Liposomal Lactoferrin Reduces Brain Neuroinflammation in Rats and Alleviates Jetlag and Improves Sleep Quality After Long-Haul Travel
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design in Rats
2.2. Sample Preparation
2.3. Experimental Protocol
- Control: Vehicle solution orally and i.p. administration of saline (n = 5);
- Poly I:C: Vehicle solution orally and i.p. administration of poly I:C (n = 5);
- L-bLF: L-bLF solution orally and i.p. administration of saline (n = 5);
- L-bLF + poly I:C: L-bLF solution orally and i.p. administration of poly I:C (n = 6).
2.4. qRT-PCR Analysis of Brain mRNA Expression in Rats
2.5. Participants and Study Design
2.6. Outcomes of the Open-Label Pilot Study in Humans
2.7. Statistical Analyses
3. Results
3.1. Animal Study Experimental Groups
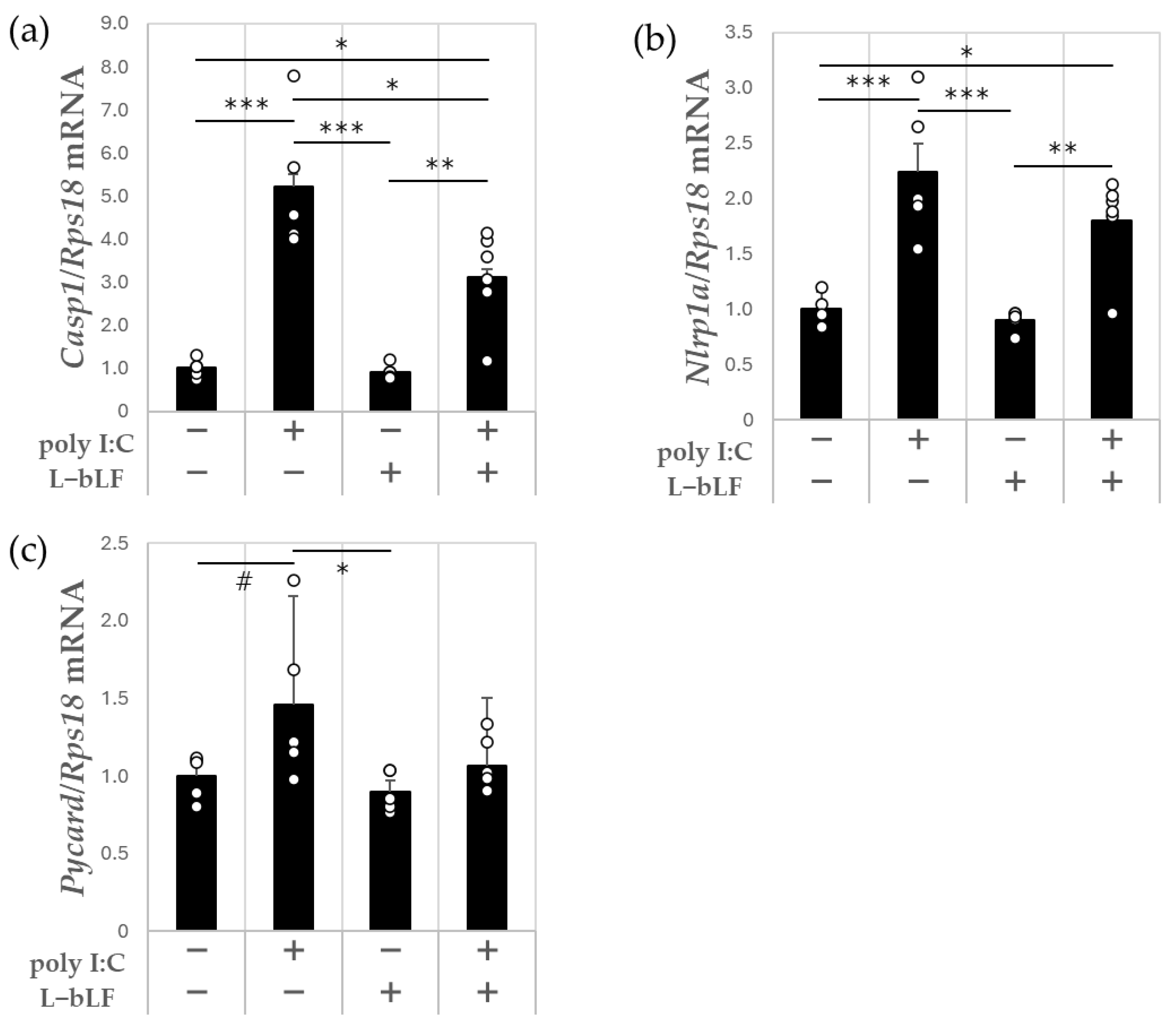
3.2. The Effects of L-bLF on Hippocampal mRNA Expression Levels in Poly I:C-Injected Rats
3.3. Characteristics of the Study Participants
3.4. The Effects of L-bLF Sleep Quality and Jetlag in Tour Conductors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Non-Intake | L-bLF | ||
|---|---|---|---|
| Mean ± SEM | Mean ± SEM | ||
| Fatigue | Day 1 | −0.38 ± 0.404 | 0.25 ± 0.734 |
| Day 2 | −1.28 ± 0.571 | −0.92 ± 0.437 | |
| Sleep | Day 1 | 0.39 ± 0.528 | 0.64 ± 0.695 |
| Day 2 | 0.70 ± 0.590 | 0.13 ± 0.601 | |
| Mental Performance and Mood | Day 1 | −0.14 ± 0.328 | 0.05 ± 0.239 |
| Day 2 | −0.36 ± 0.497 | −0.29 ± 0.395 | |
| Meals | Day 1 | −0.16 ± 0.280 | 0.22 ± 0.209 |
| Day 2 | 0.26 ± 0.246 | −0.29 ± 0.387 | |
| Bowel Activity | Day 1 | −0.88 ± 0.598 | −0.02 ± 0.392 |
| Day 2 | −0.54 ± 0.709 | −0.29 ± 0.451 |
References
- O’Callaghan, J.P.; Miller, D.B. Neuroinflammation disorders exacerbated by environmental stressors. Metabolism 2019, 100, 153951. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.P.; Drake, A.L.; Frey, D.J.; Fleshner, M.; Desouza, C.A.; Gronfier, C.; Czeisler, C.A. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav. Immun. 2015, 47, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Opp, M.R.; Krueger, J.M. Sleep and host defence. In Principles and Practice of Sleep Medicine, 6th ed.; Kryger, M., Roth, T., Dement, W.C., Eds.; Elsevier: Philadelphia, PA, USA, 2017; pp. 193–201. [Google Scholar]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory response in the CNS: Friend or foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar] [CrossRef] [PubMed]
- Mullington, J.M.; Simpson, N.S.; Meier-Ewert, H.K.; Haack, M. Sleep loss and inflammation. Best. Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 775–784. [Google Scholar] [CrossRef]
- Abdo Qaid, E.Y.; Abdullah, Z.; Zakaria, R.; Long, I. Minocycline mitigates tau pathology via modulating the TLR-4/NF-кβ signalling pathway in the hippocampus of neuroinflammation rat model. Neurol. Res. 2024, 46, 261–271. [Google Scholar] [CrossRef]
