A Systematic Review of Lifestyle Interventions for Neuropathy and Neuropathic Pain: Smoking Cessation
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion/Exclusion Criteria According to PICO Framework
2.2. Search Strategy
2.3. Study Selection
2.4. Risk of Bias and Certainty of Evidence
2.5. Meta-Analysis
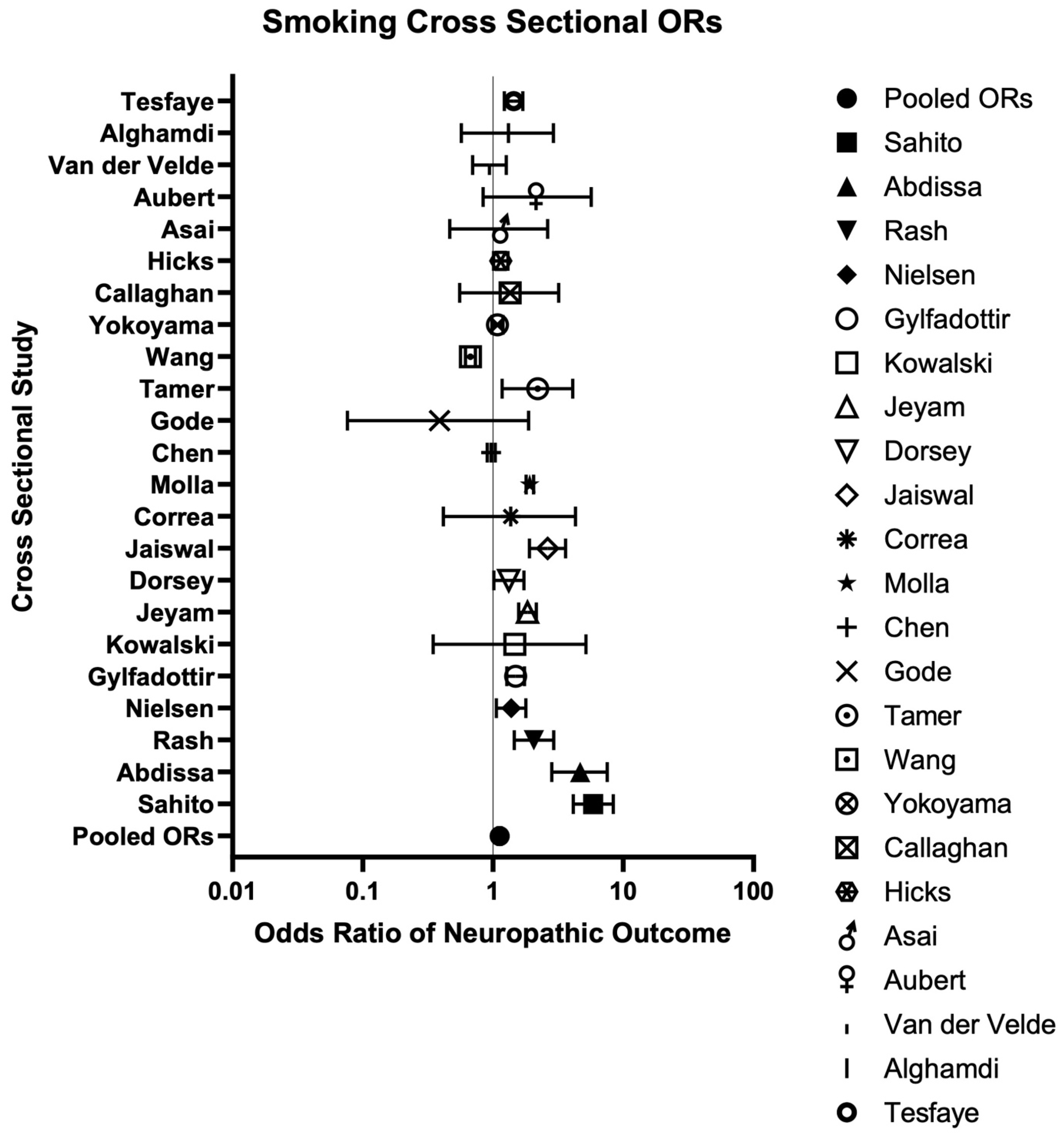
3. Results
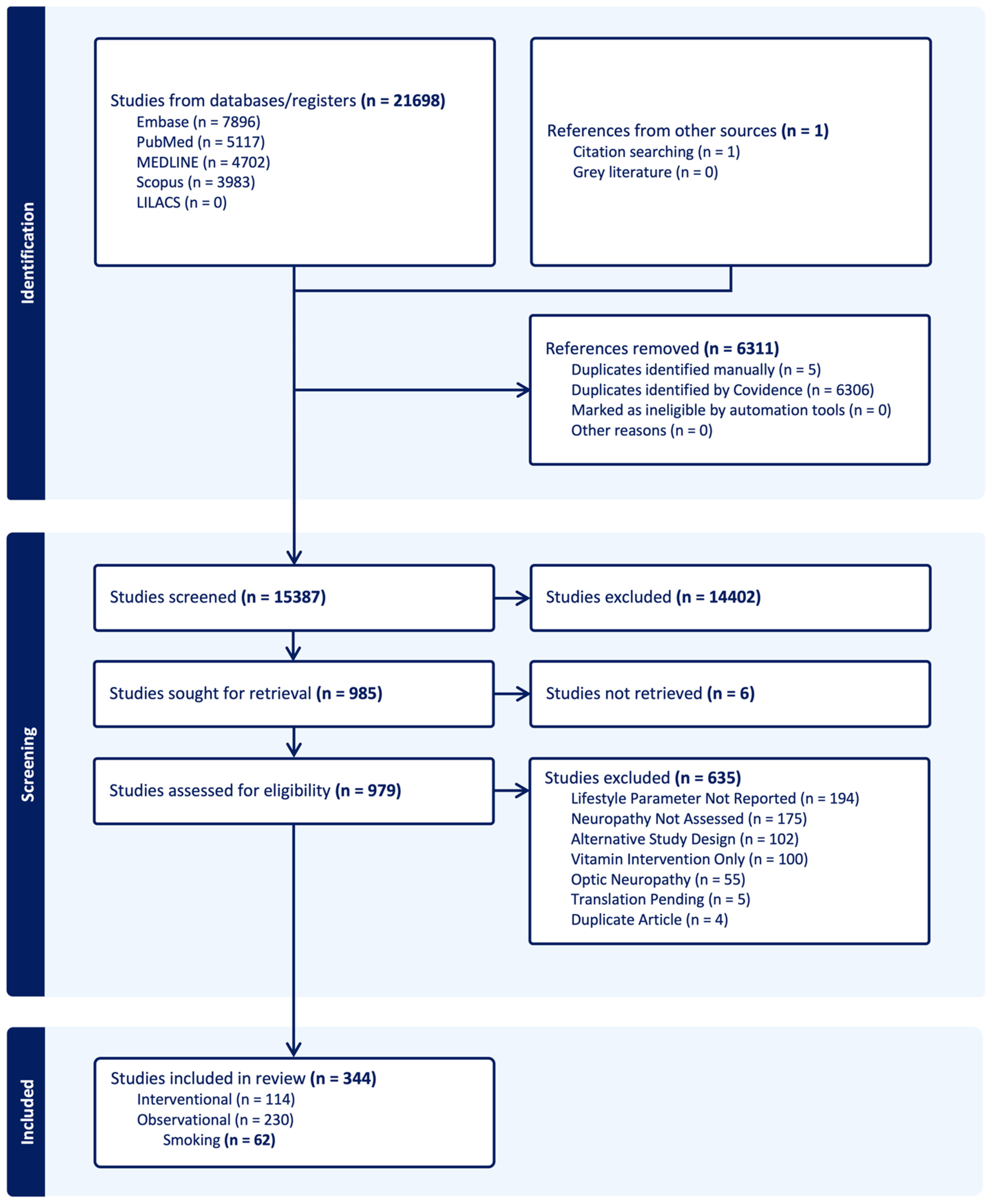
3.1. Literature Search
| Author (Year) | Study Design | Setting | N | Sex N (F:M) | Age (Mean ± SD (Range)) | Population/Etiology | Lifestyle | Outcomes |
|---|---|---|---|---|---|---|---|---|
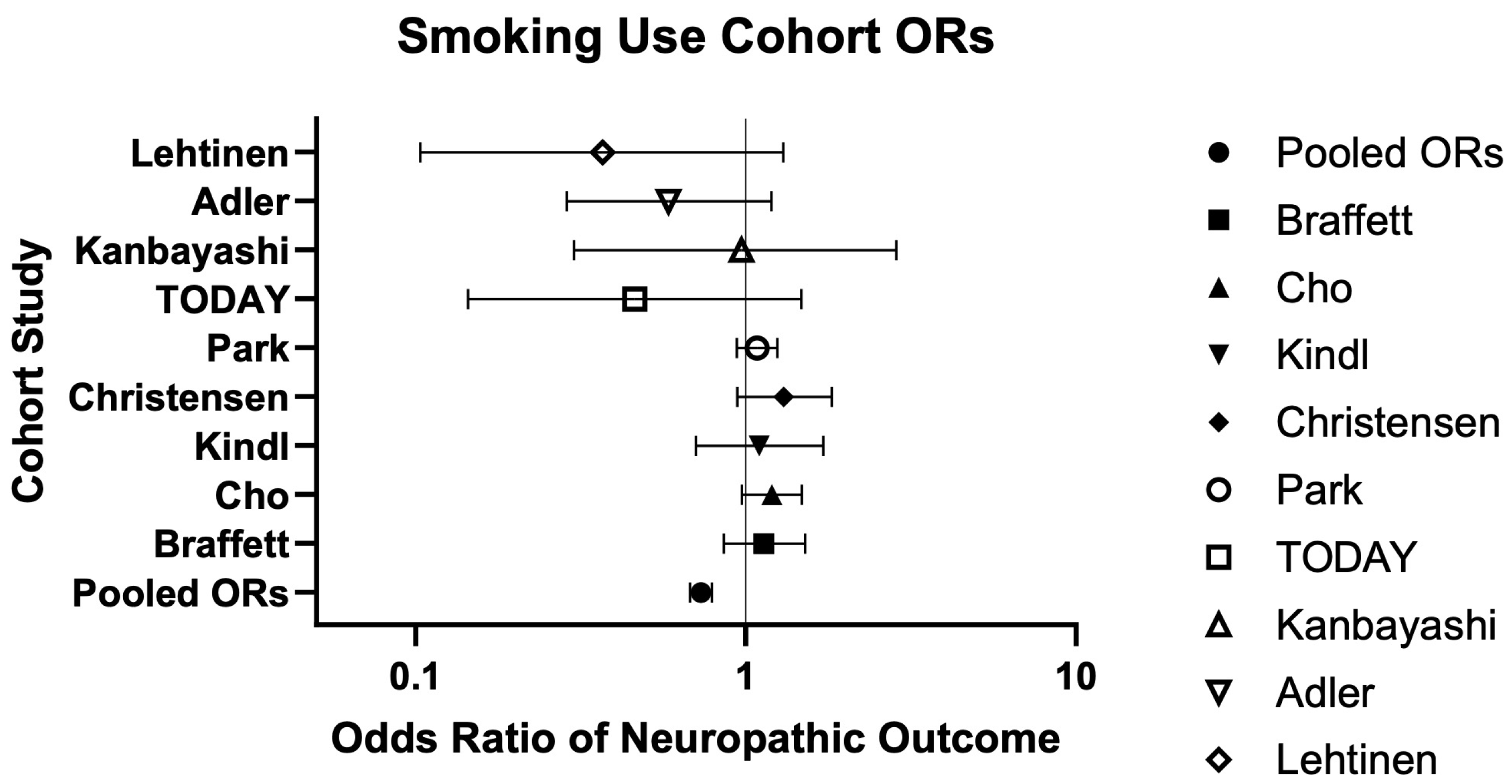
| Adler (1997) [25] | Cohort Study | US | With Ne: 58; Without Ne: 230 | With Ne: 1:57; Without Ne: 11:219 | With Ne: 64.0; Without Ne: 61.5 | DM ± Incident Ne | Smoking: ever, current | Current smoking was significantly associated with lower odds of incident Ne (10.3% vs. 26.1%, p = 0.011; β = −1.52, SE = 0.5635, 0.22 [0.07–0.66], p = 0.007). Authors suggest individuals with Ne may quit smoking due to emerging impairment (reverse causation—protopathic bias). |
| Braffett (2020) [26] | Cohort Study | US | With DPN: 455; Without DPN: 931 | With DPN: 182:273; Without DPN: 475:456 | ^ With DPN: 29 (24, 34); Without DPN 26 (21, 32) | T1DM ± DPN | Cigarette Smoker | Cigarette smoking was not significantly associated with DPN (0.98 [0.75–1.29], p = 0.89). |
| Cheng (2022) [27] | Cohort Study | China | Overall: 1091; With DPN: 793; Without DPN: 298 | Overall: 413:678; With DPN: 282:511; Without DPN: 131:167 | With DPN: 60.40 ± 11.53; Without DPN: 54.79 ± 12.32 | T2DM ± DPN | Smoking: Yes, No | Prevalence of smoking was significantly greater in those with DPN vs. those without (33.5% vs. 26.2%, p = 0.020), but was insignificant in multivariate analysis. |
| Cho (2024) [28] | Cohort Study | Korea | Overall: 2316; Non-smoker: 549; Ex-smoker: 924; Current Smoker: 843 | 0:2316 | Non-smoker: 53.5 ± 11.3; Ex-smoker: 53.9 ± 9.7; Current Smoker: 49.4 ± 9.3 | T2DM ± Ne | Smoking: Non, Ex, Current | Current smoking was significantly associated with increased odds of DN (1.38 [1.05–1.81], p = 0.021). |
| Christensen (2020) [29] | Cohort Study | Denmark | Overall: 5249; With DPoN: 938; With DPoN + Pain: 386 | Overall: 2205:3144 | ^ 65 (57, 72) | T2DM ± DPoN ± Pain | Smoking: Never, Former, Current, Discontinued, Continued | Current and former smoking were significantly associated with DPoN (aPR = 1.50 [1.24–1.81]; aPR = 1.27 [1.06–1.52]). Current smoking was also significantly associated with NP in DoPN (aPR = 1.29 [1.03–1.62]). |
| Kanbayashi (2022) [30] | Cohort Study | Japan | Overall: 38; Data Points: 76 | 76:0 | ^^ CTCAE 0: 59 (45–73); 1: 58 (34–76); 2: 61 (40–66); 3: 66 (62–70) | Breast Cancer ± CIPN (Nab-paclitaxel) | Smoking History: Yes, No | Smoking history was significantly associated with CIPN on both CTCAE and PNQ (sensory only) during univariate (4.64 [1.60–13.5], p = 0.0048; 3.80 [1.40–10.30], p = 0.0087) and multivariate (4.79 [1.65–13.92], p = 0.004; 3.65 [1.38–9.69], p = 0.0093) analyses. |
| Khan (2023) [31] | Cohort Study | US | TUD: 8009; TAUD: 1672; PSUD: 642; TUD Co: 8009; TAUD Co: 1672; PSUD Co: 642 | TUD: 4660:3349; TAUD: 582:1090; PSUD: 233:409; TUD Co: 4665:3344; TAUD Co: 584:1088; PSUD Co: 234:408 | TUD: 61.6 ± 12.1; TAUD: 61.52 ± 10.3; PSUD: 57.84 ± 8.3; TUD Co: 61.6 ± 12.1; TAUD Co: 61.42 ± 10; PSUD Co: 57.88 ± 8.1 | T2DM + Hypertension ± Neuropathy | TUD: Yes, No; TAUD: Yes, No; PSUD: Yes, No | DN was significantly higher in those with TUD vs. without (19.7% vs. 14.2%, 1.48 [1.35–1.61], p < 0.05). |
| Kindl (2021) [32] | Cohort Study | Germany | With MSK: 255; With CRPS: 223 | With MSK: 160:95; With CRPS: 173:50 | With MSK: 54.6 (20–80); With CRPS: 50.9 (18–77) | CRPS or MSK, due to trauma | Smoking: Yes, No | Smoking was significantly associated with higher current pain scores (NSRC: −0.252, SE: 0.118, SRC: −0.09, both p < 0.05) |
| Lehtinen (1993) [33] | Cohort Study | Finland | With ND: 12; Without ND: 101 | With ND: 9:3; Without ND: 46:55 | With ND: 57.2 ± 4.7; Without ND: 55.4 ± 10.4 | DM ± ND | Smoking history | Smoking history was not significantly different between ND groups (25% vs. 48%, p > 0.05). |
| Park (2023) [34] | Cohort Study | Korea | Overall: 26,673; Never Smoked: 13,426 (Group 1: 8260; Group 2: 4106; Group 3: 1060); Quitting: 3426 (Group 4: 2848; Group 5: 578); Current Smokers: 9821 (Group 6: 2876; Group 7: 6945) | 0:26,673 | Overall: 59.6 ± 9; Group 1: 61.8 ± 9.4; Group 2: 61.3 ± 9.3; Group 3: 58.2 ± 8.9; Group 4: 58.7 ± 8.7; Group 5: 57.4 ± 8.3; Group 6: 57.8 ± 8.2; Group 7: 57.5 ± 8.1 | T1DM or T2DM ± Ne | Smoking status: Group 1: never–never; Group 2: never–quitting; Group 3: never–current; Group 4: quitting–quitting; Group 5: quitting–current; Group 6: current–quitting; Group 7: current–current | Current–quitting smoking, current–current smoking, and heavy smoking (>20 pack-yrs) were significantly associated with DN (1.360 [1.076–1.719]; 1.237 [1.025–1.492]; 1.246 [1.048–1.481]). |
| Sreeram (2023) [35] | Cohort Study | US | Overall: 1034; With CIPN: 704; Without CIPN: 330 | Overall: 797:237; With CIPN: 570:134; Without CIPN: 227:103 | Overall: 57.1 ± 10.9 (27–79); With CIPN: 55.8 ± 10.8 (27–79); Without CIPN: 59.9 ± 10.4 (27–79) | Cancer survivors ± CIPN | Smoking (>100 cig/lifetime): Yes, No | Smoking was significantly different between CIPN groups (p = 0.004); however, it was not significantly associated with CIPN in multivariate analysis (1.20 [0.87–1.65], p = 0.27). |
| Today Study Group (2022) [36] | Cohort Study | US | Overall: 674; Normal MNSI: 528; Abnormal MNSI: 146; Normal Monofilament: 643; Abnormal Monofilament: 31 | Normal MNSI: 171:357; Abnormal MNSI: 65:81; Normal Monofilament: 219:424; Abnormal Monofilament: 17:14 | Normal MNSI: 13.9 ± 2; Abnormal MNSI: 14.3 ± 1.9; Normal Monofilament: 14 ± 2; Abnormal Monofilament: 14.2 ± 2.1 | T2DM ± DPN | Smoking Status: Yes (within past mo), No (never or not within past mo) | Smoking was not significantly associated with DPN via abnormal MNSI (HR: 0.99 [0.83–1.65], p = 0.97) or monofilament (HR: 1.02 [0.33–3.17], p = 0.97). |
| Voulgari (2011) [37] | Cohort Study | Greece | 193 | 97:96 | 56.4 ± 7.8 | T2DM ± PN | Smoking: Cessation vs. Continuing | Smoking cessation was significantly associated with a reduction in PN at 1 yr (p < 0.05). |
| Benbow (1997) [38] | Case–Control Study | UK | With Pain: 49 (Current Smokers: 13; Ex-smokers: 15; Non-smokers: 21); Without Pain: 23 | With Pain: 14:35 (Current Smokers: 4:9; Ex-smokers: 3:12; Non-smokers: 7:14); Without Pain: 9:14 | With Pain: (Current Smokers: 54.2 ± 3.2; Ex-smokers: 57 ± 2.9; Non-smokers: 58 ± 2.9); Without Pain: 51 ± 1.9 | DM ± PDN | Smoking: Current, Ex, Non; Pack-Yrs (20 cig/d for 1 yr = 1 pack-yr) | Current or past smoking was not significantly associated with the severity or duration of PDN (p > 0.05). |
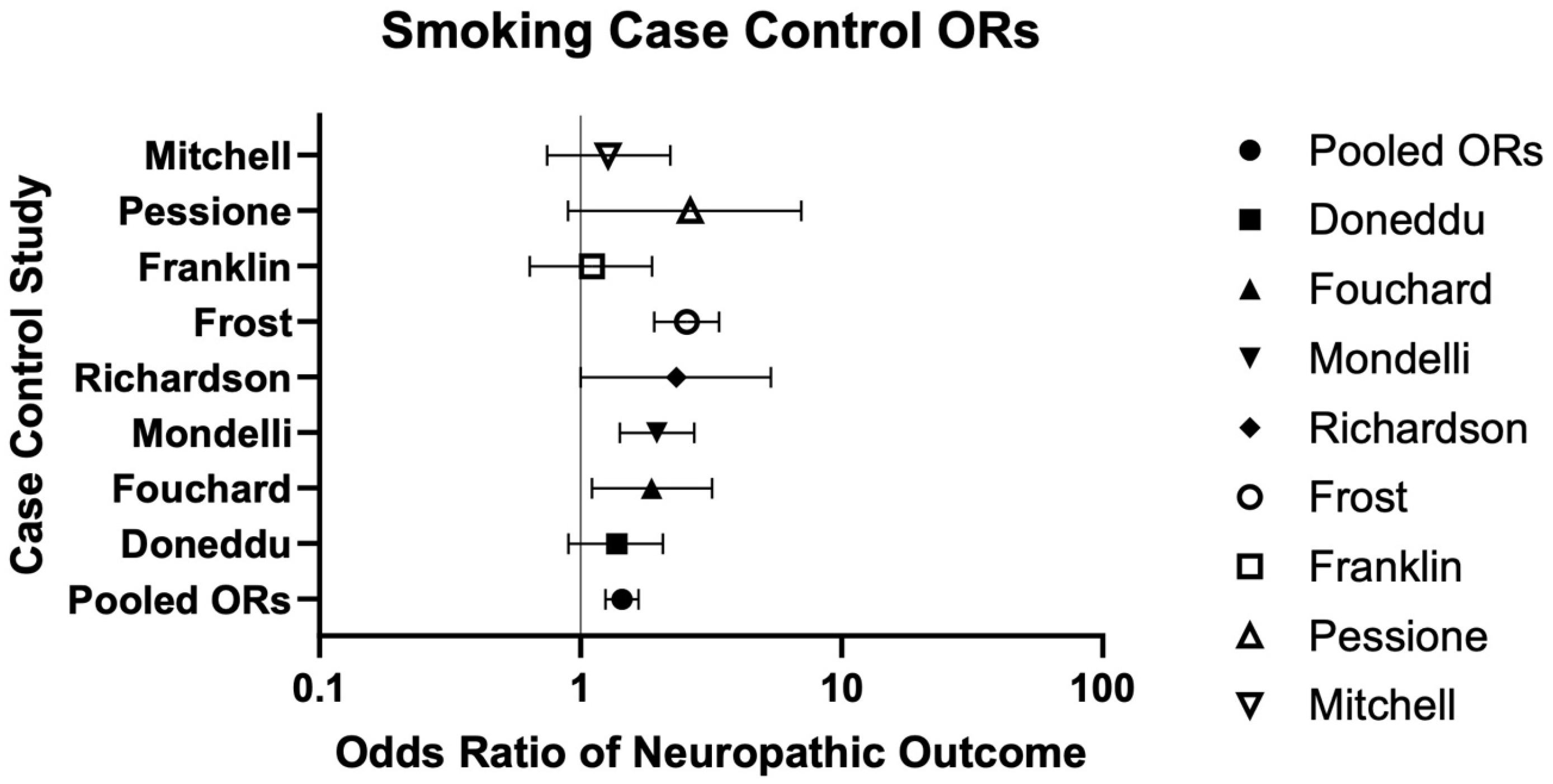
| Doneddu (2020) [39] | Case–Control Study | Italy | Ca: 195; Co: 195 | Ca: 109:86; Co: 109:86 | NR | CIDP due to any etiology and their partners | Smoking Status: Yes (Current or Past), No | Smoking was not significantly associated with CIDP (1.43 [0.93–2.20], p = 0.1056). |
