Autonomic Nervous System, Cognition, and Emotional Valence During Different Phases of the Menstrual Cycle—A Narrative Review
Abstract
1. Introduction
2. The Menstrual Cycle and Hormonal Changes
3. Menstrual Cycle and Psychopathology
3.1. Emotional Valence
3.2. Cognitive Functioning
4. Effects of the Menstrual Cycle on the Autonomic Nervous System
4.1. Assessment of the Autonomic Nervous System
4.1.1. Heart Rate Variability
4.1.2. Baroreflex Sensitivity
4.1.3. Muscle Sympathetic Nerve Activity
4.1.4. Pupil Light Reflex
4.2. Influence of the Menstrual Cycle on Parameters of the Autonomic Nervous System
4.2.1. Heart Rate Variability
4.2.2. Baroreflex Sensitivity
4.2.3. Muscle Sympathetic Nerve Activity
4.2.4. Pupillometry and Psychological Determinants of the Menstrual Cycle
5. The ANS, Cognition, and Emotional Valence During the Menstrual Cycle
6. Limitations
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Benarroch, E.E. The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clin. Proc. 1993, 68, 988–1001. [Google Scholar] [CrossRef]
- Lamotte, G.; Shouman, K.; Benarroch, E.E. Stress and central autonomic network. Auton. Neurosci. 2021, 235, 102870. [Google Scholar] [CrossRef]
- Sie, J.H.; Chen, Y.H.; Shiau, Y.H.; Chu, W.C. Gender- and Age-Specific Differences in Resting-State Functional Connectivity of the Central Autonomic Network in Adulthood. Front. Hum. Neurosci. 2019, 13, 369. [Google Scholar] [CrossRef]
- Thiyagarajan, D. Physiology, Menstrual Cycle; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Critchley, H.D.; Eccles, J.; Garfinkel, S.N. Interaction between cognition, emotion, and the autonomic nervous system. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 59–77. [Google Scholar]
- Blaser, B.L.; Weymar, M.; Wendt, J. Premenstrual syndrome is associated with differences in heart rate variability and attentional control throughout the menstrual cycle: A pilot study. Int. J. Psychophysiol. 2024, 204, 112374. [Google Scholar] [CrossRef]
- Tschudin, S.; Bertea, P.C.; Zemp, E. Prevalence and predictors of premenstrual syndrome and premenstrual dysphoric disorder in a population-based sample. Arch. Womens Ment. Health 2010, 13, 485–494. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, H.; Gao, Z.; Cheng, X.; Sun, Y.; Qiao, M.; Gao, D. Global and regional prevalence and burden for premenstrual syndrome and premenstrual dysphoric disorder: A study protocol for systematic review and meta-analysis. Medicine 2022, 101, e28528. [Google Scholar] [CrossRef]
- Koifman, R.; Dayan, L.; Ablin, J.N.; Jacob, G. Cardiovascular Autonomic Profile in Women With Premenstrual Syndrome. Front. Physiol. 2018, 9, 1384. [Google Scholar] [CrossRef]
- Du, R.; Liang, T.; Lu, G. Modulation of empathic abilities by the interplay between estrogen receptors and arginine vasopressin. Neurosci. Res. 2025, 210, 11–18. [Google Scholar] [CrossRef]
- Albert, K.M.; Newhouse, P.A. Estrogen, Stress, and Depression: Cognitive and Biological Interactions. Annu. Rev. Clin. Psychol. 2019, 15, 399–423. [Google Scholar] [CrossRef] [PubMed]
- Landry, M.; Lévesque, D.; Di Paolo, T. Estrogenic Properties of Raloxifene, but Not Tamoxifen, on D2 and D3 Dopamine Receptors in the Rat Forebrain. Neuroendocrinology 2002, 76, 214–222. [Google Scholar] [CrossRef]
