Influence of Temporal and Frequency Selective Patterns Combined with CSP Layers on Performance in Exoskeleton-Assisted Motor Imagery Tasks
Abstract
1. Introduction
2. Materials and Methods
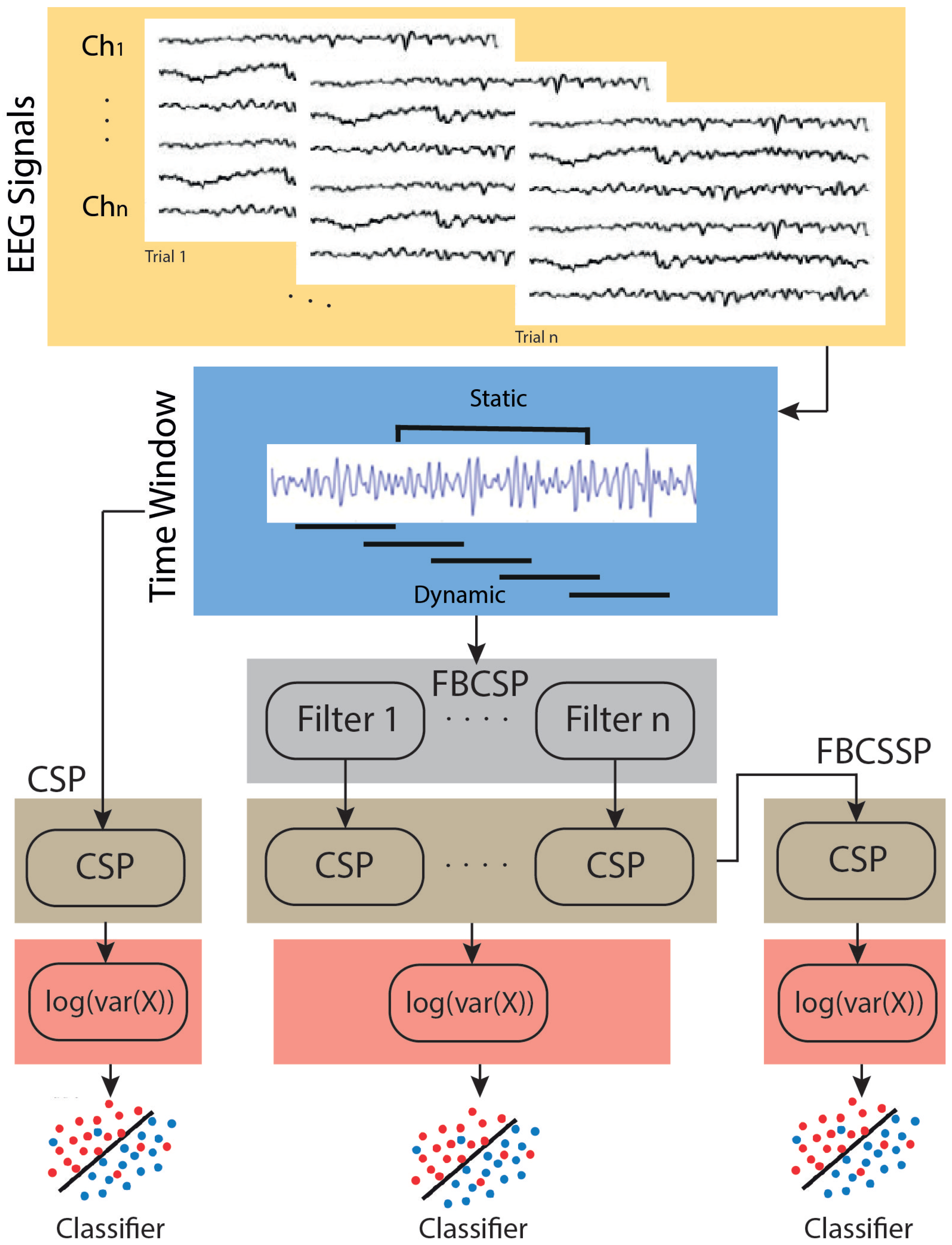
2.1. CSP-Based Methods
2.1.1. Classic CSP
2.1.2. Selective Frequency CSP
2.1.3. Combined CSP Layers with Selective Frequency
- Layer 1: This layer enables the extraction of the CSP from filtered signals after employing the filter bank. Its importance stems from its capability to emphasize differences in variances between classes for particular frequency-selective patterns [38].