| Fouchard (2023) [40] | Case–Control Study | France | Overall: 323; Ca: 162; Co: 161 | Overall: 192:131; Ca: 88:74; Co: 104:57 | Ca: 56 ± 16; Co: 69 ± 13 | Cutaneous paresthesia ± SFN via IENFD due to any etiology | Smoking: Yes, No | Smoking was significantly higher in those with SFN vs. without (26.5% vs.16.1%, p = 0.02). |
| Franklin (1994) [41] | Case–Control Study | US | Ca: 77; Co: 200 | Ca: 29:48; Co: 118:82 | Ca: 61.7; Co: 58.6 | NIDDM ± DSN | Smoking: never, pack-years (<20, >20) | Smoking (pack-years: <20, >20) was not significantly associated with DSN (1.49 [0.69–3.22] p = 0.29, 0.74 [0.29–1.84]). |
| Frost (2013) [42] | Case–Control Study | Denmark | Ca: 324; Co: 832 | Ca: 121:203; Co 317:515 | Ca Smokers: 49 ± 9.7; Ca Non-smokers: 44 ± 11.5; Co Smokers: 50 ± 9; Co Non-smokers: 48 ± 9.9 | Ca: Electroneurographically confirmed UN; Co: Without UN | Smoking: ever, never, pack-years | Pack-years (>10-<24 and >24) were significantly associated with UN (2.58 [1.48–4.51], 1.65 [1.37–1.99]). Current smoking was more significantly associated with UN (4.09 [2.43–6.86]) vs. ex-smokers (1.36 [0.83–2.23]), with highest pack-years exhibiting stronger associations in current (5.00 [2.69–9.32]) vs. ex-smokers (2.61 [1.16–5.88]). Smoking (>3 pack-years) was significantly associated with severe ulnar nerve damage, including localized demyelination (2.11 [0.95–4.72]) and axonal degeneration (1.65 [0.66–4.14]). |
| Mitchell (1990) [43] | Case–Control Study | US | IDDM: Ca: 54, Co: 56; NIDDM: Ca: 39, Co: 65 | IDDM: Ca: 31:23, Co: 35:21; NIDDM: Ca: 25:14, Co: 44:21 | IDDM: Ca: 36.1, Co: 32.7; NIDDM: 59.4: 39, Co: 57.7 | DM ± Ne | Smoking: ever, median pack-yrs | Smoking (ever) was significantly higher in those with IDDM Ne vs. those without (64.8% vs. 42.8%, p < 0.05). Smoking (>30 median pack-yrs) was significantly associated with Ne in IDDM (40 vs. 0, p < 0.001; Mantel–Haenszel OR: 5.18 [1.91–14.1], p = 0.001; β = 1.2015, SE = 0.5397, 3.32 [1.15–9.58], p = 0.026). |
| Mondelli (2020) [44] | Case–Control Study | Italy | Ca: 220; Co: 460 | Ca: 84:136; Co: 242:218 | Ca: 51.7 ± 11.8; Co: 47.8 ± 12.4 | Ca: UNE; Co: Upper-limb complaints | Tobacco: Pack-Yrs | Smoking (>25 pack-yrs) was significantly associated with both UNE and idiopathic UNE in univariate (3.3 [2–5.4]; 2.6 [1.3–5.2]) and multivariable (2.3 [1.3–4.1]; 2.2 [1–5.2]) analyses. |
| Pessione (1995) [45] | Case–Control Study | France | Ca: 32; Co: 58 | Ca: 6:26; Co: 22:36 | Ca: 49 ± 10.1; Co: 46.8 ± 9.6 | Alcoholism ± PN | Smoking (current, former, never) | Tobacco consumption did not significantly differ between PN groups (p = 0.19). |
| Richardson (2009) [46] | Case–Control Study | US | Ca: 50; Co: 50 | Ca: 18:32; Co: 34:16 | Ca: 48.4 ± 12.8; Co: 39.2 ± 12 | Ca: With UNE; Co: Without UNE | Smoking: ever, remote (quit >10 yrs), pack-years | Ever smoking and pack-yrs were significantly greater in those with UNE vs. without (25 vs. 15, p = 0.041; 0.29 ± 0.41 vs. 0.13 ± 0.27, p = 0.025). Pack-years were significantly associated with UNE (1.035 [1.001–1.070], p = 0.049). Electrophysiology including CMAP, CV, and conduction block were significantly associated with pack-yrs (p < 0.05). |
| Abdissa (2020) [47] | Cross-Sectional Study | Ethiopia | Overall: 366; With DPN: 196; Without DPN: 170 | 163:203 | 50.1 ± 14.28 | T2DM ± DPN | Smoking: Current, Former, Never | Current and former smoking were both significantly associated with higher odds of DPN (7.96 [3.23–19.64], p < 0.001; 2.65 [1.22–5.77], p = 0.013). |
| Alghamdi (2022) [48] | Cross-Sectional Study | Saudi Arabia | Overall: 306; With Ne: 102; Without Ne: 102; Co: 102 | Overall: 147:159; With Ne: 49:53; Without Ne: 49:53; Co: 49:53 | With Ne: 54.1 ± 21.8; Without Ne: 54.1 ± 21.8; Co: 53.5 ± 13.1 | T1DM or T2DM ± DSyPN | Smoking: Yes (Ever), No (Never) | Smoking was not significantly different between DSyPN groups (13.7% vs. 16.7%, p > 0.05). |
| Asai (2022) [49] | Cross-Sectional Study | Japan | Overall: 817; With CP: 35; Without CP: 782 | Overall: 431:386; With CP: 24:11; Without CP: 407:375 | With CP: 63.91 [60.11–67.72]; Without CP: 63.75 [63.02–67.72] | Chronic neck/shoulder/upper-limb pain due to any etiology | Current smoker: Yes, No | Current smoking was not significantly different between CP groups (20% vs. 18%, p = 0.77). |
| Aubert (2014) [50] | Cross-Sectional Study | France | 198 | 40:158 | 65 (43–85) | T2DM ± PN | Current Smoking | Current smoking was significantly associated with greater PN severity when NDS was considered a continuous variable (p = 0.01) but was insignificant in univariate and multivariate models (p > 0.05). |
| Billault (1991) [51] | Cross-Sectional Study | France | 157 | 45:55 | 41.6 ± 15.3 | T1DM ± Ne | Tobacco Consumption: Pack-Yrs (20 cig/d for 1 yr = 1 pack-yr) | Tobacco consumption was not significantly different between Ne groups (11.98 ± 17.43 vs. 7.19 ± 12.93, p > 0.05). |
| Callaghan (2020) [52] | Cross-Sectional Study | US | BMI < 35 kg–Ne: 45; BMI > 35 kg–Ne: 110; BMI > 35 kg + Ne: 28 | BMI < 35 kg–Ne: 37:8; BMI > 35 kg–Ne: 87:23; BMI > 35 kg + Ne: 18:10 | BMI < 35 kg–Ne: 43.8 ± 12.1; BMI > 35 kg–Ne: 43.5 ± 11.2; BMI > 35 kg + Ne: 51.4 ± 9.6 | Ca: BMI > 35 kg ± Ne; Co: BMI < 25 kg BMI < 35 kg–Ne; BMI > 35 kg–Ne; BMI > 35 kg + Ne | Tobacco: Current, Never, Former | Smoking status was not significantly different between Ne groups (BMI < 35 kg–Ne: 82.2% vs. BMI > 35 kg–Ne: 70.9% vs. BMI > 35 kg + Ne: 64.3%, p = 0.50). |
| Çelik (2017) [53] | Cross-Sectional Study | Turkey | 444 | 190:254 | F: 42.9 ± 11.2; M: 43.4 ± 14 | NP due to any etiology | Smoking Duration (packs/yr) | Smoking duration was significantly higher in NP vs. without (31.8 ± 18.3 vs. 22.4 ± 15.5, p < 0.05), and addiction severity (on Fagerström scale) was significantly associated with pain existence (1.29 [1.14–1.46]). |
| Chen (2024) [54] | Cross-Sectional Study | China | Overall: 13,315; With DPN: 5847; Without DPN: 7468 | With DPN: 3330:2517; Without DPN: 3774:3694 | With DPN: 66.3 ± 9.8; Without DPN: 61 ± 11.4 | T2DM ± DPN | Smoking: Yes (Ever), No (Never) | Smoking (ever vs. never) was not significantly associated with DPN (0.95 [0.91–1.01], p = 0.488). |
| Chukwubuzo (2022) [55] | Cross-Sectional Study | Nigeria | 422 | 289:133 | 57.6 ± 10.1 | T1DM or T2DM ± PN | Cigarette smoker: Yes, No | Smoking was not significantly associated with PDPN (0.81 [0.28–2.33], p = 0.7). |
| Correa (2023) [56] | Cross-Sectional Study | Brazil | Overall: 444; LLBP: 313; PNBP: 33; WP: 98 | Overall: 289:155; LLBP: 188:125; PNBP: 26:7; WP: 75:23 | Overall: 39.72 ± 14.68; LLBP: 37.02 ± 13.39; PNBP: 8.45 ± 14.30; WP: 48.78 ± 15.59 | Chronic BP due to any etiology | Smoking: Yes, No | Smoking was not significantly associated with pain-related interference in BP (LLBP: 5.8% vs. PNBP: 9.1% vs. WP: 10.2%, p > 0.05). |
| Dorsey (2009) [57] | Cross-Sectional Study | US | 948 | LED+: 129:236; LED−: 302:281 | !! LED+: 63.8 (0.92); LED−: 59.0 (0.54) | DM ± PN | Smoking: Never, Former, Current | Smoking status was not significant between LED groups (Never: 40.9%, Former: 33.8%, Current: 25.3% vs. 47.7%, 32.8%, 19.5%, p > 0.05). |
