Reserpine Causes Neuroendocrine Toxicity, Inducing Impairments in Cognition via Disturbing Hypothalamic–Pituitary–Thyroid Axis in Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Reagents
2.2. Zebrafish Maintenance and Chemical Exposure
2.3. Developmental Toxicity Evaluation
2.4. Neurotoxicity Evaluation
2.5. Locomotion Recording—From This Point, My Changes Are Denoted in Blue
2.6. Assessment of Thyroid Development
2.7. Real-Time Quantitative PCR (qPCR)
2.8. Statistical Analysis
3. Results
3.1. Reserpine Induces Dose-Dependent Developmental Toxicity
3.2. Effects of Reserpine on CNS Neuron Differentiation
3.3. Dose-Dependent Effects of Reserpine on Thyroid Development
3.4. Reserpine Injures Locomotor Capacity of Zebrafish in a Dose-Dependent Manner
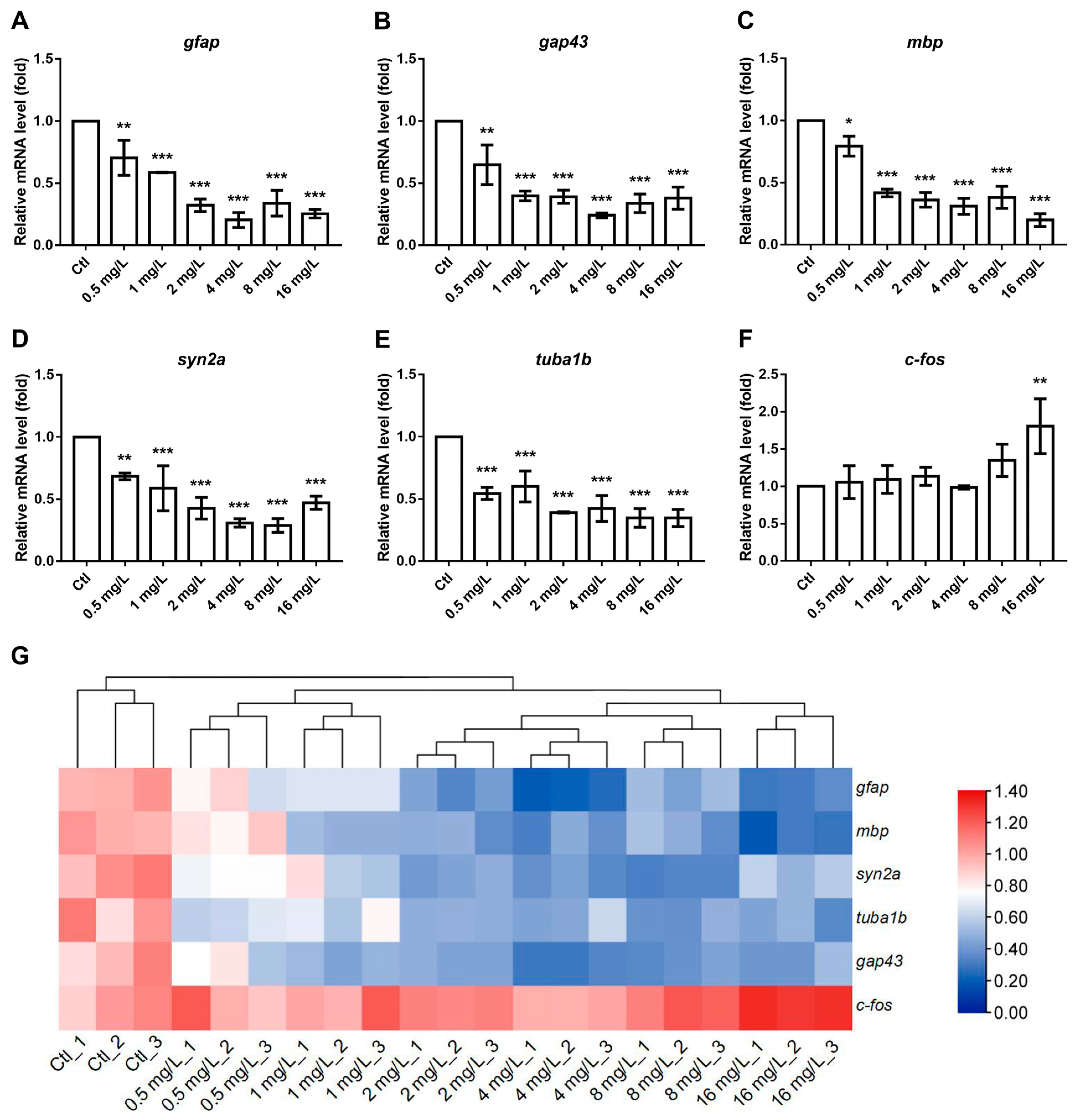
3.5. The Effects of Reserpine on Transcript Levels of NES- and Cognition-Related Genes
4. Discussion
4.1. The Dose-Dependently Developmental Toxicity of Reserpine
4.2. Reserpine Induced Neurotoxic Characteristics
4.3. The Effect of Reserpine Exposure on the Endocrine System
4.4. Mechanisms of Reserpine-Induced Neuroendocrine Toxicity That Lead to Cognitive Impairments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samad, N.; Manzoor, N.; Muneer, Z.; Bhatti, S.A.; Imran, I. Reserpine-induced altered neuro-behavioral, biochemical and histopathological assessments prevent by enhanced antioxidant defence system of thymoquinone in mice. Metab. Brain Dis. 2021, 36, 2535–2552. [Google Scholar] [PubMed]
- Siddiqui, M.; Bhatt, H.; Judd, E.K.; Oparil, S.; Calhoun, D.A. Reserpine Substantially Lowers Blood Pressure in Patients With Refractory Hypertension: A Proof-of-Concept Study. Am. J. Hypertens. 2020, 33, 741–747. [Google Scholar] [CrossRef]
- Fadanni, G.P.; Leão, A.H.F.F.; Granzotto, N.; Pereira, A.G.; de Gois, A.M.; Anjos, P.A.R.; Linder, Á.E.; Santos, J.R.; Silva, R.H.; Izídio, G.S. Genetic effects in a progressive model of parkinsonism induced by reserpine. Psychopharmacology 2023, 240, 1131–1142. [Google Scholar]
- Wu, D.; Chen, Q.; Yu, Z.; Huang, B.; Zhao, J.; Wang, Y.; Su, J.; Zhou, F.; Yan, R.; Li, N.; et al. Transport and inhibition mechanisms of human VMAT2. Nature 2024, 626, 427–434. [Google Scholar]
- Wang, S.; Duan, M.; Guan, K.; Zhou, X.; Zheng, M.; Shi, X.; Ye, M.; Guan, W.; Kuver, A.; Huang, M.; et al. Developmental neurotoxicity of reserpine exposure in zebrafish larvae (Danio rerio). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 223, 115–123. [Google Scholar]
- Akinlaja, Y.O.; Adeyemi, D.H.; Oluwatobi, J.; Oyedele; Mustapha, R.A. Evaluation of Male Reproductive Hormones in Reserpine Treated Rabbits. Int. J. Innov. Sci. Res. Technol. 2020, 5, 789–794. [Google Scholar]