- Rachon, D.; Mysliwska, J.; Suchecka-Rachon, K.; Wieckiewicz, J.; Mysliwski, A. Effects of oestrogen deprivation on interleukin-6 production by peripheral blood mononuclear cells of postmenopausal women. J. Endocrinol. 2002, 172, 387–395. [Google Scholar] [CrossRef]
- Brinton, R.D.; Thompson, R.F.; Foy, M.R.; Baudry, M.; Wang, J.; Finch, C.E.; Morgan, T.E.; Pike, C.J.; Mack, W.J.; Stanczyk, F.Z.; et al. Progesterone receptors: Form and function in brain. Front. Neuroendocrinol. 2008, 29, 313–339. [Google Scholar] [CrossRef]
- Hara, Y.; Waters, E.M.; McEwen, B.S.; Morrison, J.H. Estrogen Effects on Cognitive and Synaptic Health Over the Lifecourse. Physiol. Rev. 2015, 95, 785–807. [Google Scholar] [CrossRef]
- Wallace, M.; Luine, V.; Arellanos, A.; Frankfurt, M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 2006, 1126, 176–182. [Google Scholar] [CrossRef]
- Weber, M.T.; Maki, P.M.; McDermott, M.P. Cognition and mood in perimenopause: A systematic review and meta-analysis. J. Steroid Biochem. Mol. Biol. 2014, 142, 90–98. [Google Scholar] [CrossRef]
- Bortz, J.; Klatt, K.C.; Wallace, T.C. Perspective: Estrogen and the Risk of Cognitive Decline: A Missing Choline(rgic) Link? Adv. Nutr. 2022, 13, 376–387. [Google Scholar] [CrossRef]
- Le, J.; Thomas, N.; Gurvich, C. Cognition, The Menstrual Cycle, and Premenstrual Disorders: A Review. Brain Sci. 2020, 10, 198. [Google Scholar] [CrossRef]
- Rajendra Acharya, U.; Paul Joseph, K.; Kannathal, N.; Lim, C.M.; Suri, J.S. Heart rate variability: A review. Med. Bio. Eng. Comput. 2006, 44, 1031–1051. [Google Scholar] [CrossRef]
- Schulz, S.; Koschke, M.; Bär, K.-J.; Voss, A. The altered complexity of cardiovascular regulation in depressed patients. Physiol. Meas. 2010, 31, 303–321. [Google Scholar] [CrossRef]
- Kamath, M.V.; Fallen, E.L. Power spectral analysis of heart rate variability: A noninvasive signature of cardiac autonomic function. Crit. Rev. Biomed. Eng. 1993, 21, 245–311. [Google Scholar]
- Rimoldi, O.R.; Pierini, S.I.; Ferrari, A.N.; Cerutti, S.E.; Pagani, M.A.; Malliani, A.L. Analysis of short-term oscillations of R-R and arterial pressure in conscious dogs. Am. J. Physiol. 1990, 258, H967–H976. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E. Heart rate variability-a historical perspective. Front. Physiol. 2011, 2, 86. [Google Scholar] [CrossRef]
- Mulcahy, J.S.; Larsson, D.E.O.; Garfinkel, S.N.; Critchley, H.D. Heart rate variability as a biomarker in health and affective disorders: A perspective on neuroimaging studies. Neuroimage 2019, 202, 116072. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Åhs, F.; Fredrikson, M.; Sollers III, J.J.; Wager, T.D. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef]
- Pham, T.; Lau, Z.J.; Chen, S.H.A.; Makowski, D. Heart Rate Variability in Psychology: A Review of HRV Indices and an Analysis Tutorial. Sensors 2021, 21, 3998. [Google Scholar] [CrossRef]
- Balzarotti, S.; Biassoni, F.; Colombo, B.; Ciceri, M.R. Cardiac vagal control as a marker of emotion regulation in healthy adults: A review. Biol. Psychol. 2017, 130, 54–66. [Google Scholar] [CrossRef]
