Which One Would You Choose?—Investigation of Widely Used Housekeeping Genes and Proteins in the Spinal Cord of an Animal Model of Amyotrophic Lateral Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
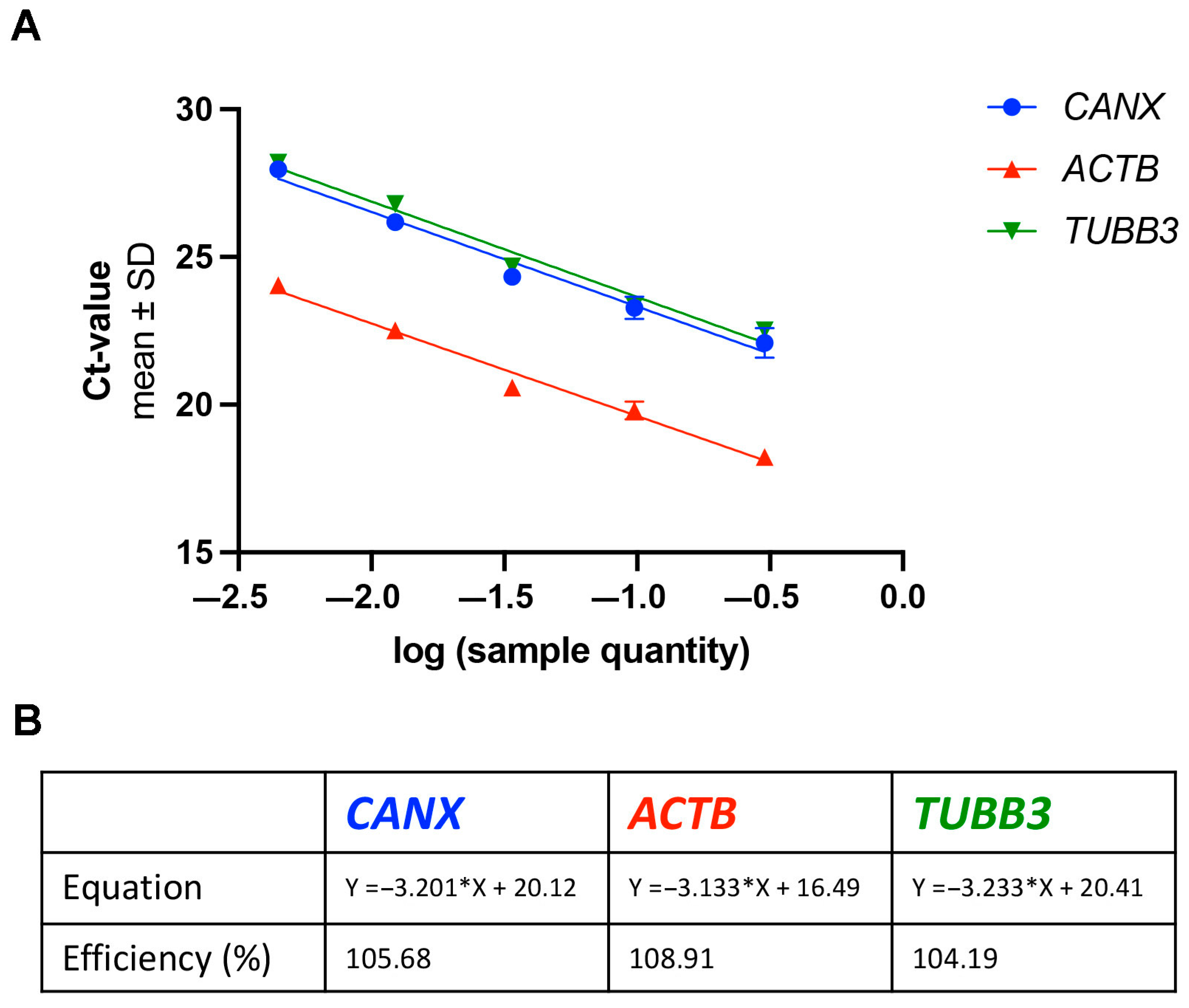
2.2. Quantitative Reverse Transcription-Polymerase Chain Reaction
2.3. Semiquantitative Protein Expression Analysis via Western Blot
2.4. Statistical Analysis
3. Results
3.1. Similar Gene Expression in the Cervical Spinal Cord of Wild Type and Wobbler Mice at the Stable Clinical Phase
3.2. Consistent Protein Expression in the Cervical Spinal Cord of Wild-Type and Wobbler Mouse Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALS | Amyotrophic lateral sclerosis |
| Vps54 | Vacuolar protein sorting-associated protein 54 |
| GARP | Golgi-associated retrograde protein complex |
| ER | Endoplasmic reticulum |
| CANX | Calnexin gene |
| ACTB | Actin beta gene |
| TUBB3 | Tubulin beta 3 class III gene |
| qPCR | Quantitative polymerase chain reaction |
| mRNA | Messenger ribonucleic acid |
| tRNA | Total RNA |
| qRT-PCR | Quantitative reverse transcription polymerase chain reaction |
| PBS | Phosphate-buffered saline |
| SDS | Sodium dodecyl sulfate |
| EDTA | Ethylenediaminetetraacetic acid |
| WT | Wild type |
| WR | Wobbler |
| Ct | Cycle threshold |
| SD | Standard deviation |
| SEM | Standard error of the mean |
| SOD1 | Superoxide dismutase 1 |
| sALS | Sporadic amyotrophic lateral sclerosis |
| BCA | Bicinchoninic acid |
| RRID | Research resource identifier |
References
- Kiernan, M.C.; Vucic, S.; Cheah, B.C.; Turner, M.R.; Eisen, A.; Hardiman, O.; Burrell, J.R.; Zoing, M.C. Amyotrophic lateral sclerosis. Lancet 2011, 377, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Schmitt-John, T.; Drepper, C.; Mußmann, A.; Hahn, P.; Kuhlmann, M.; Thiel, C.; Hafner, M.; Lengeling, A.; Heimann, P.; Jones, J.M.; et al. Mutation of Vps54 causes motor neuron disease and defective spermiogenesis in the wobbler mouse. Nat. Genet. 2005, 37, 1213–1215. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.; Walkenfort, B.; Cihankaya, H.; Hasenberg, M.; Bader, V.; Winklhofer, K.F.; Röderer, P.; Matschke, J.; Theiss, C.; Matschke, V.; et al. Increased ROS-Dependent Fission of Mitochondria Causes Abnormal Morphology of the Cell Powerhouses in a Murine Model of Amyotrophic Lateral Sclerosis. Oxidative Med. Cell. Longev. 2021, 2021, 6924251. [Google Scholar] [CrossRef] [PubMed]
- Wunsch, F.T.; Metzler-Nolte, N.; Theiss, C.; Matschke, V. Defects in Glutathione System in an Animal Model of Amyotrophic Lateral Sclerosis. Antioxidants 2023, 12, 1014. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, R.; Golfi, P.; Heimann, P.; Shaw, C.; Troakes, C.; Schmitt-John, T.; Bartsch, J.W. Endosomal accumulation of APP in wobbler motor neurons reflects impaired vesicle trafficking: Implications for human motor neuron disease. BMC Neurosci. 2011, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Ueno, N.; Kashiwagi, M.; Kanekatsu, M.; Marubashi, W.; Yamada, T. Accumulation of protein aggregates induces autolytic programmed cell death in hybrid tobacco cells expressing hybrid lethality. Sci. Rep. 2019, 9, 10223. [Google Scholar] [CrossRef] [PubMed]
