Sleep Deprivation in Rats Causes Dissociation of the Synaptic NMDA Receptor/D1 Dopamine Receptor Heterocomplex
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sleep Deprivation
2.3. Subcellular Fractioning
2.4. Immunoprecipitation
2.5. Western Blotting
2.6. F-Actin and G-Actin Fractionation
2.7. Statistical Analysis
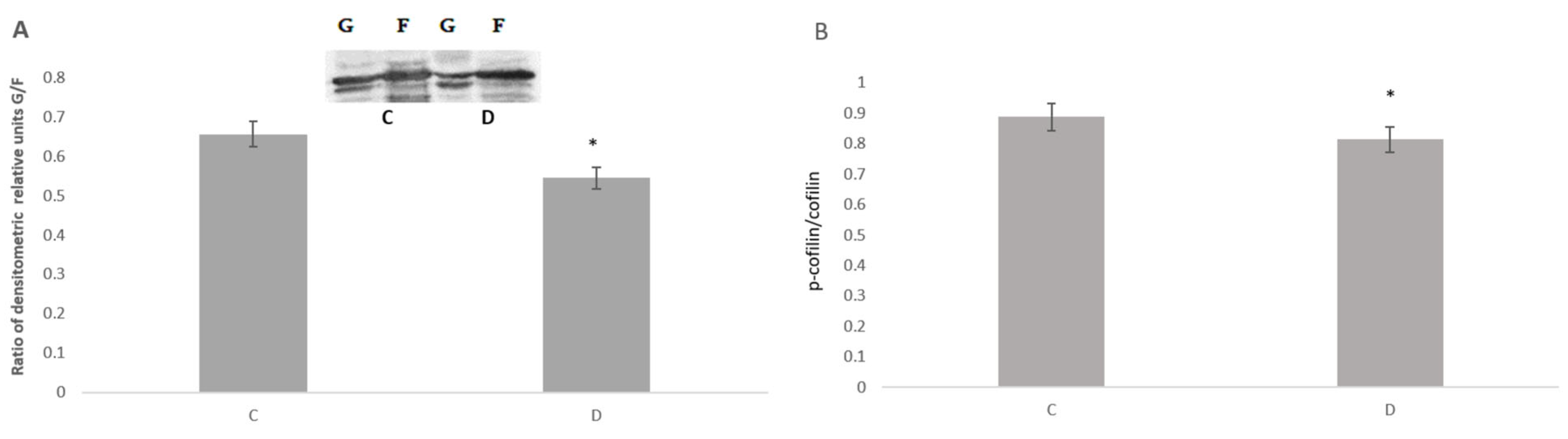
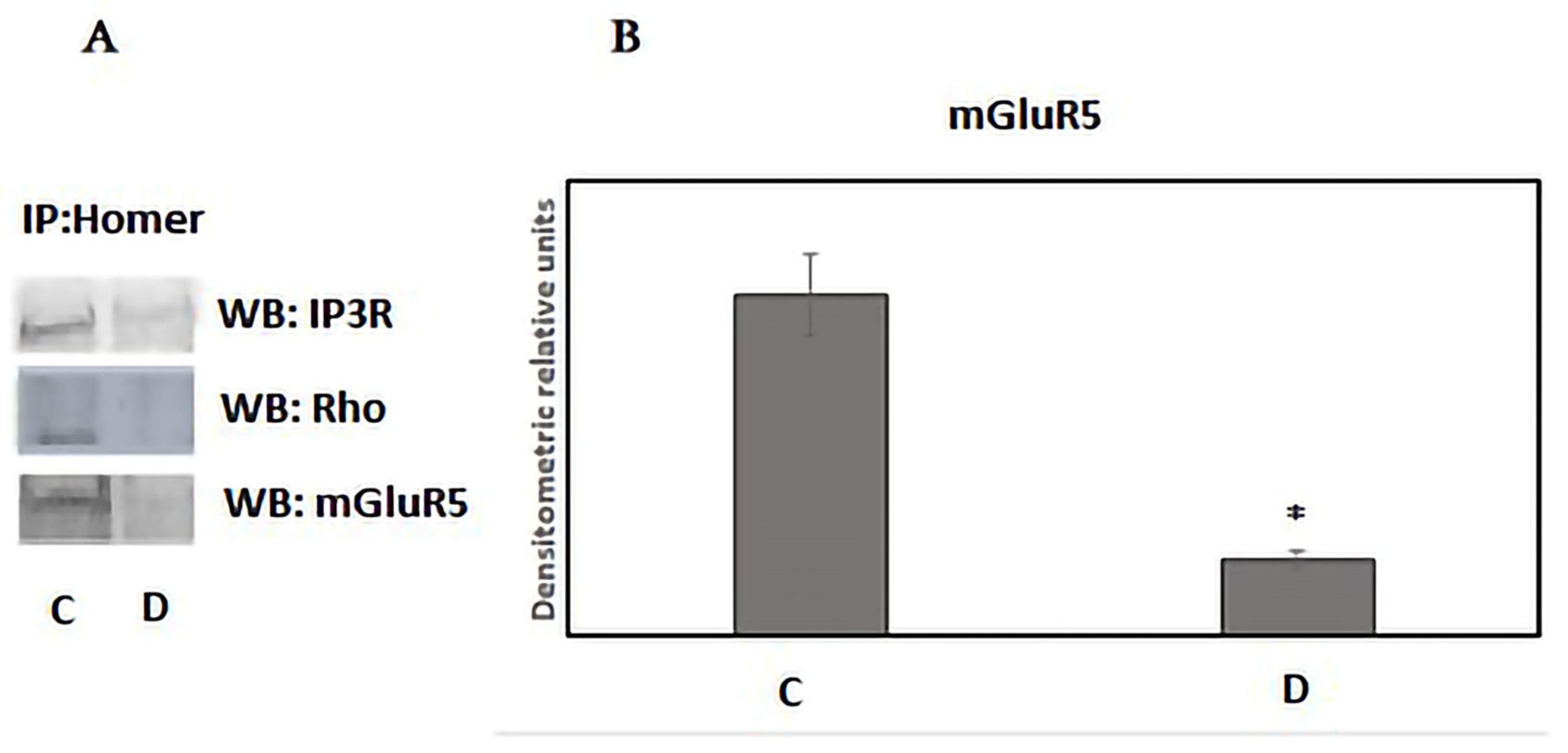
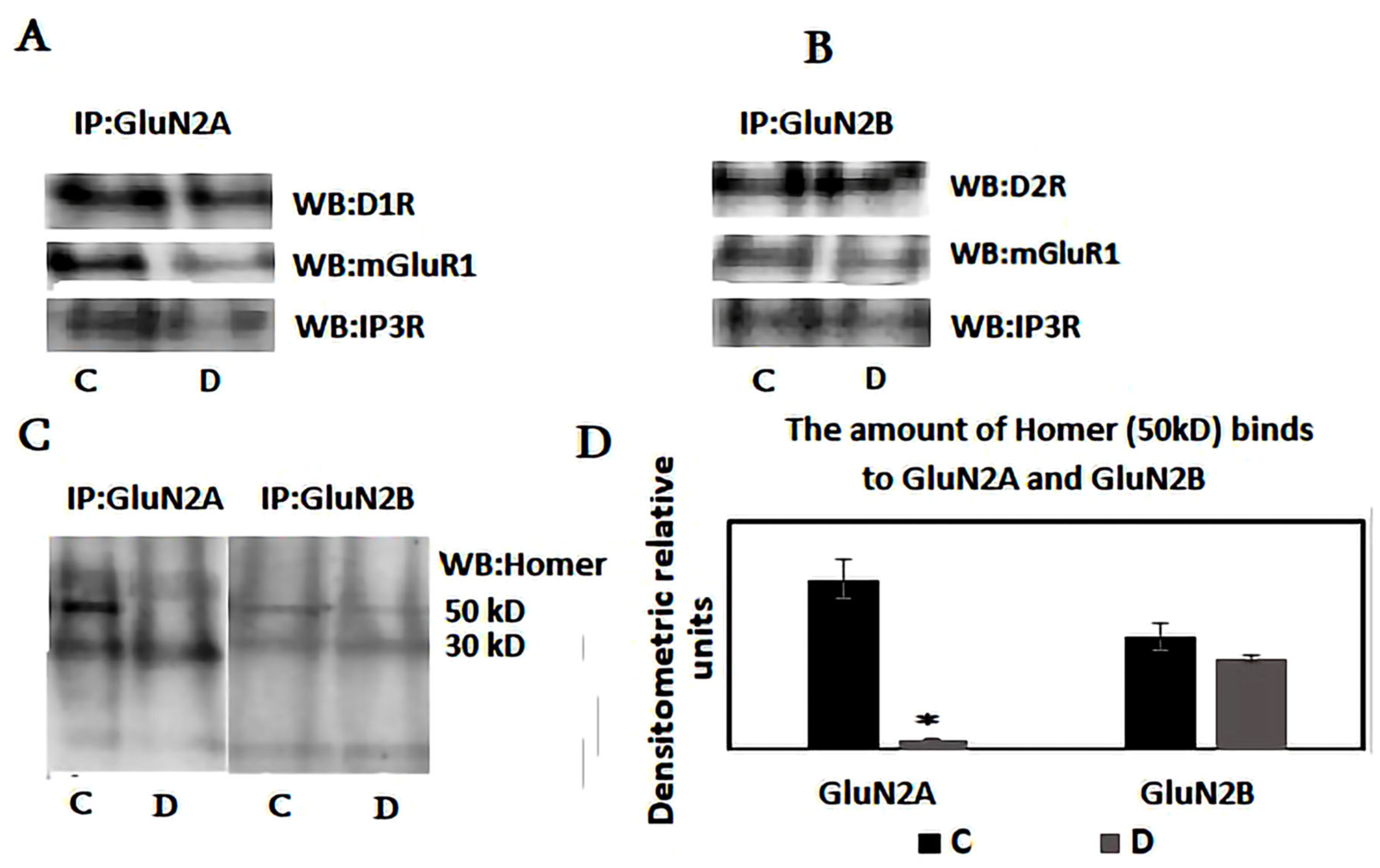
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SD | Sleep deprivation |
| NMDA | N-Methyl-D-Aspartate |
| AMPA | α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| mGluR1/5 | Metabotropic glutamate receptor type 1 |
| IP3R | Inositol trisphosphate receptor |
| DA | Dopamine |
| D1R | Dopamine1 receptor |
| D2R | Dopamine2 receptor |
| PSD | Postsynaptic density |
| GRIP | Glutamate receptor-interacting protein |
| LTD | Long-term depression |
| LTP | Long-term potentiation |
| GluN2A | GluN2A subunit of NMDA glutamate receptor |
| GluN2B | GluN2B subunit of NMDA glutamate receptor |
| GluN1(NR1) | GluN1 subunit of NMDA glutamate receptor |
References
- Shepherd, J.D. Memory, plasticity and sleep—A role for calcium permeable AMPA receptors? Front. Mol. Neurosci. 2012, 5, 2012. [Google Scholar] [CrossRef]
- Lanté, F.; Toledo-Salas, J.-C.; Ondrejcak, T.; Rowan, M.J.; Ulrich, D. Removal of Synaptic Ca2+-Permeable AMPA Receptors during Sleep. J. Neurosci. 2011, 31, 3953–3961. [Google Scholar] [CrossRef] [PubMed]
