Sensory Processing Challenges in Children with Neurodevelopmental Disorders and Genetic Conditions: An Observational Study
Abstract
1. Introduction
1.1. Sensory Integration and Sensory Integration Disorders
1.2. Sensory Integration Disorders and Neurodevelopmental Disorders
1.3. Sensory Integration Disorders in Genetic Conditions
1.4. Objective
2. Materials and Methods
2.1. Participants
2.2. Sensory Processing Measure
2.3. Procedure
2.4. Statistical Analysis
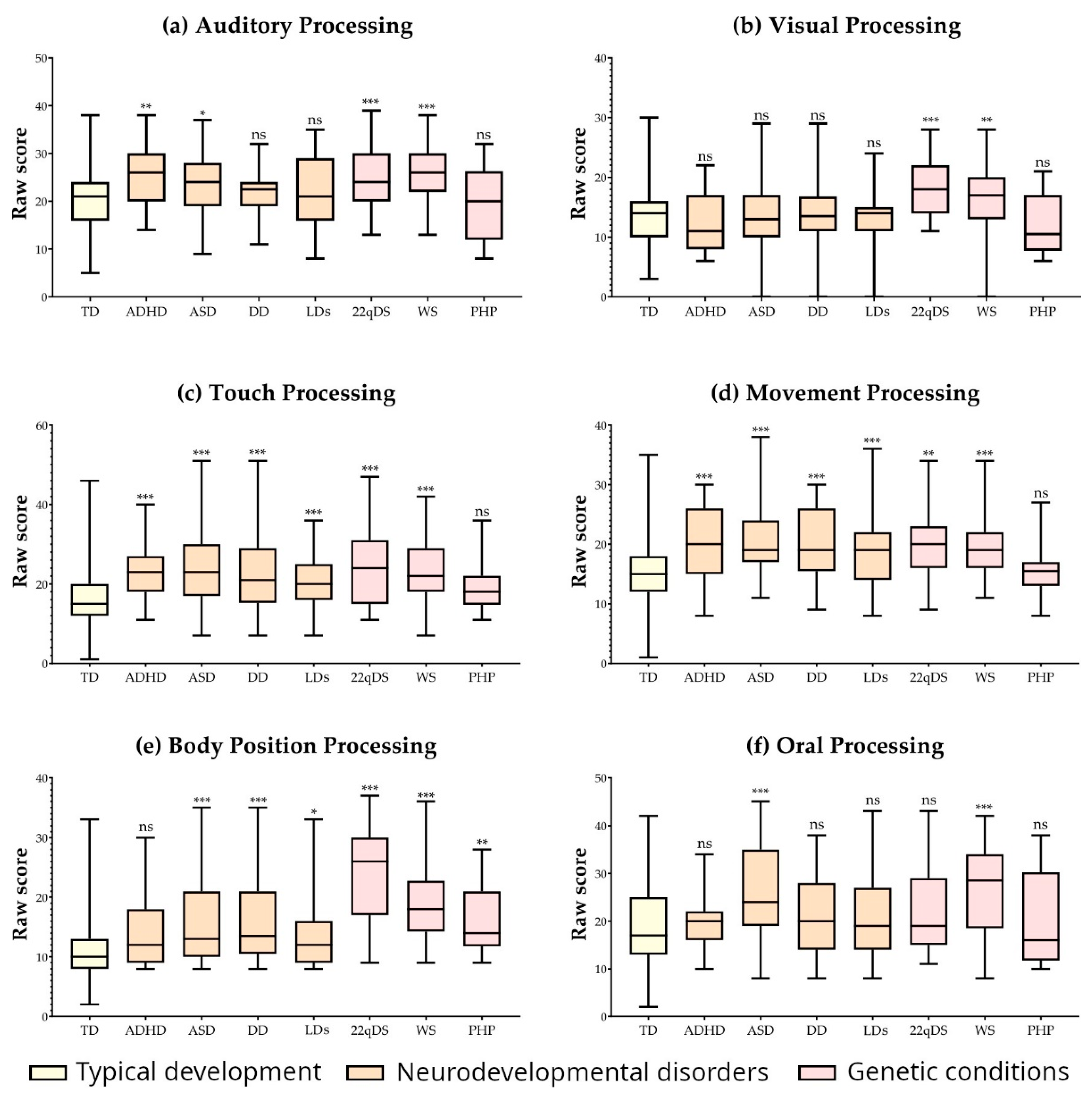
3. Results
4. Discussion
4.1. Neurodevelopmental Disorders
4.2. Genetic Conditions
4.3. Limitations and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bundy, A.C.; Lane, S.J.; Murray, E.A. Sensory Integration: Theory and Practice; FA Davis: Philadelphia, PA, USA, 2002; ISBN 0-8036-0545-5. [Google Scholar]
- Kilroy, E.; Aziz-Zadeh, L.; Cermak, S. Ayres Theories of Autism and Sensory Integration Revisited: What Contemporary Neuroscience Has to Say. Brain Sci. 2019, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.J.; Mailloux, Z.; Schoen, S.; Bundy, A.; May-Benson, T.A.; Parham, L.D.; Smith Roley, S.; Schaaf, R.C. Neural Foundations of Ayres Sensory Integration®. Brain Sci. 2019, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Ahn, R.R.; Miller, L.J.; Milberger, S.; McIntosh, D.N. Prevalence of Parents’ Perceptions of Sensory Processing Disorders among Kindergarten Children. Am. J. Occup. Ther. 2004, 58, 287–293. [Google Scholar] [CrossRef]
- Gouze, K.R.; Hopkins, J.; LeBailly, S.A.; Lavigne, J.V. Re-Examining the Epidemiology of Sensory Regulation Dysfunction and Comorbid Psychopathology. J. Abnorm. Child Psychol. 2009, 37, 1077–1087. [Google Scholar] [CrossRef]
- Engel-Yeger, B. The Applicability of the Short Sensory Profile for Screening Sensory Processing Disorders among Israeli Children. Int. J. Rehabil. Res. 2010, 33, 311–318. [Google Scholar] [CrossRef]
- Román-Oyola, R.; Reynolds, S. Prevalence of Sensory Modulation Disorder among Puerto Rican Preschoolers: An Analysis Focused on Socioeconomic Status Variables. Occup. Ther. Int. 2013, 20, 144–154. [Google Scholar] [CrossRef]
- Galiana, A.; Flores-Ripoll, J.M.; Benito-Castellanos, P.J.; Villar-Rodriguez, C.; Vela-Romero, M. Prevalence and Severity-Based Classification of Sensory Processing Issues. An Exploratory Study with Neuropsychological Implications. Appl. Neuropsychol. Child 2022, 11, 850–862. [Google Scholar] [CrossRef]
