Cytotoxic Effect of Amyloid-β1-42 Oligomers on Endoplasmic Reticulum and Golgi Apparatus Arrangement in SH-SY5Y Neuroblastoma Cells
Abstract
1. Introduction
2. Materials and Methods
3. Results
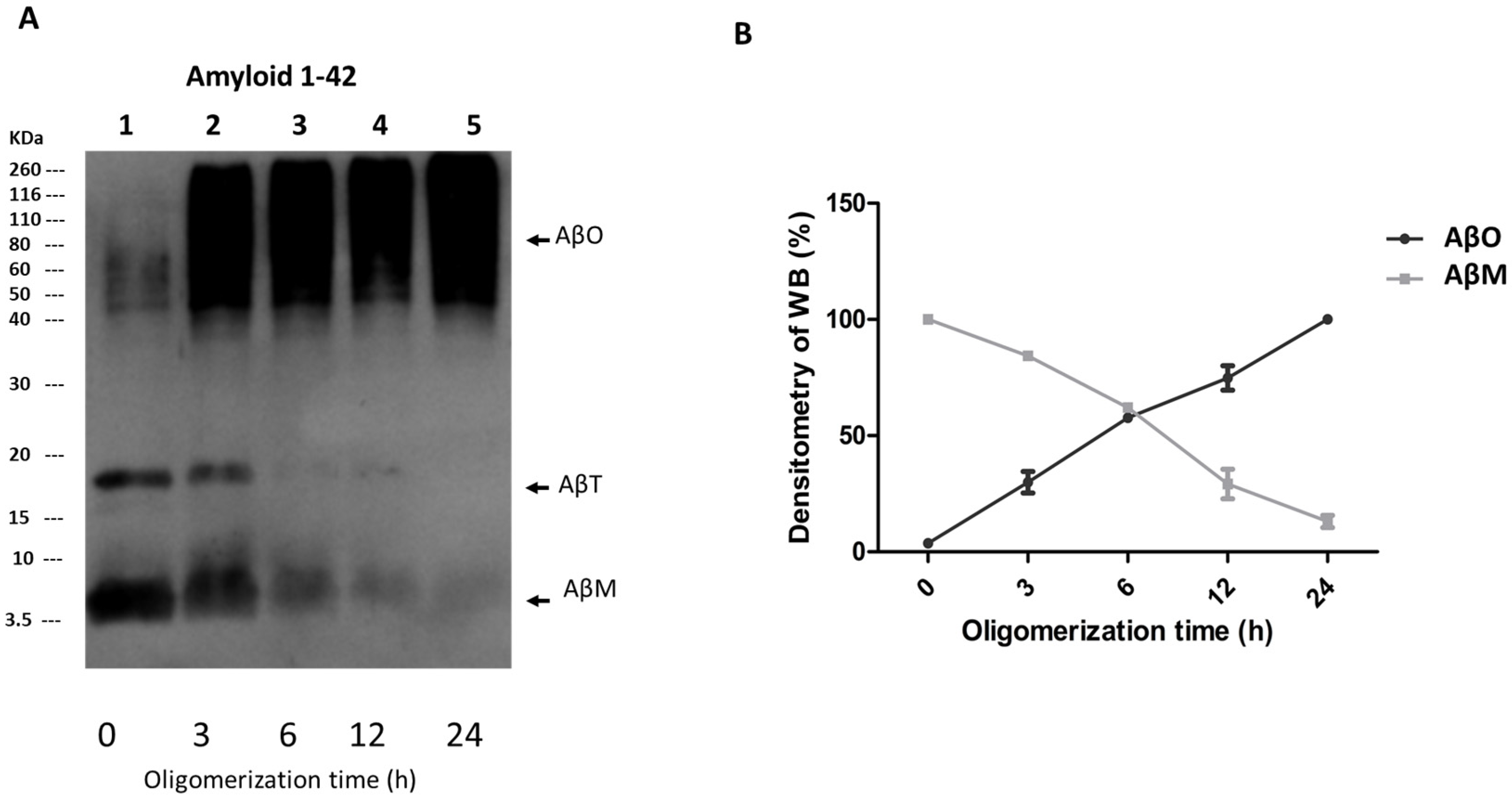
3.1. AβOs Assembled In Vitro
3.2. AβO Toxicity in SH-SY5Y Cell Cultures Is Time-Dependent
3.3. AβOs Induce Tubulin and Actin Cytoskeleton Modifications in SH-SY5Y Cells
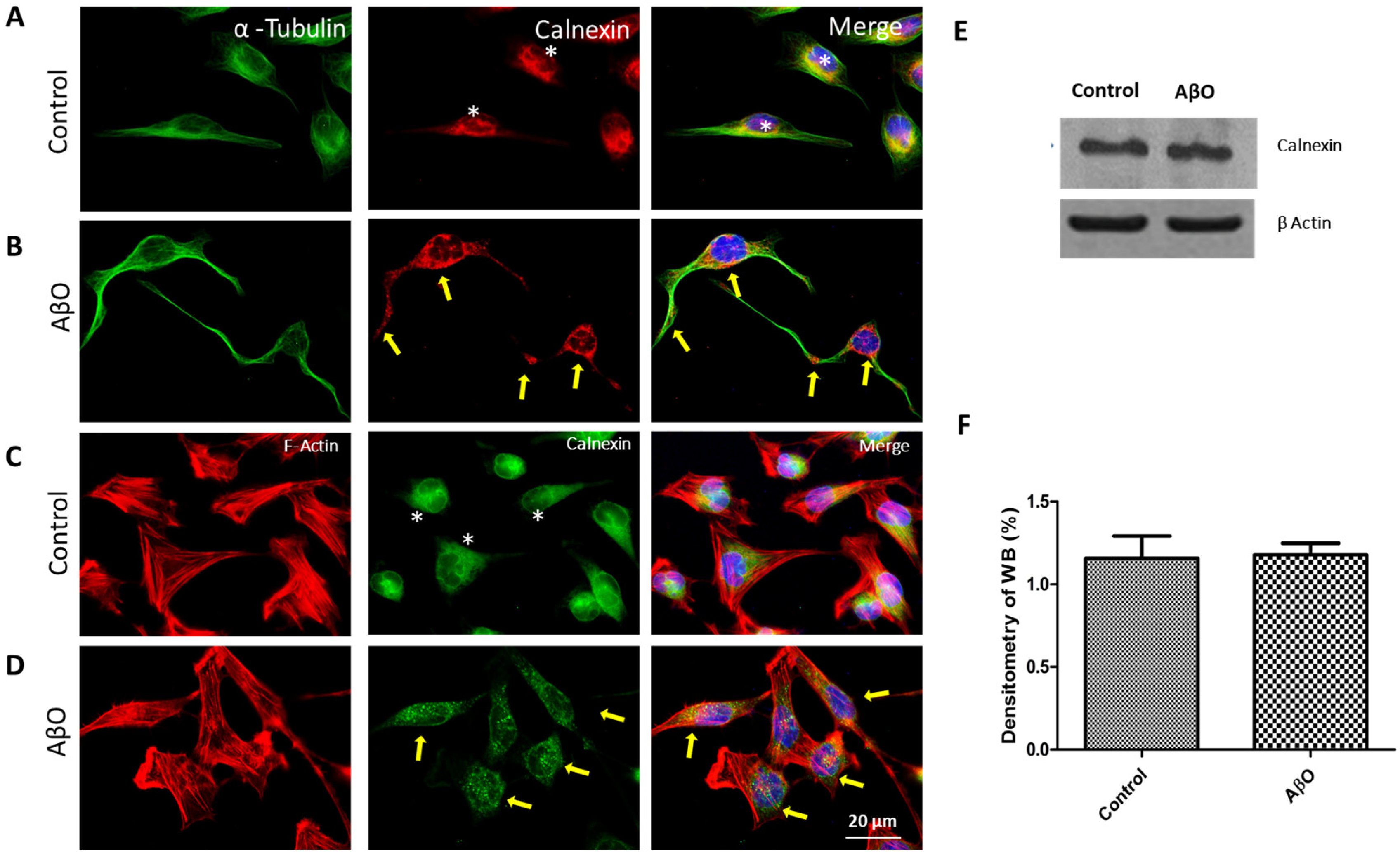
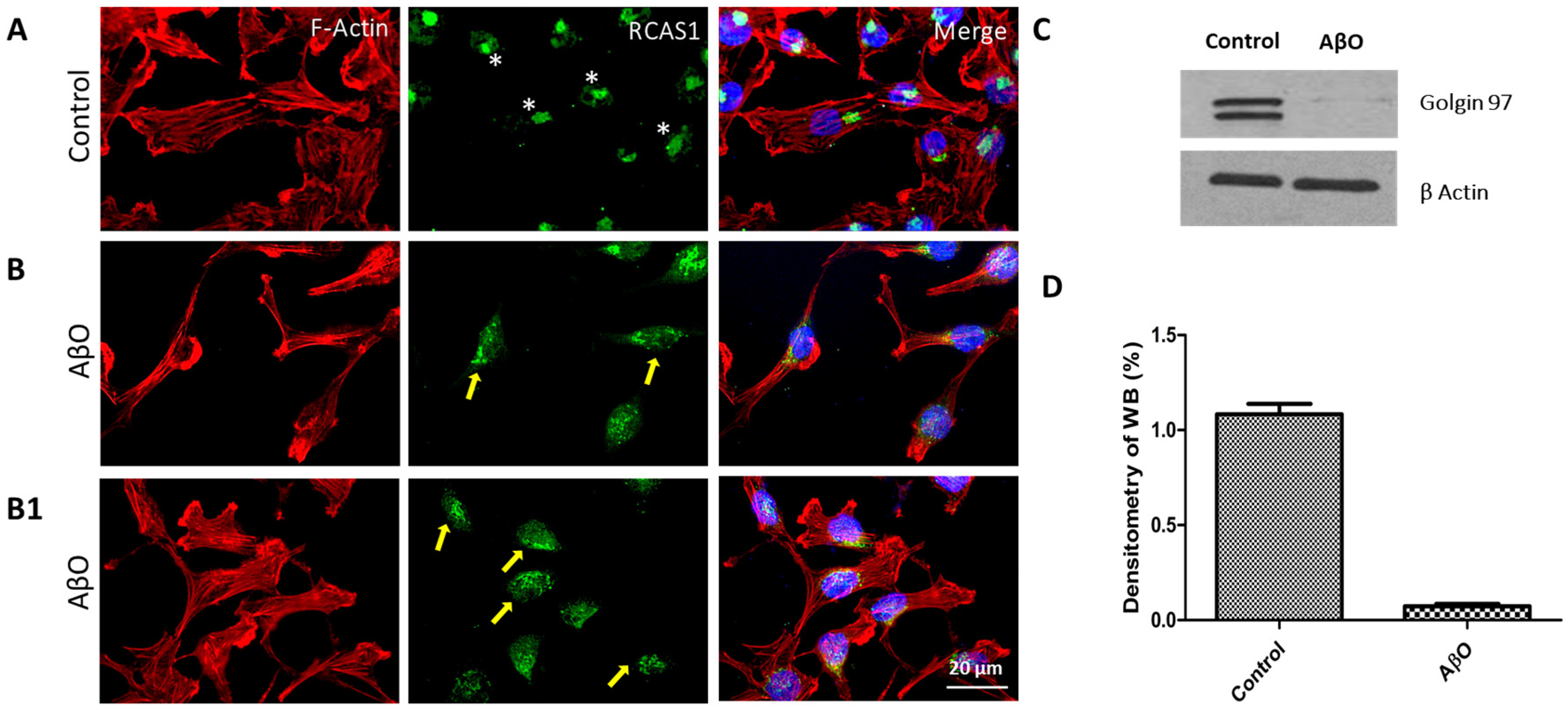
3.4. AβOs Induce Endoplasmic Reticulum and Golgi Apparatus Rearrangements in SH-SY5Y Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stakos, D.A.; Stamatelopoulos, K.; Bampatsias, D.; Sachse, M.; Zormpas, E.; Vlachogiannis, N.I.; Tual-Chalot, S.; Stellos, K. The alzheimer’s disease amyloid-beta hypothesis in cardiovascular aging and disease: Jacc focus seminar. J. Am. Coll. Cardiol. 2020, 75, 952–967. [Google Scholar] [CrossRef] [PubMed]
- Waigi, E.W.; Webb, R.C.; Moss, M.A.; Uline, M.J.; McCarthy, C.G.; Wenceslau, C.F. Soluble and insoluble protein aggregates, endoplasmic reticulum stress, and vascular dysfunction in alzheimer’s disease and cardiovascular diseases. Geroscience 2023, 45, 1411–1438. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The amyloid-beta pathway in alzheimer’s disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Niewiadomska, G.; Niewiadomski, W.; Steczkowska, M.; Gasiorowska, A. Tau oligomers neurotoxicity. Life 2021, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Vander Zanden, C.M.; Chi, E.Y. Passive immunotherapies targeting amyloid beta and tau oligomers in alzheimer’s disease. J. Pharm. Sci. 2020, 109, 68–73. [Google Scholar] [CrossRef]
- Gulisano, W.; Maugeri, D.; Baltrons, M.A.; Fa, M.; Amato, A.; Palmeri, A.; D’Adamio, L.; Grassi, C.; Devanand, D.P.; Honig, L.S.; et al. Role of amyloid-beta and tau proteins in alzheimer’s disease: Confuting the amyloid cascade. J. Alzheimers Dis. 2018, 64, S611–S631. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Mora, P.; Luna, R.; Colin-Barenque, L. Amyloid beta: Multiple mechanisms of toxicity and only some protective effects? Oxid. Med. Cell Longev. 2014, 2014, 795375. [Google Scholar] [CrossRef]
- Shankar, G.M.; Walsh, D.M. Alzheimer’s disease: Synaptic dysfunction and abeta. Mol. Neurodegener. 2009, 4, 48. [Google Scholar] [CrossRef]
- Larson, M.E.; Lesne, S.E. Soluble abeta oligomer production and toxicity. J. Neurochem. 2012, 120 (Suppl. S1), 125–139. [Google Scholar] [CrossRef]
- Nimmrich, V.; Ebert, U. Is alzheimer’s disease a result of presynaptic failure? Synaptic dysfunctions induced by oligomeric beta-amyloid. Rev. Neurosci. 2009, 20, 1–12. [Google Scholar] [CrossRef]
- Qu, X.; Yuan, F.N.; Corona, C.; Pasini, S.; Pero, M.E.; Gundersen, G.G.; Shelanski, M.L.; Bartolini, F. Stabilization of dynamic microtubules by mdia1 drives tau-dependent abeta1-42 synaptotoxicity. J. Cell Biol. 2017, 216, 3161–3178. [Google Scholar] [CrossRef] [PubMed]
- Paradis, E.; Douillard, H.; Koutroumanis, M.; Goodyer, C.; LeBlanc, A. Amyloid beta peptide of alzheimer’s disease downregulates bcl-2 and upregulates bax expression in human neurons. J. Neurosci. Off. J. Soc. Neurosci. 1996, 16, 7533–7539. [Google Scholar] [CrossRef] [PubMed]