- Layer 2: CSP is used to generate more selective features associated with the spatial and spectral domains. These parameters are computed by linearly projecting the weights with the output of each CSP in layer 1.
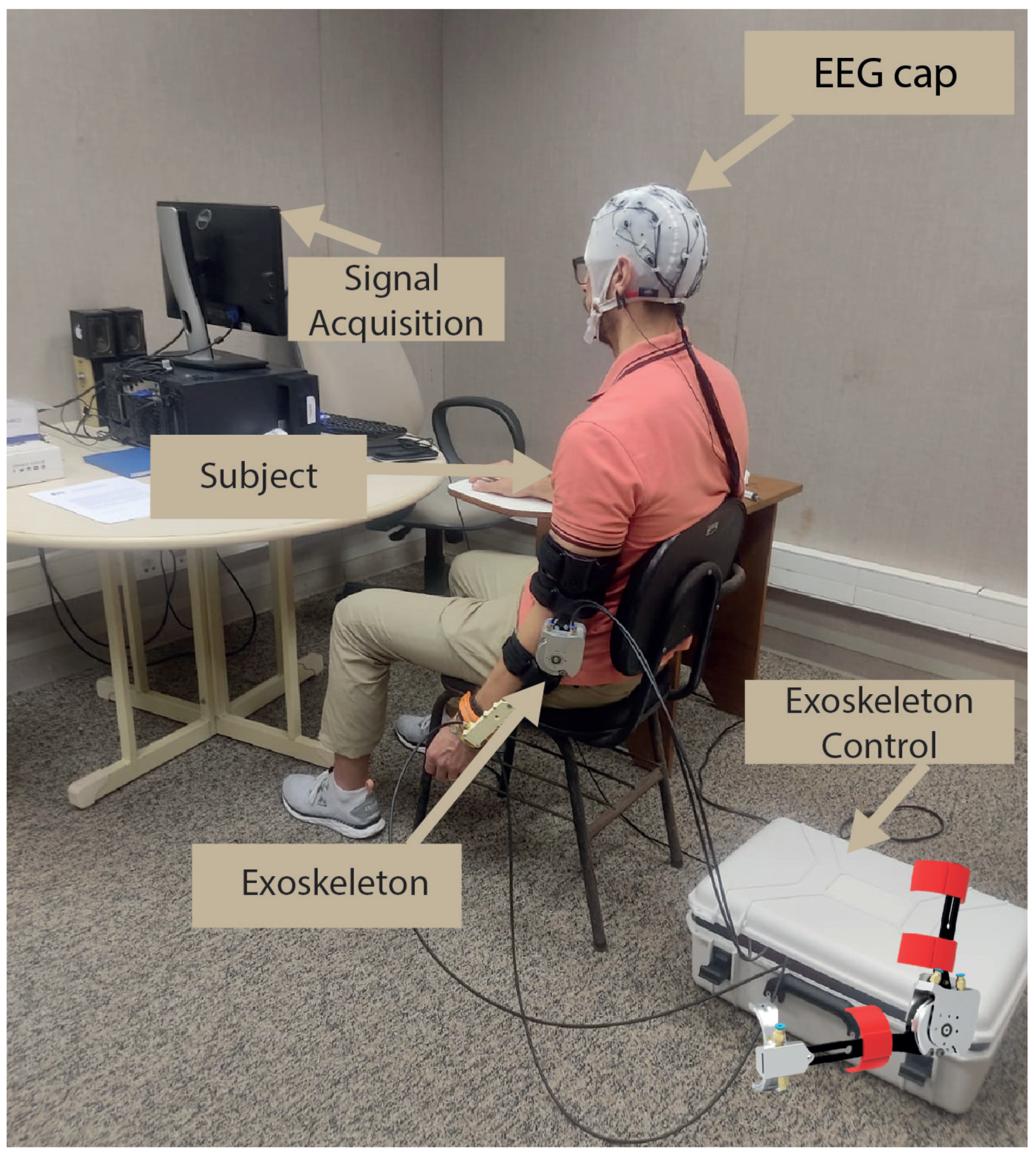
2.2. Experimental Protocol
2.2.1. Participant
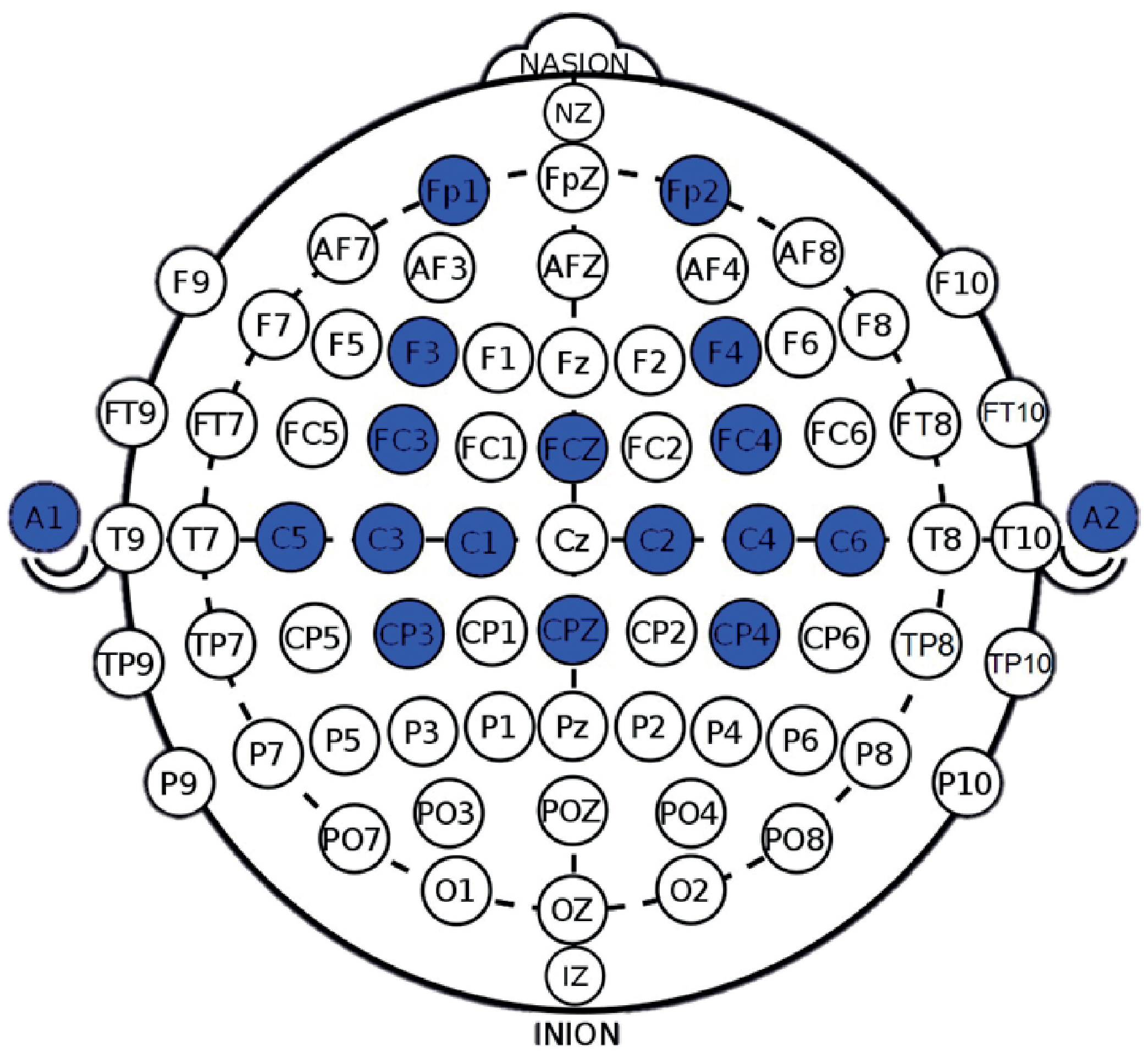
2.2.2. EEG Collection
2.2.3. Procedures
- Passive movement (baseline): The exoskeleton initiates passive movements at a minimal speed, set at 30 rotations per minute (rpm). During this phase, lasting 120 s, the subject performs passive flexion/extension tasks facilitated by the exoskeleton.
- Beep: A beep is used to signal the beginning of each trial, lasting 1.25 s. Its purpose is to inform the subject about the beginning of a new repetition cycle.
- No-action: During the no-action period, which spans from 2 to 3 s, the subject remains in a state of relaxation and rest, with no specific task or action required.