- Galiana-Simal, A.; Vela-Romero, M.; Romero-Vela, V.M.; Oliver-Tercero, N.; García-Olmo, V.; Benito-Castellanos, P.J.; Muñoz-Martinez, V.; Beato-Fernandez, L. Sensory Processing Disorder: Key Points of a Frequent Alteration in Neurodevelopmental Disorders. Cogent Med. 2020, 7, 1736829. [Google Scholar] [CrossRef]
- Narayan, A.; Rowe, M.A.; Palacios, E.M.; Wren-Jarvis, J.; Bourla, I.; Gerdes, M.; Brandes-Aitken, A.; Desai, S.S.; Marco, E.J.; Mukherjee, P. Altered Cerebellar White Matter in Sensory Processing Dysfunction Is Associated with Impaired Multisensory Integration and Attention. Front. Psychol. 2021, 11, 618436. [Google Scholar] [CrossRef]
- Chang, Y.-S.; Gratiot, M.; Owen, J.P.; Brandes-Aitken, A.; Desai, S.S.; Hill, S.S.; Arnett, A.B.; Harris, J.; Marco, E.J.; Mukherjee, P. White Matter Microstructure Is Associated with Auditory and Tactile Processing in Children with and without Sensory Processing Disorder. Front. Neuroanat. 2016, 9, 169101. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th ed.; American Psychiatric Publications: Arlington, VA, USA, 2013; ISBN 978-0-89042-557-2. [Google Scholar]
- Tavassoli, T.; Miller, L.J.; Schoen, S.A.; Nielsen, D.M.; Baron-Cohen, S. Sensory Over-Responsivity in Adults with Autism Spectrum Conditions. Autism 2014, 18, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, B.; Daly, B.P.; Nicholls, E.G.; Gullo, D.F. Assessing Sensory Processing Problems in Children With and Without Attention Deficit Hyperactivity Disorder. Phys. Occup. Ther. Pediatr. 2015, 35, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ghanizadeh, A. Sensory Processing Problems in Children with ADHD, a Systematic Review. Psychiatry Investig. 2011, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Ayres, A.J. Sensory Integration and Learning Disorders; Western Psychological Services: Los Angeles, CA, USA, 1972; ISBN 0-87424-303-3. [Google Scholar]
- May-Benson, T.A.; Koomar, J.; Teasdale, A. Incidence of Pre-, Peri-, and Post-Natal Birth and Developmental Problems of Children with Sensory Processing Disorder and Children with Autism Spectrum Disorder. Front. Integr. Neurosci. 2009, 3, 596. [Google Scholar] [CrossRef]
- Krüger, R.J.; Krüger, J.J.; Hugo, R.; Campbell, N.G. Relationship Patterns between Central Auditory Processing Disorders and Language Disorders, Learning Disabilities, and Sensory Integration Dysfunction. Commun. Disord. Q. 2001, 22, 87–98. [Google Scholar] [CrossRef]
- Armstrong, S. Sensory Processing in Children and Adults with Learning Difficulties. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2019. [Google Scholar]
