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Sirotiak, Z.; Thomas, E.B. Beyond fatigue: An intersectional analysis of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and social identities. Curr. Psychol. 2025. [Google Scholar] [CrossRef]
- Slavin, M.D.; Bailey, H.M.; Hickey, E.J.; Vasudevan, A.; Ledingham, A.; Tannenbaum, L.; Bateman, L.; Kaufman, D.L.; Peterson, D.L.; Ruhoy, I.S.; et al. Myalgic Encephalomyelitis-Chronic Fatigue Syndrome Common Data Element item content analysis. PLoS ONE 2023, 18, e0291364. [Google Scholar] [CrossRef] [PubMed]
- VanElzakker, M.B.; Brumfield, S.A.; Lara Mejia, P.S. Neuroinflammation and cytokines in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A critical review of research methods. Front. Neurol. 2019, 9, 1033. [Google Scholar] [CrossRef]
- Gottschalk, G.; Peterson, D.; Knox, K.; Maynard, M.; Whelan, R.J.; Roy, A. Elevated ATG13 in serum of patients with ME/CFS stimulates oxidative stress response in microglial cells via activation of receptor for advanced glycation end products (RAGE). Mol. Cell. Neurosci. 2022, 120, 103731. [Google Scholar] [CrossRef] [PubMed]
- Luevano-Contreras, C.; Chapman-Novakofski, K. Dietary advanced glycation end products and aging. Nutrients 2010, 2, 1247–1265. [Google Scholar] [CrossRef]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.U.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010, 110, 911–916.e12. [Google Scholar] [CrossRef]
- Maillard, L.C. Action des acides amines sur les sucres: Formation des melanoidines par voie methodique. CR Seances Soc. Biol. Fil. 1912, 154, 66–68. [Google Scholar]
- Hodge, J.E. Dehydrated foods, chemistry of browning reactions in model systems. J. Agric. Food Chem. 1953, 1, 928–943. [Google Scholar] [CrossRef]
- Rahbar, S. An abnormal hemoglobin in red cells of diabetics. Clin. Chim. Acta 1968, 22, 296–298. [Google Scholar] [CrossRef]
- Koenig, R.J.; Peterson, C.M.; Jones, R.L.; Saudek, C.; Lehrman, M.; Cerami, A. Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus. N. Engl. J. Med. 1976, 295, 417–420. [Google Scholar] [CrossRef]
- Cerami, A. The unexpected pathway to the creation of the HbA1c test and the discovery of AGE’s. J. Intern. Med. 2012, 271, 219–226. [Google Scholar] [CrossRef]
- Ramasamy, R.; Vannucci, S.J.; Yan, S.S.; Herold, K.; Yan, S.F.; Schmidt, A.M. Advanced glycation end products and RAGE: A common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology 2005, 15, 16R–28R. [Google Scholar] [CrossRef]
- Sharma, C.; Kaur, A.; Thind, S.S.; Singh, B.; Raina, S. Advanced glycation End-products (AGEs): An emerging concern for processed food industries. J. Food Sci. Technol. 2015, 52, 7561–7576. [Google Scholar] [CrossRef]
- Gaens, K.H.; Goossens, G.H.; Niessen, P.M.; van Greevenbroek, M.M.; van der Kallen, C.J.; Niessen, H.W.; Rensen, S.S.; Buurman, W.A.; Greve, J.W.; Blaak, E.E.; et al. Nε-(carboxymethyl) lysine-receptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1199–1208. [Google Scholar] [CrossRef]