- Beauchaine, T.P.; Thayer, J.F. Heart rate variability as a transdiagnostic biomarker of psychopathology. Int. J. Psychophysiol. 2015, 98, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Sgoifo, A.; Carnevali, L.; Pico Alfonso, M.D.L.A.; Amore, M. Autonomic dysfunction and heart rate variability in depression. Stress 2015, 18, 343–352. [Google Scholar] [CrossRef]
- Mankoo, A.; Roy, S.; Davies, A.; Panerai, R.B.; Robinson, T.G.; Brassard, P.; Beishon, L.C.; Minhas, J.S. The role of the autonomic nervous system in cerebral blood flow regulation in stroke: A review. Auton. Neurosci. 2023, 246, 103082. [Google Scholar] [CrossRef]
- Holwerda, S.W.; Luehrs, R.E.; DuBose, L.; Collins, M.T.; Wooldridge, N.A.; Stroud, A.K.; Fadel, P.J.; Abboud, F.M.; Pierce, G.L. Elevated Muscle Sympathetic Nerve Activity Contributes to Central Artery Stiffness in Young and Middle-Age/Older Adults. Hypertension 2019, 73, 1025–1035. [Google Scholar] [CrossRef]
- Kaufmann, H.; Biaggioni, I. Disorders of the Autonomic Nervous System. In Brocklehurst’s Textbook of Geriatric Medicine and Gerontology, 7th ed.; Elsevier: Philadelphia, PA, USA, 2010. [Google Scholar]
- Hall, C.; Chilcott, R. Eyeing up the Future of the Pupillary Light Reflex in Neurodiagnostics. Diagnostics 2018, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Woodhouse, J.M. The effect of pupil size on grating detection at various contrast levels. Vis. Res. 1975, 15, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Winston, M.; Zhou, A.; Rand, C.M.; Dunne, E.C.; Warner, J.J.; Volpe, L.J.; Pigneri, B.A.; Simon, D.; Bielawiec, T.; Gordon, S.C.; et al. Pupillometry measures of autonomic nervous system regulation with advancing age in a healthy pediatric cohort. Clin. Auton. Res. 2020, 30, 43–51. [Google Scholar] [CrossRef]
- Kaifie, A.; Reugels, M.; Kraus, T.; Kursawe, M. The pupillary light reflex (PLR) as a marker for the ability to work or drive–a feasibility study. J. Occup. Med. Toxicol. 2021, 16, 39. [Google Scholar] [CrossRef]
- Beatty, J.; Lucero-Wagoner, B. The pupillary system. In The handbook of Physiology; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Szabadi, E. Functional Organization of the Sympathetic Pathways Controlling the Pupil: Light-Inhibited and Light-Stimulated Pathways. Front. Neurol. 2018, 9, 1069. [Google Scholar] [CrossRef]
- Pinheiro, H.M.; Da Costa, R.M. Pupillary light reflex as a diagnostic aid from computational viewpoint: A systematic literature review. J. Biomed. Inform. 2021, 117, 103757. [Google Scholar] [CrossRef]
- Wong, H.K.; Epps, J. Pupillary transient responses to within-task cognitive load variation. Comput. Methods Programs Biomed. 2016, 137, 47–63. [Google Scholar] [CrossRef]
- Ebitz, R.B.; Moore, T. Selective Modulation of the Pupil Light Reflex by Microstimulation of Prefrontal Cortex. J. Neurosci. 2017, 37, 5008–5018. [Google Scholar] [CrossRef]
- Ebitz, R.B.; Pearson, J.M.; Platt, M.L. Pupil size and social vigilance in rhesus macaques. Front. Neurosci. 2014, 8, 100. [Google Scholar] [CrossRef]