- Glod, M.; Riby, D.; Rodgers, J. Sensory Processing Profiles and Autistic Symptoms as Predictive Factors in Autism Spectrum Disorder and Williams Syndrome. J. Intellect. Disabil. Res. 2020, 64, 657–665. [Google Scholar] [CrossRef]
- McDonald-McGinn, D.M.; Sullivan, K.E.; Marino, B.; Philip, N.; Swillen, A.; Vorstman, J.A.; Zackai, E.H.; Emanuel, B.S.; Vermeesch, J.R.; Morrow, B.E. 22q11. 2 Deletion Syndrome. Nat. Rev. Dis. Primers 2015, 1, 15071. [Google Scholar] [CrossRef]
- Rogdaki, M.; Gudbrandsen, M.; McCutcheon, R.A.; Blackmore, C.E.; Brugger, S.; Ecker, C.; Craig, M.C.; Daly, E.; Murphy, D.G.; Howes, O. Magnitude and Heterogeneity of Brain Structural Abnormalities in 22q11. 2 Deletion Syndrome: A Meta-Analysis. Mol. Psychiatry 2020, 25, 1704–1717. [Google Scholar] [CrossRef]
- Pober, B.R. Williams–Beuren Syndrome. N. Engl. J. Med. 2010, 362, 239–252. [Google Scholar] [CrossRef]
- Jackowski, A.P.; Rando, K.; de Araújo, C.M.; Del Cole, C.G.; Silva, I.; de Lacerda, A.L.T. Brain Abnormalities in Williams Syndrome: A Review of Structural and Functional Magnetic Resonance Imaging Findings. Eur. J. Paediatr. Neurol. 2009, 13, 305–316. [Google Scholar]
- Mantovani, G.; Bastepe, M.; Monk, D.; De Sanctis, L.; Thiele, S.; Usardi, A.; Ahmed, S.F.; Bufo, R.; Choplin, T.; De Filippo, G.; et al. Diagnosis and Management of Pseudohypoparathyroidism and Related Disorders: First International Consensus Statement. Nat. Rev. Endocrinol. 2018, 14, 476–500. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Li, Z.; Gao, Q.; Dong, L.; Lin, J.; Peng, K.; Xiang, W.; Deng, B. Characterizing Cerebral Imaging and Electroclinical Features of Five Pseudohypoparathyroidism Cases Presenting with Epileptic Seizures. Behav. Neurol. 2022, 2022, 8710989. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W. Perfil Sensorial 2. Manual de La Adaptación Española; Pearson Educación: Madrid, Spain, 2016. [Google Scholar]
- Parham, L.D.; Ecker, C.L.; Kuhaneck, H.; Henry, D.A.; Glennon, T.J. Sensory Processing Measure, Second Edition (SPM-2); Western Psychological Services: Los Angeles, CA, USA, 2021. [Google Scholar]
- Ayres, A.J. Sensory Integration and Praxis Test (SIPT); Western Psychological Services: Los Angeles, CA, USA, 1989. [Google Scholar]
- Mailloux, Z.; Parham, L.D.; Roley, S.S.; Ruzzano, L.; Schaaf, R.C. Introduction to the Evaluation in Ayres Sensory Integration® (EASI). Am. J. Occup. Ther. 2018, 72, 7201195030p1–7201195030p7. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W. The Sensory Profile: User’s Manual; The Psychological Corporation: San Antonio, TX, USA, 1999. [Google Scholar]