- Verzijl, N.; DeGroot, J.; Oldehinkel, E.; Bank, R.A.; Thorpe, S.R.; Baynes, J.W.; Bayliss, M.T.; Bijlsma, J.W.; Lafeber, F.P.; TeKoppele, J.M. Age-related accumulation of Maillard reaction products in human articular cartilage collagen. Biochem. J. 2000, 350, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Uceda, A.B.; Mariño, L.; Casasnovas, R.; Adrover, M. An overview on glycation: Molecular mechanisms, impact on proteins, pathogenesis, and inhibition. Biophys. Rev. 2024, 16, 189–218. [Google Scholar] [CrossRef] [PubMed]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced glycation end-products (AGEs): Formation, chemistry, classification, receptors, and diseases related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Simm, A.; Nass, N.; Bartling, B.; Hofmann, B.; Silber, R.E.; Navarrete Santos, A. Potential biomarkers of ageing. Biol. Chem. 2008, 389, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Koschinsky, T.; He, C.J.; Mitsuhashi, T.; Bucala, R.; Liu, C.; Buenting, C.; Heitmann, K.; Vlassara, H. Orally absorbed reactive glycation products (glycotoxins): An environmental risk factor in diabetic nephropathy. Proc. Natl. Acad. Sci. USA 1997, 94, 6474–6479. [Google Scholar] [CrossRef]
- Vlassara, H.; Uribarri, J. Advanced glycation end products (AGE) and diabetes: Cause, effect, or both? Curr. Diabetes Rep. 2014, 14, 453. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, V.; Maczurek, A.; Phan, T.; Steele, M.; Westcott, B.; Juskiw, D.; Münch, G. Advanced glycation endproducts and their receptor RAGE in Alzheimer’s disease. Neurobiol. Aging 2011, 32, 763–777. [Google Scholar] [CrossRef]
- De la Monte, S.M. Insulin resistance and neurodegeneration: Progress towards the development of new therapeutics for Alzheimer’s disease. Drugs 2017, 77, 47–65. [Google Scholar] [CrossRef]
- Zhang, R.; Jiang, L.; Li, G.; Wu, J.; Tian, P.; Zhang, D.; Qin, Y.; Shi, Z.; Gao, Z.; Zhang, N.; et al. Advanced glycosylation end products induced synaptic deficits and cognitive decline through ROS-JNK-p53/miR-34c/SYT1 axis in diabetic encephalopathy. J. Alzheimer’s Dis. 2022, 87, 843–861. [Google Scholar] [CrossRef] [PubMed]
- Snelson, M.; Coughlan, M.T. Dietary advanced glycation end products: Digestion, metabolism and modulation of gut microbial ecology. Nutrients 2019, 11, 215. [Google Scholar] [CrossRef]
- Kook, S.Y.; Seok Hong, H.; Moon, M.; Mook-Jung, I. Disruption of blood-brain barrier in Alzheimer’s disease pathogenesis. Tissue Barriers 2013, 1, 8845–8854. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Phuong-Nguyen, K.; McNeill, B.A.; Aston-Mourney, K.; Rivera, L.R. Advanced glycation end-products and their effects on gut health. Nutrients 2023, 15, 405. [Google Scholar] [CrossRef]
- Palaseweenun, P.; Hagen-Plantinga, E.A.; Schonewille, J.T.; Koop, G.; Butre, C.; Jonathan, M.; Wierenga, P.A.; Hendriks, W.H. Urinary excretion of advanced glycation end products in dogs and cats. J. Anim. Physiol. Anim. Nutr. 2021, 105, 149–156. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, X.; Li, L.; Li, B.; Yang, Z. The fate of dietary advanced glycation end products in the body: From oral intake to excretion. Crit. Rev. Food Sci. Nutr. 2020, 60, 3475–3491. [Google Scholar] [CrossRef] [PubMed]
- Koska, J.; Gerstein, H.C.; Beisswenger, P.J.; Reaven, P.D. Advanced glycation end products predict loss of renal function and high-risk chronic kidney disease in type 2 diabetes. Diabetes Care 2022, 45, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Roncero-Ramos, I.; Delgado-Andrade, C.; Tessier, F.J.; Niquet-Léridon, C.; Strauch, C.; Monnier, V.M.; Navarro, M.P. Metabolic transit of Nε-carboxymethyl-lysine after consumption of AGEs from bread crust. Food Funct. 2013, 4, 1032–1039. [Google Scholar] [CrossRef]
