Pediatric Acute Disseminated Encephalomyelitis Triggered by Concurrent Administration of Seasonal and H1N1 Influenza Vaccines: A Case Report and Review
Abstract
:1. Introduction
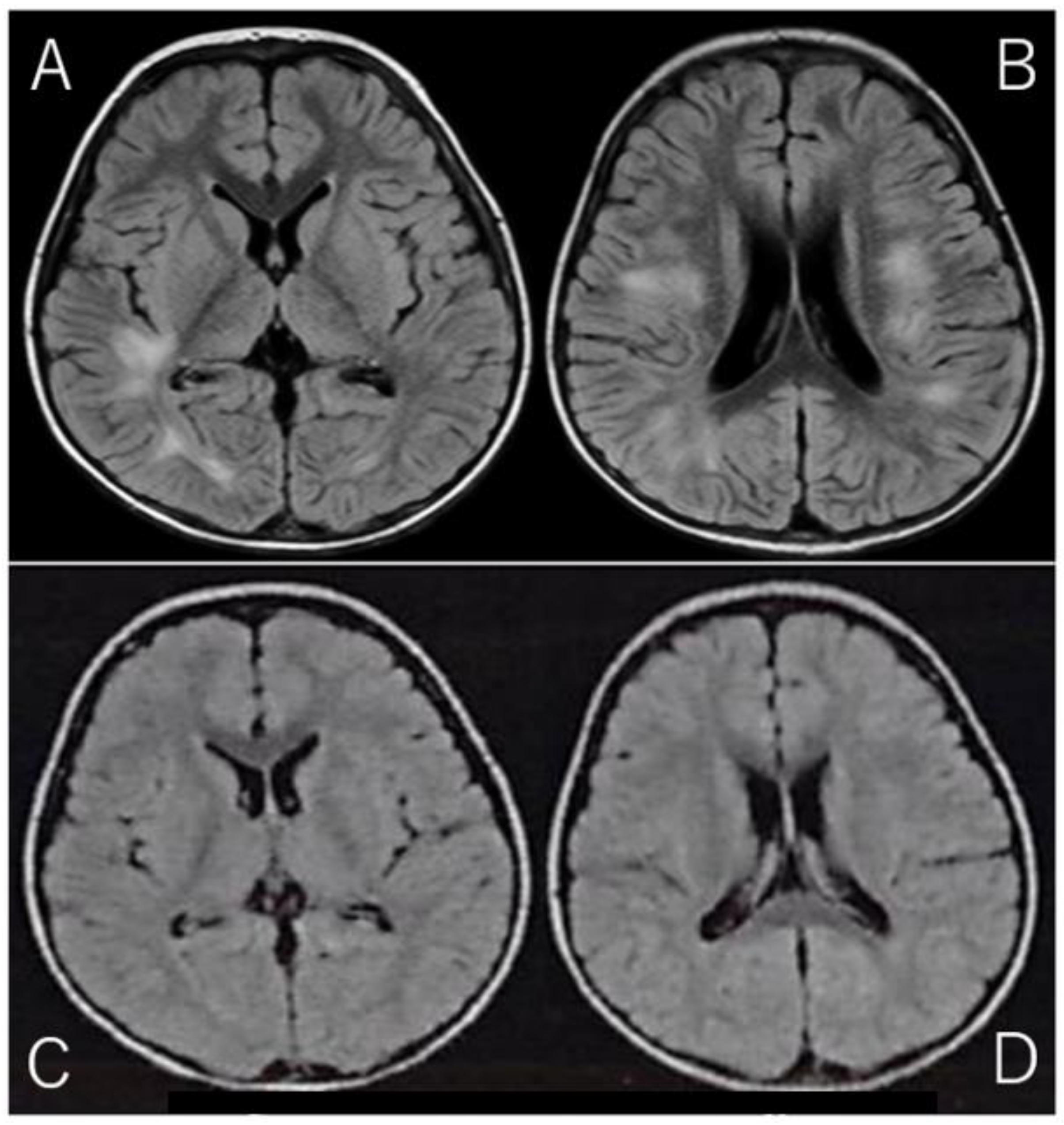
2. Case Report
3. Discussion
4. Limitation of This Case Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Massa, S.; Fracchiolla, A.; Neglia, C.; Argentiero, A.; Esposito, S. Update on Acute Disseminated Encephalomyelitis in Children and Adolescents. Children 2021, 8, 280. [Google Scholar] [CrossRef]
- Neal, A.H.; Halsey, A.; Talaat, K.R.; Greembaum, A.; Mensah, E.; Dudley, M.Z.; Proveaux, T.; Salmon, D.A. The Safety of Influenza Vaccines in Children: An Institute for Vaccine Safety White Paper. Vaccine 2015, 33, F1–F67. [Google Scholar]
- Sarkanen, T.O.; Alakuijala, A.P.; Dauvilliers, Y.A.; Partinen, M.M. Incidence of Narcolepsy After H1N1 Influenza and Vaccinations: Systematic Review and Meta-Analysis. Sleep Med. Rev. 2018, 38, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Sanz Fadrique, R.S.; Martín Arias, L.; Molina-Guarneros, J.A.; Jimeno Bulnes, N.; García Ortega, P. Guillain-Barré Syndrome and Influenza Vaccines: Current Evidence. Rev. Esp. Quimioter. 2019, 32, 288–295. [Google Scholar] [PubMed]
- Vieira, M.A.C.; Costa, C.N.; Vieira, C.P.; Cavalcanti, M.S.; Ferreira-Filho, S.P. Transverse Myelitis with Brown-Sèquard Syndrome After H1N1 Immunization. Arq. Neuropsiquiatr. 2012, 70, 555. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.C.; Mostaço-Guidolin, L.C.; Sinnock, H.; Bozat-Emre, S.; Routledge, M.; Mahmud, S.M. Pandemic H1N1 Vaccination and Incidence of Acute Disseminated Encephalomyelitis in Manitoba. Can. J. Neurol. Sci. 2016, 43, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Olberg, H.K.; Eide, G.E.; Cox, R.J.; Jul-Larsen, Å.; Lartey, S.L.; Vedeler, C.A.; Myhr, K.-M. Antibody Response to Seasonal Influenza Vaccination in Patients with Multiple Sclerosis Receiving Immunomodulatory Therapy. Eur. J. Neurol. 2018, 25, 527–534. [Google Scholar] [CrossRef]
- Al-Quliti, K.; Qureshi, A.; Quadri, M.; Abdulhameed, B.; Alanazi, A.; Alhujeily, R. Acute Demyelinating Encephalomyelitis Post-COVID-19 Vaccination: A Case Report and Literature Review. Diseases 2022, 10, 13. [Google Scholar] [CrossRef]
- Lazaro, L.G.; Perea Cossio, J.E.; Luis, M.B.; Tamagnini, F.; Paguay Mejia, D.A.; Solarz, H.; Fernandez Liguori, N.A.; Alonso, R.N. Acute Disseminated Encephalomyelitis Following Vaccination Against SARS-CoV-2: A Case Report. Brain Behav. Immun. Health 2022, 20, 100439. [Google Scholar] [CrossRef]