| Faden (1981) [58] | Cross-Sectional | USA | With COPD: 23; Without COPD: 8; Healthy Co: 12 | NR | With COPD: 56.3 ± 6.7; Without COPD: 50.3 ± 8.7; Healthy Co: 30.4 ± 3.9 | COPD ± PoN | Smoking: pack-yrs | Smoking was significantly greater in those with COPD vs. without (53.2 ± 8.76 vs. 11.1 ± 5.36, p < 0.0001), and was significantly associated with sural, ulnar, and radial sensory nerve dysfunction in COPD (rs = 0.68, p < 0.01; rs = 0.48, p < 0.01; rs = 0.38, p < 0.05). |
| Gode (2022) [59] | Cross-Sectional Study | Ethiopia | Overall: 216; With PN: 108; Without PN: 108 | Overall: 111:105; With PN: 54:54; Without PN: 57:51 | Overall: 57.9 ± 12.6; With PN: 62.2 ± 10.9; Without PN: 53.5 ± 12.7 | T2DM ± PN | Smoking: Yes, No | Cigarette smoking did not significantly differ between PN groups (28.6% vs. 71.4%, p = 0.249); very small sample sizes. |
| Gunduz (2022) [60] | Cross-Sectional Study | Turkey | Overall: 109; LANSS > 12: 8 | Overall: 41:68; LANSS > 12: 4:4 | Overall: 54 ± 13.97; LANSS > 12: 47.5 ± 10.6 | Thoracotomy ± NP | Smoking History: Pack/Yr | Smoking history was significant between LANSS > 12 vs. overall (21.8 ± 19.7 vs. 33.1 ± 14.1, p = 0.05). |
| Gylfadottir (2020) [61] | Cross-Sectional Study | Denmark | 5514 | 2355:3159 | 64.1 ± 10.9 | T2DM ± DPoN | Smoking: Active, Daily, Occasionally, Previous, Never | Smoking (ever vs. never) was significantly associated with DPoN (1.36 [1.14–1.63], p < 0.05) and painful DPoN (1.52 [1.20–1.93], p < 0.05). |
| Hicks (2022) [62] | Cross-Sectional Study | US | Overall: 6902; With PN: 1181; Without PN: 5721 | Overall: 3589:3313; With PN: 443:738; Without PN: 3101:2620 | %! 40–49: 36 (0.9); 50–59: 27.8 (0.8); 60–69: 18.2 (0.6); 70–79: 12.8 (0.4); ≥80: 5.2 (0.3) | DM ± PN | Tobacco: Never, Former, Current | Tobacco use reported between PN groups: Never: 43.4%, Former: 38.6%, Current: 18% vs. 46.8%, 32.5%, 20.7% (statistics NR). |
| Jaiswal (2017) [63] | Cross-Sectional Study | US | 1992 | 1037:955 | (14–27) | T1DM or T2DM ± DPN | Smoking: Non, Former, Current | Smoking was significantly higher in those with DPN vs. without in both T1DM (54% vs. 31%, p = 0.001) and T2DM (72% vs. 58%, p = 0.01). |
| Jeyam (2020) [64] | Cross-Sectional Study | Scotland | Overall: 5558; With DPN 715; Without DPN 4842 | Overall: 2449:3109; With DPN: 320:395; Without DPN 2129:2713 | ^ Overall: 44.7 (33, 55.2); With DPN: 50.6 (41, 59.3); Without DPN: 43.7 (32, 54.4) | T1DM ± DPN | Smoking: Ever, Never | Smoking (ever) was significantly associated with DPN (1.67 [1.37–2.03], p < 0.05). |
| Kowalski (2022) [65] | Cross-Sectional Study | US | Overall: 43; With NP: 28; Without NP: 15 | With NP: 4:24; Without NP: 4:11 | Overall: 38.65 ± 12.12; With NP: 40.12 ± 12.77; Without NP: 35.91 ± 10.67 | SCI ± NP | Smoking: Ever (1+/d for 1 yr or 20+ packs in a lifetime), Never | NP severity was significantly greater in those with a history of smoking vs. without (6.77 ± 0.65 vs. 4.65 ± 0.69, p = 0.04). |
| Li (2023) [66] | Cross-Sectional Study | China | Overall: 25,710; With PDPN: 14,699; Without PDPN: 11,011 | Overall: 10,785:14,925; With PDPN: 6240:8459; Without PDPN: 4545:6466 | ^ Overall: 63 (55, 71); With PDPN: 65 (56, 73); Without PDPN: 61 (53, 69) | T2DM ± PDPN | Smoking: Yes, No | PDPN was significantly higher in smokers vs. non-smokers (54.8% vs. 45.2%, p = 0.001); however, smoking was reported less among those with PDPN compared to those without (16.2% vs. 17.9%, p = 0.001). |
| Al-Mahroos (2007) [67] | Cross-Sectional Study | Bahrain | 1477 | 842:635 | 57.3 ± 6.32 | T2DM ± DPN | Smoking: Yes, No | DPN was significantly higher in smokers vs. non-smokers (57% vs. 16%, p = 0.05). Smoking status was significantly associated with DPN in univariate and multivariate analyses (2.18 [1.16–1.77], p = 0.002; 1.24 [1.01–1.31], p < 0.02). |
| Mick (2021) [68] | Cross-Sectional Study | France, Italy, Spain, UK | 1030 | 651:379 | 60.2 ± 15.32; ^ 61 (49–72) | Localized NP due to any etiology | Tobacco: Ex, Never, Current (≤10 yrs, >10 yrs, <10 cig/d, ≥10 cig/d | Never smoking was more frequently reported in NP (59.2%) compared to ex- and current smokers (20.55% vs. 20.25%); additional statistics for NR. |
| Mizokami-Stout (2020) [69] | Cross-Sectional Study | US | Overall: 5936; With DPN: 630; Without DPN: 5306 | Overall: 3265:2671; With DPN: 378:252; Without DPN: 2865:2441 | Overall: 39 ± 18; With DPN 51 ± 17; Without DPN: 37 ± 17 | T1DM ± DPN | Smoking: Yes, No | Smoking was significantly different between DPN groups (8% vs. 4%, p = 0.008), and was significantly associated with DPN (1.83 [1.18–2.82]). |
| Molla (2020) [70] | Cross-Sectional Study | Iran | Overall: 99,651; With T1DM: 10,390; With T2DM: 82,308 | 68,743:30,424 | 57.4 ± 12.8 | T1DM or T2DM ± Ne | Smoking: Yes (Current), No (Past/Never) | Smoking was significantly associated with Ne in both T1DM and T2DM (2.40 [1.95–2.96], p < 0.001; 1.70 [1.69–1.93], p < 0.001). |
| Moore (2019) [71] | Cross-Sectional Study | US | Normal BMI (<25): 52; Overweight BMI (25–29.9): 52; Obese BMI (>30): 39 | Normal BMI (<25): 19:33; Overweight BMI (25–29.9): 19:33; Obese BMI (>30): 21:18 | ^^ Normal BMI (<25): 66 (41–81); Overweight BMI (25–29.9): 69.5 (42–85); Obese BMI (>30): 61 (25–81) | Newly Diagnosed Multiple Myeloma ± CIPN (Bortezomib) | Smoking: Never, Former, Current | Former vs never, and current vs. never smoking were not significantly associated with CIPN (0.613 [0.285–1.317], p = 0.45; 0.750 [0.179–3.134], p = 0.45). |
| Nagakura (2023) [72] | Ecological Cross-Sectional Study | Japan | Pregabalin Reimbursement Claims per 1000 population; up to 126 million | NR | (40–74) | NP due to any etiology treated with pregabalin | Tobacco (>100 cig or >6 mo): Yes, No | Smoking was significantly associated with an increase prevalence of NP (β = 206.4 [45–367.8], p = 0.0134). |
| Nielsen (2022) [73] | Cross-Sectional Study | Denmark | 2839 | High CIPN Score: 274:146; Low CIPN Score: 1193:870 | ^^ High CIPN Score: 69; Low CIPN Score: 67; (18–99) | Cancer diagnosis at any stage of treatment ± CIPN | Tobacco: yes/no | Smoking was significantly associated with higher CIPN20 scores vs. lower scores (21% vs. 15%, p = 0.04). |
| Ponirakis (2022) [74] | Cross-Sectional Study | Qatar, Kuwait and KSA | Overall: 3021; Qatar: 1093; Kuwait: 1168; KSA: 760 | Overall: 1408:1613; Qatar: 428:665; Kuwait: 552:616; KSA: 428:332 | Overall: 57.9 ± 11.7; Qatar: 52.4 ± 11.3; Kuwait: 63.3 ± 9.9; KSA: 57.1 ± 11.2 | T2DM + DPN or NP | Smoking: Yes (1+/d over 1 yr), No | Smoking was significantly associated with PN (1.5 [1.2–1.9], p < 0.01) overall, and painful PN (1.8 [1.1–3.0], p = 0.01) in Qatar only. |
| Rash (2022) [75] | Cross-Sectional Study | US | Overall: 933; Current Smokers: 166; Former Smokers: 252; Never Smokers: 515 | Overall: 569:364; Current Smokers: 61:105; Former Smokers: 146:106; Never Smokers: 361:154 | Overall: 38 ± 16; Current Smokers: 31 ± 12; Former Smokers: 46 ± 15; Never Smokers: 37 ± 16 | T1DM ± Ne | Smoking: Never, Former, Current (Daily, <Daily) | Ne was significantly higher in daily smokers compared to < daily smokers (18% vs. 11%, p = 0.09) |