- Ngomba, R.T.; Lüttjohann, A.; Dexter, A.; Ray, S.; van Luijtelaar, G. The Metabotropic Glutamate 5 Receptor in Sleep and Wakefulness: Focus on the Cortico-Thalamo-Cortical Oscillations. Cells 2023, 12, 1761. [Google Scholar] [CrossRef]
- Holter, K.M.; Pierce, B.E.; Gould, R.W. Metabotropic Glutamate Receptor Function and Regulation of Sleep-Wake Cycles; Academic Press: Cambridge, MA, USA, 2023; pp. 93–175. [Google Scholar] [CrossRef]
- Cortese, B.M.; Mitchell, T.R.; Galloway, M.P.; Prevost, K.E.; Fang, J.; Moore, G.J.; Uhde, T.W. Region-specific alteration in brain glutamate: Possible relationship to risk-taking behavior. Physiol. Behav. 2010, 99, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Holst, S.C.; Sousek, A.; Hefti, K.; Saberi-Moghadam, S.; Buck, A.; Ametamey, S.M.; Scheidegger, M.; Franken, P.; Henning, A.; Seifritz, E.; et al. Cerebral mGluR5 availability contributes to elevated sleep need and behavioral adjustment after sleep deprivation. eLife 2017, 6, e28751. [Google Scholar] [CrossRef] [PubMed]
- Schultz, W. Multiple Dopamine Functions at Different Time Courses. Annu. Rev. Neurosci. 2007, 30, 259–288. [Google Scholar] [CrossRef]
- Broussard, J.I.; Yang, K.; Levine, A.T.; Tsetsenis, T.; Jenson, D.; Cao, F.; Garcia, I.; Arenkiel, B.R.; Zhou, F.-M.; De Biasi, M.; et al. Dopamine Regulates Aversive Contextual Learning and Associated In Vivo Synaptic Plasticity in the Hippocampus. Cell Rep. 2016, 14, 1930–1939. [Google Scholar] [CrossRef]
