1. Introduction
Recruitment of circulating immune cells, including monocytes, to inflamed tissues has been demonstrated in experimental models of nociceptive pain, such as those induced by inoculation with Freund’s complete adjuvant (FCA), which causes chronic inflammation [
1]. Several lines of evidence indicate that these recruited immune cells contribute to antinociception through enhanced production of endogenous opioids within inflamed tissues. Endogenous opioids derived from immune cells—primarily monocytes and macrophages—are also suggested to contribute to antinociception in experimental models of neuropathic pain, such as chronic constriction injury (CCI) of the sciatic nerve [
2]. Peripheral transfusion of monocytes/macrophages pretreated ex vivo to produce endogenous opioids has been investigated as a potential therapeutic strategy for nerve injury [
3]. However, the effects of pulsed radiofrequency (PRF) current on such ex vivo treatment in the context of neuropathic pain have not yet been evaluated.
PRF and continuous radiofrequency (CRF) are two clinical modalities widely used for the management of chronic pain [
4,
5,
6]. CRF was originally developed to thermally ablate nerve tissue through the continuous application of a high-frequency current, typically around 500 kHz, resulting in sustained tissue heating around the electrode tip. To prevent excessive thermal damage, CRF devices are equipped with temperature control systems that regulate the tip temperature, often maintaining it at 70–90 °C during treatment. In contrast, PRF delivers short bursts of RF current (typically 20 ms at 2 Hz), which markedly limits tissue heating while still applying high peak voltages. This intermittent stimulation leads to a reduced average power delivery—approximately 4% of that in CRF at the same voltage—thus minimizing thermal injury [
4,
5]. Importantly, PRF has been shown to modulate neural and immune function without causing neurodestructive effects, and clinical evidence supports its efficacy in treating various neuropathic pain conditions, such as postherpetic neuralgia [
7,
8], cervical radicular pain [
9], or chronic knee osteoarthritis [
10]. Despite these findings, the underlying cellular mechanisms influenced by PRF, particularly those involving immune cells like monocytes and macrophages, remain incompletely understood.
We previously demonstrated that exposure of human monocytic THP-1 cells to a PRF electric field increased mRNA expression of proopiomelanocortin (POMC), the precursor of β-endorphin, and found that thermal conditions alone, even in the absence of PRF current, were responsible for this upregulation [
5]. Interestingly, we also observed a significant increase in POMC mRNA expression under hypothermic conditions, in which THP-1 cells sedimented in test tubes were exposed to PRF current while being incubated at 20 °C [
5]. These findings suggest that PRF may enhance POMC gene expression through mechanisms independent of thermal effects.
β-Endorphin is produced as part of the amino acid sequence of the precursor protein POMC, which also gives rise to several other bioactive peptides in the pituitary gland, melanocytes, and immune cells, including monocytes [
11,
12]. Post-translational processing of POMC by tissue-specific proteases leads to the production of various hormones and bioactive compounds, such as adrenocorticotropic hormone (ACTH), lipotropins, melanocyte-stimulating hormones (MSHs), corticotropin-like intermediate peptide (CLIP), and β-endorphin. Among these, ACTH and β-lipotropin undergo further cleavage to generate smaller peptides, including α-MSH and CLIP from ACTH, and β-MSH and β-endorphin from β-lipotropin [
11]. Because β-endorphin is one of the key molecules responsible for immune cell-derived antinociception and is known to be produced by monocytes, we hypothesized that exposure of human monocytic cells to PRF current and/or heat would increase β-endorphin expression at the protein level, as we previously observed at the mRNA level.