- MI+passive movement: MI plus passive movement are incorporated, with variations in two speeds—30 and 85 rpm over a duration of 10 s. In this phase, the subject is instructed to mentally simulate (MI) the performance of flexion/extension movements while simultaneously receiving passive movements from the exoskeleton.
2.3. Signal Pre-Processing and Evaluation
2.3.1. EEG Signal Pre-Processing
2.3.2. Evaluation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padfield, N.; Zabalza, J.; Zhao, H.; Masero, V.; Ren, J. EEG-Based Brain-Computer Interfaces Using Motor-Imagery: Techniques and Challenges. Sensors 2019, 19, 1423. [Google Scholar] [CrossRef] [PubMed]
- Herranz-Gómez, A.; Gaudiosi, C.; Angulo-Díaz-Parreño, S.; Suso-Martí, L.; La Touche, R.; Cuenca-Martínez, F. Effectiveness of motor imagery and action observation on functional variables: An umbrella and mapping review with meta-meta-analysis. Neurosci. Biobehav. Rev. 2020, 118, 828–845. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Lopes da Silva, F. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, K.B.; dos Santos Cardoso, M.; da Costa Cabral, V.R.; Dos Santos, A.O.B.; da Silva, P.S.; de Castro, J.B.P.; de Souza Vale, R.G. Effects of motor imagery as a complementary resource on the rehabilitation of stroke patients: A meta-analysis of randomized trials. J. Stroke Cerebrovasc. Dis. 2021, 30, 105876. [Google Scholar] [CrossRef] [PubMed]
- Chholak, P.; Niso, G.; Maksimenko, V.A.; Kurkin, S.A.; Frolov, N.S.; Pitsik, E.N.; Hramov, A.E.; Pisarchik, A.N. Visual and kinesthetic modes affect motor imagery classification in untrained subjects. Sci. Rep. 2019, 9, 9838. [Google Scholar] [CrossRef]
- Khan, M.A.; Das, R.; Iversen, H.K.; Puthusserypady, S. Review on motor imagery based BCI systems for upper limb post-stroke neurorehabilitation: From designing to application. Comput. Biol. Med. 2020, 123, 103843. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Chin, C.; Popovic, M.R. Functional electrical stimulation therapy for restoration of motor function after spinal cord injury and stroke: A review. Biomed. Eng. Online 2020, 19, 34. [Google Scholar] [CrossRef]
- Qin, C.; Yang, S.; Chu, Y.H.; Zhang, H.; Pang, X.W.; Chen, L.; Zhou, L.Q.; Chen, M.; Tian, D.S.; Wang, W. Signaling pathways involved in ischemic stroke: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Pilla, A.; Trigili, E.; McKinney, Z.; Fanciullacci, C.; Malasoma, C.; Posteraro, F.; Crea, S.; Vitiello, N. Robotic rehabilitation and multimodal instrumented assessment of post-stroke elbow motor functions—A randomized controlled trial protocol. Front. Neurol. 2020, 11, 587293. [Google Scholar] [CrossRef]