- Eban-Rothschild, A.; Rothschild, G.; Giardino, W.J.; Jones, J.R.; de Lecea, L. VTA dopaminergic neurons regulate ethologically relevant sleep–wake behaviors. Nat. Neurosci. 2016, 19, 1356–1366. [Google Scholar] [CrossRef]
- Hasegawa, E.; Miyasaka, A.; Sakurai, K.; Cherasse, Y.; Li, Y.; Sakurai, T. Rapid eye movement sleep is initiated by basolateral amygdala dopamine signaling in mice. Science 2022, 375, 994–1000. [Google Scholar] [CrossRef]
- Wang, X. Effects of sleep deprivation on typical neurotransmitters. E3S Web Conf. 2024, 553, 05041. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, X.; Feng, S.; Freda, S.N.; Kumari, P.; Dumrongprechachan, V.; Kozorovitskiy, Y. Dopamine pathways mediating affective state transitions after sleep loss. Neuron 2024, 112, 141–154.e8. [Google Scholar] [CrossRef]
- Bethus, I.; Tse, D.; Morris, R.G.M. Dopamine and Memory: Modulation of the Persistence of Memory for Novel Hippocampal NMDA Receptor-Dependent Paired Associates. J. Neurosci. 2010, 30, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Duszkiewicz, A.J.; Sonneborn, A.; Spooner, P.A.; Yamasaki, M.; Watanabe, M.; Smith, C.C.; Fernández, G.; Deisseroth, K.; Greene, R.W.; et al. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 2016, 537, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, D.; Tortorella, C.; Marcoli, M.; Cervetto, C.; De Caro, R.; Maura, G.; Agnati, L.F. Modulation of Neuron and Astrocyte Dopamine Receptors via Receptor–Receptor Interactions. Pharmaceuticals 2023, 16, 1427. [Google Scholar] [CrossRef]