- Parham, L.D.; Ecker, C.; Miller Kuhaneck, H.; Henry, D.A.; Glennon, T.J. Sensory Processing Measure (SPM): Manual; Western Psychological Services: Los Angeles, CA, USA, 2007. [Google Scholar]
- Dunn, W.; Westman, K. The Sensory Profile: The Performance of a National Sample of Children without Disabilities. Am. J. Occup. Ther. 1997, 51, 25–34. [Google Scholar] [CrossRef]
- Rani, I.; Agarwal, V.; Arya, A.; Mahour, P. Sensory Processing in Children and Adolescents with Attention Deficit Hyperactivity Disorder. J. Atten. Disord. 2023, 27, 145–151. [Google Scholar] [CrossRef]
- Gándara-Gafo, B.; Beaudry-Bellefeuille, I. Convergent Validity of Two Sensory Questionnaires in Spain: Sensory Profile-2 and Sensory Processing Measure. Children 2023, 10, 1516. [Google Scholar] [CrossRef]
- Jussila, K.; Junttila, M.; Kielinen, M.; Ebeling, H.; Joskitt, L.; Moilanen, I.; Mattila, M.-L. Sensory Abnormality and Quantitative Autism Traits in Children with and without Autism Spectrum Disorder in an Epidemiological Population. J. Autism Dev. Disord. 2020, 50, 180–188. [Google Scholar] [CrossRef]
- Mimouni-Bloch, A.; Offek, H.; Rosenblum, S.; Posener, E.; Silman, Z.; Engel-Yeger, B. Association between Sensory Processing Disorder and Daily Function of Children with Attention Deficit/Hyperactive Disorder and Controls. Eur. J. Paediatr. Neurol. 2017, 21, e171. [Google Scholar] [CrossRef]
- Delgado-Lobete, L.; Pértega-Díaz, S.; Santos-del-Riego, S.; Montes-Montes, R. Sensory Processing Patterns in Developmental Coordination Disorder, Attention Deficit Hyperactivity Disorder and Typical Development. Res. Dev. Disabil. 2020, 100, 103608. [Google Scholar] [CrossRef]
- Fernández-Pires, P.; Valera-Gran, D.; Sánchez-Pérez, A.; Hurtado-Pomares, M.; Peral-Gómez, P.; Espinosa-Sempere, C.; Juárez-Leal, I.; Navarrete-Muñoz, E.-M. The Infancia y Procesamiento Sensorial (InProS—Childhood and Sensory Processing) Project: Study Protocol for a Cross-Sectional Analysis of Parental and Children’s Sociodemographic and Lifestyle Features and Children’s Sensory Processing. Int. J. Environ. Res. Public Health 2020, 17, 1447. [Google Scholar] [CrossRef]
- Nieto, C.; Lopez, B.; Gandia, H. Relationships between Atypical Sensory Processing Patterns, Maladaptive Behaviour and Maternal Stress in Spanish Children with Autism Spectrum Disorder. J. Intellect. Disabil. Res. 2017, 61, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Ayres, A.J. Sensory Integration and the Child: Understanding Hidden Sensory Challenges; Western Psychological Services: Los Angeles, CA, USA, 2005; ISBN 0-87424-437-4. [Google Scholar]