- Lüth, H.J.; Ogunlade, V.; Kuhla, B.; Kientsch-Engel, R.; Stahl, P.; Webster, J.; Arendt, T.; Münch, G. Age-and stage-dependent accumulation of advanced glycation end products in intracellular deposits in normal and Alzheimer’s disease brains. Cereb. Cortex 2005, 15, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Gooch, K.; Culleton, B.F.; Manns, B.J.; Zhang, J.; Alfonso, H.; Tonelli, M.; Frank, C.; Klarenbach, S.; Hemmelgarn, B.R. NSAID use and progression of chronic kidney disease. Am. J. Med. 2007, 120, 280.e1–280.e7. [Google Scholar] [CrossRef]
- Sparvero, L.J.; Asafu-Adjei, D.; Kang, R.; Tang, D.; Amin, N.; Im, J.; Rutledge, R.; Lin, B.; Amoscato, A.A.; Zeh, H.J.; et al. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J. Transl. Med. 2009, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, W. Molecular links between Alzheimer’s disease and diabetes mellitus. Neuroscience 2013, 250, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Komaroff, A.L.; Bateman, L. Will COVID-19 lead to myalgic encephalomyelitis/chronic fatigue syndrome? Front. Med. 2021, 7, 606824. [Google Scholar] [CrossRef]
- Manigrasso, M.B.; Pan, J.; Rai, V.; Zhang, J.; Reverdatto, S.; Quadri, N.; DeVita, R.J.; Ramasamy, R.; Shekhtman, A.; Schmidt, A.M. Small molecule inhibition of ligand-stimulated RAGE-DIAPH1 signal transduction. Sci. Rep. 2016, 6, 22450. [Google Scholar] [CrossRef]
- Piperi, C.; Goumenos, A.; Adamopoulos, C.; Papavassiliou, A.G. AGE/RAGE signalling regulation by miRNAs: Associations with diabetic complications and therapeutic potential. Int. J. Biochem. Cell Biol. 2015, 60, 197–201. [Google Scholar] [CrossRef]
- Xiong, X.; Dou, J.; Shi, J.; Ren, Y.; Wang, C.; Zhang, Y.; Cui, Y. RAGE inhibition alleviates lipopolysaccharides-induced lung injury via directly suppressing autophagic apoptosis of type II alveolar epithelial cells. Respir. Res. 2023, 24, 24. [Google Scholar] [CrossRef]
- Oczypok, E.A.; Perkins, T.N.; Oury, T.D. All the “RAGE” in lung disease: The receptor for advanced glycation endproducts (RAGE) is a major mediator of pulmonary inflammatory responses. Paediatr. Respir. Rev. 2017, 23, 40–49. [Google Scholar] [CrossRef]
- Rojas, A.; Gonzalez, I.; Morales, M.A. SARS-CoV-2-mediated inflammatory response in lungs: Should we look at RAGE? Inflamm. Res. 2020, 69, 641–643. [Google Scholar] [CrossRef]
- Bopp, C.; Bierhaus, A.; Hofer, S.; Bouchon, A.; Nawroth, P.P.; Martin, E.; Weigand, M.A. Bench-to-bedside review: The inflammation-perpetuating pattern-recognition receptor RAGE as a therapeutic target in sepsis. Crit. Care 2008, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Ariza, M.E.; Glaser, R.; Kaumaya, P.T.; Jones, C.; Williams, M.V. The EBV-encoded dUTPase activates NF-κB through the TLR2 and MyD88-dependent signaling pathway. J. Immunol. 2009, 182, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Ariza, M.E.; Rivailler, P.; Glaser, R.; Chen, M.; Williams, M.V. Epstein-Barr virus encoded dUTPase containing exosomes modulate innate and adaptive immune responses in human dendritic cells and peripheral blood mononuclear cells. PLoS ONE 2013, 8, e69827. [Google Scholar] [CrossRef] [PubMed]
- Ostrand-Rosenberg, S.; Huecksteadt, T.; Sanders, K. The receptor for advanced glycation endproducts (RAGE) and its ligands S100A8/A9 and high mobility group box protein 1 (HMGB1) are key regulators of myeloid-derived suppressor cells. Cancers 2023, 15, 1026. [Google Scholar] [CrossRef]
- Selvam, R.; Khan, E.; Mehta, M.K.; Singh, D.; Gupta, S.; Chandra, S. Epstein-Barr virus-encoded Latent Membrane protein-1 (LMP-1) as a Prognostic marker in OSCC and OPMDs. Oral Oncol. Rep. 2024, 9, 100187. [Google Scholar] [CrossRef]