- Cantillo-Negrete, J.; Carino-Escobar, R.I.; Carrillo-Mora, P.; Elias-Vinas, D.; Gutierrez-Martinez, J. Motor imagery-based brain-computer interface coupled to a robotic hand orthosis aimed for neurorehabilitation of stroke patients. J. Healthc. Eng. 2018, 2018, 1624637. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, A.H. EEG sensor driven assistive device for elbow and finger rehabilitation using deep learning. Expert Syst. Appl. 2024, 244, 122954. [Google Scholar] [CrossRef]
- Xu, B.; Song, A.; Zhao, G.; Xu, G.; Pan, L.; Yang, R.; Li, H.; Cui, J.; Zeng, H. Robotic neurorehabilitation system design for stroke patients. Adv. Mech. Eng. 2015, 7, 1687814015573768. [Google Scholar] [CrossRef]
- Ang, K.K.; Chua, K.S.G.; Phua, K.S.; Wang, C.; Chin, Z.Y.; Kuah, C.W.K.; Low, W.; Guan, C. A randomized controlled trial of EEG-based motor imagery brain-computer interface robotic rehabilitation for stroke. Clin. EEG Neurosci. 2015, 46, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Vourvopoulos, A.; Pardo, O.M.; Lefebvre, S.; Neureither, M.; Saldana, D.; Jahng, E.; Liew, S.L. Effects of a brain-computer interface with virtual reality (VR) neurofeedback: A pilot study in chronic stroke patients. Front. Hum. Neurosci. 2019, 13, 460405. [Google Scholar] [CrossRef] [PubMed]
- Vourvopoulos, A.; Marin-Pardo, O.; Neureither, M.; Saldana, D.; Jahng, E.; Liew, S.L. Multimodal Head-Mounted Virtual-Reality Brain-Computer Interface for Stroke Rehabilitation: A Clinical Case Study with REINVENT. In Proceedings of the Virtual, Augmented and Mixed Reality. Multimodal Interaction: 11th International Conference, VAMR 2019, Held as Part of the 21st HCI International Conference, HCII 2019, Orlando, FL, USA, 26–31 July 2019; Proceedings, Part I 21. Springer: Berlin/Heidelberg, Germany, 2019; pp. 165–179. [Google Scholar]
- Huang, Z.; Wang, M. A review of electroencephalogram signal processing methods for brain-controlled robots. Cogn. Robot. 2021, 1, 111–124. [Google Scholar] [CrossRef]
- Guerrero-Mendez, C.D.; Blanco-Díaz, C.F.; Jaramillo-Isaza, S.; Bastos-Filho, T.F.; Ruiz-Olaya, A.F. Artificial Intelligence Applied to Neuromotor Rehabilitation Engineering: Advances and Challenges. In Computational Approaches in Biomaterials and Biomedical Engineering Applications; CRC Press: Boca Raton, FL, USA, 2024; pp. 212–244. [Google Scholar]
- Koles, Z.J.; Lazar, M.S.; Zhou, S.Z. Spatial patterns underlying population differences in the background EEG. Brain Topogr. 1990, 2, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Chin, Z.Y.; Wang, C.; Guan, C.; Zhang, H. Filter Bank Common Spatial Pattern Algorithm on BCI Competition IV Datasets 2a and 2b. Front. Neurosci. 2012, 6. [Google Scholar] [CrossRef]
- Wu, W.; Chen, Z.; Gao, X.; Li, Y.; Brown, E.N.; Gao, S. Probabilistic common spatial patterns for multichannel EEG analysis. IEEE Trans. Pattern Anal. Mach. Intell. 2014, 37, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Triana-Guzman, N.; Orjuela-Cañon, A.D.; Jutinico, A.L.; Mendoza-Montoya, O.; Antelis, J.M. Decoding EEG rhythms offline and online during motor imagery for standing and sitting based on a brain-computer interface. Front. Neuroinform. 2022, 16. [Google Scholar] [CrossRef]