- Ausderau, K.; Sideris, J.; Furlong, M.; Little, L.M.; Bulluck, J.; Baranek, G.T. National Survey of Sensory Features in Children with ASD: Factor Structure of the Sensory Experience Questionnaire (3.0). J. Autism Dev. Disord. 2014, 44, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Baranek, G.T.; Little, L.M.; Parham, L.D.; Ausderau, K.K.; Sabatos-DeVito, M.G. Sensory Features in Autism Spectrum Disorders. In Handbook of Autism and Pervasive Developmental Disorders, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Ben-Sasson, A.; Hen, L.; Fluss, R.; Cermak, S.A.; Engel-Yeger, B.; Gal, E. A Meta-Analysis of Sensory Modulation Symptoms in Individuals with Autism Spectrum Disorders. J. Autism Dev. Disord. 2009, 39, 1–11. [Google Scholar] [CrossRef]
- Little, L.M.; Dean, E.; Tomchek, S.; Dunn, W. Sensory Processing Patterns in Autism, Attention Deficit Hyperactivity Disorder, and Typical Development. Phys. Occup. Ther. Pediatr. 2018, 38, 243–254. [Google Scholar] [CrossRef]
- Baranek, G.T.; David, F.J.; Poe, M.D.; Stone, W.L.; Watson, L.R. Sensory Experiences Questionnaire: Discriminating Sensory Features in Young Children with Autism, Developmental Delays, and Typical Development. J. Child Psychol. Psychiatry 2006, 47, 591–601. [Google Scholar] [CrossRef]
- Lidstone, D.E.; Mostofsky, S.H. Moving Toward Understanding Autism: Visual-Motor Integration, Imitation, and Social Skill Development. Pediatr. Neurol. 2021, 122, 98–105. [Google Scholar] [CrossRef]
- Volkmar, F.R.; Paul, R.; Rogers, S.J.; Pelphrey, K.A. (Eds.) Handbook of Autism and Pervasive Developmental Disorders. Volume 2, Assessment, Interventions, and Policy, 4th ed.; Wiley: Hoboken, NJ, USA, 2014; ISBN 978-1-118-28683-8. [Google Scholar]
- Cook, B.; Buysse, V.; Klingner, J.; Landrum, T.; McWilliam, R.; Tankersley, M.; Test, D. Council for Exceptional Children: Standards for Evidence-Based Practices in Special Education. Teach. Except. Child. 2014, 46, 206. [Google Scholar]
- Li, Q.; Li, Y.; Zheng, J.; Yan, X.; Huang, J.; Xu, Y.; Zeng, X.; Shen, T.; Xing, X.; Chen, Q. Prevalence and Trends of Developmental Disabilities among US Children and Adolescents Aged 3 to 17 Years, 2018–2021. Sci. Rep. 2023, 13, 17254. [Google Scholar] [CrossRef]
- Salari, N.; Ghasemi, H.; Abdoli, N.; Rahmani, A.; Shiri, M.H.; Hashemian, A.H.; Akbari, H.; Mohammadi, M. The Global Prevalence of ADHD in Children and Adolescents: A Systematic Review and Meta-Analysis. Ital. J. Pediatr. 2023, 49, 48. [Google Scholar] [CrossRef]
- Schaaf, R.; Mailloux, Z. Clinician’s Guide for Implementing Ayres Sensory Integration®. Promoting Participation for Children with Autism; AOTA Press: Bethesta, MD, USA, 2015. [Google Scholar]