- Diering, G.H.; Huganir, R.L. The AMPA Receptor Code of Synaptic Plasticity. Neuron 2018, 100, 314–329. [Google Scholar] [CrossRef]
- Brakeman, P.R.; Lanahan, A.A.; O’BRien, R.; Roche, K.; Barnes, C.A.; Huganir, R.L.; Worley, P.F. Homer: A protein that selectively binds metabotropic glutamate receptors. Nature 1997, 386, 284–288. [Google Scholar] [CrossRef]
- Martin, S.C.; Monroe, S.K.; Diering, G.H. Homer1a and mGluR1/5 Signaling in Homeostatic Sleep Drive and Output. Yale J. Biol. Med. 2019, 92, 93–101. [Google Scholar]
- Clifton, N.E.; Trent, S.; Thomas, K.L.; Hall, J. Regulation and Function of Activity-Dependent Homer in Synaptic Plasticity. Complex Psychiatry 2019, 5, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Diering, G.H.; Nirujogi, R.S.; Roth, R.H.; Worley, P.F.; Pandey, A.; Huganir, R.L. Homer1a drives homeostatic scaling-down of excitatory synapses during sleep. Science 2017, 355, 511–515. [Google Scholar] [CrossRef]
- O’Callaghan, V.S.; Couvy-Duchesne, B.; Strike, L.T.; McMahon, K.L.; Byrne, E.M.; Wright, M.J. A meta-analysis of the relationship between subjective sleep and depressive symptoms in adolescence. Sleep Med. 2021, 79, 134–144. [Google Scholar] [CrossRef]
- Noya, S.B.; Colameo, D.; Brüning, F.; Spinnler, A.; Mircsof, D.; Opitz, L.; Mann, M.; Tyagarajan, S.K.; Robles, M.S.; Brown, S.A. The forebrain synaptic transcriptome is organized by clocks but its proteome is driven by sleep. Science 2019, 366, eaav2642. [Google Scholar] [CrossRef]