| Revesz (2022) [76] | Cross-Sectional Study | The Netherlands | Overall: 1516; With PN: 980; Without PN: 536 | Overall: 634:882; With PN: 445:535; Without PN: 189:347 | Overall: 69.1 ± 9.4; With PN: 70.1 ± 9.4; Without PN: 67.2 ± 9.2 | Colorectal cancer survivors ± PN | Smoking: Never, Former, Current | Smoking was significantly associated with higher motor, autonomic, and total PN |
| Sahito (2022) [77] | Cross-Sectional Study | Pakistan | Overall: 1057; With PN: 607; Without PN: 450 | Overall: 414:643; With PN: 230:377; Without PN: 184:266 | 30–40: 119; 41–50: 316; 51–60: 324; 61–70: 165; >70 yrs: 133 | T2DM ± PN | History of smoking: Yes, No | Smoking was significantly higher in those with DPN vs. without (41.8% vs. 37.2%, statistics NR). |
| Srivastava (2022) [78] | Cross-Sectional Study | India | 98 | 79:19 | 51.63 ± 10.68 | Cancer survivors ± CIPN | History of smoking: Yes (current, former), No | Smoking history was not significantly associated with sensory, motor, or autonomic CIPN severity (p > 0.05). |
| Tamer (2005) [79] | Cross-Sectional Study | Turkey | 191 | 109:82 | 58.7 ± 10 | T2DM ± DSPoN | Smoking: Yes, No | Smoking was significantly associated with electromyography supported DSPoN (2.330 [1.222–4.445]). |
| Tesfaye (1996) [80] | Cross-Sectional Study | Europe | 3250 | 1582:1668 | 32.7 ± 10.2 (15–61) | IDDM ± DPN | Smoking (current, former, never) | Current smoking was significantly associated with DPN (RR: 2.4 [1.1–5], p = 0.02) in multivariate analysis. |
| Trendowski (2021) [81] | Cross-Sectional Study | US | With CIPN: 550; Without CIPN: 495 | With CIPN: 440:110; Without CIPN: 355:140 | ^^ With CIPN: 56 (23–79); Without CIPN: 58 (21–79) | African American cancer survivors ± CIPN | Tobacco: Never, Ever (≥100 cig/lifetime), Current; Smoking Frequency (cig/d): 1–9, 10–19, ≥20 | Ever and current smoking were not significantly associated with CIPN (1.04 [0.80–1.35], p = 0.76; 1.28 [0.90–1.82], p = 0.18). |
| Van der Velde (2019) [82] | Cross-Sectional Study | The Netherlands | Overall: 2401; High Sural SNAPA: 793; Med Sural SNAPA: 796; Low Sural SNAPA 812 | Overall: 1174:1227; High Sural SNAPA: 464:329; Med Sural SNAPA: 377:419; Low Sural SNAPA: 334:478 | Overall: 59.3 ± 8.2; High Sural SNAPA: 56.4 ± 8.2; Med Sural SNAPA: 59.4 ± 7.9; Low Sural SNAPA: 62 ± 7.5 | T2DM ± PN | Smoking: Current, Former, Never | Current smoking was associated with worse nerve function (β = −0.11 [−0.17–−0.04]) and higher VPT (β = 0.17 [0.06–0.28]). Former smoking was associated with lower peroneal NCV (β = −0.12 [−0.20–−0.05]). Smoking (ever) was associated with NP (2.13 [1.38–3.29]). |
| Wang (2023) [83] | Cross-Sectional Study | China | Overall: 14,908; With DPN: 10,084; Without DPN: 4824 | Overall: 6322:8586; With DPN: 4365:5719; Without DPN: 1957:2867 | Overall: 61.3 ± 13, ^ 62 (53, 70); With DPN: 62.6 ± 12.5, ^ 63 (55, 71); Without DPN: 58.5 ± 13.5, ^ 59 (50, 67) | T2DM ± DPN | Tobacco: Current | Smoking was significantly lower in those with DPN compared to without (14.9% vs. 20.7%, p < 0.001). |
| Weinberger (2018) [84] | Cross-Sectional Study | US | Overall: 103; With Pain: 70; Without Pain: 33 | With Pain: 34:36; Without Pain: 17:16 | With Pain: 50.5 ± 8.2; Without Pain: 47.7 ± 9.7 | HIV/AIDS ± NP | Smoking habits: daily vs. <daily, cig/d (>10 vs. <10), Quit attempts (number and d of longest) | All smoking habits were not significantly associated with NP (p > 0.05). |
| Yokoyama (2020) [85] | Cross-Sectional Study | Japan | Overall: 9914; Without DPoN: 6180; With DPoN: 2745 (with DPoNS: 1689; with UDoPN: 989) | Overall: 3715:6139; Without DPoN: 2273:3904; With DPoN: 1041:1705 (with DPoNS: 664:1025, with UDPoN: 397:530) | ^^ Overall: 66 (69–73); Without DPoN: 65 (57–71); With DPoN: 70 (63–77) (with DPoNS: 69 (63–76), with UDPoN: 67 (59–75)) | T2DM ± DPoN | Tobacco: Current, Former, Never | Current and former smoking were significantly associated with DPoN (2.04 [1.42–2.91], p < 0.001; 1.64 [1.19–2.26], p = 0.002). |
| Richards (2005) [86] | Case Series | US | 2 | 0:2 | 38 and 55 | SCI + NP | Smoking abstinence prior to surgery | Subjective improvement of NP during smoking abstinence, and NP returned when smoking re-endorsed (additional statistics NR). |
3.2. Included Studies
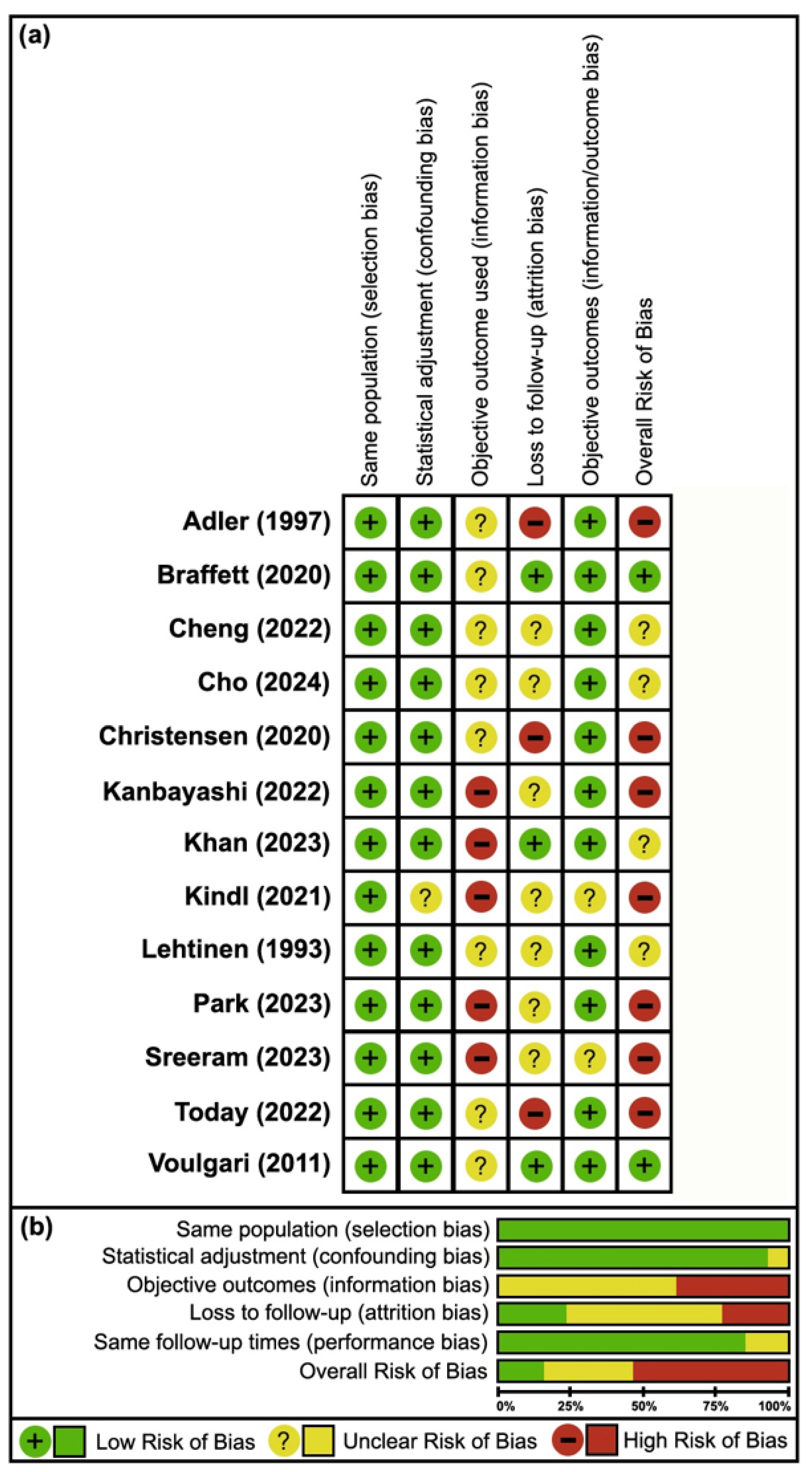
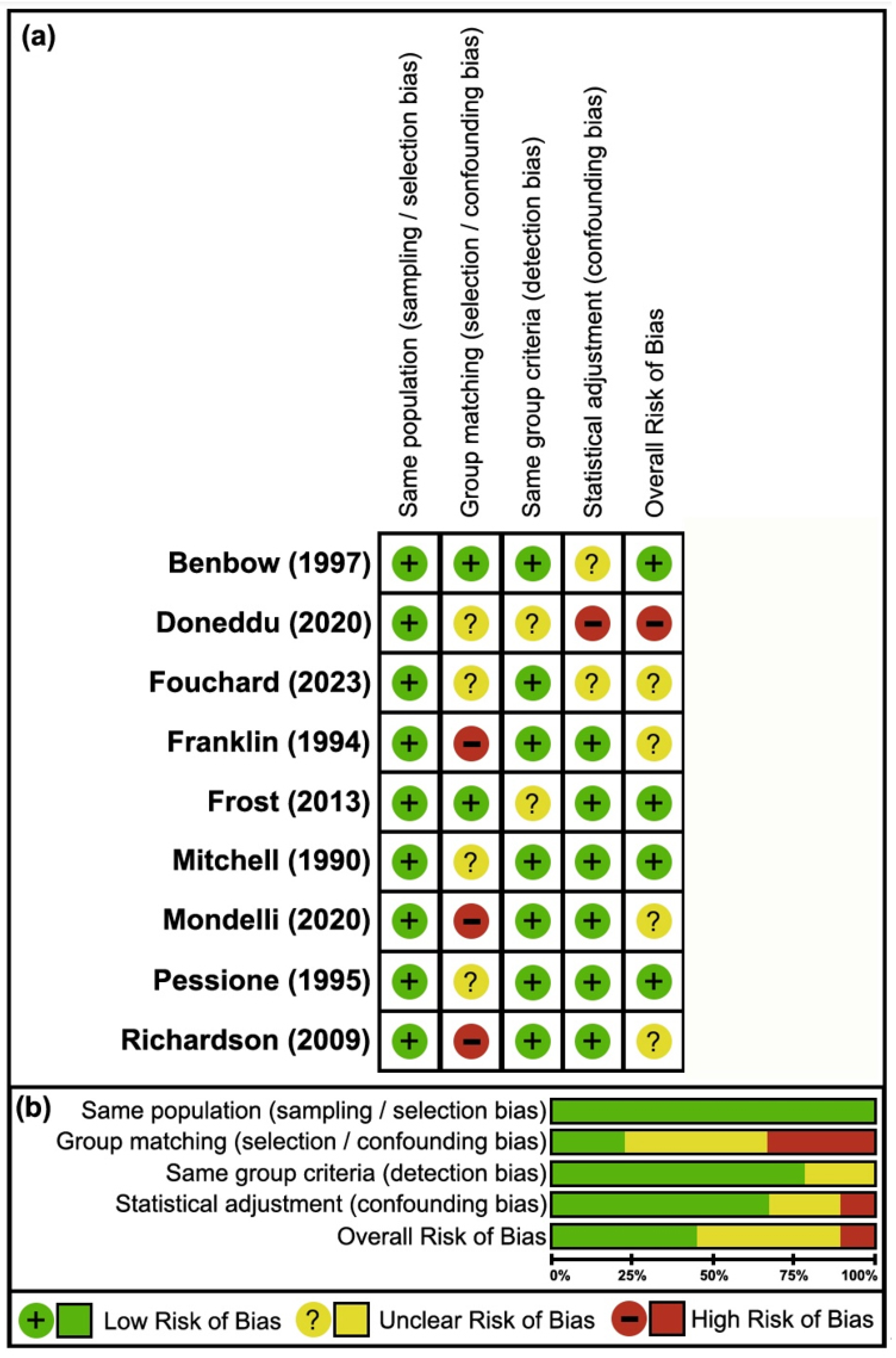
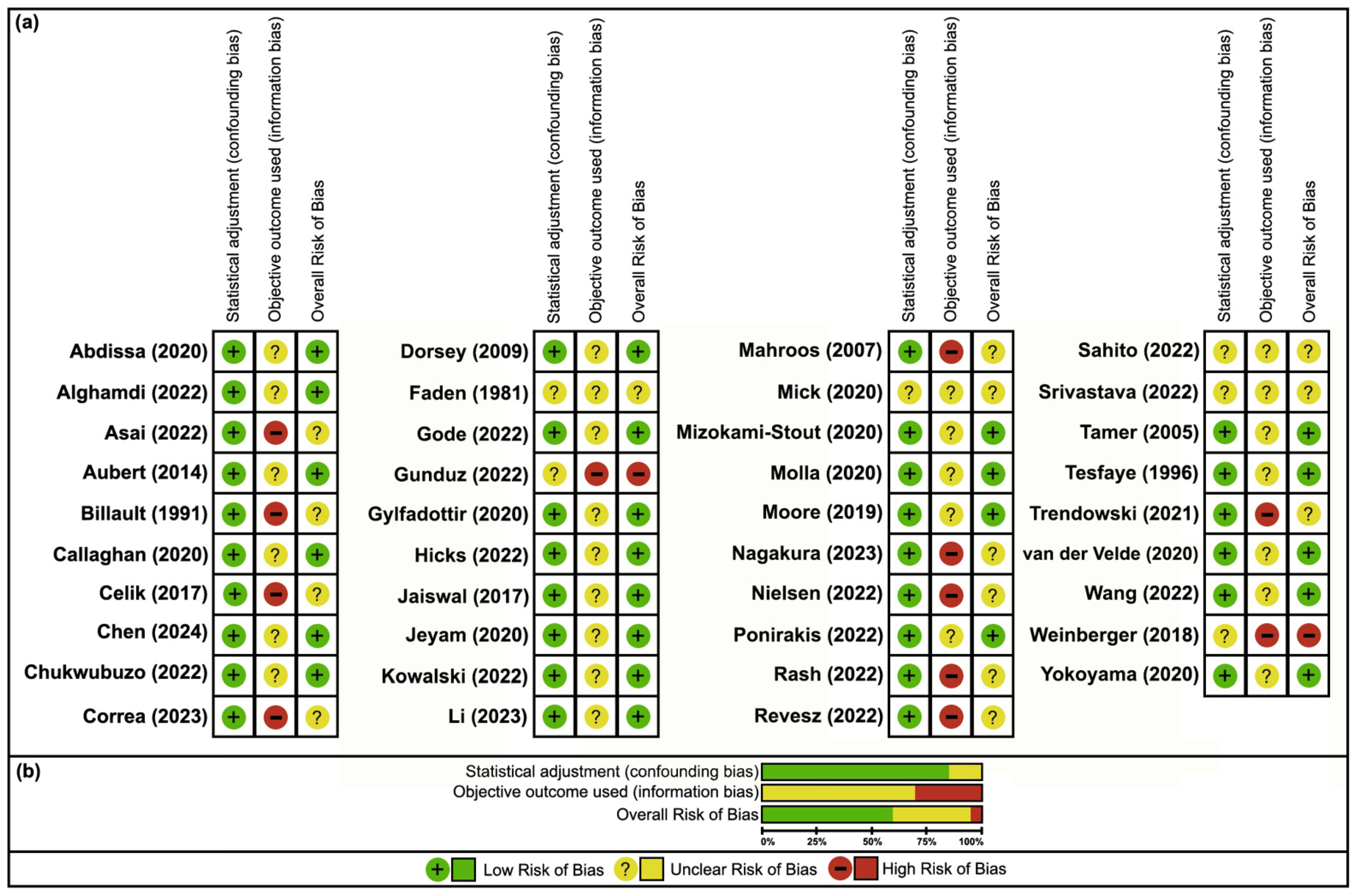
3.3. Risk of Bias
| Incidence of Neuropathy/NP With/Without Smoking | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Population(s): DM ± PN; DM ± Ne; CRPS or MSK; T2DM ± DPoN ± Pain; CIPN; DM ± ND | |||||||||||
| Exposure: Smoking | |||||||||||
| Comparison: No smoking | |||||||||||
| Outcome: Neuropathy incidence | |||||||||||
| Setting: Denmark, Finland, Germany, Japan, Korea, the USA | |||||||||||
| Study Design: Cohort studies | |||||||||||
| Stratification | No. of studies | Neuropathy-positive (%) | Neuropathy-negative (%) | Odds ratio (95% CI) | Relative risk (95% CI) | Risk of bias | Inc | Ind | Imp | Certainty of evidence (GRADE) | References |
| Current/Former Smoking | 9 | 1734/2957 (58.64%) | 19,303/29,261 (65.97%) | 0.73 (0.68–0.79) | 0.89 (0.86–0.92) | Very serious | Low risk | Low risk | Low risk | Very Low ⨁◯◯◯ | Braffett (2020) [26], Cho (2024) [28], Kindl (2021) [32], Christensen (2020) [29], Park (2023) [34], TODAY (2022) [36], Kanbayashi (2022) [30], Adler (1997) [25], Lehtinen (1993) [33] |
| Smoking Dependence | 1 | 2129/3266 (65.19%) | 8194/15,066 (54.39%) | 1.57 (1.45–1.70) | 1.20 (1.16–1.23) | Serious | NA | Low risk | Low risk | Very Low ⨁◯◯◯ | Khan (2023) [31] |
| Lifetime Smoking | 1 | 328/700 (46.86%) | 185/328 (56.40%) | 0.68 (0.52–0.89) | 0.83 (0.73–0.94) | Very serious | NA | Low risk | Low risk | Very Low ⨁◯◯◯ | Sreeram (2023) [35] |
| Smoking Cessation | 1 | 22/35 (62.86%) | 98/158 (62.03%) | 1.04 (0.51–2.16) | 1.01 (0.76–1.34) | Not serious | NA | Low risk | High risk | Very Low ⨁◯◯◯ | Voulgari (2011) [37] |
| Association Between Neuropathy/NP and Smoking | |||||||||||
| Population: CIDP; Paresthesia ± SFN; UNE; NIDDM ± DSN; Alcoholism ± PN; DM ± Ne; healthy controls | |||||||||||
| Exposure: Smoking | |||||||||||
| Comparison: No smoking | |||||||||||
| Outcome: Neuropathy association | |||||||||||
| Setting: Denmark, France, Italy, US | |||||||||||
| Study Design: Case–control studies | |||||||||||
| Stratification | No. of studies | Neuropathy-positive (%) | Neuropathy-negative (%) | Odds ratio (95% CI) | Relative risk (95% CI) | Risk of bias | Inc | Ind | Imp | Certainty of evidence (GRADE) | References |
| Current/Former Smoking | 8 | 664/1153 (57.59%) | 1007/2075 (48.53%) | 1.44 (1.25–1.67) | --- | Serious | Low risk | Low risk | Low risk | Very Low ⨁◯◯◯ | Doneddu (2020) [39], Fouchard (2023) [40], Mondelli (2020) [44], Richardson (2009) [46], Frost (2013) [42], Franklin (1994) [41], Pessione (1995) [45], Mitchell (1990) [43] |
| Prevalence of Neuropathy/NP with/without Smoking | |||||||||||
| Population: DM ± PN; CIPN; T2DM ± DPoN; SCI ± NP; Chronic BP; T2DM ± DSPoN; BMI > 35 kg ± Ne; Chronic neck/shoulder/upper-limb pain; DM ± DSyPN; IDDM ± DPN; HIV/AIDS ± NP | |||||||||||
| Exposure: Smoking | |||||||||||
| Comparison: No smoking | |||||||||||
| Outcome: Neuropathy prevalence | |||||||||||