- Kou, J.; Wang, M.; Shi, J.; Zhang, H.; Pu, X.; Song, S.; Yang, C.; Yan, Y.; Döring, Y.; Xie, X.; et al. Curcumin reduces cognitive deficits by inhibiting neuroinflammation through the endoplasmic reticulum stress pathway in apolipoprotein E4 transgenic mice. ACS Omega 2021, 6, 6654–6662. [Google Scholar] [CrossRef]
- You, M.M.; Chen, Y.F.; Pan, Y.M.; Liu, Y.C.; Tu, J.; Wang, K.; Hu, F.L. Royal jelly attenuates LPS-induced inflammation in BV-2 microglial cells through modulating NF-κB and p38/JNK signaling pathways. Mediators Inflamm. 2018, 2018, 7834381. [Google Scholar] [CrossRef]
- Khan, A.; Ikram, M.; Muhammad, T.; Park, J.; Kim, M.O. Caffeine modulates cadmium-induced oxidative stress, neuroinflammation, and cognitive impairments by regulating Nrf-2/HO-1 in vivo and in vitro. J. Clin. Med. 2019, 8, 680. [Google Scholar] [CrossRef]
- Legrand, D. Overview of lactoferrin as a natural immune modulator. J. Pediatr. 2016, 173, S10–S15. [Google Scholar] [CrossRef]
- Li, Y.Q.; Guo, C. A review on lactoferrin and central nervous system diseases. Cells 2021, 10, 1810. [Google Scholar] [CrossRef]
- Bolat, E.; Eker, F.; Kaplan, M.; Duman, H.; Arslan, A.; Saritaş, S.; Şahutoğlu, A.S.; Karav, S. Lactoferrin for COVID-19 prevention, treatment, and recovery. Front. Nutr. 2022, 9, 992733. [Google Scholar] [CrossRef] [PubMed]
- Cutone, A.; Rosa, L.; Ianiro, G.; Lepanto, M.S.; Bonaccorsi di Patti, M.C.; Valenti, P.; Musci, G. Lactoferrin’s anti-cancer properties: Safety, selectivity, and wide range of action. Biomolecules 2020, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Abdelmoneem, M.A.; Hassanin, I.A.; Abd Elwakil, M.M.; Elnaggar, M.A.; Mokhtar, S.; Fang, J.Y.; Elkhodairy, K.A. Lactoferrin, a multi-functional glycoprotein: Active therapeutic, drug nanocarrier & targeting ligand. Biomaterials 2020, 263, 120355. [Google Scholar] [CrossRef]
- Steijns, J.M.; van Hooijdonk, A.C. Occurrence, structure, biochemical properties and technological characteristics of lactoferrin. Br. J. Nutr. 2000, 84 (Suppl. 1), 11–17. [Google Scholar] [CrossRef] [PubMed]
- Presti, S.; Manti, S.; Parisi, G.F.; Papale, M.; Barbagallo, I.A.; Li Volti, G.; Leonardi, S. Lactoferrin: Cytokine modulation and application in clinical practice. J. Clin. Med. 2021, 10, 5482. [Google Scholar] [CrossRef]
- Siqueiros-Cendón, T.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F.; García-Montoya, I.A.; Salazar-Martínez, J.; Rascón-Cruz, Q. Immunomodulatory effects of lactoferrin. Acta Pharmacol. Sin. 2014, 35, 557–566. [Google Scholar] [CrossRef]
- Zimecki, M.; Actor, J.K.; Kruzel, M.L. The potential for lactoferrin to reduce SARS-CoV-2 induced cytokine storm. Int. Immunopharmacol. 2021, 95, 107571. [Google Scholar] [CrossRef]