- Rithwik, P.; Benzy, V.; Vinod, A. High accuracy decoding of motor imagery directions from EEG-based brain computer interface using filter bank spatially regularised common spatial pattern method. Biomed. Signal Process. Control 2022, 72, 103241. [Google Scholar] [CrossRef]
- Cantillo-Negrete, J.; Carino-Escobar, R.I.; Carrillo-Mora, P.; Rodriguez-Barragan, M.A.; Hernandez-Arenas, C.; Quinzaños-Fresnedo, J.; Hernandez-Sanchez, I.R.; Galicia-Alvarado, M.A.; Miguel-Puga, A.; Arias-Carrion, O. Brain-computer interface coupled to a robotic hand orthosis for stroke patients’ neurorehabilitation: A crossover feasibility study. Front. Hum. Neurosci. 2021, 15, 656975. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Chin, Z.Y.; Zhang, H.; Guan, C. Filter bank common spatial pattern (FBCSP) in brain-computer interface. In Proceedings of the 2008 IEEE International Joint Conference on Neural Networks (IEEE World Congress on Computational Intelligence), Hong Kong, China, 1–8 June 2008; pp. 2390–2397. [Google Scholar]
- Yu, N.; Yang, R.; Huang, M. Deep Common Spatial Pattern based Motor Imagery Classification with Improved Objective Function. Int. J. Netw. Dyn. Intell. 2022, 1, 73–84. [Google Scholar] [CrossRef]
- Blanco-Diaz, C.F.; Antelis, J.M.; Ruiz-Olaya, A.F. Comparative analysis of spectral and temporal combinations in CSP-based methods for decoding hand motor imagery tasks. J. Neurosci. Methods 2022, 371, 109495. [Google Scholar] [CrossRef] [PubMed]
- Martin-Clemente, R.; Olias, J.; Cruces, S.; Zarzoso, V. Unsupervised Common Spatial Patterns. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhu, Z.; Zhang, B.; Feng, B.; Yu, T.; Li, Z. The CSP-Based New Features Plus Non-Convex Log Sparse Feature Selection for Motor Imagery EEG Classification. Sensors 2020, 20, 4749. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Lam, H.K.; Ling, S.H. Multi-classification for EEG motor imagery signals using data evaluation-based auto-selected regularized FBCSP and convolutional neural network. Neural Comput. Appl. 2023, 35, 12001–12027. [Google Scholar] [CrossRef]
- Padfield, N.; Camilleri, K.; Camilleri, T.; Fabri, S.; Bugeja, M. A Comprehensive Review of Endogenous EEG-Based BCIs for Dynamic Device Control. Sensors 2022, 22, 5802. [Google Scholar] [CrossRef] [PubMed]
- Dong, E.; Li, C.; Li, L.; Du, S.; Belkacem, A.N.; Chen, C. Classification of multi-class motor imagery with a novel hierarchical SVM algorithm for brain-computer interfaces. Med. Biol. Eng. Comput. 2017, 55, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Mendez, C.D.; Blanco-Diaz, C.F.; Ruiz-Olaya, A.F.; López-Delis, A.; Jaramillo-Isaza, S.; Milanezi Andrade, R.; Ferreira De Souza, A.; Delisle-Rodriguez, D.; Frizera-Neto, A.; Bastos-Filho, T.F. EEG motor imagery classification using deep learning approaches in naïve BCI users. Biomed. Phys. Eng. Express 2023, 9, 045029. [Google Scholar] [CrossRef]