- Critchley, H.D.; Tang, J.; Glaser, D.; Butterworth, B.; Dolan, R.J. Anterior cingulate activity during error and autonomic response. NeuroImage 2005, 27, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Bitsios, P.; Szabadi, E.; Bradshaw, C.M. Sensitivity of the fear-inhibited light reflex to diazepam. Psychopharmacology 1998, 135, 93–98. [Google Scholar] [CrossRef]
- Crombie, D.; Spacek, M.A.; Leibold, C.; Busse, L. Spiking activity in the visual thalamus is coupled to pupil dynamics across temporal scales. PLoS Biol. 2024, 22, e3002614. [Google Scholar] [CrossRef]
- Samuels, E.; Szabadi, E. Functional Neuroanatomy of the Noradrenergic Locus Coeruleus: Its Roles in the Regulation of Arousal and Autonomic Function Part II: Physiological and Pharmacological Manipulations and Pathological Alterations of Locus Coeruleus Activity in Humans. Curr. Neuropharmacol. 2008, 6, 254–285. [Google Scholar] [CrossRef] [PubMed]
- Mathôt, S.; Van Der Linden, L.; Grainger, J.; Vitu, F. The Pupillary Light Response Reveals the Focus of Covert Visual Attention. PLoS ONE 2013, 8, e78168. [Google Scholar] [CrossRef] [PubMed]
- Schmalenberger, K.M.; Eisenlohr-Moul, T.A.; Jarczok, M.N.; Eckstein, M.; Schneider, E.; Brenner, I.G.; Duffy, K.; Schweizer, S.; Kiesner, J.; Thayer, J.F.; et al. Menstrual Cycle Changes in Vagally-Mediated Heart Rate Variability Are Associated with Progesterone: Evidence from Two Within-Person Studies. J. Clin. Med. 2020, 9, 617. [Google Scholar] [CrossRef]
- Schmalenberger, K.M.; Eisenlohr-Moul, T.A.; Würth, L.; Schneider, E.; Thayer, J.F.; Ditzen, B.; Jarczok, M.N. A Systematic Review and Meta-Analysis of Within-Person Changes in Cardiac Vagal Activity across the Menstrual Cycle: Implications for Female Health and Future Studies. J. Clin. Med. 2019, 8, 1946. [Google Scholar] [CrossRef]
- Blake, E.F.; Eagan, L.E.; Ranadive, S.M. Heart rate variability between hormone phases of the menstrual and oral contraceptive pill cycles of young women. Clin. Auton. Res. 2023, 33, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Brar, T.K. Effect of Different Phases of Menstrual Cycle on Heart Rate Variability (HRV). J. Clin. Diagn. Res. JCDR 2015, 9, CC01. [Google Scholar] [CrossRef]
- Hamidovic, A.; Davis, J.; Wardle, M.; Naveed, A.; Soumare, F. Periovulatory Subphase of the Menstrual Cycle Is Marked by a Significant Decrease in Heart Rate Variability. Biology 2023, 12, 785. [Google Scholar] [CrossRef] [PubMed]
- Shankhwar, V.; Urvec, J.; Steuber, B.; Schmid Zalaudek, K.; Saloň, A.; Hawliczek, A.; Bergauer, A.; Aljasmi, K.; Abdi, A.; Naser, A.; et al. Effects of menstrual cycle on hemodynamic and autonomic responses to central hypovolemia. Front. Cardiovasc. Med. 2024, 11, 1290703. [Google Scholar] [CrossRef]
- Ramesh, S.; James, M.T.; Holroyd-Leduc, J.M.; Wilton, S.B.; Sola, D.Y.; Ahmed, S.B. Heart rate variability as a function of menopausal status, menstrual cycle phase, and estradiol level. Physiol. Rep. 2022, 10, e15298. [Google Scholar] [CrossRef] [PubMed]