- Machnicki, M.; Zimecki, M.; Zagulski, T. Lactoferrin regulates the release of tumour necrosis factor alpha and interleukin 6 in vivo. Int. J. Exp. Pathol. 1993, 74, 433–439. [Google Scholar]
- Håversen, L.; Ohlsson, B.G.; Hahn-Zoric, M.; Hanson, L.A.; Mattsby-Baltzer, I. Lactoferrin down-regulates the LPS-induced cytokine production in monocytic cells via NF-kappa B. Cell Immunol. 2002, 220, 83–95. [Google Scholar] [CrossRef]
- Inubushi, T.; Kawazoe, A.; Miyauchi, M.; Kudo, Y.; Ao, M.; Ishikado, A.; Makino, T.; Takata, T. Molecular mechanisms of the inhibitory effects of bovine lactoferrin on lipopolysaccharide-mediated osteoclastogenesis. J. Biol. Chem. 2012, 287, 23527–23536. [Google Scholar] [CrossRef]
- Fillebeen, C.; Ruchoux, M.M.; Mitchell, V.; Vincent, S.; Benaïssa, M.; Pierce, A. Lactoferrin is synthesized by activated microglia in the human substantia nigra and its synthesis by the human microglial CHME cell line is upregulated by tumor necrosis factor alpha or 1-methyl-4-phenylpyridinium treatment. Brain Res. Mol. Brain Res. 2001, 96, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Tsatsanis, A.; McCorkindale, A.N.; Wong, B.X.; Patrick, E.; Ryan, T.M.; Evans, R.W.; Bush, A.I.; Sutherland, G.T.; Sivaprasadarao, A.; Guennewig, B.; et al. The acute phase protein lactoferrin is a key feature of Alzheimer’s disease and predictor of Aβ burden through induction of APP amyloidogenic processing. Mol. Psychiatry 2021, 26, 5516–5531. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yang, Z.H.; Zhang, S.; Chai, R.; Xue, H.; Zhang, Y.H.; Li, J.Y.; Wang, Z.Y. Intranasal lactoferrin enhances α-secretase-dependent amyloid precursor protein processing via the ERK1/2-CREB and HIF-1α pathways in an Alzheimer’s disease mouse model. Neuropsychopharmacology 2017, 42, 2504–2515. [Google Scholar] [CrossRef]
- Kawamata, T.; Tooyama, I.; Yamada, T.; Walker, D.G.; McGeer, P.L. Lactotransferrin immunocytochemistry in Alzheimer and normal human brain. Am. J. Pathol. 1993, 142, 1574–1585. [Google Scholar]
- Yong, S.J.; Veerakumarasivam, A.; Lim, W.L.; Chew, J. Neuroprotective effects of lactoferrin in Alzheimer’s and Parkinson’s diseases: A narrative review. ACS Chem. Neurosci. 2023, 14, 1342–1355. [Google Scholar] [CrossRef]
- Mohamed, W.A.; Salama, R.M.; Schaalan, M.F. A pilot study on the effect of lactoferrin on Alzheimer’s disease pathological sequelae: Impact of the p-Akt/PTEN pathway. Biomed. Pharmacother. 2019, 111, 714–723. [Google Scholar] [CrossRef]
- Zheng, J.; Xie, Y.; Li, F.; Zhou, Y.; Qi, L.; Liu, L.; Chen, Z. Lactoferrin improves cognitive function and attenuates brain senescence in aged mice. J. Funct. Foods 2020, 65, 103736. [Google Scholar] [CrossRef]