- Blokhuis, A.M.; Groen, E.J.N.; Koppers, M.; Van Den Berg, L.H.; Pasterkamp, R.J. Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol. 2013, 125, 777–794. [Google Scholar] [CrossRef] [PubMed]
- Dahlke, C.; Saberi, D.; Ott, B.; Brand-Saberi, B.; Schmitt-John, T.; Theiss, C. Inflammation and neuronal death in the motor cortex of the wobbler mouse, an ALS animal model. J. Neuroinflamm. 2015, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Geloso, M.C.; Corvino, V.; Marchese, E.; Serrano, A.; Michetti, F.; D’Ambrosi, N. The dual role of microglia in ALS: Mechanisms and therapeutic approaches. Front. Aging Neurosci. 2017, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Pehar, M.; Harlan, B.A.; Killoy, K.M.; Vargas, M.R. Role and Therapeutic Potential of Astrocytes in Amyotrophic Lateral Sclerosis. Curr. Pharm. Des. 2018, 23, 5010–5021. [Google Scholar] [CrossRef] [PubMed]
- Saberi, D.; Ott, B.; Dahlke, C.; Matschke, V.; Schmitt-John, T.; Theiss, C. The spatiotemporal pattern of degeneration in the cerebellum of the wobbler mouse. J. Neuropathol. Exp. Neurol. 2016, 75, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Moser, J.M.; Bigini, P.; Schmitt-John, T. The wobbler mouse, an ALS animal model. Mol. Genet. Genom. 2013, 288, 207–229. [Google Scholar] [CrossRef] [PubMed]
- Ott, B.; Dahlke, C.; Meller, K.; Napirei, M.; Schmitt-John, T.; Brand-Saberi, B.; Theiss, C.; Saberi, D. Implementation of a manual for working with wobbler mice and criteria for discontinuation of the experiment. Ann. Anat. 2015, 200, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Klatt, C.L.; Theis, V.; Hahn, S.; Theiss, C.; Matschke, V. Deregulated miR-29b-3p correlates with tissue-specific activation of intrinsic apoptosis in an animal model of amyotrophic lateral sclerosis. Cells 2019, 8, 1077. [Google Scholar] [CrossRef] [PubMed]
- Cihankaya, H.; Bader, V.; Winklhofer, K.F.; Vorgerd, M.; Matschke, J.; Stahlke, S.; Theiss, C.; Matschke, V. Elevated NLRP3 Inflammasome Activation Is Associated with Motor Neuron Degeneration in ALS. Cells 2024, 13, 995. [Google Scholar] [CrossRef] [PubMed]
- Röderer, P.; Klatt, L.; John, F.; Theis, V.; Winklhofer, K.F.; Theiss, C.; Matschke, V. Increased ROS Level in Spinal Cord of Wobbler Mice due to Nmnat2 Downregulation. Mol Neurobiol. 2018, 55, 8414–8424. [Google Scholar] [CrossRef] [PubMed]
- Junghans, M.; John, F.; Cihankaya, H.; Schliebs, D.; Winklhofer, K.F.; Bader, V.; Matschke, J.; Theiss, C.; Matschke, V. ROS scavengers decrease γH2ax spots in motor neuronal nuclei of ALS model mice in vitro. Front. Cell. Neurosci. 2022, 16, 963169. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Higgins, P.J.; Crawford, D.R. Control selection for RNA quantitation. Biotechniques 2000, 29, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bergen, J.A.; Miles, D.C.; Sinclair, A.H.; Western, P.S. Normalizing gene expression levels in mouse fetal germ cells. Biol. Reprod. 