- Manczak, M.; Anekonda, T.S.; Henson, E.; Park, B.S.; Quinn, J.; Reddy, P.H. Mitochondria are a direct site of a beta accumulation in alzheimer’s disease neurons: Implications for free radical generation and oxidative damage in disease progression. Hum. Mol. Genet. 2006, 15, 1437–1449. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H.; Manczak, M.; Mao, P.; Calkins, M.J.; Reddy, A.P.; Shirendeb, U. Amyloid-beta and mitochondria in aging and alzheimer’s disease: Implications for synaptic damage and cognitive decline. J. Alzheimer’s Dis. JAD 2010, 20 (Suppl. S2), S499–S512. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, A.L.; Giodini, A.; Cresswell, P. A role for the endoplasmic reticulum protein retrotranslocation machinery during crosspresentation by dendritic cells. Immunity 2006, 25, 607–617. [Google Scholar] [CrossRef]
- Stieber, A.; Mourelatos, Z.; Gonatas, N.K. In alzheimer’s disease the golgi apparatus of a population of neurons without neurofibrillary tangles is fragmented and atrophic. Am. J. Pathol. 1996, 148, 415–426. [Google Scholar]
- Ghanta, J.; Shen, C.L.; Kiessling, L.L.; Murphy, R.M. A strategy for designing inhibitors of beta-amyloid toxicity. J. Biol. Chem. 1996, 271, 29525–29528. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, M.G.; Palade, G.E. The golgi apparatus: 100 years of progress and controversy. Trends Cell Biol. 1998, 8, 2–10. [Google Scholar] [CrossRef]
- Klein, W.L. Abeta toxicity in alzheimer’s disease: Globular oligomers (addls) as new vaccine and drug targets. Neurochem. Int. 2002, 41, 345–352. [Google Scholar] [CrossRef]
- Resende, R.; Ferreiro, E.; Pereira, C.; Resende de Oliveira, C. Neurotoxic effect of oligomeric and fibrillar species of amyloid-beta peptide 1-42: Involvement of endoplasmic reticulum calcium release in oligomer-induced cell death. Neuroscience 2008, 155, 725–737. [Google Scholar] [CrossRef]
- Picone, P.; Carrotta, R.; Montana, G.; Nobile, M.R.; San Biagio, P.L.; Di Carlo, M. Abeta oligomers and fibrillar aggregates induce different apoptotic pathways in lan5 neuroblastoma cell cultures. Biophys. J. 2009, 96, 4200–4211. [Google Scholar] [CrossRef] [PubMed]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Tarozzi, A.; Merlicco, A.; Morroni, F.; Bolondi, C.; Di Iorio, P.; Ciccarelli, R.; Romano, S.; Giuliani, P.; Hrelia, P. Guanosine protects human neuroblastoma cells from oxidative stress and toxicity induced by amyloid-beta peptide oligomers. J. Biol. Regul. Homeost. Agents 2010, 24, 297–306. [Google Scholar]
- Liu, L.; Zhang, C.; Kalionis, B.; Wan, W.; Murthi, P.; Chen, C.; Li, Y.; Xia, S. Egb761 protects against abeta1-42 oligomer-induced cell damage via endoplasmic reticulum stress activation and hsp70 protein expression increase in sh-sy5y cells. Exp. Gerontol. 2016, 75, 56–63. [Google Scholar] [CrossRef]
- de Medeiros, L.M.; De Bastiani, M.A.; Rico, E.P.; Schonhofen, P.; Pfaffenseller, B.; Wollenhaupt-Aguiar, B.; Grun, L.; Barbe-Tuana, F.; Zimmer, E.R.; Castro, M.A.A.; et al. Cholinergic differentiation of human neuroblastoma sh-sy5y cell line and its potential use as an in vitro model for alzheimer’s disease studies. Mol. Neurobiol. 2019, 56, 7355–7367. [Google Scholar] [CrossRef] [PubMed]
- Gomes, G.M.; Dalmolin, G.D.; Bar, J.; Karpova, A.; Mello, C.F.; Kreutz, M.R.; Rubin, M.A. Inhibition of the polyamine system counteracts beta-amyloid peptide-induced memory impairment in mice: Involvement of extrasynaptic nmda receptors. PLoS ONE 2014, 9, e99184. [Google Scholar] [CrossRef][Green Version]
- Fabbretti, E.; Antognolli, G.; Tongiorgi, E. Amyloid-beta impairs dendritic trafficking of golgi-like organelles in the early phase preceding neurite atrophy: Rescue by mirtazapine. Front. Mol. Neurosci. 2021, 14, 661728. [Google Scholar] [CrossRef]
- SanMartin, C.D.; Veloso, P.; Adasme, T.; Lobos, P.; Bruna, B.; Galaz, J.; Garcia, A.; Hartel, S.; Hidalgo, C.; Paula-Lima, A.C. Ryr2-mediated Ca2+ release and mitochondrial ros generation partake in the synaptic dysfunction caused by amyloid beta peptide oligomers. Front. Mol. Neurosci. 2017, 10, 115. [Google Scholar] [CrossRef]