| Setting: Brazil, China, Denmark, Ethiopia, Europe, France, Iran, Japan, the Netherlands, Pakistan, Saudi Arabia, Scotland, Turkey, the US | |||||||||||
| Study Design: Cross-sectional studies | |||||||||||
| Stratification | No. of studies | Neuropathy-positive (%) | Neuropathy-negative (%) | Odds ratio (95% CI) | Relative risk (95% CI) | Risk of bias | Inc | Ind | Imp | Certainty of evidence (GRADE) | References |
| Current/Former Smoking | 23 | 9238/52,595 (17.56%) | 18,298/114,710 (15.95%) | 1.12 (1.09–1.15) | --- | Not serious | High risk | Mod risk | Low risk | Very Low ⨁◯◯◯ | Sahito (2022) [77], Abdissa (2020) [47], Rash (2022) [75], Nielsen (2022) [73], Gylfadottir (2020) [61], Kowalski (2022) [65], Jeyam (2020) [64], Dorsey (2009) [57], Jaiswal (2017) [63], Molla (2020) [70], Chen (2024) [54], Correa (2023) [56], Gode (2022) [59], Tamer (2005) [79], Wang (2023) [83], Yokoyama (2020) [85], Callaghan (2020) [52], Hicks (2022) [62], Asai (2022) [49], Aubert (2014) [50], van der Velde (2019) [82], Alghamdi (2022) [48], Tesfaye (1996) [80] |
| Lifetime Smoking | 1 | 32/66 (48.49%) | 15/33 (45.46%) | 1.13 (0.50–2.64) | --- | Very serious | NA | Low risk | High risk | Very Low ⨁◯◯◯ | Weinberger (2018) [84] |
3.4. Summary of Findings
4. Discussion
4.1. Cohort Studies
4.2. Case–Control Studies
4.3. Cross-Sectional Studies
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| Ca | Cases |
| CIDP | Chronic inflammatory demyelinating polyradiculoneuropathy |
| CIPN | Chemotherapy-induced peripheral neuropathy |
| CIPN20 | European Organization for Research and Treatment of Cancer 20-item scale |
| CMAP | Compound muscle action potential |
| Co | Controls |
| COPD | Chronic obstructive pulmonary disease |
| CP | Chronic pain |
| CRPS | Complex regional pain syndrome |
| CTCAE | National Cancer Institute Common Terminology Criteria for Adverse Events |
| CV | Conduction velocity |
| DM | Diabetes mellitus |
| DN | Diabetic neuropathy |
| DPN | Diabetic peripheral neuropathy |
| DPoN | Diabetic polyneuropathy |
| DPoNS | Diabetic polyneuropathy-related sensory symptoms/signs |
| DSN | Distal symmetric neuropathy |
| DSPoN | Distal sensory polyneuropathy |
| DSyPN | Distal symmetrical peripheral neuropathy |
| GABA | Gamma-aminobutyric acid |
| IDDM | Insulin-dependent diabetes mellitus |
| IEFND | Intraepidermal nerve fiber density |
| KSA | Kingdom of Saudi Arabia |
| LANSS | Leeds assessment of neuropathic symptoms and signs |
| LED | Lower-extremity disease |
| LLBP | Localized lower-back pain |
| MNSI | Michigan neuropathy screening instrument |
| MSK | Musculoskeletal pain |
| NCV | Nerve conduction velocity |
| NDS | Neuropathy disability score |
| ND | Neurophysiologically deteriorated |
| NIDDM | Non-insulin-dependent diabetes mellitus |
| NNT | Number needed to treat |
| NP | Neuropathic pain |
| NSRC | Non-standardized regression coefficient |
| PDN | Painful diabetic neuropathy |
| PDPN | Painful diabetic peripheral neuropathy |
| PN | Peripheral neuropathy |
| PNBP | Peripheral neuropathic back pain |
| PNQ | Patient neurotoxicity questionnaire |
| PoN | Polyneuropathy |
| PSUD | Polysubstance use disorder group (tobacco, alcohol, +1 other) |
| PY | Person-years |
| SCI | Spinal cord injury |
| SFN | Small-fiber neuropathy |
| SNAPA | Sensory nerve action potential amplitude |
| SRC | Standardized regression coefficient |
| T1DM | Type 1 diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| TAUD | Tobacco and alcohol use disorder group |
| TUD | Tobacco use disorder group |
| UDPoN | Unknown-status diabetic polyneuropathy |
| UN | Ulnar neuropathy |
| UNE | Ulnar neuropathy of the elbow |
| VPT | Vibration perception threshold |
| WP | Widespread pain |
References
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.B. Neuropathic Pain Quality-of-Life Impact, Costs and Cost Effectiveness of Therapy. Pharmacoeconomics 2009, 27, 95–112. [Google Scholar]
- Attal, N.; Bouhassira, D.; Colvin, L. Advances and challenges in neuropathic pain: A narrative review and future directions. Br. J. Anaesth. 2023, 131, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Sindrup, S.H.; Jensen, T.S. The evidence for pharmacological treatment of neuropathic pain. Pain 2010, 150, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Di Stefano, G.; Di Lionardo, A.; Di Pietro, G.; Cruccu, G.; Truini, A. Pharmacotherapeutic Options for Managing Neuropathic Pain: A Systematic Review and Meta-Analysis. Pain Res. Manag. 2021, 2021, 6656863. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Haroutounian, S.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpaa, M.; Jensen, T.S.; Kamerman, P.R.; McNicol, E.; Moore, A.; et al. Neuropathic pain clinical trials: Factors associated with decreases in estimated drug efficacy. Pain 2018, 159, 2339–2346. [Google Scholar] [CrossRef]
- Bunner, A.E.; Wells, C.L.; Gonzales, J.; Agarwal, U.; Bayat, E.; Barnard, N.D. A dietary intervention for chronic diabetic neuropathy pain: A randomized controlled pilot study. Nutr. Diabetes 2015, 5, e158. [Google Scholar] [CrossRef]
- Arnold, R.; Pianta, T.J.; Pussell, B.A.; Kirby, A.; O’bRien, K.; Sullivan, K.; Holyday, M.; Cormack, C.; Kiernan, M.C.; Krishnan, A.V. Randomized, Controlled Trial of the Effect of Dietary Potassium Restriction on Nerve Function in CKD. Clin. J. Am. Soc. Nephrol. 2017, 12, 1569–1577. [Google Scholar] [CrossRef]
- Torlak, M.S.; Bagcaci, S.; Akpinar, E.; Okutan, O.; Nazli, M.S.; Kuccukturk, S. The effect of intermittent diet and/or physical therapy in patients with chronic low back pain: A single-blinded randomized controlled trial. Explore 2022, 18, 76–81. [Google Scholar] [CrossRef]
- Klowak, M.; Boggild, A.K. The efficacy of a whole foods, plant-based dietary lifestyle intervention for the treatment of peripheral neuropathic pain in leprosy: A randomized control trial protocol. Front. Nutr. 2023, 10, 1196470. [Google Scholar] [CrossRef]
- Klowak, M.; Lau, R.; Mohammed, M.N.; Birago, A.; Samson, B.; Ahmed, L.; Renee, C.; Meconnen, M.; Sam, M.; Boggild, A.K. A Systematic Review of Dietary Lifestyle Interventions for Neuropathic Pain. J. Clin. Med. 2024, 13, 6766. [Google Scholar] [CrossRef]
- Sibille, K.T.; Steingrímsdóttir, Ó.A.; Fillingim, R.B.; Stubhaug, A.; Schirmer, H.; Chen, H.; McEwen, B.S.; Nielsen, C.S. Investigating the Burden of Chronic Pain: An Inflammatory and Metabolic Composite. Pain Res. Manag. 2016, 2016, 7657329. [Google Scholar] [CrossRef]
- Iida, H.; Yamaguchi, S.; Goyagi, T.; Sugiyama, Y.; Taniguchi, C.; Matsubara, T.; Yamada, N.; Yonekura, H.; Iida, M. Consensus statement on smoking cessation in patients with pain. J. Anesthesia 2022, 36, 671–687. [Google Scholar] [CrossRef]
- Ditre, J.W.; Brandon, T.H.; Zale, E.L.; Meagher, M.M. Pain, Nicotine, and Smoking: Research Findings and Mechanistic Considerations. Psychol. Bull. 2011, 137, 1065–1093. [Google Scholar] [CrossRef]