- Ishikado, A.; Imanaka, H.; Kotani, M.; Fujita, A.; Mitsuishi, Y.; Kanemitsu, T.; Tamura, Y.; Makino, T. Liposomal lactoferrin induced significant increase of the interferon-alpha (IFN-alpha) producibility in healthy volunteers. Biofactors 2004, 21, 69–72. [Google Scholar] [CrossRef]
- Ishikado, A.; Imanaka, H.; Takeuchi, T.; Harada, E.; Makino, T. Liposomalization of lactoferrin enhanced it’s anti-inflammatory effects via oral administration. Biol. Pharm. Bull. 2005, 28, 1717–1721. [Google Scholar] [CrossRef]
- Yamano, E.; Miyauchi, M.; Furusyo, H.; Kawazoe, A.; Ishikado, A.; Makino, T.; Tanne, K.; Tanaka, E.; Takata, T. Inhibitory effects of orally administrated liposomal bovine lactoferrin on the LPS-induced osteoclastogenesis. Lab. Investig. 2010, 90, 1236–1246. [Google Scholar] [CrossRef]
- Ishikado, A.; Uesaki, S.; Suido, H.; Nomura, Y.; Sumikawa, K.; Maeda, M.; Miyauchi, M.; Takata, T.; Makino, T. Human trial of liposomal lactoferrin supplementation for periodontal disease. Biol. Pharm. Bull. 2010, 33, 1758–1762. [Google Scholar] [CrossRef] [PubMed]
- Uesaki, S.; Imanaka, H.; Suido, H.; Kondo, S.; Suwa, M.; Matsumoto, M. Effects of lactoferrin supplementation on sleep quality, mood states, and enteric environment in poor sleepers-randomized, placebo-controlled, double-blind study. Jpn. Pharmacol. Ther. 2018, 46, 55–63. [Google Scholar]
- Yamato, M.; Tamura, Y.; Eguchi, A.; Kume, S.; Miyashige, Y.; Nakano, M.; Watanabe, Y.; Kataoka, Y. Brain interleukin-1β and the intrinsic receptor antagonist control peripheral toll-like receptor 3-mediated suppression of spontaneous activity in rats. PLoS ONE 2014, 9, e90950. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.O.; Oh, S.Y.; Matsui, T.; Oh, M.J.; Nishizawa, T. RSIV is probably insensitive to the transient innate immune response induced by administration of poly(I:C), a synthetic double-stranded RNA. Fish. Pathology 2012, 47, 137–142. [Google Scholar] [CrossRef][Green Version]
- Katafuchi, T.; Kondo, T.; Yasaka, T.; Kubo, K.; Take, S.; Yoshimura, M. Prolonged effects of polyriboinosinic: Polyribocytidylic acid on spontaneous running wheel activity and brain interferon-alpha mRNA in rats: A model for immunologically induced fatigue. Neuroscience 2003, 120, 837–845. [Google Scholar] [CrossRef]
- Ketelauri, P.; Scharov, K.; von Gall, C.; Johann, S. Acute circadian disruption due to constant light promotes caspase 1 activation in the mouse hippocampus. Cells 2023, 12, 1836. [Google Scholar] [CrossRef]
- Frank, M.G.; Barrientos, R.M.; Watkins, L.R.; Maier, S.F. Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. J. Neuroimmunol. 2010, 226, 181–184. [Google Scholar] [CrossRef]
- Barrientos, R.M.; Frank, M.G.; Hein, A.M.; Higgins, E.A.; Watkins, L.R.; Rudy, J.W.; Maier, S.F. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav. Immun. 2009, 23, 46–54. [Google Scholar] [CrossRef]