- Wang, L.; Cao, J.; Shi, Z.; Fan, W.; Liu, H.; Deng, J.; Deng, J. Experimental study on the neurotoxic effect of beta-amyloid on the cytoskeleton of pc12 cells. Int. J. Mol. Med. 2018, 41, 2764–2770. [Google Scholar]
- Pchitskaya, E.; Rakovskaya, A.; Chigray, M.; Bezprozvanny, I. Cytoskeleton protein eb3 contributes to dendritic spines enlargement and enhances their resilience to toxic effects of beta-amyloid. Int. J. Mol. Sci. 2022, 23, 2274. [Google Scholar] [CrossRef]
- Wu, J.C.; Liang, Z.Q.; Qin, Z.H. Quality control system of the endoplasmic reticulum and related diseases. Acta Biochim. Biophys. Sin. 2006, 38, 219–226. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siddiqi, M.K.; Malik, S.; Majid, N.; Alam, P.; Khan, R.H. Cytotoxic species in amyloid-associated diseases: Oligomers or mature fibrils. Adv. Protein Chem. Struct. Biol. 2019, 118, 333–369. [Google Scholar] [PubMed]
- Pimplikar, S.W. Reassessing the amyloid cascade hypothesis of alzheimer’s disease. Int. J. Biochem. Cell Biol. 2009, 41, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.P.; Clark, I.A.; Vissel, B. Inconsistencies and controversies surrounding the amyloid hypothesis of alzheimer’s disease. Acta Neuropathol. Commun. 2014, 2, 135. [Google Scholar] [CrossRef]
- Toledo, J.P.; Fernandez-Perez, E.J.; Ferreira, I.L.; Marinho, D.; Riffo-Lepe, N.O.; Pineda-Cuevas, B.N.; Pinochet-Pino, L.F.; Burgos, C.F.; Rego, A.C.; Aguayo, L.G. Boldine attenuates synaptic failure and mitochondrial deregulation in cellular models of alzheimer’s disease. Front. Neurosci. 2021, 15, 617821. [Google Scholar] [CrossRef]
- Rudrabhatla, P. Regulation of neuronal cytoskeletal protein phosphorylation in neurodegenerative diseases. J. Alzheimers Dis. 2014, 41, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Tang, D.; Lu, S.Y.; Jiang, Z.F. Endoplasmic reticulum stress as a novel neuronal mediator in alzheimer’s disease. Neurol. Res. 2015, 37, 366–374. [Google Scholar] [CrossRef]
- Narayan, P.; Krishnarjuna, B.; Vishwanathan, V.; Jagadeesh Kumar, D.; Babu, S.; Ramanathan, K.V.; Easwaran, K.R.; Nagendra, H.G.; Raghothama, S. Does aluminium bind to histidine? An nmr investigation of amyloid beta12 and amyloid beta16 fragments. Chem. Biol. Drug Des. 2013, 82, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.T.; Abner, E.L.; Schmitt, F.A.; Kryscio, R.J.; Jicha, G.A.; Santacruz, K.; Smith, C.D.; Patel, E.; Markesbery, W.R. Brains with medial temporal lobe neurofibrillary tangles but no neuritic amyloid plaques are a diagnostic dilemma but may have pathogenetic aspects distinct from alzheimer disease. J. Neuropathol. Exp. Neurol. 2009, 68, 774–784. [Google Scholar] [CrossRef]
- Malaplate-Armand, C.; Florent-Bechard, S.; Youssef, I.; Koziel, V.; Sponne, I.; Kriem, B.; Leininger-Muller, B.; Olivier, J.L.; Oster, T.; Pillot, T. Soluble oligomers of amyloid-beta peptide induce neuronal apoptosis by activating a cpla2-dependent sphingomyelinase-ceramide pathway. Neurobiol. Dis. 2006, 23, 178–189. [Google Scholar] [CrossRef]
- Monroy-Ramirez, H.C.; Basurto-Islas, G.; Mena, R.; Cisneros, B.; Binder, L.I.; Avila, J.; Garcia-Sierra, F. Alterations in the nuclear architecture produced by the overexpression of tau protein in neuroblastoma cells. J. Alzheimers Dis. 2013, 36, 503–520. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Neuropathological stageing of alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer’s disease is a synaptic failure. Science 2002, 298, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Munoz, M.J.; Gerson, J.; Castillo-Carranza, D.L. Tau oligomers: The toxic player at synapses in alzheimer’s disease. Front. Cell Neurosci. 2015, 9, 464. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.; Grutzendler, J.; Duff, K.; Gan, W.B. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat. Neurosci. 2004, 7, 1181–1183. [Google Scholar] [CrossRef] [PubMed]