There is mounting experimental evidence that PRF induces neuromodulation via electric field-dependent increases in intracellular Ca
2+ levels. For instance, Mercadal et al. demonstrated that exposure of HEK-293 cells to electric fields generated by PRF current-elevated intracellular Ca
2+ concentrations in a field strength-dependent manner [
13]. Meanwhile, Sauer et al. reported that transient stimulation of human monocytes with lipopolysaccharide (LPS) promotes β-endorphin release via Toll-like receptor 4 and that this release requires elevated intracellular Ca
2+ levels [
14]. These findings may suggest a convergence in mechanism, whereby PRF may similarly enhance endogenous opioid production in monocytes by modulating intracellular Ca
2+ dynamics. However, the transcriptional upregulation and time course observed in our study [
5] imply that PRF-induced β-endorphin production may also reflect a sustained cellular response rather than merely transient peptide release.
Given the capacity of monocytes to produce β-endorphin and their established role in peripheral antinociception, there is growing interest in harnessing these cells as therapeutic vehicles. A previous study by Pannell et al. has explored the transfusion of monocytes/macrophages pretreated ex vivo to enhance opioid peptide production as a potential approach for pain management [
3]. In this context, our study serves as a foundational investigation into the ability of PRF and thermal modulation to upregulate β-endorphin production in monocytic cells under controlled conditions. The findings may provide a basis for future development of ex vivo monocyte-based therapies for neuropathic pain.
4. Discussion
We have previously reported that exposure of human monocytic THP-1 cells to a PRF electric field for 15 min using the NT500 RF generator, which maintains the temperature around the electrode tip below 43 °C, increased mRNA expression of POMC, compared with non-exposed cells [
5]. In the present study, we confirmed that PRF application also enhanced the extracellular release of β-endorphin, a final bioactive product derived from POMC, as shown by ELISA.
Several earlier studies have demonstrated that leukocytes, including monocytes, can produce endogenous opioids in inflamed tissues or around injured peripheral nerves. Rittner et al. showed in a rat model of chronic inflammatory pain induced by Freund’s complete adjuvant (FCA) inoculation that granulocytes expressing opioids were recruited to inflamed tissue and regional lymph nodes in the early phase of inflammation [
1]. These granulocytes were later replaced by monocytes and macrophages as the dominant opioid-producing leukocytes. Analgesia, evaluated by pressure threshold after cold water swim stress, a surrogate of opioid-mediated pain relief, was enhanced in the affected limb. Similarly, Labuz et al. demonstrated that opioid-containing immune cells, including monocytes, accumulated at sites of nerve injury in a chronic constriction injury (CCI) model [
2]. They further showed that these cells expressed corticotropin-releasing factor (CRF) receptors, and that local CRF injection suppressed mechanical hyperalgesia in a naloxone-reversible manner, indicating a role for leukocyte-derived opioids in CRF-induced analgesia [
2]. Machelska et al. extended this concept by delivering vectors carrying the POMC gene into the inflamed paw in the FCA model, thereby promoting local production of endogenous opioids [
19]. Although promising, this gene therapy approach has not been widely adopted due to limitations in reliability and feasibility. In a follow-up study, the same group stimulated murine bone marrow-derived monocytes to differentiate into macrophages ex vivo, and polarized them into M1 or M2 phenotypes. They found that M2 macrophages, but not M0 or M1, contained higher levels of intracellular opioid peptides and attenuated tactile allodynia in the CCI model [
3].
Our study sought to investigate whether stimulation of human monocytic cells with PRF current, under thermally controlled conditions, could enhance β-endorphin production without inducing cell injury. In the field of interventional pain medicine, conventional RF (i.e., continuous RF current) is used to ablate peripheral nerves via thermal coagulation [
20]. In the mid-1990s, PRF was introduced as a less-destructive alternative, delivering 20 ms bursts of RF current at 2 Hz [
4,
20,
21]. Because RF-induced heat is proportional to the square of the voltage and the duration of exposure, PRF greatly reduces heat accumulation—down to 4% compared to continuous RF—allowing higher voltage use without causing thermal injury [
4].