- Alazrai, R.; Abuhijleh, M.; Alwanni, H.; Daoud, M.I. A Deep Learning Framework for Decoding Motor Imagery Tasks of the Same Hand Using EEG Signals. IEEE Access 2019, 7, 109612–109627. [Google Scholar] [CrossRef]
- Barios, J.A.; Ezquerro, S.; Bertomeu-Motos, A.; Nann, M.; Badesa, F.J.; Fernandez, E.; Soekadar, S.R.; Garcia-Aracil, N. Synchronization of slow cortical rhythms during motor imagery-based brain–machine interface control. Int. J. Neural Syst. 2019, 29, 1850045. [Google Scholar] [CrossRef] [PubMed]
- González-Cely, A.X.; Blanco-Díaz, C.F.; Guerrero-Mendez, C.D.; Bastos-Filho, T.F. Hand Motor Imagery Identification Using Machine Learning Approaches in a Protocol Based on Visual Stimuli and Passive Movement. In Proceedings of the 2023 IEEE Colombian Caribbean Conference (C3), Barranquilla, Colombia, 22–25 November 2023; pp. 1–6. [Google Scholar] [CrossRef]
- Guggenberger, R.; Heringhaus, M.; Gharabaghi, A. Brain-machine neurofeedback: Robotics or electrical stimulation? Front. Bioeng. Biotechnol. 2020, 8, 639. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Wada, K.; Kurata, M.; Seki, N. Enhancement of motor-imagery ability via combined action observation and motor-imagery training with proprioceptive neurofeedback. Neuropsychologia 2018, 114, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Díaz, C.F.; Guerrero-Mendez, C.D.; Delisle-Rodriguez, D.; Jaramillo-Isaza, S.; Ruiz-Olaya, A.F.; Frizera-Neto, A.; Ferreira de Souza, A.; Bastos-Filho, T. Evaluation of temporal, spatial and spectral filtering in CSP-based methods for decoding pedaling-based motor tasks using EEG signals. Biomed. Phys. Eng. Express 2024, 10, 035003. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Shalchyan, V. State-Based Decoding of Continuous Hand Movements using EEG Signals. IEEE Access 2023, 11, 42764–42778. [Google Scholar] [CrossRef]
- Dias, E.; Ulhoa, P.; Andrade, R. Design of a 3 Degree-of-Freedom Upper-Limb Active Exoskeleton with Cable-Driven Actuators for Neuromotor Rehabilitation. In Proceedings of the 2023 IEEE Colombian Caribbean Conference (C3), Barranquilla, Colombia, 22–25 November 2023. [Google Scholar] [CrossRef]
- Dias, E.A.F.; Andrade, R.M. Órtese Robótica de Membro Superior Movida por Cabos de Aço para Reabilitação Neuromotora. Brazil (BR) BR2020230213729, 16 October 2023. [Google Scholar]
- Lotte, F.; Bougrain, L.; Cichocki, A.; Clerc, M.; Congedo, M.; Rakotomamonjy, A.; Yger, F. A review of classification algorithms for EEG-based brain–computer interfaces: A 10 year update. J. Neural Eng. 2018, 15, 031005. [Google Scholar] [CrossRef]