- Kayacan, Y.; Makaracı, Y.; Ozgocer, T.; Ucar, C.; Yıldız, S. Cortisol Awakening Response and Heart Rate Variability in the Menstrual Cycle of Sportswomen. Res. Q. Exerc. Sport 2021, 92, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Mascone, S.E.; Jacob, D.W.; Eagan, L.E.; Harper, J.L.; Limberg, J.K.; Ranadive, S.M. Naturally menstruating women exhibit lower cardiovagal baroreflex sensitivity than oral contraceptive users during the lower hormone phase. Exp. Physiol. 2023, 108, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Sato, M.; Umehara, S.; Nishikawa, T. Influence of menstrual cycle on baroreflex control of heart rate: Comparison with male volunteers. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2003, 285, R1091–R1097. [Google Scholar] [CrossRef] [PubMed]
- Minson, C.T.; Halliwill, J.R.; Young, T.M.; Joyner, M.J. Influence of the Menstrual Cycle on Sympathetic Activity, Baroreflex Sensitivity, and Vascular Transduction in Young Women. Circulation 2000, 101, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.R.; Lawrence, J.E. Effects of the menstrual cycle on sympathetic neural responses to mental stress in humans. J. Physiol. 2007, 585, 635–641. [Google Scholar] [CrossRef]
- Anand, N.S.; Ridhi, S. The influence of different phases of menstrual cycle on autonomic reactivity to a cognitive and emotional stressor-A cross sectional study: Menstrual cycle influencing emotion and cognition. Ind. J. Physiol. Allied. Sci. 2022, 73, 10–15. [Google Scholar] [CrossRef]
- Wang, C.A.; Baird, T.; Huang, J.; Coutinho, J.D.; Brien, D.C.; Munoz, D.P. Arousal Effects on Pupil Size, Heart Rate, and Skin Conductance in an Emotional Face Task. Front. Neurol. 2018, 9, 1029. [Google Scholar] [CrossRef]
- Charkoudian, N. Influences of female reproductive hormones on sympathetic control of the circulation in humans. Clin. Auton. Res. 2001, 11, 295–301. [Google Scholar] [CrossRef]
- Bradley, M.M.; Miccoli, L.; Escrig, M.A.; Lang, P.J. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology 2008, 45, 602–607. [Google Scholar] [CrossRef]
- Unsworth, N.; Robison, M.K. Pupillary correlates of lapses of sustained attention. Cogn. Affect. Behav. Neurosci. 2016, 16, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Ferencová, N.; Višňovcová, Z.; Bona Olexová, L.; Tonhajzerová, I. Eye pupil–a window into central autonomic regulation via emotional/cognitive processing. Physiol. Res. 2021, 70, S669–S682. [Google Scholar] [CrossRef] [PubMed]
- Einhäuser, W. The Pupil as Marker of Cognitive Processes. In Computational and Cognitive Neuroscience of Vision; Zhao, Q., Ed.; Springer: Singapore, 2017; pp. 141–169. [Google Scholar]
- Thayer, J.F.; Hansen, A.L.; Saus-Rose, E.; Johnsen, B.H. Heart Rate Variability, Prefrontal Neural Function, and Cognitive Performance: The Neurovisceral Integration Perspective on Self-regulation, Adaptation, and Health. Ann. Behav. Med. 2009, 37, 141–153. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Lischke, A.; Pahnke, R.; Mau-Moeller, A.; Behrens, M.; Grabe, H.J.; Freyberger, H.J.; Hamm, A.O.; Weippert, M. Inter-individual differences in heart rate variability are associated with inter-individual differences in empathy and alexithymia. Front. Psychol. 2018, 9, 229. [Google Scholar] [CrossRef]
- Mauersberger, H.; Tune, J.L.; Kastendieck, T.; Czarna, A.Z.; Hess, U. Higher heart rate variability predicts better affective interaction quality in non-intimate social interactions. Psychophysiology 2022, 59, e14084. [Google Scholar] [CrossRef]