The clinical efficacy of PRF has been supported by meta-analyses, particularly in the treatment of postherpetic neuralgia (PHN) involving the trigeminal nerve [
7], as well as in the cervical, thoracic, and lumbosacral regions [
8], and in cases of cervical radiculopathy [
9]. A randomized controlled trial also demonstrated that PRF combined with exercise provided superior pain relief for patients with chronic knee osteoarthritis compared to exercise alone [
10].
In contrast to the growing body of clinical evidence supporting the use of PRF to alleviate pain in various conditions, its exact mechanism of action remains unclear. Our initial goal was to clarify whether β-endorphin production in monocytes contributes to the clinical efficacy of PRF, which is typically delivered via an electrode placed near injured peripheral nerves in patients with neuropathic pain or around regional nerves in those with osteoarthritis. However, our interest has gradually shifted toward exploring whether monocytes stimulated ex vivo with PRF might exert antinociceptive effects in vivo through β-endorphin release.
Other strategies to induce opioid production in monocytes, such as POMC gene transduction [
19] or cytokine stimulation [
3], pose ethical or technical challenges for clinical application. PRF, on the other hand, has been used safely for more than 30 years in clinical settings [
4,
20,
21]. Therefore, our model—applying PRF current to suspended human monocytes ex vivo—may represent a more practical and ethically acceptable approach to promoting endogenous opioid release for pain management.
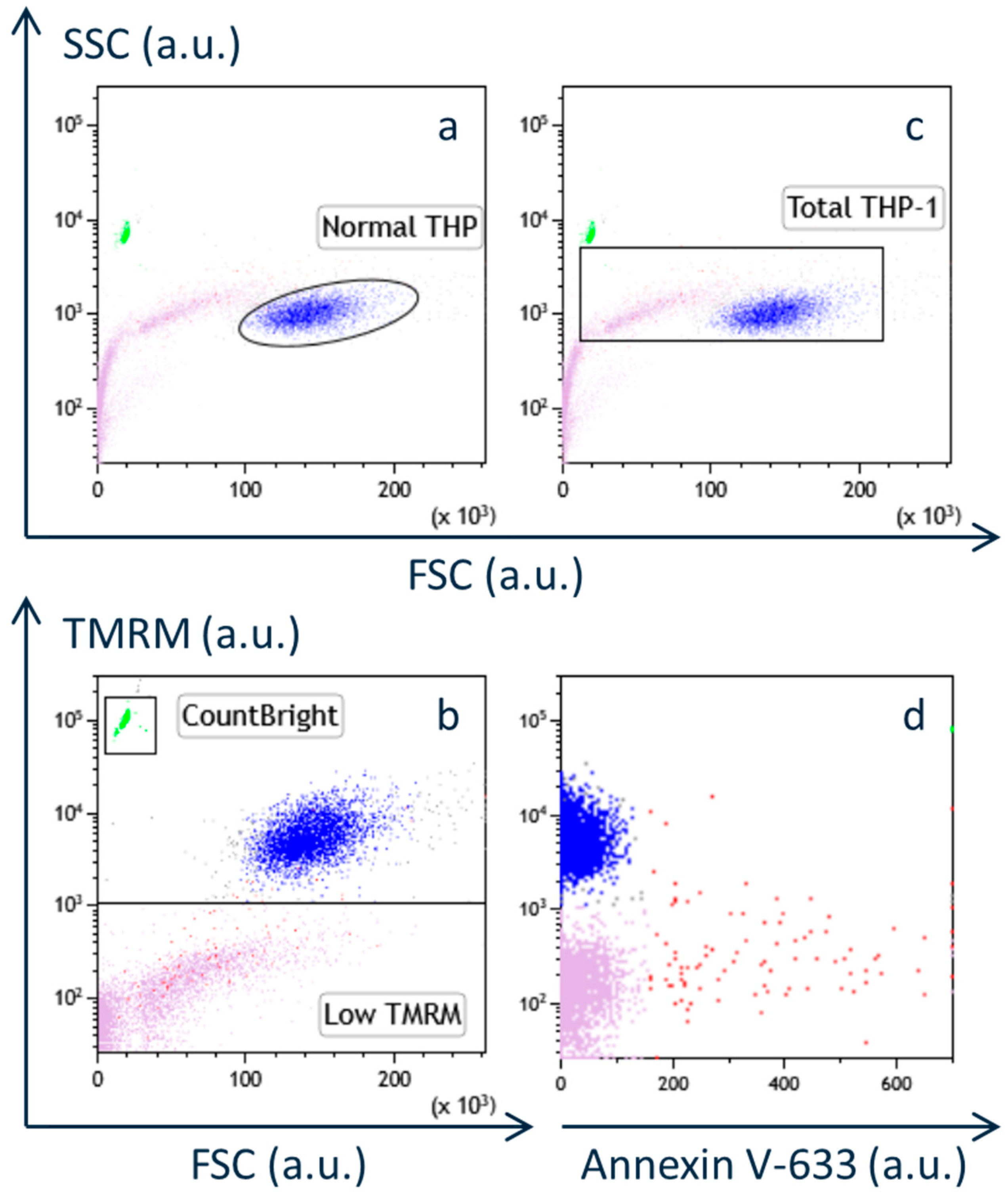
We used the Abbott NT500 RF generator, which limits the electrode tip temperature to below 43 °C. A newer Abbott device, the Ionic RF generator, allows this temperature to rise up to 49 °C. However, the classical PRF protocol (e.g., 45 V for 2 min at 42 °C [
22]) lacks safety data at higher temperatures. Therefore, in this study, we first confirmed the safety of heat-only conditions (i.e., without PRF current) for up to 3 min at temperatures below 46 °C, in alignment with conventional PRF parameters. Under these conditions, no decrease in viable cell number or apoptosis was observed, supporting our earlier finding that classical PRF does not cause cytotoxicity [
5,
6].
In contrast, the duration of PRF exposure in our current study was extended to 15 min, consistent with our previous work [