- Ripoli, C.; Piacentini, R.; Riccardi, E.; Leone, L.; Li Puma, D.D.; Bitan, G.; Grassi, C. Effects of different amyloid beta-protein analogues on synaptic function. Neurobiol. Aging 2013, 34, 1032–1044. [Google Scholar] [CrossRef]
- Lasagna-Reeves, C.A.; Castillo-Carranza, D.L.; Sengupta, U.; Clos, A.L.; Jackson, G.R.; Kayed, R. Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild-type mice. Mol. Neurodegener. 2011, 6, 39. [Google Scholar] [CrossRef]
- Fa, M.; Puzzo, D.; Piacentini, R.; Staniszewski, A.; Zhang, H.; Baltrons, M.A.; Li Puma, D.D.; Chatterjee, I.; Li, J.; Saeed, F.; et al. Extracellular tau oligomers produce an immediate impairment of ltp and memory. Sci. Rep. 2016, 6, 19393. [Google Scholar] [CrossRef]
- Calingasan, N.Y.; Chen, J.; Kiaei, M.; Beal, M.F. Beta-amyloid 42 accumulation in the lumbar spinal cord motor neurons of amyotrophic lateral sclerosis patients. Neurobiol. Dis. 2005, 19, 340–347. [Google Scholar] [CrossRef]
- Green, K.N.; Steffan, J.S.; Martinez-Coria, H.; Sun, X.; Schreiber, S.S.; Thompson, L.M.; LaFerla, F.M. Nicotinamide restores cognition in alzheimer’s disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of thr231-phosphotau. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 11500–11510. [Google Scholar] [CrossRef]
- Pianu, B.; Lefort, R.; Thuiliere, L.; Tabourier, E.; Bartolini, F. The abeta1-42 peptide regulates microtubule stability independently of tau. J. Cell Sci. 2014, 127, 1117–1127. [Google Scholar] [PubMed]
- Torres-Cruz, F.M.; Rodriguez-Cruz, F.; Escobar-Herrera, J.; Barragan-Andrade, N.; Basurto-Islas, G.; Ripova, D.; Avila, J.; Garcia-Sierra, F. Expression of tau produces aberrant plasma membrane blebbing in glial cells through rhoa-rock-dependent f-actin remodeling. J. Alzheimers Dis. 2016, 52, 463–482. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyama, Y.; Zhang, B.; Bruce, J.; Trojanowski, J.Q.; Lee, V.M. Reduction of detyrosinated microtubules and golgi fragmentation are linked to tau-induced degeneration in astrocytes. J. Neurosci. 2003, 23, 10662–10671. [Google Scholar] [CrossRef] [PubMed]
- Chesarone, M.A.; DuPage, A.G.; Goode, B.L. Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat. Rev. Mol. Cell Biol. 2010, 11, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Yamana, N.; Arakawa, Y.; Nishino, T.; Kurokawa, K.; Tanji, M.; Itoh, R.E.; Monypenny, J.; Ishizaki, T.; Bito, H.; Nozaki, K.; et al. The rho-mdia1 pathway regulates cell polarity and focal adhesion turnover in migrating cells through mobilizing apc and c-src. Mol. Cell. Biol. 2006, 26, 6844–6858. [Google Scholar] [CrossRef]
- Hotulainen, P.; Llano, O.; Smirnov, S.; Tanhuanpaa, K.; Faix, J.; Rivera, C.; Lappalainen, P. Defining mechanisms of actin polymerization and depolymerization during dendritic spine morphogenesis. J. Cell Biol. 2009, 185, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Huang, J.; Balasubramanian, M.K. The yeast actin cytoskeleton. FEMS Microbiol. Rev. 2014, 38, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.L.; Yang, F.; Calon, F.; Ubeda, O.J.; Hansen, J.E.; Weisbart, R.H.; Beech, W.; Frautschy, S.A.; Cole, G.M. P21-activated kinase-aberrant activation and translocation in alzheimer disease pathogenesis. J. Biol. Chem. 2008, 283, 14132–14143. [Google Scholar] [PubMed]