- Sateia, M.J. International classification of sleep disorders-third edition: Highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef]
- Cao, D.; Zhao, Y.; Wang, Y.; Wei, D.; Yan, M.; Su, S.; Pan, H.; Wang, Q. Effects of sleep deprivation on anxiety-depressive-like behavior and neuroinflammation. Brain Res. 2024, 1836, 148916. [Google Scholar] [CrossRef]
- Jerigova, V.; Zeman, M.; Okuliarova, M. Circadian disruption and consequences on innate immunity and inflammatory response. Int. J. Mol. Sci. 2022, 23, 13722. [Google Scholar] [CrossRef] [PubMed]
- Akkaoui, M.A.; Palagini, L.; Geoffroy, P.A. Sleep immune cross talk and insomnia. Adv. Exp. Med. Biol. 2023, 1411, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Veler, H. Sleep and inflammation: Bidirectional relationship. Sleep. Med. Clin. 2023, 18, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Minowa, M.; Uchiyama, M.; Okawa, M.; Kim, K.; Shibui, K.; Kamei, Y. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res. 2000, 97, 165–172. [Google Scholar] [CrossRef]
- Waterhouse, J.; Reilly, T.; Atkinson, G.; Edwards, B. Jet lag: Trends and coping strategies. Lancet 2007, 369, 1117–1129. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Tanaka, H.; Maeda, M.; Yamazaki, K.; Shirakawa, S. Characteristics of personality affecting on sleep feeling: Study for neuroticism and extroversion-introversion. Jpn. J. Health Psychol. 2000, 13, 13–22. [Google Scholar]
- Huang, W.C.; Liou, C.J.; Shen, S.C.; Hu, S.; Chao, J.C.; Hsiao, C.Y.; Wu, S.J. Urolithin A inactivation of TLR3/TRIF signaling to block the NF-κB/STAT1 axis reduces inflammation and enhances antioxidant defense in poly(I:C)-induced RAW264.7 cells. Int. J. Mol. Sci. 2022, 23, 4697. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. TLR signaling. Cell Death Differ. 2006, 13, 816–825. [Google Scholar] [CrossRef]
- Essam, R.M.; Saadawy, M.A.; Gamal, M.; Abdelsalam, R.M.; El-Sahar, A.E. Lactoferrin averts neurological and behavioral impairments of thioacetamide-induced hepatic encephalopathy in rats via modulating HGMB1/TLR-4/MyD88/Nrf2 pathway. Neuropharmacology 2023, 236, 109575. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Y.; Meng, J.; Wu, M.; Bi, F.; Chang, C.; Li, H.; Zhang, L. Ablation of caspase-1 protects against TBI-induced pyroptosis in vitro and in vivo. J. Neuroinflamm. 2018, 15, 48. [Google Scholar] [CrossRef]