- Li, Y.; Yin, Q.; Wang, B.; Shen, T.; Luo, W.; Liu, T. Preclinical reserpine models recapitulating motor and non-motor features of Parkinson’s disease: Roles of epigenetic upregulation of alpha-synuclein and autophagy impairment. Front. Pharmacol. 2022, 13, 944376. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.B.; Zhao, R.; Li, X.S.; Guo, H.J.; Tian, Z.; Zhang, N.; Gao, G.D.; Zhao, M.G. Attenuation of reserpine-induced pain/depression dyad by gentiopicroside through downregulation of GluN2B receptors in the amygdala of mice. Neuromolecular. Med. 2014, 16, 350–359. [Google Scholar]
- Toni, R. The neuroendocrine system: Organization and homeostatic role. J. Endocrinol. Investig. 2004, 27, 35–47. [Google Scholar]
- Schimmack, S.; Svejda, B.; Lawrence, B.; Kidd, M.; Modlin, I.M. The diversity and commonalities of gastroenteropancreatic neuroendocrine tumors. Langenbecks Arch. Surg. 2011, 396, 273–298. [Google Scholar]
- Zacharjasz, J.; Sztachera, M.; Smuszkiewicz, M.; Piwecka, M. Micromanaging the neuroendocrine system—A review on miR-7 and the other physiologically relevant miRNAs in the hypothalamic-pituitary axis. FEBS Lett. 2024, 598, 1557–1575. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Niu, L.; Guo, H.; Sun, X.; Chen, L.; Tu, W.; Dai, Q.; Ye, J.; Liu, W.; Liu, J. Long-term exposure to the non-steroidal anti-inflammatory drug (NSAID) naproxen causes thyroid disruption in zebrafish at environmentally relevant concentrations. Sci. Total Environ. 2019, 676, 387–395. [Google Scholar] [CrossRef]
- Miller, D.B.; O’Callaghan, J.P. Neuroendocrine aspects of the response to stress. Metabolism 2002, 51 (Suppl. 1), 5–10. [Google Scholar] [CrossRef] [PubMed]
- León-Olea, M.; Martyniuk, C.J.; Orlando, E.F.; Ottinger, M.A.; Rosenfeld, C.; Wolstenholme, J.; Trudeau, V.L. Current concepts in neuroendocrine disruption. Gen. Comp. Endocrinol. 2014, 203, 158–173. [Google Scholar] [CrossRef]
- Joffe, R.T.; Sokolov, S.T. Thyroid hormones, the brain, and affective disorders. Crit. Rev. Neurobiol. 1994, 8, 45–63. [Google Scholar] [PubMed]
- Yap, Y.W.; Onyekwelu, E.; Alam, U. Thyroid disease in pregnancy. Clin. Med. 2023, 23, 125–128. [Google Scholar] [CrossRef]
- Oerbeck, B.; Sundet, K.; Kase, B.F.; Heyerdahl, S. Congenital hypothyroidism: No adverse effects of high dose thyroxine treatment on adult memory, attention, and behaviour. Arch. Dis. Child. 2005, 90, 132–137. [Google Scholar] [CrossRef]
- Grüters, A.; Krude, H. Detection and treatment of congenital hypothyroidism. Nat. Rev. Endocrinol. 2011, 8, 104–113. [Google Scholar] [CrossRef]