5]. This protocol was based on expert opinion from pain physicians, suggesting enhanced analgesia with longer PRF application. The NT500 generator suspends PRF delivery when the tissue temperature exceeds 42 °C, resuming only when it drops below this threshold. As a result, when temperatures rise above 42 °C, it takes more time to deliver the same cumulative energy. To address this limitation, the newer Ionic RF generator offers a protocol in which the total number of 20 ms RF bursts can be selected, rather than relying solely on a fixed duration of application.
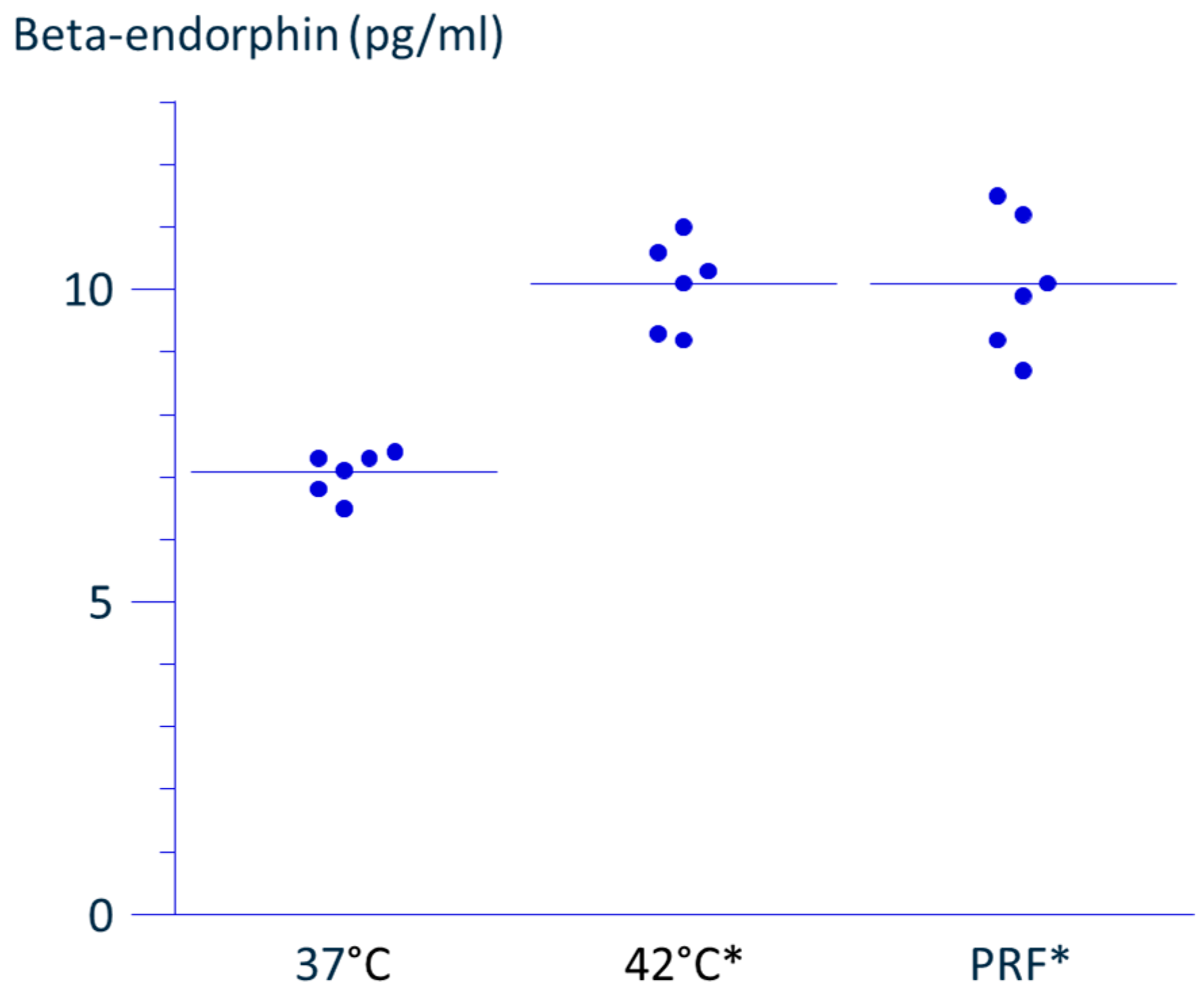
Taken together with our previous findings, the current results indicate that applying 70 V of PRF current for 15 min at just under 43 °C enhances POMC mRNA [
5], and increases β-endorphin release in THP-1 cells, without inducing cytotoxic effects [
5,
6].
We confirmed that PRF current and heat stress enhanced the expression of β-endorphin from monocytic cells in vitro. Although previous studies have suggested that monocyte-derived β-endorphin contributes to the alleviation of neuropathic pain [
1,
2,
3], it remains unclear whether β-endorphin production induced by ex vivo PRF stimulation of monocytic cells is sufficient to alleviate pain in patients with neuropathic or nociceptive pain.