- Ramírez, E.; Ortega, A.R.; Del Paso, G.A. Anxiety, attention, and decision making: The moderating role of heart rate variability. Int. J. Psychophysiol. 2015, 98, 490–496. [Google Scholar] [CrossRef]
- Diamond, L.M.; Fagundes, C.P.; Butterworth, M.R. Attachment Style, Vagal Tone, and Empathy During Mother–Adolescent Interactions. J. Res. Adolesc. 2012, 22, 165–184. [Google Scholar] [CrossRef]
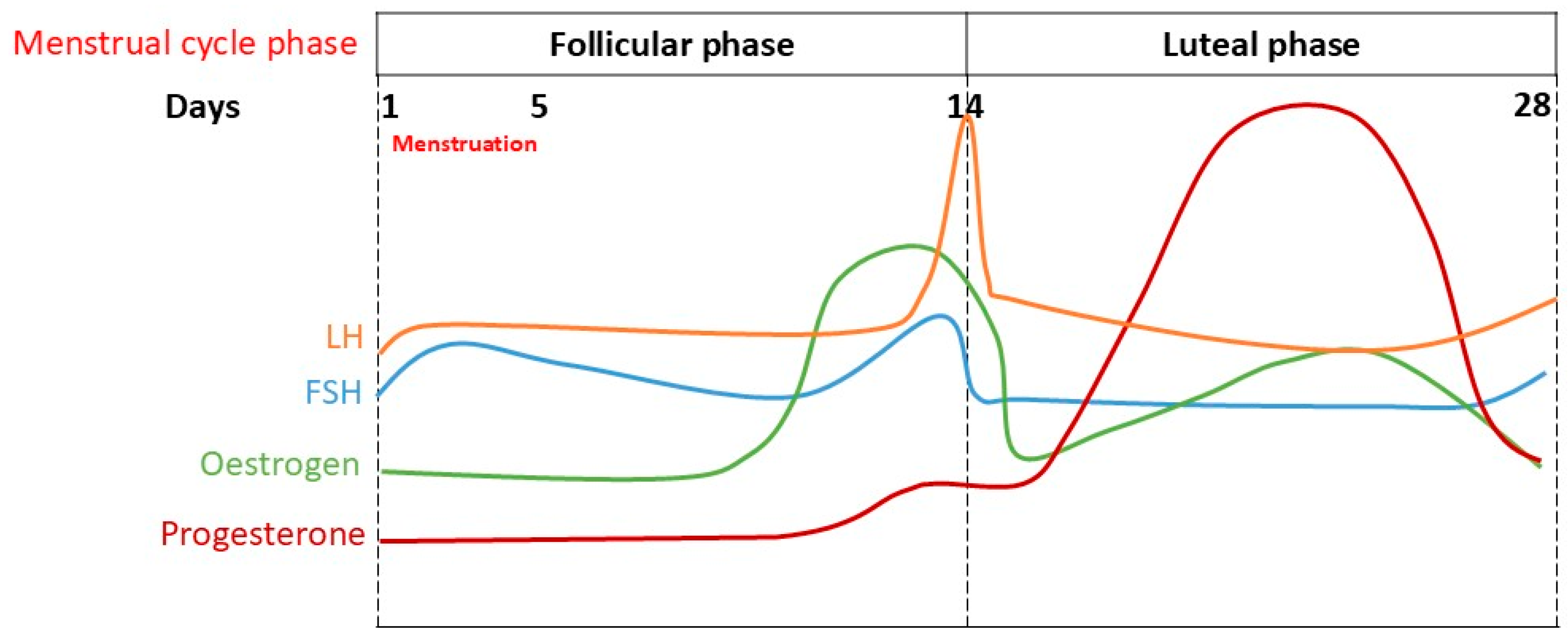

| Menstrual Stage | Key Hormonal Changes | Physiological and Psychological Characteristics |
|---|---|---|
| Menstruation and early follicular phase | Low oestrogen; low progesterone. | This phase is characterised by higher sympathetic activity and is associated with a lower baroreflex sensitivity (BRS), higher mean heart rate (HR), and higher low-frequency (LF) components of heart rate variability (HRV) compared to the late follicular phase. This phase may involve lower mood and increased muscle sympathetic nerve activity (MSNA). Reduced vagal modulation is common. |
| Follicular Phase | Oestrogen production gradually increases, peaking before ovulation. Some progesterone is also produced. Surge in luteinising hormone (LH) and follicle-stimulating hormone (FSH) at the end of this phase. | As oestrogen rises, there is an association with increased parasympathetic activity, but also reports of reduced vagal modulation. Studies show mixed results for HRV measures in this phase. MSNA can be higher early in this phase compared to the luteal phase, and a lower BRS is seen during its early part. Oestrogen’s impact on memory tasks has been observed, and it interacts with the cholinergic system. The LH surge, occurring just before ovulation, is linked to elevated pupillary light reflex (PLR) activity, suggesting a more active autonomic response. |
| Ovulation | Peak oestrogen, surge in luteinising hormone (LH) and follicle-stimulating hormone (FSH). | This is the release of a mature egg from the ovary, triggered by the LH and FSH surge. It is associated with high oestrogen levels. Additionally, an elevated PLR activity has been linked to the high LH levels, indicating a more active autonomic response during this phase. |
| Luteal Phase | Gradual increase in progesterone levels and an initial drop in the oestrogen levels followed by a gradual rise. | Characterised by reduced parasympathetic activity. Increased cardio-vagal activity is associated with positive affect, while decreased activity is linked to stress. A lower BRS is observed, especially in the late luteal phase for women with premenstrual syndrome (PMS). Some studies indicate higher vagally mediated heart rate variability (vmHRV), while others report reduced vmHRV in women with more severe cases of PMS. The rise in progesterone is linked to increased sympathetic activity and larger pupil sizes. |
| Topic | Finding | Source |
|---|---|---|
| Emotional Valence | Phases with low oestrogen levels are associated with low mood in healthy biological women and depression in PMDD patients. Oestrogen affects dopamine metabolism, potentially reducing depression, and has anti-inflammatory effects related to depression. | [11,12,13] |