- Rand, D.; Cooper, I. Caspase-1: An important player and possible target for repair of the blood-brain barrier underlying neurodegeneration. Neural Regen. Res. 2021, 16, 2390–2392. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhang, L.L.; Li, D.; Wu, J.; Guo, Y.X.; Fan, J.; Wu, Q.; Wang, H.P.; Wan, Z.; Xu, J.Y.; et al. Lactoferrin alleviates Western diet-induced cognitive impairment through the microbiome-gut-brain axis. Curr. Res. Food Sci. 2023, 7, 100533. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. NRF2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Innamorato, N.G.; Rojo, A.I.; García-Yagüe, A.J.; Yamamoto, M.; de Ceballos, M.L.; Cuadrado, A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J. Immunol. 2008, 181, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Harashima, N.; Minami, T.; Uemura, H.; Harada, M. Transfection of poly(I:C) can induce reactive oxygen species-triggered apoptosis and interferon-β-mediated growth arrest in human renal cell carcinoma cells via innate adjuvant receptors and the 2-5A system. Mol. Cancer 2014, 13, 217. [Google Scholar] [CrossRef]
- Mohan, S.; Gupta, D. Crosstalk of toll-like receptors signaling and Nrf2 pathway for regulation of inflammation. Biomed. Pharmacother. 2018, 108, 1866–1878. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Huang, R.Q.; Ke, W.L.; Qu, Y.H.; Zhu, J.H.; Pei, Y.Y.; Jiang, C. Characterization of lactoferrin receptor in brain endothelial capillary cells and mouse brain. J. Biomed. Sci. 2007, 14, 121–128. [Google Scholar] [CrossRef]
- Yoshise, R.E.; Ueda, N.; Matsuyama, H.; Serizawa, A. Suppressive effect of lactoferrin with 70 irons via oral administration on dysmenorrheal. Milk. Sci. 2010, 59, 115–123. [Google Scholar]
- Kamemori, N.; Takeuchi, T.; Sugiyama, A.; Miyabayashi, M.; Kitagawa, H.; Shimizu, H.; Ando, K.; Harada, E. Trans-endothelial and trans-epithelial transfer of lactoferrin into the brain through BBB and BCSFB in adult rats. J. Vet. Med. Sci. 2008, 70, 313–315. [Google Scholar] [CrossRef]
- Zielinski, M.R.; Gibbons, A.J. Neuroinflammation, Sleep, and Circadian Rhythms. Front. Cell Infect. Microbiol. 2022, 12, 853096. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.L.; Wright, K.P. Shift work, shift-work disorder, and jet lag. In Principles and Practice of Sleep Medicine, 6th ed.; Kryger, M., Roth, T., Dement, W.C., Eds.; Elsevier: Philadelphia, PA, USA, 2017; pp. 721–725. [Google Scholar]
- Guzmán-Mejía, F.; Vega-Bautista, A.; Molotla-Torres, D.E.; Aguirre-Garrido, J.F.; Drago-Serrano, M.E. Bovine lactoferrin as a modulator of neuroendocrine components of stress. Curr. Mol. Pharmacol. 2021, 14, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Moriya, T.; Sawauchi, M.; Kobayashi, T.; Tsushima, C.; Kuwata, H.; Hirasawa, N.; Harada, E. The potentiating action of bovine lactoferrin on the light entrainment of the circadian clock in mice. Lactoferrin 2017, 2017, 43–48. [Google Scholar]
- Li, Y.; Shao, L.; Mou, Y.; Zhang, Y.; Ping, Y. Sleep, circadian rhythm and gut microbiota: Alterations in Alzheimer’s disease and their potential links in the pathogenesis. Gut Microbes 2021, 13, 1957407. [Google Scholar] [CrossRef]