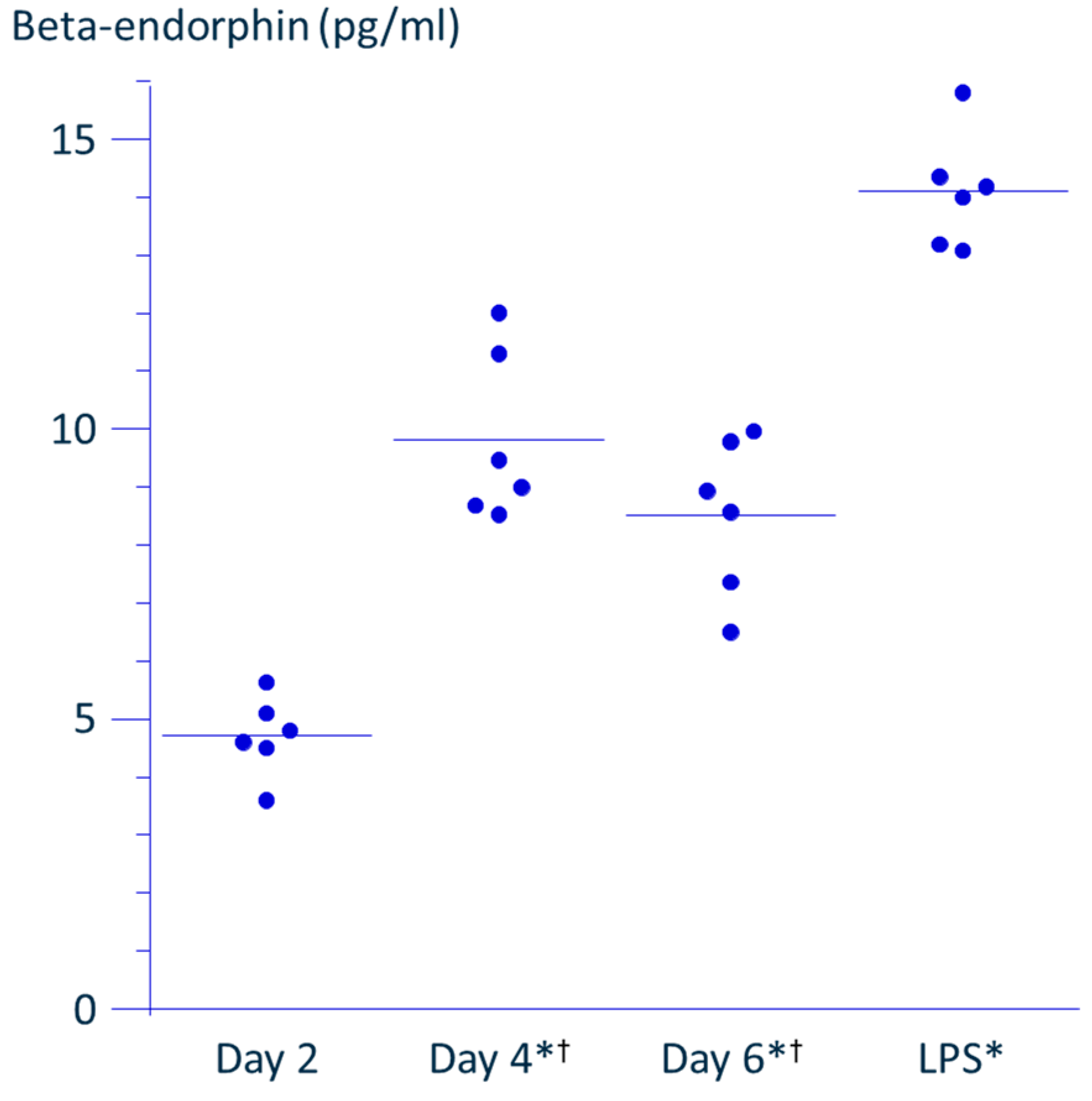
To address this issue, we compared the effects of PRF application with those of LPS, a known inducer of β-endorphin production from monocytes. Since THP-1 cells are proliferative and undergo macrophage-like differentiation upon LPS activation, incubation of cells was adjusted so that the cell density reached the permissible maximum by day 4, corresponding to the time when LPS-induced activation is completed. This approach allowed us to match the cell density as closely as possible to that in the PRF application experiments. As shown in
Figure 4, the concentration of β-endorphin in the culture supernatant peaked on day 4, suggesting that the cells had reached confluence. On day 4, the LPS-stimulated wells were rinsed and fresh medium was added; the accumulation of β-endorphin over the subsequent 48 h was then measured to match the PRF experiment protocol. The concentration of β-endorphin induced by LPS stimulation was 1.6-fold higher than the maximal level observed in the absence of stimulation, while PRF application induced a 1.4-fold increase over control. These findings suggest that PRF stimulation leads to a comparable enhancement of β-endorphin production to that induced by LPS.