- Aboushaar, N.; Serrano, N. The mutually reinforcing dynamics between pain and stress: Mechanisms, impacts and management strategies. Front. Pain Res. 2024, 5, 1445280. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, Y.; Schneider, C.; Balle, T. The Anti-Nociceptive Effects of Nicotine in Humans: A Systematic Review and Meta-Analysis. Pharmaceuticals 2023, 16, 1665. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Y.; Song, H.; Gou, H.; Zhao, Q.; Hong, W.; Piao, Y.; Chen, Y.; Chen, Y.; Wen, S.; et al. The interaction of oxytocin and nicotine addiction on psychosocial stress: An fMRI study. Transl. Psychiatry 2024, 14, 348. [Google Scholar] [CrossRef]
- Wang, F.; Li, H.; Mu, Q.; Shan, L.; Kang, Y.; Yang, S.; Chang, H.-C.; Su, K.-P.; Liu, Y. Association of Acute Postoperative Pain and Cigarette Smoking With Cerebrospinal Fluid Levels of Beta-Endorphin and Substance P. Front. Mol. Neurosci. 2022, 14, 755799. [Google Scholar] [CrossRef]
- O’nEill, J.; Diaz, M.P.; Alger, J.R.; Pochon, J.-B.; Ghahremani, D.; Dean, A.C.; Tyndale, R.F.; Petersen, N.; Marohnic, S.; Karaiskaki, A.; et al. Smoking, tobacco dependence, and neurometabolites in the dorsal anterior cingulate cortex. Mol. Psychiatry 2023, 28, 4756–4765. [Google Scholar] [CrossRef]
- Ahn, Y.H.; Park, H.; Kweon, S.S. Causes of death among persons affected by leprosy in Korea, 2010–2013. Am. J. Trop. Med. Hyg. 2020, 102, 42–47. [Google Scholar] [CrossRef]
- Silva, R.V.G.; de Araújo, R.S.; Aarão, T.L.S.; da Silva Costa, P.D.; Sousa, J.R.; Quaresma, J.A.S. Correlation between therapy and lipid profile of leprosy patients: Is there a higher risk for developing cardiovascular diseases after treatment? Infect. Dis. Poverty 2017, 6, 28–34. [Google Scholar] [CrossRef]
- Upputuri, B.; Srikantam, A.; Mamidi, R.S. Comorbidities associated with non-healing of plantar ulcers in leprosy patients. PLoS Negl. Trop. Dis. 2020, 14, e0008393. [Google Scholar] [CrossRef]
- Behrend, C.; Schonbach, E.; Coombs, A.; Coyne, E.; Prasarn, M.; Rechtine, G. Smoking Cessation Related to Improved Patient-Reported Pain Scores Following Spinal Care in Geriatric Patients. Geriatr. Orthop. Surg. Rehabil. 2014, 5, 191–194. [Google Scholar] [CrossRef]
- Adler, A.I.; Boyko, E.J.; Ahroni, J.H.; Stensel, V.; Forsberg, R.C.; Smith, D.G. Risk Factors for Diabetic Peripheral Sensory Neuropathy Results of the Seattle Prospective Diabetic Foot Study. Diabetes Care 1997, 20, 1162–1167. [Google Scholar] [CrossRef]
- Braffett, B.H.; Gubitosi-Klug, R.A.; Albers, J.W.; Feldman, E.L.; Martin, C.L.; White, N.H.; Orchard, T.J.; Lopes-Virella, M.; Lachin, J.M.; Pop-Busui, R.; et al. Risk factors for diabetic peripheral neuropathy and cardiovascular autonomic neuropathy in the Diabetes Control and Complications Trial/Epidemiology of Diabetes interventions and Complications (DCCT/EDIC) study. Diabetes 2020, 69, 1000–1010. [Google Scholar] [CrossRef]
- Cheng, Y.; Cao, W.; Zhang, J.; Wang, J.; Liu, X.; Wu, Q.; Lin, Q. Determinants of Diabetic Peripheral Neuropathy and Their Clinical Significance: A Retrospective Cohort Study. Front. Endocrinol. 2022, 13, 934020. [Google Scholar] [CrossRef]
- Cho, Y.; Park, H.-S.; Seo, D.H.; Ahn, S.H.; Hong, S.; Suh, Y.J.; Chon, S.; Woo, J.-T.; Baik, S.H.; Lee, K.W.; et al. The Association of Smoking Status with Diabetic Microvascular Complications in Korean Patients with Type 2 Diabetes. Yonsei Med. J. 2024, 65, 427–433. [Google Scholar] [CrossRef]
- Christensen, D.H.; Knudsen, S.T.; Gylfadottir, S.S.; Christensen, L.B.; Nielsen, J.S.; Beck-Nielsen, H.; Sørensen, H.T.; Andersen, H.; Callaghan, B.C.; Feldman, E.L.; et al. Metabolic Factors, Lifestyle Habits, and Possible Polyneuropathy in Early Type 2 Diabetes: A Nationwide Study of 5,249 Patients in the Danish Centre for Strategic Research in Type 2 Diabetes (DD2) Cohort. Diabetes Care 2020, 43, 1266–1275. [Google Scholar] [CrossRef]
- Kanbayashi, Y.; Sakaguchi, K.; Ishikawa, T.; Tabuchi, Y.; Takagi, R.; Yokota, I.; Katoh, N.; Takayama, K.; Taguchi, T. Predictors of the development of nab-paclitaxel-induced peripheral neuropathy in breast cancer patients: Post hoc analysis of a prospective, phase II, self-controlled clinical trial. Med. Oncol. 2022, 39, 1–8. [Google Scholar] [CrossRef]
- Khan, M.T.F.; Lewis, D.; Kaelber, D.C.; Winhusen, T.J. Health outcomes associated with patterns of substance use disorders among patients with type 2 diabetes and hypertension: Electronic health record findings. Prim. Care Diabetes 2023, 17, 43–47. [Google Scholar] [CrossRef]
- Kindl, G.; Teichmüller, K.; Escolano-Lozano, F.; Birklein, F.; Rittner, H.L. Pain, disability, and lifestyle: Patients with complex regional pain syndrome compared to chronic musculoskeletal pain—A retrospective analysis. Eur. J. Pain 2022, 26, 719–728. [Google Scholar] [CrossRef]
- Lehtinen, J.M.; Niskanen, L.; Hyv6nen, K.; Siitonen, O.; Uusitupa, M. Nerve function and its determinants in patients with newly-diagnosed Type 2 (non-insulin-dependent) diabetes mellitus and in control subjects-a 5-year follow-up. Diabetologia 1993, 36, 68–72. [Google Scholar] [CrossRef]
- Park, S.K.; Kim, M.; Jung, J.Y.; Oh, C.; Ha, E.; Nam, D.J.; Yang, E.H.; Hwang, W.Y.; Lee, S.; Ryoo, J. Changes in smoking status, amount of smoking and their relation to the risk of microvascular complications in men with diabetes mellitus. Diabetes/Metab. Res. Rev. 2023, 39, e3697. [Google Scholar] [CrossRef]
- Sreeram, K.; Seaton, R.; Greenwald, M.K.; Kamgar, M.; Assad, H.; Baird, T.; Schwartz, A.G.; Ruterbusch, J.; Simon, M.S. Chemotherapy-induced peripheral neuropathy in the detroit research on cancer survivors (ROCS) cohort. Cancer Causes Control 2023, 34, 459–468. [Google Scholar] [CrossRef]
- TODAY Study Group. Risk factors for diabetic peripheral neuropathy in adolescents and young adults with type 2 diabetes: Results from the TODAY study. Diabetes Care 2022, 45, 1065–1072. [Google Scholar] [CrossRef]
- Voulgari, C.; Katsilambros, N.; Tentolouris, N. Smoking cessation predicts amelioration of microalbuminuria in newly diagnosed type 2 diabetes mellitus: A 1-year prospective study. Metabolism 2011, 60, 1456–1464. [Google Scholar] [CrossRef]
- Benbow, S.J.; Williams, G.; Macfarlane, I.A. Smoking Habits and Painful Diabetic Neuropathy. J. Diabetes Complicat. 1997, 11, 334–337. [Google Scholar] [CrossRef]
- Doneddu, P.E.; Bianchi, E.; Cocito, D.; Manganelli, F.; Fazio, R.; Filosto, M.; Mazzeo, A.; Cosentino, G.; Cortese, A.; Jann, S.; et al. Risk factors for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): Antecedent events, lifestyle and dietary habits. Data from the Italian CIDP Database. Eur. J. Neurol. 2019, 27, 136–143. [Google Scholar] [CrossRef]
- Fouchard, M.; Brenaut, E.; Genestet, S.; Ficheux, A.S.; Marcorelles, P.; Misery, L. Observational case-control study of small-fiber neuropathies, with regards on smoking and vitamin D deficiency and other possible causes. Front. Med. 2023, 9, 1051967. [Google Scholar] [CrossRef]