- Baranek, G.T.; Boyd, B.A.; Poe, M.D.; David, F.J.; Watson, L.R. Hyperresponsive Sensory Patterns in Young Children with Autism, Developmental Delay, and Typical Development. Am. J. Ment. Retard. 2007, 112, 233–245. [Google Scholar] [CrossRef]
- Ayres, A.J. Tactile Functions. Their Relation to Hyperactive and Perceptual Motor Behavior. Am. J. Occup. Ther. Off. Publ. Am. Occup. Ther. Assoc. 1964, 18, 6–11. [Google Scholar]
- Schaaf, R.C.; Benevides, T.W.; Blanche, E.; Brett-Green, B.A.; Burke, J.; Cohn, E.; Koomar, J.; Lane, J.S.; Miller, L.J.; May-Benson, T.A.; et al. Parasympathetic Functions in Children with Sensory Processing Disorder. Front. Integr. Neurosci. 2010, 4, 594. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J.; Ziviani, J.; Rodger, S. Sensory Processing and Classroom Emotional, Behavioral, and Educational Outcomes in Children with Autism Spectrum Disorder. Am. J. Occup. Ther. 2008, 62, 564–573. [Google Scholar] [CrossRef]
- Liss, M.; Saulnier, C.; Fein, D.; Kinsbourne, M. Sensory and Attention Abnormalities in Autistic Spectrum Disorders. Autism 2006, 10, 155–172. [Google Scholar] [CrossRef]
- Hitoglou, M.; Ververi, A.; Antoniadis, A.; Zafeiriou, D.I. Childhood Autism and Auditory System Abnormalities. Pediatr. Neurol. 2010, 42, 309–314. [Google Scholar] [CrossRef]
- Kientz, M.A.; Dunn, W. A Comparison of the Performance of Children with and without Autism on the Sensory Profile. Am. J. Occup. Ther. 1997, 51, 530–537. [Google Scholar] [CrossRef]
- Lucker, J.R. Auditory Hypersensitivity in Children With Autism Spectrum Disorders. Focus Autism Other Dev Disabl 2013, 28, 184–191. [Google Scholar] [CrossRef]
- Dalpatadu, M.; Wijetunga, S.; Kapugama, K.; Kotalawala, S.; Suraweera, C. Sensory Processing in Children with and without Attention Deficit Hyperactivity Disorder: A Comparative Study Using the Short Sensory Profile. Eur. Psychiatr. 2017, 41, S435. [Google Scholar] [CrossRef]
- Lanzetta-Valdo, B.; Oliveira, G.; Ferreira, J.; Palacios, E. Auditory Processing Assessment in Children with Attention Deficit Hyperactivity Disorder: An Open Study Examining Methylphenidate Effects. Int. Arch. Otorhinolaryngol. 2016, 21, 72–78. [Google Scholar] [CrossRef][Green Version]
- Wuang, Y.; Tsai, H. Sensorimotor and Visual Perceptual Functioning in School-aged Children with Williams Syndrome. J. Intellect. Disabil. Res. 2017, 61, 348–362. [Google Scholar] [CrossRef]