- Castanon-Cervantes, O.; Wu, M.; Ehlen, J.C.; Paul, K.; Gamble, K.L.; Johnson, R.L.; Besing, R.C.; Menaker, M.; Gewirtz, A.T.; Davidson, A.J. Dysregulation of inflammatory responses by chronic circadian disruption. J. Immunol. 2010, 185, 5796–5805. [Google Scholar] [CrossRef]




| Gene | Forward | Reverse |
|---|---|---|
| Il1b | CCCTGAACTCAACTGTGAAATAGCA | CCCAAGTCAAGGGCTTGGAA |
| Il6 | ATTGTATGAACAGCGATGATGCAC | CCAGGTAGAAACGGAACTCCAGA |
| Tnf | TCAGTTCCATGGCCCAGAC | GTTGTCTTTGAGATCCATGCCATT |
| Hmox1 | ATTTGTCCGAGGCCTTGAA | CCAGGGCCGTATAGATATGGTA |
| Nfe2l2 | GATGATGCCAGCCAGCTGAA | GCGACTGACTAATGGCAGCAGA |
| Nlrp1a | GCCCTGGAGACAAAGAATCC | AGTGGGCATCGTCATGTGT |
| Pycard | TCTGTGCTTAGAGACATGGGCATAC | GCCATACAGAGCATCCAGCAA |
| Casp1 | CTAGACTACAGATGCCAACCACTGA | GCATGATTCCCAACACAGGTACA |
| Nqo1 | TGAGCCCGGATATTGTAGCTGA | GCATACGTGTAGGCGAATCCTG |
| Sod2 | GGTGTGAGCTGCTCTTGATTGA | TTGATGGCCTTATGATGACAGTGA |
| Gclm | TGAATGGAGCTCCCAAATCAG | CATGGGACATGGTACATTCCAA |
| Rps18 | CTTCCACAGGAGGCCTACAC | GATGGTGATCACACGCTCCA |
| Age | Experience as an International Tour Guide (yrs) | Global PSQI Score | Non-Intake Period | L-bLF Period | Notes |
|---|---|---|---|---|---|
| 43 | 20 | 7 | Eastward | Eastward | Direction matched |
| 50 | 20 | 7 | Eastward | Eastward | Direction matched |
| 50 | 28 | 7 | Eastward | Eastward | Direction matched |
| 53 | 20 | - | Eastward | Eastward | Direction matched |
| 54 | 10 | 8 | Eastward | Eastward | Direction matched |
| 57 | 34 | 12 | Eastward | Eastward | Direction matched |
| 57 | 30 | 7 | Eastward | Eastward | Direction matched |
| 41 | 15 | 9 | Westward | Eastward | Direction mismatched |
| 45 | 23 | 12 | Eastward | Westward | Direction mismatched |
| 46 | 21 | 3 | Westward | Eastward | Direction mismatched |
| 46 | 10 | 6 | Eastward | Westward | Direction mismatched |
| 49 | 25 | 6 | Eastward | Westward | Direction mismatched |
| 50 | 24 | 8 | Westward | Eastward | Direction mismatched |
| 53 | 20 | - | Westward | Eastward | Direction mismatched |
| Average time difference (hours) | 9.29 ± 0.82 | 9.57 ± 0.84 | |||
| Excluded participants | |||||
| 43 | 17 | 10 | Eastward | Eastward | Drop-out: delay in intake of test tablets during L-bLF period |
| 53 | 20 | - | Westward | Westward | Drop-out: missing actigraphic data during non-intake period |
| 53 | 20 | 7 | - | Westward | Drop-out: tour cancellation during non-intake period |
| Mean ± SEM | ||
|---|---|---|
| Sleep latency (minutes) | Non-intake | 5.98 ± 0.27 |
| L-bLF | 4.20 ± 0.33 *** | |
| Total counts | Non-intake | 35,270.64 ± 4300.03 |
| L-bLF | 20,998.01 ± 1863.39 *** | |
| Sleep efficiency (%) | Non-intake | 78.72 ± 2.83 |
| L-bLF | 85.64 ± 2.33 *** | |