- Nam, K.N.; Mounier, A.; Wolfe, C.M.; Fitz, N.F.; Carter, A.Y.; Castranio, E.L.; Kamboh, H.I.; Reeves, V.L.; Wang, J.; Han, X.; et al. Effect of high fat diet on phenotype, brain transcriptome and lipidome in alzheimer’s model mice. Sci. Rep. 2017, 7, 4307. [Google Scholar] [CrossRef]
- Popugaeva, E.; Bezprozvanny, I. Role of endoplasmic reticulum ca2+ signaling in the pathogenesis of alzheimer disease. Front. Mol. Neurosci. 2013, 6, 29. [Google Scholar] [CrossRef]
- Saito, T.; Matsuba, Y.; Mihira, N.; Takano, J.; Nilsson, P.; Itohara, S.; Iwata, N.; Saido, T.C. Single app knock-in mouse models of alzheimer’s disease. Nat. Neurosci. 2014, 17, 661–663. [Google Scholar] [PubMed]
- Aihara, Y.; Inoue, T.; Tashiro, T.; Okamoto, K.; Komiya, Y.; Mikoshiba, K. Movement of endoplasmic reticulum in the living axon is distinct from other membranous vesicles in its rate, form, and sensitivity to microtubule inhibitors. J. Neurosci. Res. 2001, 65, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Bannai, H.; Inoue, T.; Nakayama, T.; Hattori, M.; Mikoshiba, K. Kinesin dependent, rapid, bi-directional transport of er sub-compartment in dendrites of hippocampal neurons. J. Cell Sci. 2004, 117, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, N.; Talwar, P.; Parimisetty, A.; Lefebvre d’Hellencourt, C.; Ravanan, P. A molecular web: Endoplasmic reticulum stress, inflammation, and oxidative stress. Front. Cell Neurosci. 2014, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Mollereau, B. Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat. Rev. Neurosci. 2014, 15, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Chiti, F.; Dobson, C.M. Protein misfolding, amyloid formation, and human disease: A summary of progress over the last decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, U.; Nilson, A.N.; Kayed, R. The role of amyloid-beta oligomers in toxicity, propagation, and immunotherapy. EBioMedicine 2016, 6, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, T.; Bieger, S.C.; Bruhl, B.; Tienari, P.J.; Ida, N.; Allsop, D.; Roberts, G.W.; Masters, C.L.; Dotti, C.G.; Unsicker, K.; et al. Distinct sites of intracellular production for alzheimer’s disease a beta40/42 amyloid peptides. Nat. Med. 1997, 3, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.S.; Hong, H.; Kim, C.; Mook-Jung, I. Acute er stress regulates amyloid precursor protein processing through ubiquitin-dependent degradation. Sci. Rep. 2015, 5, 8805. [Google Scholar] [CrossRef]
- Sakono, M.; Zako, T. Amyloid oligomers: Formation and toxicity of abeta oligomers. FEBS J. 2010, 277, 1348–1358. [Google Scholar] [CrossRef]
- Gorbatyuk, M.S.; Gorbatyuk, O.S. The molecular chaperone grp78/bip as a therapeutic target for neurodegenerative disorders: A mini review. J. Genet. Syndr. Gene Ther. 2013, 4, 128. [Google Scholar] [CrossRef] [PubMed]
- Nagele, R.G.; D’Andrea, M.R.; Anderson, W.J.; Wang, H.Y. Intracellular accumulation of beta-amyloid1-42 in neurons is facilitated by the alpha 7 nicotinic acetylcholine receptor in alzheimer’s disease. Neuroscience 2002, 110, 199–211. [Google Scholar] [CrossRef]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid-beta protein dimers isolated directly from alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef]
- Musiek, E.S.; Holtzman, D.M. Three dimensions of the amyloid hypothesis: Time, space and ‘wingmen’. Nat. Neurosci. 2015, 18, 800–806. [Google Scholar] [CrossRef]
- Lee, S.J.; Nam, E.; Lee, H.J.; Savelieff, M.G.; Lim, M.H. Towards an understanding of amyloid-beta oligomers: Characterization, toxicity mechanisms, and inhibitors. Chem. Soc. Rev. 2017, 46, 310–323. [Google Scholar] [CrossRef]