- Gulyássy, P.; Todorov-Völgyi, K.; Tóth, V.; Györffy, B.A.; Puska, G.; Simor, A.; Juhász, G.; Drahos, L.; Kékesi, K.A. The Effect of Sleep Deprivation and Subsequent Recovery Period on the Synaptic Proteome of Rat Cerebral Cortex. Mol. Neurobiol. 2022, 59, 1301–1319. [Google Scholar] [CrossRef] [PubMed]
- Baucum, A.J. Proteomic Analysis of Postsynaptic Protein Complexes Underlying Neuronal Plasticity. ACS Chem. Neurosci. 2017, 8, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Gardoni, F.; Di Luca, M. Protein-protein interactions at the NMDA receptor complex: From synaptic retention to synaptonuclear protein messengers. Neuropharmacology 2021, 190, 108551. [Google Scholar] [CrossRef]
- Lautz, J.D.; Tsegay, K.B.; Zhu, Z.; Gniffke, E.P.; Welsh, J.P.; Smith, S.E.P. Synaptic protein interaction networks encode experience by assuming stimulus-specific and brain-region-specific states. Cell Rep. 2021, 37, 110076. [Google Scholar] [CrossRef] [PubMed]
- Colavito, V.; Fabene, P.F.; Grassi-Zucconi, G.; Pifferi, F.; Lamberty, Y.; Bentivoglio, M.; Bertini, G. Experimental sleep deprivation as a tool to test memory deficits in rodents. Front. Syst. Neurosci. 2013, 7, 106. [Google Scholar] [CrossRef]
- Won, S.; Incontro, S.; Nicoll, R.A.; Roche, K.W. PSD-95 stabilizes NMDA receptors by inducing the degradation of STEP61. Proc. Natl. Acad. Sci. USA 2016, 113, E4736–E4744. [Google Scholar] [CrossRef]
- Western Blot Protocol. 2020. Available online: https://www.abcam.com/protocols/general-western-blot-protocol (accessed on 6 February 2024).