- Galton, V.A. The roles of the iodothyronine deiodinases in mammalian development. Thyroid 2005, 15, 823–834. [Google Scholar] [CrossRef]
- Rivkees, S.A.; Bode, H.H.; Crawford, J.D. Long-term growth in juvenile acquired hypothyroidism: The failure to achieve normal adult stature. N. Engl. J. Med. 1988, 318, 599–602. [Google Scholar] [CrossRef]
- Choi, T.Y.; Choi, T.I.; Lee, Y.R.; Choe, S.K.; Kim, C.H. Zebrafish as an animal model for biomedical research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [PubMed]
- Giacomini, A.C.; Bueno, B.W.; Marcon, L.; Scolari, N.; Genario, R.; Demin, K.A.; Kolesnikova, T.O.; Kalueff, A.V.; de Abreu, M.S. An acetylcholinesterase inhibitor, donepezil, increases anxiety and cortisol levels in adult zebrafish. J. Psychopharmacol. 2020, 34, 1449–1456. [Google Scholar] [CrossRef]
- Van der Ven, K.; Keil, D.; Moens, L.N.; Van Leemput, K.; van Remortel, P.; De Coen, W.M. Neuropharmaceuticals in the environment: Mianserin-induced neuroendocrine disruption in zebrafish (Danio rerio) using cDNA microarrays. Environ. Toxicol. Chem. 2006, 25, 2645–2652. [Google Scholar]
- Geng, Y.; Zou, H.; Guo, Y.; Huang, M.; Wu, Y.; Hou, L. Chronic exposure to cortisone induces thyroid endocrine disruption and retinal dysfunction in adult female zebrafish (Danio rerio). Sci. Total Environ. 2023, 905, 167022. [Google Scholar] [PubMed]
- Park, H.C.; Kim, C.H.; Bae, Y.K.; Yeo, S.Y.; Kim, S.H.; Hong, S.K.; Shin, J.; Yoo, K.W.; Hibi, M.; Hirano, T.; et al. Analysis of upstream elements in the HuC promoter leads to the establishment of transgenic zebrafish with fluorescent neurons. Dev. Biol. 2000, 227, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Collymore, C.; Porelli, G.; Lieggi, C.; Lipman, N.S. Evaluation of 5 cleaning and disinfection methods for nets used to collect zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 657–660. [Google Scholar]
- Karlsson, J.; von Hofsten, J.; Olsson, P.E. Generating transparent zebrafish: A refined method to improve detection of gene expression during embryonic development. Mar. Biotechnol. 2001, 3, 522–527. [Google Scholar]
- Olanrewaju, J.A.; Owolabi, J.O.; Awodein, I.P.; Enya, J.I.; Adelodun, S.T.; Olatunji, S.Y.; Fabiyi, S.O. Zingiber officinale Ethanolic Extract Attenuated Reserpine-Induced Depression-Like Condition and Associated Hippocampal Aberrations in Experimental Wistar Rats. J. Exp. Pharmacol. 2020, 12, 439–446. [Google Scholar] [CrossRef]
- Pype, C.; Verbueken, E.; Saad, M.A.; Casteleyn, C.R.; Van Ginneken, C.J.; Knapen, D.; Van Cruchten, S.J. Incubation at 32.5 °C and above causes malformations in the zebrafish embryo. Reprod. Toxicol. 2015, 56, 56–63. [Google Scholar]