- Nishikawa, T.; Fujiwara, M.; Takei, H. Acute Disseminated Encephalomyelitis following Seasonal Influenza Vaccination in an Elderly Patient. Clin. Vaccine Immunol. 2013, 20, 1166–1169. [Google Scholar]
- Pellegrino, P.; Carnovale, C.; Perrone, V.; Salvati, D.; Gentili, M.; Radice, S.; Clementi, E. Acute Disseminated Encephalomyelitis Onset: Evaluation Based on Vaccine Adverse Event Reporting Systems. PLoS ONE 2013, 8, e77766. [Google Scholar] [CrossRef]
- Segal, Y.; Shoenfeld, Y. Vaccine-induced autoimmunity: The role of molecular mimicry and immune crossreaction. Cell. Mol. Immunol. 2018, 15, 586–594. [Google Scholar] [CrossRef]
- Murthy, J.M. Acute disseminated encephalomyelitis. Neurol. India 2002, 50, 238–243. [Google Scholar] [PubMed]
- Vellozzi, C.; Iqbal, S.; Broder, K. Guillain-Barré Syndrome, Influenza, and Influenza Vaccination: The Epidemiologic Evidence. Clin. Infect. Dis. 2014, 58, 1149–1155. [Google Scholar] [CrossRef]
- Agmon-Levin, N.; Kivity, S.; Shoenfeld, Y. Transverse myelitis and vaccines: A multi-analysis. Lupus 2009, 18, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Huynh, W.; Cordato, D.J.; Kehdi, E.; Masters, L.T.; Dedousis, C. Post-vaccination encephalomyelitis: Literature review and illustrative case. J. Clin. Neurosci. 2008, 15, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.; Bakshi, N.; Fireman, B.; Lewis, E.; Ray, P.; Vellozzi, C.; Klein, N.P. Lack of association of Guillain-Barré syndrome with vaccinations. Clin. Infect. Dis. 2013, 57, 197–204. [Google Scholar] [CrossRef]
- Haber, P.; Sejvar, J.; Mikaeloff, Y.; DeStefano, F. Vaccines and Guillain-Barré syndrome. Drug Saf. 2009, 32, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Shoenfeld, Y.; Aron-Maor, A. Vaccination and autoimmunity—‘vaccinosis’: A dangerous liaison? J. Autoimmun. 2000, 14, 1–10. [Google Scholar]
- King, J.C., Jr.; Stoddard, J.J.; Gaglani, M.J.; Moore, K.A.; Magder, L.; McClure, E.; Rubin, J.D.; Englund, J.A.; Neuzil, K. Effectiveness of school-based influenza vaccination. N. Eng. J. Med. 2006, 355, 2523–2532. [Google Scholar] [CrossRef] [PubMed]
- Englund, J.A.; Walter, E.B.; Fairchok, M.P.; Monto, A.S.; Neuzil, K.M. A comparison of 2 influenza vaccine schedules in 6- to 23-month-old children. Pediatrics 2005, 115, 1039–1047. [Google Scholar] [CrossRef]