- Powell, B.; Van Herwegen, J. Sensory Processing in Williams Syndrome: Individual Differences and Changes over Time. J. Autism Dev. Disord. 2022, 52, 3129–3141. [Google Scholar] [CrossRef] [PubMed]
- O’Hora, K.P.; Kushan-Wells, L.; Schleifer, C.H.; Cruz, S.; Hoftman, G.D.; Jalbrzikowski, M.; Gur, R.E.; Gur, R.C.; Bearden, C.E. Distinct Neurocognitive Profiles and Clinical Phenotypes Associated with Copy Number Variation at the 22q11.2 Locus. Autism Res. 2023, 16, 2247–2262. [Google Scholar] [CrossRef] [PubMed]
- Roley, S.S.; Mailloux, Z.; Parham, L.D.; Schaaf, R.C.; Lane, C.J.; Cermak, S. Sensory Integration and Praxis Patterns in Children with Autism. Am. J. Occup. Ther. 2015, 69, 6901220010p1–6901220010p8. [Google Scholar] [CrossRef]

| Group | Total | Boys | Girls | z-Value | p | ||
|---|---|---|---|---|---|---|---|
| TD | N | 342 | 169 | 173 | −0.22 | 0.829 | (ns) |
| % | 100 | 49.42 | 50.58 | ||||
| Age (mean) | 7.18 | 7.14 | 7.21 | −0.02 | 0.985 | (ns) | |
| Age (sd) | 2.19 | 2.06 | 2.31 | ||||
| ADHD | N | 35 | 33 | 2 | 2.43 | 0.015 | * |
| % | 100 | 94.29 | 5.71 | ||||
| Age (mean) | 8.34 | 8.15 | 11.50 | −0.74 | 0.457 | (ns) | |
| Age (sd) | 2.71 | 2.68 | 0.50 | ||||
| ASD | N | 47 | 37 | 10 | 3.22 | 0.001 | ** |
| % | 100 | 78.72 | 21.28 | ||||
| Age (mean) | 6.00 | 5.51 | 7.80 | −0.62 | 0.537 | (ns) | |
| Age (sd) | 3.64 | 2.75 | 5.49 | ||||
| DD | N | 36 | 28 | 8 | 2.77 | 0.006 | ** |
| % | 100 | 77.78 | 22.22 | ||||
| Age (mean) | 3.56 | 3.43 | 4.00 | −0.21 | 0.834 | (ns) | |
| Age (sd) | 1.40 | 0.98 | 2.29 | ||||
| LDs | N | 65 | 46 | 19 | 3.05 | 0.002 | ** |
| % | 100 | 70.77 | 29.23 | ||||
| Age (mean) | 6.48 | 6.57 | 6.26 | 0.08 | 0.933 | (ns) | |
| Age (sd) | 3.11 | 3.25 | 2.71 | ||||
| 22qDS | N | 35 | 15 | 20 | −0.84 | 0.403 | (ns) |
| % | 100 | 42.86 | 57.14 | ||||
| Age (mean) | 9.31 | 8.67 | 9.80 | −0.26 | 0.792 | (ns) | |
| Age (sd) | 5.27 | 4.09 | 5.95 | ||||
| WS | N | 40 | 25 | 15 | 1.53 | 0.126 | (ns) |
| % | 100 | 62.50 | 37.50 | ||||
| Age (mean) | 7.68 | 7.80 | 7.47 | 0.09 | 0.932 | (ns) | |
| Age (sd) | 3.86 | 3.76 | 4.00 | ||||
| PHP | N | 14 | 8 | 6 | 0.53 | 0.597 | (ns) |
| % | 100 | 57.14 | 42.86 | ||||
| Age (mean) | 9.21 | 9.25 | 9.17 | 0.02 | 0.985 | (ns) | |
| Age (sd) | 3.84 | 3.99 | 3.62 | ||||
| SP2 Sensory Sections | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Auditory processing | Visual processing | Touch processing | Movement processing | Body position processing | Oral sensory processing | |||||||
| Group | mean (sd) | p/dCliff (vs. TD) | mean (sd) | p/dClif (vs. TD) | mean (sd) | p/dClif (vs. TD) | mean (sd) | p/dClif (vs. TD) | mean (sd) | p/dClif (vs. TD) | mean (sd) | p/dClif (vs. TD) |