- Song, Y.; Liu, S.; Jiang, X.; Ren, Q.; Deng, H.; Paudel, Y.N.; Wang, B.; Liu, K.; Jin, M. Benzoresorcinol induces developmental neurotoxicity and injures exploratory, learning and memorizing abilities in zebrafish. Sci. Total Environ. 2022, 834, 155268. [Google Scholar]
- Schnörr, S.J.; Steenbergen, P.J.; Richardson, M.K.; Champagne, D.L. Measuring thigmotaxis in larval zebrafish. Behav. Brain Res. 2012, 228, 367–374. [Google Scholar]
- Laurent, S. Antihypertensive drugs. Pharmacol. Res. 2017, 124, 116–125. [Google Scholar]
- Buelke-Sam, J.; Kimmel, G.L.; Webb, P.J.; Slikker, W., Jr.; Newport, G.D.; Nelson, C.J.; Kimmel, C.A. Postnatal toxicity following prenatal reserpine exposure in rats: Effects of dose and dosing schedule. Fundam. Appl. Toxicol. 1984, 4, 983–991. [Google Scholar] [PubMed]
- Goldman, A.S.; Yakovac, W.C. Teratogenic action in rats of reserpine alone and in combination with salicylate and immobilization. Proc. Soc. Exp. Biol. Med. 1965, 118, 857–862. [Google Scholar]
- Pauli, R.M.; Pettersen, B.J. Is reserpine a human teratogen? J. Med. Genet. 1986, 23, 267–268. [Google Scholar] [CrossRef] [PubMed]
- Van Onselen, R.; Downing, T.G. Neonatal Reserpine Administration Produces Widespread Neuronal Losses and α-Synuclein Inclusions in a Rat Model. Neurotox. Res. 2021, 39, 1762–1770. [Google Scholar] [PubMed]
- Legradi, J.; el Abdellaoui, N.; van Pomeren, M.; Legler, J. Comparability of behavioural assays using zebrafish larvae to assess neurotoxicity. Environ. Sci. Pollut. Res. Int. 2015, 22, 16277–16289. [Google Scholar]
- Huang, Z. Evidence that Alzheimer’s Disease Is a Disease of Competitive Synaptic Plasticity Gone Awry. J. Alzheimers Dis. 2024, 99, 447–470. [Google Scholar]
- Zheng, S.; Huang, W.; Liu, C.; Xiao, J.; Wu, R.; Wang, X.; Cai, Z.; Wu, K. Behavioral change and transcriptomics reveal the effects of 2, 2′, 4, 4′-tetrabromodiphenyl ether exposure on neurodevelopmental toxicity to zebrafish (Danio rerio) in early life stage. Sci. Total Environ. 2021, 752, 141783. [Google Scholar]
- Kao, H.T.; Porton, B.; Czernik, A.J.; Feng, J.; Yiu, G.; Häring, M.; Benfenati, F.; Greengard, P. A third member of the synapsin gene family. Proc. Natl. Acad. Sci. USA 1998, 95, 4667–4672. [Google Scholar]
- Jin, M.; Dang, J.; Paudel, Y.N.; Wang, X.; Wang, B.; Wang, L.; Li, P.; Sun, C.; Liu, K. The possible hormetic effects of fluorene-9-bisphenol on regulating hypothalamic-pituitary-thyroid axis in zebrafish. Sci. Total Environ. 2021, 776, 145963. [Google Scholar]