- Ferreira, I.L.; Ferreiro, E.; Schmidt, J.; Cardoso, J.M.; Pereira, C.M.; Carvalho, A.L.; Oliveira, C.R.; Rego, A.C. Abeta and nmdar activation cause mitochondrial dysfunction involving er calcium release. Neurobiol. Aging 2015, 36, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Stefani, I.C.; Wright, D.; Polizzi, K.M.; Kontoravdi, C. The role of er stress-induced apoptosis in neurodegeneration. Curr. Alzheimer Res. 2012, 9, 373–387. [Google Scholar] [CrossRef]
- Pannaccione, A.; Secondo, A.; Molinaro, P.; D’Avanzo, C.; Cantile, M.; Esposito, A.; Boscia, F.; Scorziello, A.; Sirabella, R.; Sokolow, S.; et al. A new concept: Abeta1-42 generates a hyperfunctional proteolytic ncx3 fragment that delays caspase-12 activation and neuronal death. J. Neurosci. 2012, 32, 10609–10617. [Google Scholar] [CrossRef] [PubMed]
- Mota, S.I.; Ferreira, I.L.; Pereira, C.; Oliveira, C.R.; Rego, A.C. Amyloid-beta peptide 1-42 causes microtubule deregulation through n-methyl-d-aspartate receptors in mature hippocampal cultures. Curr. Alzheimer Res. 2012, 9, 844–856. [Google Scholar] [CrossRef]
- Merriam, E.B.; Millette, M.; Lumbard, D.C.; Saengsawang, W.; Fothergill, T.; Hu, X.; Ferhat, L.; Dent, E.W. Synaptic regulation of microtubule dynamics in dendritic spines by calcium, f-actin, and drebrin. J. Neurosci. 2013, 33, 16471–16482. [Google Scholar] [CrossRef]
- Sergeeva, M.; Ubl, J.J.; Reiser, G. Disruption of actin cytoskeleton in cultured rat astrocytes suppresses atp- and bradykinin-induced [Ca2+]i oscillations by reducing the coupling efficiency between Ca2+ release, capacitative Ca2+ entry, and store refilling. Neuroscience 2000, 97, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, S.; Zhang, Q.; Shao, W.; Han, X.; Wang, Y.; Du, Y. Endoplasmic reticulum stress mediates jnk-dependent irs-1 serine phosphorylation and results in tau hyperphosphorylation in amyloid beta oligomer-treated pc12 cells and primary neurons. Gene 2016, 587, 183–193. [Google Scholar] [CrossRef]
- Li, H.D.; Liu, W.X.; Michalak, M. Enhanced clathrin-dependent endocytosis in the absence of calnexin. PLoS ONE 2011, 6, e21678. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, T.; Qi, H.; Yap, M.C.; Tahbaz, N.; Milburn, L.A.; Lucchinetti, E.; Lou, P.H.; Zaugg, M.; LaPointe, P.G.; Mercier, P.; et al. The er chaperone calnexin controls mitochondrial positioning and respiration. Sci. Signal 2020, 13, eaax6660. [Google Scholar] [CrossRef] [PubMed]
- Paskevicius, T.; Farraj, R.A.; Michalak, M.; Agellon, L.B. Calnexin, more than just a molecular chaperone. Cells 2023, 12, 403. [Google Scholar] [CrossRef]
- Lakkaraju, A.K.; van der Goot, F.G. Calnexin controls the stat3-mediated transcriptional response to egf. Mol. Cell 2013, 51, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Chevet, E.; Wong, H.N.; Gerber, D.; Cochet, C.; Fazel, A.; Cameron, P.H.; Gushue, J.N.; Thomas, D.Y.; Bergeron, J.J. Phosphorylation by ck2 and mapk enhances calnexin association with ribosomes. EMBO J. 1999, 18, 3655–3666. [Google Scholar] [CrossRef]
- Cameron, P.H.; Chevet, E.; Pluquet, O.; Thomas, D.Y.; Bergeron, J.J. Calnexin phosphorylation attenuates the release of partially misfolded alpha1-antitrypsin to the secretory pathway. J. Biol. Chem. 2009, 284, 34570–34579. [Google Scholar] [CrossRef]
- Ontiveros-Torres, M.A.; Labra-Barrios, M.L.; Diaz-Cintra, S.; Aguilar-Vazquez, A.R.; Moreno-Campuzano, S.; Flores-Rodriguez, P.; Luna-Herrera, C.; Mena, R.; Perry, G.; Floran-Garduno, B.; et al. Fibrillar amyloid-beta accumulation triggers an inflammatory mechanism leading to hyperphosphorylation of the carboxyl-terminal end of tau polypeptide in the hippocampal formation of the 3xtg-ad transgenic mouse. J. Alzheimers Dis. 2016, 52, 243–269. [Google Scholar] [CrossRef]