- Šimičić, P.; Batović, M.; Stojanović Marković, A.; Židovec-Lepej, S. Deciphering the role of Epstein–Barr virus latent membrane protein 1 in immune modulation: A multifaced Signalling perspective. Viruses 2024, 16, 564. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Sun, X.; Lu, L.; Yong, Y.; Kong, X.; Song, J. Suppressing neuroinflammation by Shenfu injection against ischemic stroke in mice via inhibiting RAGE-PI3K-Akt pathway. Phytomedicine 2025, 144, 156940. [Google Scholar] [CrossRef]
- Liu, Y.; Lui, K.S.; Ye, Z.; Chen, L.; Cheung, A.K. Epstein–Barr Virus BRRF1 Induces Butyrophilin 2A1 in Nasopharyngeal Carcinoma NPC43 Cells via the IL-22/JAK3-STAT3 Pathway. Int. J. Mol. Sci. 2024, 25, 13452. [Google Scholar] [CrossRef]
- Sivagurunathan, N.; Calivarathan, L. SARS-CoV-2 infection to premature neuronal aging and neurodegenerative diseases: Is there any connection with Hypoxia? CNS Neurol. Disord. Drug Targets 2024, 23, 431–448. [Google Scholar] [CrossRef]
- Shirato, K.; Kizaki, T. SARS-CoV-2 spike protein S1 subunit induces pro-inflammatory responses via toll-like receptor 4 signaling in murine and human macrophages. Heliyon 2021, 7, e06187. [Google Scholar] [CrossRef] [PubMed]
- van Beijnum, J.R.; Buurman, W.A.; Griffioen, A.W. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis 2008, 11, 91–99. [Google Scholar] [CrossRef]
- Shankar, V.; Wilhelmy, J.; Curtis, E.J.; Michael, B.; Cervantes, L.; Mallajosyula, V.; Davis, R.W.; Snyder, M.; Younis, S.; Robinson, W.H.; et al. Oxidative stress is a shared characteristic of ME/CFS and Long COVID. Proc. Natl. Acad. Sci. USA 2025, 122, e2426564122. [Google Scholar] [CrossRef]
- DeOre, B.J.; Tran, K.A.; Andrews, A.M.; Ramirez, S.H.; Galie, P.A. SARS-CoV-2 spike protein disrupts blood–brain barrier integrity via RhoA activation. J. Neuroimmune Pharmacol. 2021, 16, 722–728. [Google Scholar] [CrossRef]
- Martinez-Rojas, M.A.; Vega-Vega, O.; Bobadilla, N.A. Is the kidney a target of SARS-CoV-2? Am. J. Physiol.-Ren. Physiol. 2020, 318, F1454–F1462. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, X.; Hu, B.; Li, D.; Chen, L.; Li, Y.; Tu, Y.; Xiong, S.; Wang, G.; Deng, J.; et al. Mechanisms of SARS-CoV-2 infection-induced kidney injury: A literature review. Front. Cell. Infect. Microbiol. 2022, 12, 838213. [Google Scholar] [CrossRef]
- Iqbal, A.; Hafeez Kamran, S.; Siddique, F.; Ishtiaq, S.; Hameed, M.; Manzoor, M. Modulatory effects of rutin and vitamin A on hyperglycemia induced glycation, oxidative stress and inflammation in high-fat-fructose diet animal model. PLoS ONE 2024, 19, e0303060. [Google Scholar] [CrossRef]
- Levi, B.; Werman, M.J. Long-term fructose consumption accelerates glycation and several age-related variables in male rats. J. Nutr. 1998, 128, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- de Lima, N.C., Jr.; Fernandes-Batista, T.; Ferreira-Serra, L.; Paes-Dias, A.L.; Matta-Pereira, L.; Medeiros, F.H.; Pazos-Moura, C.C.; Fortunato, R.S.; Carvalho, D.P.; Dias, G.R.; et al. Oxidative Stress Parameters are Differentially Regulated in Visceral and Subcutaneous Adipose Tissue by Western Diet and Intermittent Fasting. Horm. Metab. Res. 2025, 57, 396–404. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Z.; Colucci, M.; Deng, L.; Yang, M.; Huang, X.; Zhou, X.; Jin, Y.; Lazzarini, E.; Balbi, C.; et al. The mixed effect of Endocrine-Disrupting chemicals on biological age Acceleration: Unveiling the mechanism and potential intervention target. Environ. Int. 2024, 184, 108447. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.V.; Aschner, M.; Santamaria, A.; Lu, R.; Rocha, J.B.; Zalavina, S.V.; Korchin, V.I.; Ke, T.; Tinkov, A.A. The Differential Role of Toxic and Essential Metals in Formation and Toxicity of Advanced Glycation End-Products. In Toxicology of Essential and Xenobiotic Metals; CRC Press: London, UK, 2024; pp. 160–198. [Google Scholar]