- Lindå, H.; Piehl, F.; Dagerlind, A.; Verge, V.M.; Arvidsson, U.; Cullheim, S.; Risling, M.; Ulfhake, B.; Hökfelt, T. Expression of GAP-43 mRNA in the adult mammalian spinal cord under normal conditions and after different types of lesions, with special reference to motoneurons. Exp. Brain Res. 1992, 91, 284–295. [Google Scholar]
- Zhao, J.C.; Zhang, L.X.; Zhang, Y.; Shen, Y.F. The differential regulation of Gap43 gene in the neuronal differentiation of P19 cells. J. Cell. Physiol. 2012, 227, 2645–2653. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.Y.; Zhang, Y.; Zhu, X.Y.; Zhou, J.L.; Li, J.; Li, C.Q.; Wu, L.R. Developmental neurotoxicity and toxic mechanisms induced by olaquindox in zebrafish. J. Appl. Toxicol. 2021, 41, 549–560. [Google Scholar]
- Johnson, K.; Barragan, J.; Bashiruddin, S.; Smith, C.J.; Tyrrell, C.; Parsons, M.J.; Doris, R.; Kucenas, S.; Downes, G.B.; Velez, C.M.; et al. Gfap-positive radial glial cells are an essential progenitor population for later-born neurons and glia in the zebrafish spinal cord. Glia 2016, 64, 1170–1189. [Google Scholar] [PubMed]
- Brösamle, C.; Halpern, M.E. Characterization of myelination in the developing zebrafish. Glia 2002, 39, 47–57. [Google Scholar]
- Yoshida, M.; Macklin, W.B. Oligodendrocyte development and myelination in GFP-transgenic zebrafish. J. Neurosci. Res. 2005, 81, 1–8. [Google Scholar] [PubMed]
- Hagihara, H.; Shoji, H.; Otabi, H.; Toyoda, A.; Katoh, K.; Namihira, M.; Miyakawa, T. Protein lactylation induced by neural excitation. Cell Rep. 2021, 37, 109820. [Google Scholar]
- Dyrvig, M.; Hansen, H.H.; Christiansen, S.H.; Woldbye, D.P.; Mikkelsen, J.D.; Lichota, J. Epigenetic regulation of Arc and c-Fos in the hippocampus after acute electroconvulsive stimulation in the rat. Brain Res. Bull. 2012, 88, 507–513. [Google Scholar]
- Moon, R.C.; Turner, C.W. A Mode of Action for Thyroid Inhibition by Reserpine. Proc. Soc. Exp. Biol. Med. 1959, 102, 134–136. [Google Scholar]
- Stolk, J. Thyroxine metabolism and thyroid function during reserpine treatment. Pharmacology 1971, 5, 351–358. [Google Scholar] [PubMed]
- Calabrese, E.J.; Bachmann, K.A.; Bailer, A.J.; Bolger, P.M.; Borak, J.; Cai, L.; Cedergreen, N.; Cherian, M.G.; Chiueh, C.C.; Clarkson, T.W.; et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol. Appl. Pharmacol. 2007, 222, 122–128. [Google Scholar] [CrossRef]
- Thompson, C.C.; Potter, G.B. Thyroid hormone action in neural development. Cereb. Cortex. 2000, 10, 939–945. [Google Scholar] [PubMed]
- Gothié, J.D.; Vancamp, P.; Demeneix, B.; Remaud, S. Thyroid hormone regulation of neural stem cell fate: From development to ageing. Acta Physiol. 2020, 228, e13316. [Google Scholar]
- Martin, E.; Velayudhan, L. Neuropsychiatric Symptoms in Mild Cognitive Impairment: A Literature Review. Dement. Geriatr. Cogn. Disord. 2020, 49, 146–155. [Google Scholar] [PubMed]