- Úbeda, A.; Azorín, J.M.; Chavarriaga, R.; Millán, J.d.R. Classification of upper limb center-out reaching tasks by means of EEG-based continuous decoding techniques. J. Neuroeng. Rehabil. 2017, 14, 9. [Google Scholar] [CrossRef]
- Allison, B.Z.; Neuper, C. Could Anyone Use a BCI? In Human-Computer Interaction Series; Springer: London, UK, 2010; pp. 35–54. [Google Scholar] [CrossRef]
- Edelman, B.J.; Baxter, B.; He, B. EEG Source Imaging Enhances the Decoding of Complex Right-Hand Motor Imagery Tasks. IEEE Trans. Biomed. Eng. 2016, 63, 4–14. [Google Scholar] [CrossRef]

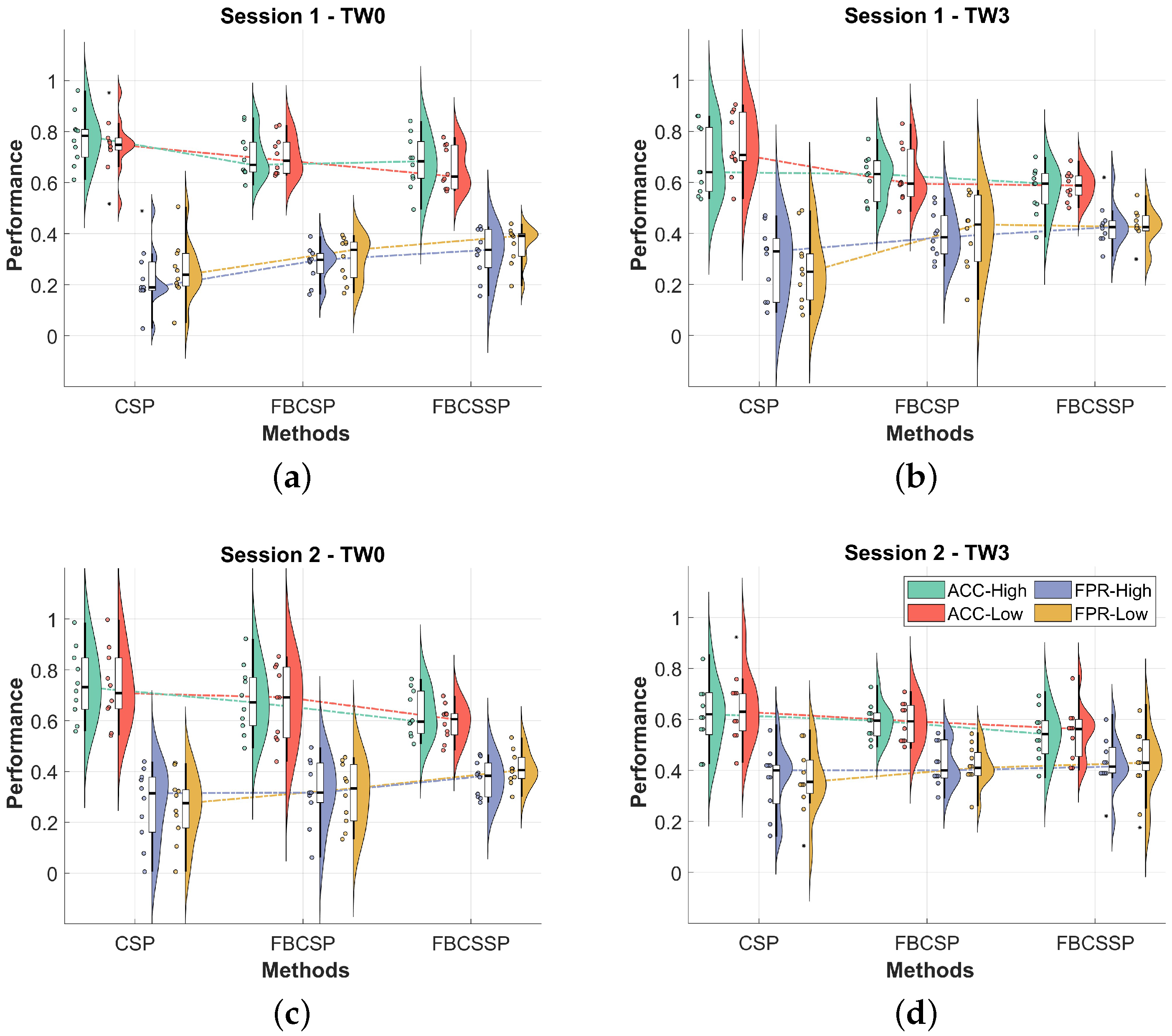
| TW0 | TW3 | |||||||
|---|---|---|---|---|---|---|---|---|
| ACC-H | ACC-L | FPR-H | FPR-L | ACC-H | ACC-L | FPR-H | FPR-L | |
| CSP vs. FBCSP | ∼ | ∼ | ∼ | ∼ | ∼ | † | † | † |
| CSP vs. FBCSSP | † | † | † | † | † | † | † | † |
| FBCSP vs. FBCSSP | ∼ | ∼ | ∼ | ∼ | ∼ | ∼ | ∼ | ∼ |
| TW0 | TW3 | |||||||
|---|---|---|---|---|---|---|---|---|
| ACC-H | ACC-L | FPR-H | FPR-L | ACC-H | ACC-L | FPR-H | FPR-L | |
| CSP vs. FBCSP | ∼ | ∼ | ∼ | ∼ | ∼ | ∼ | ∼ | ∼ |
| CSP vs. FBCSSP | † | † | † | † | ∼ | ∼ | ∼ | ∼ |
| FBCSP vs. FBCSSP | ∼ | ∼ | ∼ | † | ∼ | ∼ | ∼ | ∼ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero-Mendez, C.D.; Blanco-Diaz, C.F.; Rivera-Flor, H.; Fabriz-Ulhoa, P.H.; Fragoso-Dias, E.A.; de Andrade, R.M.; Delisle-Rodriguez, D.; Bastos-Filho, T.F. Influence of Temporal and Frequency Selective Patterns Combined with CSP Layers on Performance in Exoskeleton-Assisted Motor Imagery Tasks. NeuroSci 2024, 5, 169-183. https://doi.org/10.3390/neurosci5020012
Guerrero-Mendez CD, Blanco-Diaz CF, Rivera-Flor H, Fabriz-Ulhoa PH, Fragoso-Dias EA, de Andrade RM, Delisle-Rodriguez D, Bastos-Filho TF. Influence of Temporal and Frequency Selective Patterns Combined with CSP Layers on Performance in Exoskeleton-Assisted Motor Imagery Tasks. NeuroSci. 2024; 5(2):169-183. https://doi.org/10.3390/neurosci5020012
Chicago/Turabian StyleGuerrero-Mendez, Cristian David, Cristian Felipe Blanco-Diaz, Hamilton Rivera-Flor, Pedro Henrique Fabriz-Ulhoa, Eduardo Antonio Fragoso-Dias, Rafhael Milanezi de Andrade, Denis Delisle-Rodriguez, and Teodiano Freire Bastos-Filho. 2024. "Influence of Temporal and Frequency Selective Patterns Combined with CSP Layers on Performance in Exoskeleton-Assisted Motor Imagery Tasks" NeuroSci 5, no. 2: 169-183. https://doi.org/10.3390/neurosci5020012
APA StyleGuerrero-Mendez, C. D., Blanco-Diaz, C. F., Rivera-Flor, H., Fabriz-Ulhoa, P. H., Fragoso-Dias, E. A., de Andrade, R. M., Delisle-Rodriguez, D., & Bastos-Filho, T. F. (2024). Influence of Temporal and Frequency Selective Patterns Combined with CSP Layers on Performance in Exoskeleton-Assisted Motor Imagery Tasks. NeuroSci, 5(2), 169-183. https://doi.org/10.3390/neurosci5020012