Exploring β-endorphin production across different cell densities might have strengthened the consistency of the findings. However, the cell density used was already near the upper limit for THP-1 culture, while the amount of β-endorphin in the supernatant was close to the detection limit of the ELISA. This technical limitation made such evaluation difficult. Similarly, investigating the time course of β-endorphin production after PRF exposure could provide insights into the duration of PRF’s effects. However, due to the proliferative nature of THP-1 cells, prolonged observation would likely exceed optimal culture conditions.
Sauer et al. demonstrated that intraplantar injection of LPS in a CFA-induced pain model in rats produced antinociception via M2 macrophages, and this effect was antagonized by naloxone [
14]. In the same study, LPS stimulation also increased β-endorphin production from monocytes in vitro. Taken together, these findings suggest that the β-endorphin produced by monocytes in response to LPS contributes to the analgesic effects observed in the CFA model, and that the levels produced are sufficient for meaningful pain relief. Based on this analogy, the PRF-induced enhancement of β-endorphin production from monocytic cells ex vivo may also represent a promising mechanism of analgesia.
As suggested by theoretical estimations, including those by Cosman et al., high-intensity electric fields are generated in the vicinity of the RF electrode [
23]. Although the electric field itself does not directly generate heat, higher field strength increases current density within the surrounding tissues, leading to localized, time-dependent temperature elevation via Joule heating (I
2R or V
2/R) [
4,
23]. Since the electric field strength decays inversely proportional to the square of the distance, thermal effects at regions farther from the electrode tip are expected to decrease accordingly. Both theoretical modeling [
23] and experimental data using egg white (albumen) [
24] have demonstrated that, when RF currents near 500 kHz are applied, the critical region of temperature elevation does not exceed 2–3 mm from the shaft of the electrode. In albumen-based models, when the PRF temperature setting was raised above 60 °C, visible thermal coagulation occurred; even at a maximum setting of 70 °C, the extent of coagulation remained within 3 mm of the shaft [
24].
In our own experiments, the maximum temperature of the medium surrounding the cell pellet in conical tubes during PRF application under identical conditions was 40.4 ± 0.7 °C (
Figure S2). Based on these observations, it is presumed that the individual THP-1 cells sedimented in the tube were exposed to a thermal gradient ranging from 40 °C to 43 °C.
In a previous report, we demonstrated that PRF current applied to THP-1 cells in tubes pre-cooled to 20 °C led to an upregulation of POMC mRNA expression comparable to that observed during incubation at 37 °C [
5]. These findings suggest that PRF stimulation may enhance β-endorphin production through mechanisms independent of thermal effects.
Mercadal et al. cultured HEK-293 cells on coverslips and loaded them with the calcium indicator dye Calcium Green-1 to monitor intracellular Ca
2+ levels at the single-cell level [
13]. When exposed to a 500 kHz PRF electric field, they observed transient increases in intracellular calcium concentrations, which were shown to correlate with the electric field strength calculated based on the distance from the electrode. These findings suggest that the cellular response to PRF varies depending on the distance from the electrode, and thus the field strength experienced by each cell. Theoretically, it is also expected that the temperature distribution within the solution is not uniform.
Based on this context, it remains uncertain whether the observed upregulation of β-endorphin production in our study was confined to a subpopulation of THP-1 cells that was exposed to temperatures approaching 42 °C, or whether PRF-induced effects also occurred in cells experiencing minimal thermal input but significant electric field exposure.
To date, no experimental study has definitively established a mechanism by which PRF current modulates cellular function independently of thermal effects. Cosman et al. proposed that the high electric field strengths generated by clinically available RF generators may lead to membrane depolarization or even electroporation, potentially altering ion channel activity [
23]. Although Mercadal et al. provided experimental support for these hypotheses [
13], it should be noted—as discussed in the previous section—that local temperature elevations are inherently coupled with electric field strength due to Joule heating, and thus difficult to isolate experimentally.