- Bhambhvani, H.P.; Mueller, T.M.; Simmons, M.S.; Meador-Woodruff, J.H. Actin polymerization is reduced in the anterior cingulate cortex of elderly patients with schizophrenia. Transl. Psychiatry 2017, 7, 1278. [Google Scholar] [CrossRef]
- Cao, Y.-Y.; Wu, L.-L.; Li, X.-N.; Yuan, Y.-L.; Zhao, W.-W.; Qi, J.-X.; Zhao, X.-Y.; Ward, N.; Wang, J. Molecular Mechanisms of AMPA Receptor Trafficking in the Nervous System. Int. J. Mol. Sci. 2023, 25, 111. [Google Scholar] [CrossRef]
- Nishimune, A.; Isaac, J.T.; Molnar, E.; Noel, J.; Nash, S.; Tagaya, M.; Collingridge, G.L.; Nakanishi, S.; Henley, J.M. NSF Binding to GluR2 Regulates Synaptic Transmission. Neuron 1998, 21, 87–97. [Google Scholar] [CrossRef]
- Keith, D. Excitation control: Balancing PSD-95 function at the synapse. Front. Mol. Neurosci. 2008, 1, 200. [Google Scholar] [CrossRef]
- Baranovic, J. AMPA receptors in the synapse: Very little space and even less time. Neuropharmacology 2021, 196, 108711. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.-I.; Nagai, T.; Miyawaki, A.; Hayashi, Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat. Neurosci. 2004, 7, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Bourne, J.N.; Harris, K.M. Balancing Structure and Function at Hippocampal Dendritic Spines. Annu. Rev. Neurosci. 2008, 31, 47–67. [Google Scholar] [CrossRef]
- Malinow, R.; Malenka, R.C. AMPA Receptor Trafficking and Synaptic Plasticity. Annu. Rev. Neurosci. 2002, 25, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Hayashi, Y. Structural plasticity of dendritic spines. Curr. Opin. Neurobiol. 2012, 22, 383–388. [Google Scholar] [CrossRef]
- Lisman, J.; Yasuda, R.; Raghavachari, S. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 2012, 13, 169–182. [Google Scholar] [CrossRef]
- Havekes, R.; Park, A.J.; Tudor, J.C.; Luczak, V.G.; Hansen, R.T.; Ferri, S.L.; Bruinenberg, V.M.; Poplawski, S.G.; Day, J.P.; Aton, S.J.; et al. Sleep deprivation causes memory deficits by negatively impacting neuronal connectivity in hippocampal area CA1. eLife 2016, 5, e13424. [Google Scholar] [CrossRef]
- Beaulieu, J.; Espinoza, S.; Gainetdinov, R.R. Dopamine receptors—IUPHAR Review 13. Br. J. Pharmacol. 2015, 172, 1–23. [Google Scholar] [CrossRef]
- Hansen, H.D.; Schain, M.; Deng, H.P.; Mandeville, J.B.; Rosen, B.R.; Sander, C.Y. Differential D1 and D2 receptor internalization and recycling induced by amphetamine in vivo. bioRxiv 2022. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Romero-Fernandez, W.; Tarakanov, A.O.; Ciruela, F.; Agnati, L.F.; Fuxe, K. On the Existence of a Possible A2A–D2–β-Arrestin2 Complex: A2A Agonist Modulation of D2 Agonist-Induced β-Arrestin2 Recruitment. J. Mol. Biol. 2011, 406, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Perroy, J.; Raynaud, F.; Homburger, V.; Rousset, M.-C.; Telley, L.; Bockaert, J.; Fagni, L. Direct Interaction Enables Cross-talk between Ionotropic and Group I Metabotropic Glutamate Receptors. J. Biol. Chem. 2008, 283, 6799–6805. [Google Scholar] [CrossRef]
- Ledonne, A.; Mercuri, N.B. Insights on the Functional Interaction between Group 1 Metabotropic Glutamate Receptors (mGluRI) and ErbB Receptors. Int. J. Mol. Sci. 2020, 21, 7913. [Google Scholar] [CrossRef] [PubMed]
- Rasch, B.; Born, J. About Sleep’s Role in Memory. Physiol. Rev. 2013, 93, 681–766. [Google Scholar] [CrossRef] [PubMed]