- Franklin, G.M.; Shetterly, S.M.; Cohen, J.A.; Baxter, J.; Hamman, R.F. Risk Factors for Distal Symmetric Neuropathy in The San Luis Valley Diabetes Study. Diabetes Care 1994, 17, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Frost, P.; Johnsen, B.; Fuglsang-Frederiksen, A.; Svendsen, S.W. Lifestyle risk factors for ulnar neuropathy and ulnar neuropathy-like symptoms. Muscle Nerve 2013, 48, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, B.D.; Hawthorne, V.M.; Vinik, A.I. Short Reports Cigarette Smoking and Neuropathy in Diabetic Patients. Diabetes Care 1990, 13, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Mondelli, M.; Mattioli, S.; Vinciguerra, C.; Ciaramitaro, P.; Aretini, A.; Greco, G.; Sicurelli, F.; Giorgi, S.; Curti, S. Comorbidities, anthropometric, demographic, and lifestyle risk factors for ulnar neuropathy at the elbow: A case control study. J. Peripher. Nerv. Syst. 2020, 25, 401–412. [Google Scholar] [CrossRef]
- Pessione, F.; Louis Gerchstein, J.; Rueff, B. Parental history of alcoholism: A risk factor for alcohol-related peripheral neuropathies. Alcohol Alcohol. 1995, 30, 749–754. Available online: https://academic.oup.com/alcalc/article-abstract/30/6/749/173444 (accessed on 3 June 2025). [CrossRef]
- Richardson, J.K.; Ho, S.; Wolf, J.; Spiegelberg, T. The nature of the relationship between smoking and ulnar neuropathy at the elbow. Am. J. Phys. Med. Rehabil. 2009, 88, 711–718. [Google Scholar] [CrossRef]
- Abdissa, D.; Hamba, N.; Kene, K.; Bedane, D.A.; Etana, G.; Muleta, D.; Gerbi, A. Prevalence and Determinants of Peripheral Neuropathy among Type 2 Adult Diabetes Patients Attending Jimma University Medical Center, Southwest Ethiopia, 2019, an Institutional-Based Cross-Sectional Study. J. Diabetes Res. 2020, 2020, 9562920. [Google Scholar] [CrossRef]
- Alghamdi, M.; Owolabi, L.F.; Adamu, B.; Taura, M.G.; Jibo, A.; Almansour, M.; Alaklabi, S.N.; Alghamdi, M.A.; Imam, I.A.; Abdelrazak, R.; et al. Disease-specific quality of life in patients with diabetic neuropathy. Saudi Med. J. 2022, 43, 408–417. [Google Scholar] [CrossRef]
- Asai, A.; Suzuki, F.; Tsujiguchi, H.; Hara, A.; Miyagi, S.; Kannon, T.; Suzuki, K.; Nakamura, M.; Shimizu, Y.; Nguyen, T.T.T.; et al. Relationship between fatty acid intake and chronic neck/shoulder/upper limb pain without elevated CRP in a Japanese population: A cross-sectional analysis of the Shika study. J. Nutr. Sci. 2022, 11, e38. [Google Scholar] [CrossRef]
- Aubert, C.; Michel, P.; Gillery, P.; Jaisson, S.; Fonfrede, M.; Morel, F.; Hartemann, A.; Bourron, O. Association of peripheral neuropathy with circulating advanced glycation end products, soluble receptor for advanced glycation end products and other risk factors in patients with type 2 diabetes. Diabetes/Metab. Res. Rev. 2014, 30, 679–685. [Google Scholar] [CrossRef]
- Billault, B.M.; Passa, P.L. Factors Associated With Diabetic Microangiopathy: A Study of 157 Type I (Insulin-Dependent) Diabetic Patients. J. Diabet. Complicat. 1991, 5, 238–243. [Google Scholar] [CrossRef]
- Callaghan, B.C.; Reynolds, E.; Banerjee, M.; Chant, E.; Villegas-Umana, E.; Feldman, E.L. Central Obesity is Associated With Neuropathy in the Severely Obese. Mayo Clin. Proc. 2020, 95, 1342–1353. [Google Scholar] [CrossRef]
- Çelik, S.B.; Can, H.; Sözmen, M.K.; Sengezer, T.; Kaplan, Y.C.; Utlu, G.; Sener, A.; Yilmaz, A.A.; Aygun, O. Evaluation of the neuropathic pain in the smokers. Agri 2017, 29, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xiao, S.; Chen, Z.; Yang, Y.; Yang, B.; Liu, N. Risk factors for peripheral artery disease and diabetic peripheral neuropathy among patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2024, 207, 111079. [Google Scholar] [CrossRef] [PubMed]
- Chukwubuzo, O.T.; Chibuike, O.P.; Philip, O.C.; Sunday, E.M.; Ekpunobi, C.P.; Sochima, E.E.; Obumneme, O.A.; Gerald, E.U.; Ifeoma, O.D.; Ugwu, A.R. Prevalence And Asso-ciations Of Neuropathic Pain Among Subjects With Diabetes Mellitus In The Enugu Diabetic Peripheral Neuropathy (Edipen) Study. J. Pharm. Negat. Results 2022, 13, 10104–10117. [Google Scholar]
- Corrêa, L.A.; Bittencourt, J.V.; Mathieson, S.; Nogueira, L.A.C. Pain-related interference and pain-related psychosocial factors of three different subgroups of patients with chronic low back pain. Musculoskelet. Sci. Pract. 2023, 63, 102718. [Google Scholar] [CrossRef]
- Dorsey, R.R.; Eberhardt, M.S.; Gregg, E.W.; Geiss, L.S. Control of risk factors among people with diagnosed diabetes, by lower extremity disease status. Prev. Chronic Dis. 2009, 6, A114. [Google Scholar]
- Faden, A.; Mendoza, E.; Flynn, F. Subclinical neuropathy associated with chronic obstructive pulmonary disease: Possible pathophysiologic role of smoking. Arch. Neurol. 1981, 38, 639–642. Available online: http://archneur.jamanetwork.com/ (accessed on 3 June 2025). [CrossRef]
- Gode, M.; Aga, F.; Hailu, A. Self-Care Practices Among Adult Type 2 Diabetes Patients With and Without Peripheral Neuropathy: A Cross-Sectional Study at Tertiary Healthcare Settings in Ethiopia. Can. J. Nurs. Res. 2022, 54, 345–356. [Google Scholar] [CrossRef]
- Gündüz, E. Neuropathic Pain After Thoracotomy: Risk Factors and Incidence. J. Cardıo-Vasc.-Thoracıc Anaesthesıa Intensıve Care Soc. 2022, 28, 312–317. [Google Scholar] [CrossRef]
- Gylfadottir, S.S.; Christensen, D.H.; Nicolaisen, S.K.; Andersen, H.; Callaghan, B.C.; Itani, M.; Khan, K.S.; Kristensen, A.G.; Nielsen, J.S.; Sindrup, S.H.; et al. Diabetic polyneuropathy and pain, prevalence, and patient characteristics: A cross-sectional questionnaire study of 5,514 patients with recently diagnosed type 2 diabetes. PAIN® 2020, 161, 574–583. [Google Scholar] [CrossRef]
- Hicks, C.W.; Wang, D.; Lin, F.R.; Reed, N.; Windham, B.G.; Selvin, E. Peripheral Neuropathy and Vision and Hearing Impairment in US Adults With and Without Diabetes. Am. J. Epidemiol. 2023, 192, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Divers, J.; Dabelea, D.; Isom, S.; Bell, R.A.; Martin, C.L.; Pettitt, D.J.; Saydah, S.; Pihoker, C.; Standiford, D.A.; et al. Prevalence of and Risk Factors for Diabetic Peripheral Neuropathy in Youth With Type 1 and Type 2 Diabetes: SEARCH for Diabetes in Youth Study. Diabetes Care 2017, 40, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Jeyam, A.; McGurnaghan, S.J.; Blackbourn, L.A.; McKnight, J.M.; Green, F.; Collier, A.; McKeigue, P.M.; Colhoun, H.M. Diabetic Neuropathy Is a Substantial Burden in People With Type 1 Diabetes and Is Strongly Associated With Socioeconomic Disadvantage: A Population-Representative Study From Scotland. Diabetes Care 2020, 43, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, J.L.; Nguyen, N.; Battaglino, R.A.; Falci, S.P.; Charlifue, S.; Morse, L.R. miR-338-5p Levels and Cigarette Smoking are Associated With Neuropathic Pain Severity in Individuals With Spinal Cord Injury: Preliminary Findings From a Genome-Wide microRNA Expression Profiling Screen. Arch. Phys. Med. Rehabil. 2022, 103, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, W.; Ji, Q.; Ran, X.; Kuang, H.; Yu, X.; Fang, H.; Yang, J.; Liu, J.; Xue, Y.; et al. Prevalence of painful diabetic peripheral neuropathy in type 2 diabetes mellitus and diabetic peripheral neuropathy: A nationwide cross-sectional study in mainland China. Diabetes Res. Clin. Pr. 2023, 198, 110602. [Google Scholar] [CrossRef]
- Al-Mahroos, F.; Al-Roomi, K. Diabetic neuropathy, foot ulceration, peripheral vascular disease and potential risk factors among patients with diabetes in Bahrain—A nationwide primary care diabetes clinic-based study. Ann. Saudi Med. 2007, 27, 25–31. [Google Scholar]