- Maugars, G.; Mauvois, X.; Martin, P.; Aroua, S.; Rousseau, K.; Dufour, S. New Insights Into the Evolution of Corticotropin-Releasing Hormone Family With a Special Focus on Teleosts. Front. Endocrinol. 2022, 13, 937218. [Google Scholar]
- Reul, J.M.; Labeur, M.S.; Grigoriadis, D.E.; De Souza, E.B.; Holsboer, F. Hypothalamic-pituitary-adrenocortical axis changes in the rat after long-term treatment with the reversible monoamine oxidase-A inhibitor moclobemide. Neuroendocrinology 1994, 60, 509–519. [Google Scholar]
- Kersting, A.; Kroker, K.; Horstmann, J.; Baune, B.T.; Hohoff, C.; Mortensen, L.S.; Neumann, L.C.; Arolt, V.; Domschke, K. Association of MAO-A variant with complicated grief in major depression. Neuropsychobiology 2007, 56, 191–196. [Google Scholar]
- Hsieh, Y.W.; Tsai, Y.W.; Lai, H.H.; Lai, C.Y.; Lin, C.Y.; Her, G.M. Depletion of Alpha-Melanocyte-Stimulating Hormone Induces Insatiable Appetite and Gains in Energy Reserves and Body Weight in Zebrafish. Biomedicines 2021, 9, 941. [Google Scholar] [CrossRef]
- Grattan, D.R.; Steyn, F.J.; Kokay, I.C.; Anderson, G.M.; Bunn, S.J. Pregnancy-induced adaptation in the neuroendocrine control of prolactin secretion. J. Neuroendocrinol. 2008, 20, 497–507. [Google Scholar]
- Joshi, S.; Virdi, S.; Etard, C.; Geisler, R.; Strähle, U. Mutation of a serine near the catalytic site of the choline acetyltransferase a gene almost completely abolishes motility of the zebrafish embryo. PLoS ONE 2018, 13, e0207747. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, S.D.; Malek-Ahmadi, M.H.; Alldred, M.J.; Che, S.; Elarova, I.; Chen, Y.; Jeanneteau, F.; Kranz, T.M.; Chao, M.V.; Counts, S.E.; et al. Selective decline of neurotrophin and neurotrophin receptor genes within CA1 pyramidal neurons and hippocampus proper: Correlation with cognitive performance and neuropathology in mild cognitive impairment and Alzheimer’s disease. Hippocampus. 2019, 29, 422–439. [Google Scholar] [PubMed]
- Russo, P.; Kisialiou, A.; Moroni, R.; Prinzi, G.; Fini, M. Effect of Genetic Polymorphisms (SNPs) in CHRNA7 Gene on Response to Acetylcholinesterase Inhibitors (AChEI) in Patients with Alzheimer’s Disease. Curr. Drug Targets. 2017, 18, 1179–1190. [Google Scholar] [PubMed]
- Almostafa, M.M.; Mohamed, M.E.; Younis, N.S. Ameliorative effects of vanillin against pentylenetetrazole-induced epilepsy and associated memory loss in mice: The role of Nrf2/HO-1/NQO1 and HMGB1/RAGE/TLR4/NFκB pathways. Int. Immunopharmacol. 2024, 129, 111657. [Google Scholar]





| Gene Symbol | Forward Primer Sequence (5'→3') | Reverse Primer Sequence (5'→3') |