- Cirelli, C. Sleep and synaptic changes. Curr. Opin. Neurobiol. 2013, 23, 841–846. [Google Scholar] [CrossRef]
- Chater, T.E.; Goda, Y. The role of AMPA receptors in postsynaptic mechanisms of synaptic plasticity. Front. Cell. Neurosci. 2014, 8, 401. [Google Scholar] [CrossRef]
- Cirelli, C.; Tononi, G. The why and how of sleep-dependent synaptic down-selection. Semin. Cell Dev. Biol. 2022, 125, 91–100. [Google Scholar] [CrossRef]
- Squarcio, F.; Tononi, G.; Cirelli, C. Effects of non-rapid eye movement sleep on the cortical synaptic expression of GluA1-containing AMPA receptors. Eur. J. Neurosci. 2024, 60, 3961–3972. [Google Scholar] [CrossRef]
- Vyazovskiy, V.V.; Cirelli, C.; Pfister-Genskow, M.; Faraguna, U.; Tononi, G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat. Neurosci. 2008, 11, 200–208. [Google Scholar] [CrossRef]
- Liu, Z.-W.; Faraguna, U.; Cirelli, C.; Tononi, G.; Gao, X.-B. Direct Evidence for Wake-Related Increases and Sleep-Related Decreases in Synaptic Strength in Rodent Cortex. J. Neurosci. 2010, 30, 8671–8675. [Google Scholar] [CrossRef]
- Bjorness, T.E.; Kulkarni, A.; Rybalchenko, V.; Suzuki, A.; Bridges, C.; Harrington, A.J.; Cowan, C.W.; Takahashi, J.S.; Konopka, G.; Greene, R.W.; et al. An essential role for MEF2C in the cortical response to loss of sleep in mice. eLife 2020, 9, e58331. [Google Scholar] [CrossRef]
- Vogt, K.E.; Kulkarni, A.; Pandey, R.; Dehnad, M.; Konopka, G.; Greene, R.W. Sleep need driven oscillation of glutamate synaptic phenotype. eLife 2025, 13, RP98280. [Google Scholar] [CrossRef] [PubMed]
- Zablah, Y.B.; Zhang, H.; Gugustea, R.; Jia, Z. LIM-Kinases in Synaptic Plasticity, Memory, and Brain Diseases. Cells 2021, 10, 2079. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.B.; Starck, S.R.; Roberts, R.W.; Schuman, E.M. Dopaminergic Stimulation of Local Protein Synthesis Enhances Surface Expression of GluR1 and Synaptic Transmission in Hippocampal Neurons. Neuron 2005, 45, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.A.; Ito, H.T.; Cressy, P.; Kempf, C.; Woo, J.C.; Schuman, E.M. Miniature Neurotransmission Stabilizes Synaptic Function via Tonic Suppression of Local Dendritic Protein Synthesis. Cell 2006, 125, 785–799. [Google Scholar] [CrossRef]
- Greenblatt, J.F.; Alberts, B.M.; Krogan, N.J. Discovery and significance of protein-protein interactions in health and disease. Cell 2024, 187, 6501–6517. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Chu, X.-P.; Mao, L.-M.; Wang, M.; Lan, H.-X.; Li, M.-H.; Zhang, G.-C.; Parelkar, N.K.; Fibuch, E.E.; Haines, M.; et al. Modulation of D2R-NR2B Interactions in Response to Cocaine. Neuron 2006, 52, 897–909. [Google Scholar] [CrossRef]
- Lee, F.J.; Xue, S.; Pei, L.; Vukusic, B.; Chéry, N.; Wang, Y.; Wang, Y.T.; Niznik, H.B.; Yu, X.-M.; Liu, F. Dual Regulation of NMDA Receptor Functions by Direct Protein-Protein Interactions with the Dopamine D1 Receptor. Cell 2002, 111, 219–230. [Google Scholar] [CrossRef]
- Pei, L.; Lee, F.J.S.; Moszczynska, A.; Vukusic, B.; Liu, F. Regulation of Dopamine D1 Receptor Function by Physical Interaction with the NMDA Receptors. J. Neurosci. 2004, 24, 1149–1158. [Google Scholar] [CrossRef]