2009, 81, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Dzhalilova, D.S.; Kosyreva, A.M.; Vyshnyakova, P.A.; Tsvetkov, I.S.; Zolotova, N.A.; Miroshnichenko, E.A.; Makarova, O.V. Age-Specific Features of the Levels of Reference Proteins Actin, Tubulin, and GAPDH in Different Organs of Male Wistar Rats. Bull. Exp. Biol. Med. 2022, 173, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, G.; Gehring, K. Calnexin cycle—structural features of the ER chaperone system. FEBS J. 2020, 287, 4322–4340. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Liu, S.; Wang, J.; Sun, M.Z.; Greenaway, F.T. ACTB in cancer. Clin. Chim. Acta 2013, 417, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.; Karki, R.; Spennato, M.; Pandya, D.; He, S.; Andreoli, M.; Fiedler, P.; Ferlini, C. Clas-III β-tubulin in normal and cancer tissues. Gene 2015, 563, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.C.; Moreno-Igoa, M.; Manzano, R.; Ordovás, L.; Yagüe, G.; Oliván, S.; Muñoz, M.J.; Zaragoza, P.; Osta, R. Determination of protein and RNA expression levels of common housekeeping genes in a mouse model of neurodegeneration. Proteomics 2008, 8, 4338–4343. [Google Scholar] [CrossRef] [PubMed]
- Boillée, S.; Peschanski, M.; Junier, M.P. The Wobbler Mouse: A Neurodegeneration Jigsaw Puzzle. Mol. Neurobiol. 2003, 28, 65–106. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Iwasaki, Y.; Kaji, R. Neuroprotective effect of ultra-high dose methylcobalamin in wobbler mouse model of amyotrophic lateral sclerosis. J. Neurol. Sci. 2015, 354, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Kinoshita, M.; Iwasaki, Y.; Tagaya, N.; Shiojima, T. Lecithinized superoxide dismutase retards wobbler mouse motoneuron disease. Neuromuscul. Disord. 1995, 5, 383–390. [Google Scholar] [CrossRef] [PubMed]
- DiFebo, F.; Curti, D.; Botti, F.; Biella, G.; Bigini, P.; Mennini, T.; Toselli, M. Neural precursors (NPCs) from adult L967Q mice display early commitment to “in vitro” neuronal differentiation and hyperexcitability. Exp. Neurol. 2012, 236, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Hounkpe, B.W.; Chenou, F.; de Lima, F.; de Paula, E.V. HRT Atlas v1.0 database: Redefining human and mouse housekeeping genes and candidate reference transcripts by mining massive RNA-seq datasets. Nucleic Acids Res. 2021, 49, D947–D955. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.C.; Berkelman, T.; Yadav, G.; Hammond, M. A defined methodology for reliable quantification of western blot data. Mol. Biotechnol. 2013, 55, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Usarek, E.; Barańczyk-Kuźma, A.; Kaźmierczak, B.; Gajewska, B.; Kuźma-Kozakiewicz, M. Validation of qPCR reference genes in lymphocytes from patients with amyotrophic lateral sclerosis. PLoS ONE 2017, 12, e0174317. [Google Scholar] [CrossRef] [PubMed]
- Gurney, M.E.; Pu, H.; Chiu, A.Y.; Dal Canto, M.C.; Polchow, C.Y.; Alexander, D.D.; Caliendo, J.; Hentati, A.; Kwon, Y.W.; Deng, H.X.; et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 1994, 264, 1772–1775. [Google Scholar] [CrossRef] [PubMed]