- Suhartono, E.; Triawanti, A.S.; Djati, M.S. The Role of Cadmium in Proteins Glycation by Glucose: Formation of Methylglyoxal and Hydrogen Peroxide In Vitro. J. Med. Bioeng. 2014, 3, 59–62. [Google Scholar] [CrossRef][Green Version]
- Wu, H.; Liao, Q.; Chillrud, S.N.; Yang, Q.; Huang, L.; Bi, J.; Yan, B. Environmental exposure to cadmium: Health risk assessment and its associations with hypertension and impaired kidney function. Sci. Rep. 2016, 6, 29989. [Google Scholar] [CrossRef]
- Ma, L.; Bi, K.D.; Fan, Y.M.; Jiang, Z.Y.; Zhang, X.Y.; Zhang, J.W.; Zhao, J.; Jiang, F.L.; Dong, J.X. In vitro modulation of mercury-induced rat liver mitochondria dysfunction. Toxicol. Res. 2018, 7, 1135–1143. [Google Scholar] [CrossRef]
- Wu, L.; Xu, Y.; Lv, X.; Chang, X.; Ma, X.; Tian, X.; Shi, X.; Li, X.; Kong, X. Impacts of an azo food dye tartrazine uptake on intestinal barrier, oxidative stress, inflammatory response and intestinal microbiome in crucian carp (Carassius auratus). Ecotoxicol. Environ. Saf. 2021, 223, 112551. [Google Scholar] [CrossRef]
- Gupta, R.; Yadav, R.K. Impact of chemical food preservatives on human health. Palarch’s J. Archaeol. Egypt/Egyptol. 2021, 18, 811–818. [Google Scholar]
- Mandal, D. Food preservative chemistry: Effects and side effects. J. Indian Chem. Soc. 2019, 96, 1519–1528. [Google Scholar]
- Naimi, S.; Viennois, E.; Gewirtz, A.T.; Chassaing, B. Direct impact of commonly used dietary emulsifiers on human gut microbiota. Microbiome 2021, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Burstein, A.H.; Sabbagh, M.; Andrews, R.; Valcarce, C.; Dunn, I.; Altstiel, L. Development of Azeliragon, an oral small molecule antagonist of the receptor for advanced glycation endproducts, for the potential slowing of loss of cognition in mild Alzheimer’s disease. J. Prev. Alzheimer’s Dis. 2018, 5, 149–154. [Google Scholar] [CrossRef]
- Magna, M.; Hwang, G.H.; McIntosh, A.; Drews-Elger, K.; Takabatake, M.; Ikeda, A.; Mera, B.J.; Kwak, T.; Miller, P.; Lippman, M.E.; et al. RAGE inhibitor TTP488 (Azeliragon) suppresses metastasis in triple-negative breast cancer. NPJ Breast Cancer 2023, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, W.; Chen, Q.; Cao, Q.; Di, W.; Lan, R.; Chen, Z.; Bai, J.; Han, Z.; Xu, W. Inhibition of RAGE by FPS-ZM1 alleviates renal injury in spontaneously hypertensive rats. Eur. J. Pharmacol. 2020, 882, 173228. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, T.; Yang, Y.; Lu, D.; Xu, A.; Li, K. FPS-ZM1 alleviates neuroinflammation in focal cerebral ischemia rats via blocking ligand/RAGE/DIAPH1 pathway. ACS Chem. Neurosci. 2020, 12, 63–78. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, Y.; Huang, Y.; Deng, H. Pathophysiology of RAGE in inflammatory diseases. Front. Immunol. 2022, 13, 931473. [Google Scholar] [CrossRef]
- Inan-Eroglu, E.; Ayaz, A.; Buyuktuncer, Z. Formation of advanced glycation endproducts in foods during cooking process and underlying mechanisms: A comprehensive review of experimental studies. Nutr. Res. Rev. 2020, 33, 77–89. [Google Scholar] [CrossRef]