- Ladepeche, L.; Dupuis, J.P.; Bouchet, D.; Doudnikoff, E.; Yang, L.; Campagne, Y.; Bézard, E.; Hosy, E.; Groc, L. Single-molecule imaging of the functional crosstalk between surface NMDA and dopamine D1 receptors. Proc. Natl. Acad. Sci. USA 2013, 110, 18005–18010. [Google Scholar] [CrossRef]
- Nai, Q.; Li, S.; Wang, S.-H.; Liu, J.; Lee, F.J.; Frankland, P.W.; Liu, F. Uncoupling the D1-N-Methyl-D-Aspartate (NMDA) Receptor Complex Promotes NMDA-Dependent Long-Term Potentiation and Working Memory. Biol. Psychiatry 2010, 67, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Bénac, N.; Saraceno, G.E.; Butler, C.; Kuga, N.; Nishimura, Y.; Yokoi, T.; Su, P.; Sasaki, T.; Petit-Pedrol, M.; Galland, R.; et al. Non-canonical interplay between glutamatergic NMDA and dopamine receptors shapes synaptogenesis. Nat. Commun. 2024, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.K.Y.; Zhai, D.; Su, P.; Jiang, A.; Boychuk, J.; Liu, F. The receptor-receptor interaction between mGluR1 receptor and NMDA receptor: A potential therapeutic target for protection against ischemic stroke. FASEB J. 2019, 33, 14423–14439. [Google Scholar] [CrossRef]
- Sapkota, K.; Dore, K.; Tang, K.; Irvine, M.; Fang, G.; Burnell, E.S.; Malinow, R.; Jane, D.E.; Monaghan, D.T. The NMDA receptor intracellular C-terminal domains reciprocally interact with allosteric modulators. Biochem. Pharmacol. 2019, 159, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Petit-Pedrol, M.; Groc, L. Regulation of membrane NMDA receptors by dynamics and protein interactions. J. Cell Biol. 2021, 220, e202006101. [Google Scholar] [CrossRef]
- Stillman, M.; Lautz, J.D.; Johnson, R.S.; MacCoss, M.J.; Smith, S.E.P. Activity dependent dissociation of the Homer1 interactome. Sci. Rep. 2022, 12, 3207. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, T.-X.; Hallett, P.J.; Watanabe, M.; Grant, S.G.N.; Isacson, O.; Yao, W.-D. PSD-95 Uncouples Dopamine–Glutamate Interaction in the D1/PSD-95/NMDA Receptor Complex. J. Neurosci. 2009, 29, 2948–2960. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiknadze, N.; Narmania, N.; Sepashvili, M.; Barbakadze, T.; Zhuravliova, E.; Shetekauri, T.; Tkemaladze, N.; Oniani, N.; Mikeladze, D. Sleep Deprivation in Rats Causes Dissociation of the Synaptic NMDA Receptor/D1 Dopamine Receptor Heterocomplex. NeuroSci 2025, 6, 61. https://doi.org/10.3390/neurosci6030061
Kiknadze N, Narmania N, Sepashvili M, Barbakadze T, Zhuravliova E, Shetekauri T, Tkemaladze N, Oniani N, Mikeladze D. Sleep Deprivation in Rats Causes Dissociation of the Synaptic NMDA Receptor/D1 Dopamine Receptor Heterocomplex. NeuroSci. 2025; 6(3):61. https://doi.org/10.3390/neurosci6030061
Chicago/Turabian StyleKiknadze, Natalia, Nana Narmania, Maia Sepashvili, Tamar Barbakadze, Elene Zhuravliova, Tamar Shetekauri, Nino Tkemaladze, Nikoloz Oniani, and David Mikeladze. 2025. "Sleep Deprivation in Rats Causes Dissociation of the Synaptic NMDA Receptor/D1 Dopamine Receptor Heterocomplex" NeuroSci 6, no. 3: 61. https://doi.org/10.3390/neurosci6030061
APA StyleKiknadze, N., Narmania, N., Sepashvili, M., Barbakadze, T., Zhuravliova, E., Shetekauri, T., Tkemaladze, N., Oniani, N., & Mikeladze, D. (2025). Sleep Deprivation in Rats Causes Dissociation of the Synaptic NMDA Receptor/D1 Dopamine Receptor Heterocomplex. NeuroSci, 6(3), 61. https://doi.org/10.3390/neurosci6030061