| TD | 19.94 (6.69) | - | 13.51 (4.34) | - | 17.05 (6.48) | - | 15.82 (5.67) | - | 11.09 (4.25) | - | 19.61 (7.80) | - |
| ADHD | 24.54 (6.12) | 0.001/−0.382 | 11.94 (4.76) | 0.035/0.158 | 22.91 (6.97) | <0.001/−0.507 | 20.48 (6.36) | <0.001/−0.462 | 13.65 (5.74) | 0.013/−0.377 | 19.65 (5.07) | 0.474/−0.14 |
| ASD | 24.10 (6.55) | 0.002/−0.318 | 13.42 (4.97) | 0.799/−0.034 | 23.78 (8.64) | <0.001/−0.524 | 20.38 (5.66) | <0.001/−0.514 | 15.36 (7.32) | <0.001/−0.463 | 25.34 (10.05) | <0.001/−0.367 |
| DD | 21.30 (5.56) | 0.342/−0.156 | 13.94 (5.95) | 0.976/−0.054 | 23.33 (9.35) | <0.001/−0.493 | 20.08 (5.74) | <0.001/−0.462 | 16.83 (7.79) | <0.001/−0.569 | 21.41 (7.98) | 0.160/−0.188 |
| LDs | 22.00 (7.41) | 0.104/−0.154 | 12.96 (0.99) | 0.389/0.012 | 20.29 (6.48) | <0.001/−0.358 | 19.16 (6.26) | <0.001/−0.371 | 13.43 (5.47) | 0.001/−0.373 | 21.16 (8.08) | 0.146/0.154 |
| 22qDS | 25.51 (6.75) | <0.001/−0.432 | 18.22 (4.67) | <0.001/−0.566 | 24.42 (9.41) | <0.001/−0.499 | 19.60 (6.12) | <0.001/−0.414 | 23.80 (8.04) | <0.001/−0.837 | 22.60 (8.94) | 0.068/−0.228 |
| WS | 26.30 (6.25) | <0.001/−0.512 | 16.60 (5.72) | <0.001/−0.407 | 23.02 (7.24) | <0.001/−0.528 | 19.63 (5.11) | <0.001/−0.463 | 19.07 (5.84) | <0.001/−0.808 | 26.77 (9.07) | <0.001/−0.462 |
| PHP | 19.64 (7.35) | 0.765/0.014 | 12.00 (4.72) | 0.225/0.135 | 19.14 (6.43) | 0.229/−0.282 | 15.35 (4.95) | 0.764/−0.028 | 16.35 (5.66) | <0.001/−0.647 | 19.92 (9.16) | 0.817/−0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Armendariz, E.; Vela-Romero, M.; Galiana, A. Sensory Processing Challenges in Children with Neurodevelopmental Disorders and Genetic Conditions: An Observational Study. NeuroSci 2024, 5, 339-353. https://doi.org/10.3390/neurosci5030027
Rodríguez-Armendariz E, Vela-Romero M, Galiana A. Sensory Processing Challenges in Children with Neurodevelopmental Disorders and Genetic Conditions: An Observational Study. NeuroSci. 2024; 5(3):339-353. https://doi.org/10.3390/neurosci5030027
Chicago/Turabian StyleRodríguez-Armendariz, Ekaine, María Vela-Romero, and Adrián Galiana. 2024. "Sensory Processing Challenges in Children with Neurodevelopmental Disorders and Genetic Conditions: An Observational Study" NeuroSci 5, no. 3: 339-353. https://doi.org/10.3390/neurosci5030027
APA StyleRodríguez-Armendariz, E., Vela-Romero, M., & Galiana, A. (2024). Sensory Processing Challenges in Children with Neurodevelopmental Disorders and Genetic Conditions: An Observational Study. NeuroSci, 5(3), 339-353. https://doi.org/10.3390/neurosci5030027