Our previous literature review, along with earlier reports, comprehensively examined the neuromodulatory effects of PRF [
5]. As of the present manuscript, no substantial new findings have emerged that would significantly alter the current understanding of PRF-induced cellular modulation.
However, a pathway reported in 2018 may offer a new perspective. The discovery of the itaconate-mediated intracellular protective pathway activated in monocytes and macrophages by LPS stimulation [
25] may provide a potential link between PRF stimulation and cellular modulation. LPS is classically known to stimulate monocytes to produce proinflammatory cytokines such as IL-1β and IL-6 and is widely used to induce an M1 macrophage phenotype [
3]. Nevertheless, LPS also upregulates the expression of immunoresponsive gene 1 (Irg1), which encodes aconitate decarboxylase 1, leading to the intracellular accumulation of itaconate [
25].
Itaconate, in turn, alkylates KEAP1, resulting in the activation of the Nrf2 pathway. This activation enhances antioxidant activity in monocytes/macrophages and promotes the production of anti-inflammatory cytokines such as IL-10, thereby counteracting acute inflammatory responses [
25]. Although several studies have reported that anti-inflammatory M2 macrophages produce higher levels of β-endorphin than their proinflammatory M1 counterparts, M1 macrophages are not entirely devoid of β-endorphin-producing capacity [
3,
14]. Moreover, spinal application of 4-octyl itaconate (4-OI), a cell-permeable derivative of itaconate, has been shown to increase endorphin activity in a chronic constriction injury (CCI) mouse model in an IL-10-dependent manner [
26]. These findings suggest that itaconate pathways may serve as a regulatory axis for endogenous opioid production.
Whether PRF stimulation enhances intracellular itaconate accumulation or activates the Nrf2 pathway remains unknown. However, future investigations into the relationship between PRF exposure and itaconate metabolism may be key to elucidating the cellular mechanisms underlying PRF-induced modulation of immune cell function.
Mild hyperthermia generally induces the expression of heat shock proteins (HSPs), which play cytoprotective roles by refolding denatured proteins, preventing aggregation, and aiding in the degradation of irreversibly damaged proteins [
27]. Given that PRF application induces mild thermal stress, it is plausible that HSP expression is also modulated during PRF exposure, potentially protecting THP-1 cells from thermal damage. Recent evidence has shown that HSPB8, upregulated in hyperthermia-treated triple-negative breast cancer (TNBC) cells, can be transferred via exosomes into THP-1 cells and modulate macrophage polarization [
28]. This suggests that HSP expression induced by PRF might influence monocyte/macrophage polarization. However, while HSPs like HSPB8 may be involved in immune modulation, several studies have shown that HSP expression is predominantly upregulated in M1-polarized monocytes/macrophages, but not in M2 phenotypes [
29,
30]. Since M2 macrophages are considered the major producers of opioid peptides such as β-endorphin [
3], the role of HSPs in PRF-induced β-endorphin production remains ambiguous. Taken together, HSPs may have multiple roles in this context, including cytoprotection, regulation of monocyte polarization, and possibly modulation of β-endorphin production. However, this study did not evaluate the expression of HSPs nor examine whether PRF alters the polarization state of monocytes. Future studies should assess cytokine profiles and HSP expression following PRF exposure to clarify these mechanisms.
We used the THP-1 cell line rather than primary human monocytes to ensure a reliable supply and to avoid ethical concerns. While THP-1 cells are widely used as a model of human monocytes, future studies should include primary cells to validate the translational relevance of the findings. Finally, clinical translation of this concept requires investigator-initiated trials to determine optimal cell doses and therapeutic efficacy in humans.
This study serves as a preliminary step toward a novel therapeutic concept in which PRF-stimulated monocytes could be reintroduced near peripheral nerves to alleviate neuropathic pain—leveraging a technique already used clinically, warranting further investigation.