- Troy, C.M.; Rabacchi, S.A.; Friedman, W.J.; Frappier, T.F.; Brown, K.; Shelanski, M.L. Caspase-2 mediates neuronal cell death induced by beta-amyloid. J. Neurosci. 2000, 20, 1386–1392. [Google Scholar] [CrossRef]
- Gamblin, T.C.; Chen, F.; Zambrano, A.; Abraha, A.; Lagalwar, S.; Guillozet, A.L.; Lu, M.; Fu, Y.; Garcia-Sierra, F.; LaPointe, N.; et al. Caspase cleavage of tau: Linking amyloid and neurofibrillary tangles in alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2003, 100, 10032–10037. [Google Scholar] [CrossRef] [PubMed]
- Ayala, I.; Colanzi, A. Alterations of golgi organization in alzheimer’s disease: A cause or a consequence? Tissue Cell 2017, 49, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Menarguez, J.A.; Tomas, M.; Martinez-Martinez, N.; Martinez-Alonso, E. Golgi fragmentation in neurodegenerative diseases: Is there a common cause? Cells 2019, 8, 748. [Google Scholar] [CrossRef] [PubMed]
- Jungk, L.; Franke, H.; Salameh, A.; Dhein, S. Golgi fragmentation in human patients with chronic atrial fibrillation: A new aspect of remodeling. Thorac. Cardiovasc. Surg. 2019, 67, 98–106. [Google Scholar] [CrossRef]
- Kinoshita, A.; Fukumoto, H.; Shah, T.; Whelan, C.M.; Irizarry, M.C.; Hyman, B.T. Demonstration by fret of bace interaction with the amyloid precursor protein at the cell surface and in early endosomes. J. Cell Sci. 2003, 116, 3339–3346. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.; Wang, Y. Golgi defects enhance app amyloidogenic processing in alzheimer’s disease. Bioessays 2015, 37, 240–247. [Google Scholar] [CrossRef]
- Anton-Fernandez, A.; Merchan-Rubira, J.; Avila, J.; Hernandez, F.; DeFelipe, J.; Munoz, A. Phospho-tau accumulation and structural alterations of the golgi apparatus of cortical pyramidal neurons in the p301s tauopathy mouse model. J. Alzheimers Dis. 2017, 60, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Baloyannis, S.J. Golgi apparatus and protein trafficking in alzheimer’s disease. J. Alzheimers Dis. 2014, 42 (Suppl. S3), S153–S162. [Google Scholar] [CrossRef]
- Cole, N.B.; Sciaky, N.; Marotta, A.; Song, J.; Lippincott-Schwartz, J. Golgi dispersal during microtubule disruption: Regeneration of golgi stacks at peripheral endoplasmic reticulum exit sites. Mol. Biol. Cell 1996, 7, 631–650. [Google Scholar] [CrossRef]
- Ballatore, C.; Lee, V.M.; Trojanowski, J.Q. Tau-mediated neurodegeneration in alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007, 8, 663–672. [Google Scholar] [CrossRef]
- Cohen, T.J.; Guo, J.L.; Hurtado, D.E.; Kwong, L.K.; Mills, I.P.; Trojanowski, J.Q.; Lee, V.M. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat. Commun. 2011, 2, 252. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Cruz, F.; Torres-Cruz, F.M.; Monroy-Ramirez, H.C.; Escobar-Herrera, J.; Basurto-Islas, G.; Avila, J.; Garcia-Sierra, F. Fragmentation of the golgi apparatus in neuroblastoma cells is associated with tau-induced ring-shaped microtubule bundles. J. Alzheimers Dis. 2018, 65, 1185–1207. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Tai, G.; Hong, W. Autoantigen golgin-97, an effector of arl1 gtpase, participates in traffic from the endosome to the trans-golgi network. Mol. Biol. Cell 2004, 15, 4426–4443. [Google Scholar] [CrossRef]