| Cognitive Functioning | Cognitive function is influenced by the menstrual cycle, with both progesterone and oestrogen affecting cognition. Oestrogen impacts memory tasks and interacts with the cholinergic system, while progesterone has neuroprotective effects. Inconsistent findings exist. | [14,15,16,17,18,19] |
| Heart Rate Variability (HRV) | A meta-analysis suggested a significant decrease in cardiac vagal activity from the follicular to the luteal phase. Other studies show increased vmHRV during the luteal phase; a higher mean HR, LF, and HF during menstruation compared to the follicular phase; and reduced vmHRV during the luteal phase in women with more severe cases of PMS. | [6,49,50,51,53,54,55,56] |
| Baroreflex Sensitivity (BRS) | BRS was lower in naturally menstruating women during the early follicular/placebo pill phases compared to women on OCP, and higher oestrogen (but not progesterone) predicted a lower BRS. Higher progesterone predicted a lower BRS during the late follicular to early luteal/active pill phase. A lower BRS was found in the late luteal phase in women with PMS. | [9,57,58] |
| Muscle Sympathetic Nerve Activity (MSNA) | Some studies found significantly higher MSNA during the early follicular phase compared to the mid-luteal phase. Other studies found no difference in MSNA at rest or during mental stress between the early follicular and mid-luteal phases. | [32,59,60] |
| Pupillary Light Reflex (PLR) | Oestrogen rise in the follicular phase is linked to increased parasympathetic activity and smaller pupil sizes. Progesterone rise in the luteal phase is linked to increased sympathetic activity and larger pupil sizes. More research is needed due to inconsistent results. | [48,54,62,63,64,65,66,67,68] |
| ANS, Cognition, and Emotional Valence | Reduced vagal modulation is often observed during the luteal phase. Increased cardio-vagal activity is associated with positive affect, while a decrease is linked to stress. The HF component of HRV (a measure of cardio-vagal modulation) correlates positively with empathy, social interaction, attention, and executive function. | [28,68,69,70,71,72,73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, S.; Agordati, E.; Wilcockson, T.D.W. Autonomic Nervous System, Cognition, and Emotional Valence During Different Phases of the Menstrual Cycle—A Narrative Review. NeuroSci 2025, 6, 78. https://doi.org/10.3390/neurosci6030078
Roy S, Agordati E, Wilcockson TDW. Autonomic Nervous System, Cognition, and Emotional Valence During Different Phases of the Menstrual Cycle—A Narrative Review. NeuroSci. 2025; 6(3):78. https://doi.org/10.3390/neurosci6030078
Chicago/Turabian StyleRoy, Sankanika, Elettra Agordati, and Thomas D. W. Wilcockson. 2025. "Autonomic Nervous System, Cognition, and Emotional Valence During Different Phases of the Menstrual Cycle—A Narrative Review" NeuroSci 6, no. 3: 78. https://doi.org/10.3390/neurosci6030078
APA StyleRoy, S., Agordati, E., & Wilcockson, T. D. W. (2025). Autonomic Nervous System, Cognition, and Emotional Valence During Different Phases of the Menstrual Cycle—A Narrative Review. NeuroSci, 6(3), 78. https://doi.org/10.3390/neurosci6030078