|---|---|---|
| gfap | 5'-GGATGCAGCCAATCGTAAT | 5'-TTCCAGGTCACAGGTCAG |
| gap43 | 5'-CAGCCGACGTGCCTGAA | 5'-GGATTCCTCAGCAGCGTCTG |
| mbp | AATCAGCAGGTTCTTCGGAGGAGA | AAGAAATGCACGACAGGGTTGACG |
| syn2a | GTGACCATGCCAGCATTTC | TGGTTCTCCACTTTCACCTT |
| tuba1b | AATCACCAATGCTTGCTTCGAGCC | TTCACGTCTTTGGGTACCACG |
| c-fos | GCTCCTGGCTAAAGCGGAGCTG | GACGTGTAGGTGGTGCAGGCTGG |
| crh | ATCTCAAGGAAGGCGAATAGA | AACATCGATGGAAAGTGATGA |
| hhex | TGTGGTCTCCGTTCATCCAG | TTTGACCTGTCTCTCGCTGA |
| pax8 | GAAGATCGCGGAGTACAAGC | CTGCACTTTAGTGCGGATGA |
| tra | CAATGTACCATTTCGCGTTG | GCTCCTGCTCTGTGTTTTCC |
| dio2 | TTCTCCTTGCCTCCTCAGTG | AGCCACCTCCGAACATCTTT |
| nkx2.1 | AGGACGGTAAACCGTGTCAG | CACCATGCTGCTCGTGTACT |
| tg | CCAGCCGAAAGGATAGAGTTG | ATGCTGCCGTGGAATAGGA |
| tshβ | GCAGATCCTCACTTCACCTACC | GCACAGGTTTGGAGCATCTCA |
| chata | AGGGAATAGTGCTTGTGCAG | GCTGGAAGTTCACTCATGCT |
| ngfb | CGCCATTGGAACTCATATTG | CACGCAAGCTACATTGATCC |
| creb1a | ATTAGCCAATAACGGGACGG | CCACTACTTGATTGCTGGGAAC |
| chrna7a | CTCCTGGACGTATGGAGGAT | ACTTCCACAAGGTCCCACTC |
| nqo1 | CTCAAGGATTTGCCTTCAGC | CGCAGCACTCCATTCTGTAA |
| Crhb (crf) | ATCTCAAGGAAGGCGAATAGA | AACATCGATGGAAAGTGATGA |
| mao | CAACAACCTCTGGAGGACA | GTTGCTGCATGGTCATCTT |
| mc2r | TGAGTCACGCTGTTATTGATCC | AGATCCTTGAAGCTGAGGACAG |
| pomc | GAATCCGCCGAAACGCTTCC | GGGTCCTTCTTTCCAAGTGGGTTT |
| prl | GCTCGGTCTCTGCTGTTG | GGTGTTGCGTTCTGGATGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, F.; Xia, L.; Wang, B.; Liu, Y.; Cui, X.; Kang, H.; Stoika, R.; Liu, K.; Jin, M. Reserpine Causes Neuroendocrine Toxicity, Inducing Impairments in Cognition via Disturbing Hypothalamic–Pituitary–Thyroid Axis in Zebrafish. NeuroSci 2025, 6, 28. https://doi.org/10.3390/neurosci6020028
Sun F, Xia L, Wang B, Liu Y, Cui X, Kang H, Stoika R, Liu K, Jin M. Reserpine Causes Neuroendocrine Toxicity, Inducing Impairments in Cognition via Disturbing Hypothalamic–Pituitary–Thyroid Axis in Zebrafish. NeuroSci. 2025; 6(2):28. https://doi.org/10.3390/neurosci6020028
Chicago/Turabian StyleSun, Fengzhi, Lijie Xia, Baokun Wang, Yanao Liu, Xiaotong Cui, Huijun Kang, Rostyslav Stoika, Kechun Liu, and Meng Jin. 2025. "Reserpine Causes Neuroendocrine Toxicity, Inducing Impairments in Cognition via Disturbing Hypothalamic–Pituitary–Thyroid Axis in Zebrafish" NeuroSci 6, no. 2: 28. https://doi.org/10.3390/neurosci6020028
APA StyleSun, F., Xia, L., Wang, B., Liu, Y., Cui, X., Kang, H., Stoika, R., Liu, K., & Jin, M. (2025). Reserpine Causes Neuroendocrine Toxicity, Inducing Impairments in Cognition via Disturbing Hypothalamic–Pituitary–Thyroid Axis in Zebrafish. NeuroSci, 6(2), 28. https://doi.org/10.3390/neurosci6020028