- Mick, G.; Serpell, M.; Baron, R.; Mayoral, V.; Hans, G.; Mendez, I.; Artime, E.; Qizilbash, N.; Sohns, M. Localised neuropathic pain in the primary care setting: A cross-sectional study of prevalence, clinical characteristics, treatment patterns, quality of life and sleep performance. Curr. Med Res. Opin. 2020, 37, 293–302. [Google Scholar] [CrossRef]
- Mizokami-Stout, K.R.; Li, Z.; Foster, N.C.; Shah, V.; Aleppo, G.; McGill, J.B.; Pratley, R.; Toschi, E.; Ang, L.; Pop-Busui, R. The Contemporary Prevalence of Diabetic Neuropathy in Type 1 Diabetes: Findings From the T1D Exchange. Diabetes Care 2020, 43, 806–812. [Google Scholar] [CrossRef]
- Molla, G.J.; Ismail-Beigi, F.; Larijani, B.; Khaloo, P.; Moosaie, F.; Alemi, H.; Mansournia, M.A.; Ghadimi, T.; Ghaemi, F.; Nakhjavani, M.; et al. Smoking and Diabetes Control in Adults With Type 1 and Type 2 Diabetes: A Nationwide Study From the 2018 National Program for Prevention and Control of Diabetes of Iran. Can. J. Diabetes 2020, 44, 246–252. [Google Scholar] [CrossRef]
- Moore, D.C.; Ringley, J.T.; Nix, D.; Muslimani, A. Impact of Body Mass Index on the Incidence of Bortezomib-induced Peripheral Neuropathy in Patients With Newly Diagnosed Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2020, 20, 168–173. [Google Scholar] [CrossRef]
- Nagakura, Y.; Hayashi, M.; Kajioka, S. Analysis of Japanese nationwide health datasets: Association between lifestyle habits and prevalence of neuropathic pain and fibromyalgia with reference to dementia-related diseases and Parkinson’s disease. Scand. J. Pain. 2023, 23, 662–669. [Google Scholar] [CrossRef]
- Nielsen, S.W.; Eckhoff, L.; Ruhlmann, C.H.B.; Herrstedt, J.; Dalton, S.O. The prevalence, distribution and impact of peripheral neuropathy among Danish patients with cancer–a population-based cross-sectional study. Acta Oncol. 2022, 61, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Ponirakis, G.; Elhadd, T.; Al Ozairi, E.; Brema, I.; Chinnaiyan, S.; Taghadom, E.; Al Kandari, J.; Al Wotayan, R.; Al Ozairi, A.; Aljohani, N.; et al. Prevalence and risk factors for diabetic peripheral neuropathy, neuropathic pain and foot ulceration in the Arabian Gulf region. J. Diabetes Investig. 2022, 13, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Rash, C.J.; Alessi, S.M.; Foster, N.; Tamborlane, W.; Van Name, M.A.; Wagner, J.A. Tobacco use patterns and clinical outcomes in the T1D exchange. J. Diabetes Complicat. 2022, 36, 108128. [Google Scholar] [CrossRef] [PubMed]
- Révész, D.; Bonhof, C.S.; Bours, M.J.; Weijenberg, M.P.; Vreugdenhil, G.; van de Poll-Franse, L.V.; Mols, F. Sociodemographic, Clinical, Lifestyle, and Psychological Correlates of Peripheral Neuropathy among 2- to 12-Year Colorectal Cancer Survivors. Oncol. Res. Treat. 2022, 45, 480–493. [Google Scholar] [CrossRef]
- Sahito, D.A.; Rahu, S.A.; Samad, A.; Hayee, P.A.; Zaman Shaikh, M.; Naseem, S. Assessment of Prevalence and the Risk Factors of Diabetic Peripheral Neuropathy at Primary Health Care Level at Nawab Shah. Pak. J. Med. Health Sci. 2022, 16, 330–332. [Google Scholar] [CrossRef]
- Srivastava, S.P.; Sinha, A.P.; Sharma, K.K.; Malik, P.S. Severity, Risk Factors and Quality of Life of Patients associated with Chemotherapy-Induced Peripheral Neuropathy. Clin. Nurs. Res. 2022, 31, 1080–1090. [Google Scholar] [CrossRef]
- Tamer, A.; Yildiz, S.; Yildiz, N.; Kanat, M.; Gunduz, H.; Tahtaci, M.; Celebi, H. The Prevalence of Neuropathy and Relationship with Risk Factors in Diabetic Patients: A Single-Center Experience. Med Princ. Pr. 2006, 15, 190–194. [Google Scholar] [CrossRef]
- Tesfaye, S.; Stevens, L.K.; Stephenson, J.M.; Fuller, J.H.; Plater, M.; Ionescu-Tirgoviste, C.; Nuber, A.; Pozza, G.; Ward, J.D.; the EURODIAB IDDM Complications Study Group. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: The EURODIAB IDDM Complications Study. Diabetologia 1996, 39, 1377–1384. [Google Scholar] [CrossRef]
- Trendowski, M.R.; Lusk, C.M.; Ruterbusch, J.J.; Seaton, R.; Simon, M.S.; Greenwald, M.K.; Harper, F.W.K.; Beebe-Dimmer, J.L.; Schwartz, A.G. Chemotherapy-induced peripheral neuropathy in African American cancer survivors: Risk factors and quality of life outcomes. Cancer Med. 2021, 10, 8151–8161. [Google Scholar] [CrossRef]
- van der Velde, J.H.P.M.; Koster, A.; Strotmeyer, E.S.; Mess, W.H.; Hilkman, D.; Reulen, J.P.H.; Stehouwer, C.D.A.; Henry, R.M.A.; Schram, M.T.; van der Kallen, C.J.H.; et al. Cardiometabolic risk factors as determinants of peripheral nerve function: The Maastricht Study. Diabetologia 2020, 63, 1648–1658. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ji, Q.; Ran, X.; Li, C.; Kuang, H.; Yu, X.; Fang, H.; Yang, J.; Liu, J.; Xue, Y.; et al. Prevalence and risk factors of diabetic peripheral neuropathy: A population-based cross-sectional study in China. Diabetes/Metab. Res. Rev. 2023, 39, e3702. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, A.H.; Seng, E.K.; Ditre, J.W.; Willoughby, M.; Shuter, J. Perceived interrelations of pain and cigarette smoking in a sample of adult smokers living with HIV/AIDS. Nicotine Tob. Res. 2019, 21, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Tsuji, T.; Hayashi, S.; Kabata, D.; Shintani, A. Factors associated with diabetic polyneuropathy-related sensory symptoms and signs in patients with polyneuropathy: A cross-sectional Japanese study (JDDM 52) using a non-linear model. J. Diabetes Investig. 2020, 11, 450–457. [Google Scholar] [CrossRef]
- Richards, J.S.; Kogos, S.C.; Ness, T.J.; Oleson, C.V. Effects of Smoking on Neuropathic Pain in Two People With Spinal Cord Injury. J. Spinal Cord Med. 2005, 28, 330–332. [Google Scholar] [CrossRef]
- MacRae, C.; Kopalakrishnan, S.; Faust, L.; Klowak, M.; Showler, A.; Klowak, S.A.; Boggild, A.K. Evaluation of safety tool for ambulatory leprosy patients at risk of adverse outcome. Trop. Dis. Travel Med. Vaccines 2018, 4, 1–7. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klowak, M.; Lau, R.; Mohammed, M.N.; Birago, A.; Samson, B.; Ahmed, L.; Renee, C.; Meconnen, M.; Bado, E.; Reid-John, A.; et al. A Systematic Review of Lifestyle Interventions for Neuropathy and Neuropathic Pain: Smoking Cessation. NeuroSci 2025, 6, 74. https://doi.org/10.3390/neurosci6030074
Klowak M, Lau R, Mohammed MN, Birago A, Samson B, Ahmed L, Renee C, Meconnen M, Bado E, Reid-John A, et al. A Systematic Review of Lifestyle Interventions for Neuropathy and Neuropathic Pain: Smoking Cessation. NeuroSci. 2025; 6(3):74. https://doi.org/10.3390/neurosci6030074
Chicago/Turabian StyleKlowak, Michael, Rachel Lau, Mariyam N. Mohammed, Afia Birago, Bethel Samson, Layla Ahmed, Camille Renee, Milca Meconnen, Ezra Bado, Aquilla Reid-John, and et al. 2025. "A Systematic Review of Lifestyle Interventions for Neuropathy and Neuropathic Pain: Smoking Cessation" NeuroSci 6, no. 3: 74. https://doi.org/10.3390/neurosci6030074
APA StyleKlowak, M., Lau, R., Mohammed, M. N., Birago, A., Samson, B., Ahmed, L., Renee, C., Meconnen, M., Bado, E., Reid-John, A., & Boggild, A. K. (2025). A Systematic Review of Lifestyle Interventions for Neuropathy and Neuropathic Pain: Smoking Cessation. NeuroSci, 6(3), 74. https://doi.org/10.3390/neurosci6030074