- van Zundert, B.; Brown, R.H. Silencing strategies for therapy of SOD1-mediated ALS. Neurosci. Letters. 2017, 636, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Morrice, J.R.; Gregory-Evans, C.Y.; Shaw, C.A. Animal models of amyotrophic lateral sclerosis: A comparison of model validity. Neural Regen. Res. 2018, 13, 2050–2054. [Google Scholar] [CrossRef] [PubMed]
- Joyce, P.I.; Fratta, P.; Fisher, E.M.C.; Acevedo-Arozena, A. SOD1 and TDP-43 animal models of amyotrophic lateral sclerosis: Recent advances in understanding disease toward the development of clinical treatments. Mamm. Genome 2011, 22, 420–448. [Google Scholar] [CrossRef] [PubMed]
- Nardo, G.; Trolese, M.C.; Tortarolo, M.; Vallarola, A.; Freschi, M.; Pasetto, L.; Bonetto, V.; Bendotti, C. New insights on the mechanisms of disease course variability in ALS from mutant SOD1 mouse models. Brain Pathol. 2016, 26, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.F.; Wong, W.T. Roles of the actin cytoskeleton in aging and age-associated diseases. Ageing Res. Rev. 2020, 58, 101021. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Re, D.B.; Nagata, T.; Chalazonitis, A.; Jessell, T.M.; Wichterle, H.; Przedborski, S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat. Neurosci. 2007, 10, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Heimann, P.; Laage, S.; Jockusch, H. Defect of sperm assembly in a neurological mutant of the mouse, wobbler (WR). Differentiation 1991, 47, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Achi, E.Y.; Rudnicki, S.A. ALS and frontotemporal dysfunction: A review. Neurol. Res. Int. 2012, 2012, 8063063. [Google Scholar] [CrossRef] [PubMed]
- Zwiegers, P.; Lee, G.; Shaw, C.A. Reduction in hSOD1 copy number significantly impacts ALS phenotype presentation in G37R (line 29) mice: Implications for the assessment of putative therapeutic agents. J. Negat. Results Biomed. 2014, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Lutz, C. Mouse models of ALS: Past, present and future. Brain Res. 2018, 1693, 1–10. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Epplen, A.S.C.; Stahlke, S.; Theiss, C.; Matschke, V. Which One Would You Choose?—Investigation of Widely Used Housekeeping Genes and Proteins in the Spinal Cord of an Animal Model of Amyotrophic Lateral Sclerosis. NeuroSci 2025, 6, 69. https://doi.org/10.3390/neurosci6030069
Epplen ASC, Stahlke S, Theiss C, Matschke V. Which One Would You Choose?—Investigation of Widely Used Housekeeping Genes and Proteins in the Spinal Cord of an Animal Model of Amyotrophic Lateral Sclerosis. NeuroSci. 2025; 6(3):69. https://doi.org/10.3390/neurosci6030069
Chicago/Turabian StyleEpplen, Aimo Samuel Christian, Sarah Stahlke, Carsten Theiss, and Veronika Matschke. 2025. "Which One Would You Choose?—Investigation of Widely Used Housekeeping Genes and Proteins in the Spinal Cord of an Animal Model of Amyotrophic Lateral Sclerosis" NeuroSci 6, no. 3: 69. https://doi.org/10.3390/neurosci6030069
APA StyleEpplen, A. S. C., Stahlke, S., Theiss, C., & Matschke, V. (2025). Which One Would You Choose?—Investigation of Widely Used Housekeeping Genes and Proteins in the Spinal Cord of an Animal Model of Amyotrophic Lateral Sclerosis. NeuroSci, 6(3), 69. https://doi.org/10.3390/neurosci6030069