- Lewis, K.D.C.; Ortiz, J.R.; Rahman, M.Z.; Levine, M.Z.; Rudenko, L.; Wright, P.F.; Katz, J.M.; Dally, L.; Rahman, M.; Isakova-Sivak, I.; et al. Immunogenicity and viral shedding of Russian-backbone, seasonal, trivalent, live, attenuated influenza vaccine in a phase II, randomized, placebo-controlled trial among preschool-aged children in urban Bangladesh. Clin. Infect. Dis. 2019, 69, 777–785. [Google Scholar] [CrossRef]
- Diallo, A.; Victor, J.C.; Feser, J.; Ortiz, J.R.; Kanesa-Thasan, N.; Ndiaye, M.; Diarra, B.; Cheikh, S.; Diene, D.; Ndiaye, T.; et al. Immunogenicity and safety of MF59-adjuvanted and full-dose unadjuvanted trivalent inactivated influenza vaccines among vaccine-naïve children in a randomized clinical trial in rural Senegal. Vaccine 2018, 36, 6424–6432. [Google Scholar] [CrossRef]
- Brooks, W.A.; Zaman, K.; Lewis, K.D.; Ortiz, J.R.; Goswami, D.; Feser, J.; Sharmeen, A.T.; Nahar, K.; Rahman, M.; Rahman, M.Z.; et al. Efficacy of a Russian-backbone live attenuated influenza vaccine among young children in Bangladesh: A randomized, double-blind, placebo-controlled trial. Lancet Glob. Health 2016, 4, e946–e954. [Google Scholar] [CrossRef] [PubMed]
- Shoamanesh, A.; Traboulsee, A. Acute disseminated encephalomyelitis following influenza vaccination. Vaccine 2010, 29, 318–321. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imataka, G.; Shiraishi, H.; Yoshihara, S. Pediatric Acute Disseminated Encephalomyelitis Triggered by Concurrent Administration of Seasonal and H1N1 Influenza Vaccines: A Case Report and Review. NeuroSci 2025, 6, 1. https://doi.org/10.3390/neurosci6010001
Imataka G, Shiraishi H, Yoshihara S. Pediatric Acute Disseminated Encephalomyelitis Triggered by Concurrent Administration of Seasonal and H1N1 Influenza Vaccines: A Case Report and Review. NeuroSci. 2025; 6(1):1. https://doi.org/10.3390/neurosci6010001
Chicago/Turabian StyleImataka, George, Hideaki Shiraishi, and Shigemi Yoshihara. 2025. "Pediatric Acute Disseminated Encephalomyelitis Triggered by Concurrent Administration of Seasonal and H1N1 Influenza Vaccines: A Case Report and Review" NeuroSci 6, no. 1: 1. https://doi.org/10.3390/neurosci6010001
APA StyleImataka, G., Shiraishi, H., & Yoshihara, S. (2025). Pediatric Acute Disseminated Encephalomyelitis Triggered by Concurrent Administration of Seasonal and H1N1 Influenza Vaccines: A Case Report and Review. NeuroSci, 6(1), 1. https://doi.org/10.3390/neurosci6010001