| Total minutes in bed (minutes) | Non-intake | 365.35 ± 17.51 |
| L-bLF | 332.96 ± 13.25 # | |
| Total sleep time (minutes) | Non-intake | 288.70 ± 17.40 |
| L-bLF | 286.67 ± 14.61 | |
| Wake after sleep onset (minutes) | Non-intake | 70.59 ± 11.80 |
| L-bLF | 42.02 ± 7.31 *** | |
| Number of awakenings | Non-intake | 17.49 ± 1.73 |
| L-bLF | 13.51 ± 1.12 *** | |
| Average awakening length (minutes) | Non-intake | 4.30 ± 0.51 |
| L-bLF | 3.12 ± 0.30 *** |
| Non-Intake | L-bLF | |
|---|---|---|
| Mean ± SEM | Mean ± SEM | |
| Sleepiness on rising | ||
| Concentrated | 53.13 ± 3.32 | 56.75 ± 4.31 |
| Unstressed | 48.07 ± 2.86 | 47.51 ± 2.80 |
| Respond to the survey quickly | 54.92 ± 3.68 | 57.09 ± 4.27 |
| Quite awake | 46.56 ± 3.06 | 50.15 ± 3.64 |
| Initiation and maintenance of sleep | ||
| Had a sound sleep | 53.48 ± 5.66 | 65.40 ± 5.02 * |
| Went to sleep quickly | 64.08 ± 5.04 | 71.51 ± 4.81 |
| Had no trouble falling asleep | 63.20 ± 4.90 | 72.88 ± 4.25 * |
| Had no arousal during sleep | 51.01 ± 5.31 | 56.92 ± 4.85 |
| Slept deeply | 46.42 ± 4.50 | 56.82 ± 4.78 * |
| Frequent dreaming | ||
| Had no nightmares | 53.48 ± 5.66 | 65.40 ± 5.02 |
| Had few dreams | 64.08 ± 5.04 | 71.51 ± 4.81 |
| Feeling of refreshment | ||
| Not tired | 33.10 ± 2.78 | 39.38 ± 2.49 # |
| Vigorousness | 37.88 ± 2.99 | 38.85 ± 3.31 |
| Pleasantly refreshed | 44.69 ± 2.31 | 49.18 ± 2.64 |
| Motivated to answer surveys | 54.92 ± 3.68 | 57.09 ± 4.27 |
| Sleep length | ||
| Hungry on awakening | 53.00 ± 3.80 | 52.48 ± 4.91 |
| Had prolonged sleep | 37.50 ± 4.42 | 39.11 ± 3.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uesaki, S.; Yamato, M.; Ishikado, A.; Suekawa, Y.; Tamura, Y.; Kataoka, Y. Liposomal Lactoferrin Reduces Brain Neuroinflammation in Rats and Alleviates Jetlag and Improves Sleep Quality After Long-Haul Travel. NeuroSci 2025, 6, 19. https://doi.org/10.3390/neurosci6010019
Uesaki S, Yamato M, Ishikado A, Suekawa Y, Tamura Y, Kataoka Y. Liposomal Lactoferrin Reduces Brain Neuroinflammation in Rats and Alleviates Jetlag and Improves Sleep Quality After Long-Haul Travel. NeuroSci. 2025; 6(1):19. https://doi.org/10.3390/neurosci6010019
Chicago/Turabian StyleUesaki, Shoko, Masanori Yamato, Atsushi Ishikado, Yutaka Suekawa, Yasuhisa Tamura, and Yosky Kataoka. 2025. "Liposomal Lactoferrin Reduces Brain Neuroinflammation in Rats and Alleviates Jetlag and Improves Sleep Quality After Long-Haul Travel" NeuroSci 6, no. 1: 19. https://doi.org/10.3390/neurosci6010019
APA StyleUesaki, S., Yamato, M., Ishikado, A., Suekawa, Y., Tamura, Y., & Kataoka, Y. (2025). Liposomal Lactoferrin Reduces Brain Neuroinflammation in Rats and Alleviates Jetlag and Improves Sleep Quality After Long-Haul Travel. NeuroSci, 6(1), 19. https://doi.org/10.3390/neurosci6010019