- Giannopoulos, P.F.; Joshi, Y.B.; Pratico, D. Novel lipid signaling pathways in alzheimer’s disease pathogenesis. Biochem. Pharmacol. 2014, 88, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Rabouille, C.; Watson, R.; Nilsson, T.; Hui, N.; Slusarewicz, P.; Kreis, T.E.; Warren, G. Characterization of a cis-golgi matrix protein, gm130. J. Cell Biol. 1995, 131, 1715–1726. [Google Scholar] [CrossRef] [PubMed]
- Marra, P.; Salvatore, L.; Mironov, A., Jr.; Di Campli, A.; Di Tullio, G.; Trucco, A.; Beznoussenko, G.; Mironov, A.; De Matteis, M.A. The biogenesis of the golgi ribbon: The roles of membrane input from the er and of gm130. Mol. Biol. Cell 2007, 18, 1595–1608. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.H.; de Pablo, Y.; Vincent, F.; Johnson, E.O.; Chavers, A.K.; Shah, K. Novel genetic tools reveal cdk5’s major role in golgi fragmentation in alzheimer’s disease. Mol. Biol. Cell 2008, 19, 3052–3069. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Li, X.; Zhu, X. The role of gm130 in nervous system diseases. Front. Neurol. 2021, 12, 743787. [Google Scholar] [CrossRef]
- Puthenveedu, M.A.; Bachert, C.; Puri, S.; Lanni, F.; Linstedt, A.D. Gm130 and grasp65-dependent lateral cisternal fusion allows uniform golgi-enzyme distribution. Nat. Cell Biol. 2006, 8, 238–248. [Google Scholar] [CrossRef]


| Antibody | Epitope | Host-Class | Procedure | Reference |
|---|---|---|---|---|
| Anti-β-Amyloid 1-42 | 1-42 aa | rabbit IgG | IC, WB | Abcam Inc., Waltham, MA, USA |
| α-Tubulin | α-Tubulin | mouse IgG | IC | Santa Cruz Biotechnology, Inc., Dallas, TX, USA |
| β-Actin | β-Actin | mouse IgG | WB | Santa Cruz Biotechnology, Inc., Dallas, TX, USA |
| RCAS 1 | Gly 147 | rabbit IgG | IC | Cell Signaling Technology, Danvers, MA, USA |
| Golgin-97 (CDF4) | Human Golgin-97 | mouse IgG | IC, WB | Invitrogen molecular probes, Eugene, OR, USA |
| Calnexin (C5C9) | Human Calnexin | rabbit IgG | IC, WB | Cell Signaling Technology, Danvers, MA, USA |
| Organelle | Fluorescent marker | Excitation/emission | Procedure | Reference |
| Nuclei | Hoechst-33258 | 350/461 | FL | Invitrogen Molecular Probes, Eugene, OR, USA |
| F-Actin cytoskeleton | Rhodamine–phalloidin | 540/565 | FL | Invitrogen Molecular Probes, OR, USA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarero-Basulto, J.J.; Gasca-Martínez, Y.; Rivera-Cervantes, M.C.; Gasca-Martínez, D.; Carrillo-González, N.J.; Beas-Zárate, C.; Gudiño-Cabrera, G. Cytotoxic Effect of Amyloid-β1-42 Oligomers on Endoplasmic Reticulum and Golgi Apparatus Arrangement in SH-SY5Y Neuroblastoma Cells. NeuroSci 2024, 5, 141-157. https://doi.org/10.3390/neurosci5020010
Jarero-Basulto JJ, Gasca-Martínez Y, Rivera-Cervantes MC, Gasca-Martínez D, Carrillo-González NJ, Beas-Zárate C, Gudiño-Cabrera G. Cytotoxic Effect of Amyloid-β1-42 Oligomers on Endoplasmic Reticulum and Golgi Apparatus Arrangement in SH-SY5Y Neuroblastoma Cells. NeuroSci. 2024; 5(2):141-157. https://doi.org/10.3390/neurosci5020010
Chicago/Turabian StyleJarero-Basulto, José J., Yadira Gasca-Martínez, Martha C. Rivera-Cervantes, Deisy Gasca-Martínez, Nidia Jannette Carrillo-González, Carlos Beas-Zárate, and Graciela Gudiño-Cabrera. 2024. "Cytotoxic Effect of Amyloid-β1-42 Oligomers on Endoplasmic Reticulum and Golgi Apparatus Arrangement in SH-SY5Y Neuroblastoma Cells" NeuroSci 5, no. 2: 141-157. https://doi.org/10.3390/neurosci5020010
APA StyleJarero-Basulto, J. J., Gasca-Martínez, Y., Rivera-Cervantes, M. C., Gasca-Martínez, D., Carrillo-González, N. J., Beas-Zárate, C., & Gudiño-Cabrera, G. (2024). Cytotoxic Effect of Amyloid-β1-42 Oligomers on Endoplasmic Reticulum and Golgi Apparatus Arrangement in SH-SY5Y Neuroblastoma Cells. NeuroSci, 5(2), 141-157. https://doi.org/10.3390/neurosci5020010