- Goldberg, T.; Cai, W.; Peppa, M.; Dardaine, V.; Baliga, B.S.; Uribarri, J.; Vlassara, H. Advanced glycoxidation end products in commonly consumed foods. J. Am. Diet. Assoc. 2004, 104, 1287–1291. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Yen, G.C. Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts. J. Agric. Food Chem. 2005, 53, 3167–3173. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Sukhorukov, V.N.; Surkova, R.; Orekhov, N.A.; Orekhov, A.N. Glycation of LDL: AGEs, impact on lipoprotein function, and involvement in atherosclerosis. Front. Cardiovasc. Med. 2023, 10, 1094188. [Google Scholar] [CrossRef]
- Tsigalou, C.; Konstantinidis, T.; Paraschaki, A.; Stavropoulou, E.; Voidarou, C.; Bezirtzoglou, E. Mediterranean diet as a tool to combat inflammation and chronic diseases. An overview. Biomedicines 2020, 8, 201. [Google Scholar] [CrossRef]
- Fang, Y.W.; Chen, C.W.; Su, T.C.; Wang, C.; Lin, C.Y. Investigating the associations of blood lead and cadmium with smoking-related DNA methylation and mortality among US adults. Ecotoxicol. Environ. Saf. 2025, 299, 118360. [Google Scholar] [CrossRef] [PubMed]
| Tissue | Why Vulnerable? | Example of AGE Effects |
|---|---|---|
| Kidneys | High glucose filtration; impaired clearance | Nephropathy, glomerular basement membrane thickening |
| Eyes | High metabolic rate; excessive glucose uptake | Retinopathy, blood–retinal barrier (BRB) breakdown |
| Peripheral nerves | High collagen content prone to AGE cross-linking | Atherosclerosis, vascular stiffening |
| Heart | High mitochondrial stress; susceptibility to inflammation | Diabetic cardiomyopathy |
| Brain | Low molecular weight AGE peptides cross the blood–brain barrier (BBB); circulating AGEs and RAGE at the BBB activating Nf-κB and microglia; increasing BBB permeability | Cognitive decline; increased Alzheimer’s disease risk |
| Event | Diabetes (Type 2) | Alzheimer’s Disease (Type 3) |
|---|---|---|
| AGE accumulation | ↑ due to chronic hyperglycemia | ↑ in brain with aging; further elevated in diabetic individuals |
| RAGE activation | Chronic in vascular tissues | Chronic in neurons, glia, and cerebral vasculature |
| Inflammation/ROS | ↑ vascular inflammation, oxidative stress | ↑ neuroinflammation, mitochondrial ROS, redox imbalance |
| Protein aggregation | AGEs contribute to vascular and renal protein deposits | Aβ plaque formation & tau hyperphosphorylation |
| Clearance impairment | Impaired proteostasis and AGE clearance with diabetes/aging | Impaired clearance of Aβ and AGEs, exacerbated by diabetes-induced dysfunction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pomroy, H.J.; Mote, A.; Mathew, S.; Chanasseril, S.; Lu, V.; Cheema, A.K. From Fork to Brain: The Role of AGE–RAGE Signaling and the Western Diet in Neurodegenerative Disease. NeuroSci 2025, 6, 89. https://doi.org/10.3390/neurosci6030089
Pomroy HJ, Mote A, Mathew S, Chanasseril S, Lu V, Cheema AK. From Fork to Brain: The Role of AGE–RAGE Signaling and the Western Diet in Neurodegenerative Disease. NeuroSci. 2025; 6(3):89. https://doi.org/10.3390/neurosci6030089
Chicago/Turabian StylePomroy, Haylie J., Arjun Mote, Simeon Mathew, Stebin Chanasseril, Victor Lu, and Amanpreet K. Cheema. 2025. "From Fork to Brain: The Role of AGE–RAGE Signaling and the Western Diet in Neurodegenerative Disease" NeuroSci 6, no. 3: 89. https://doi.org/10.3390/neurosci6030089
APA StylePomroy, H. J., Mote, A., Mathew, S., Chanasseril, S., Lu, V., & Cheema, A. K. (2025). From Fork to Brain: The Role of AGE–RAGE Signaling and the Western Diet in Neurodegenerative Disease. NeuroSci, 6(3), 89. https://doi.org/10.3390/neurosci6030089







