Moving towards an Understanding of the Role of the Inferior Fronto-Occipital Fasciculus in Language Processing
Abstract
1. Introduction
1.1. Diffusion Tensor Imaging and White Matter Tractography
1.2. Structural Framework of the Inferior Frontal Occipital Fasciculus
1.3. Functional Framework of the Inferior Frontal Occipital Fasciculus
1.4. Hypothesis #1: Phonological vs. Semantic/Orthographic Processing
1.5. Hypothesis #2: Difficult vs. Non-Difficult Processing
1.6. Hypothesis #3: Automatic vs. Non-Automatic Processing
1.7. Aims of the Study
2. Materials and Methods
2.1. DTI Collection
2.2. DTI Analysis
2.3. Statistical Analysis
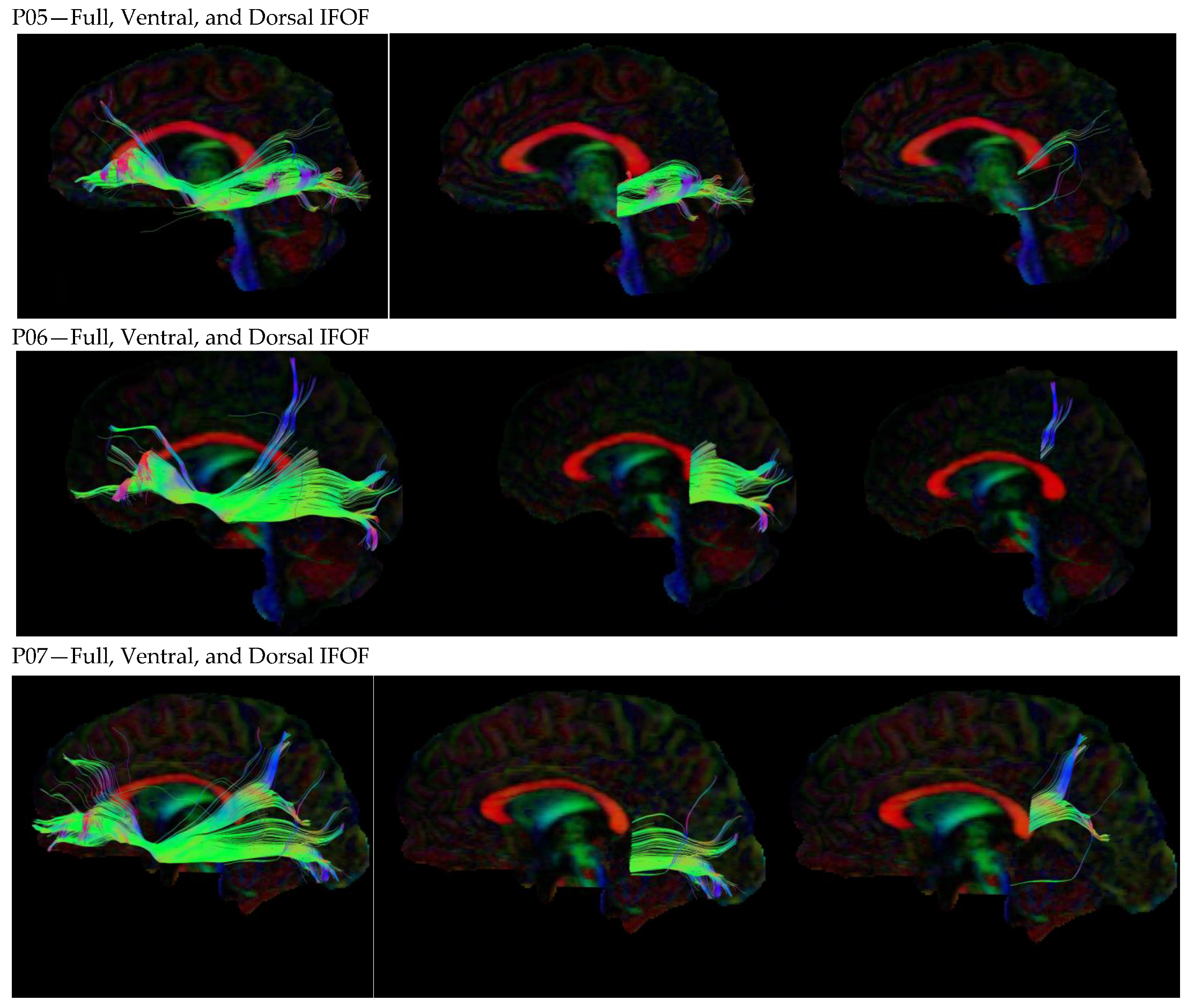
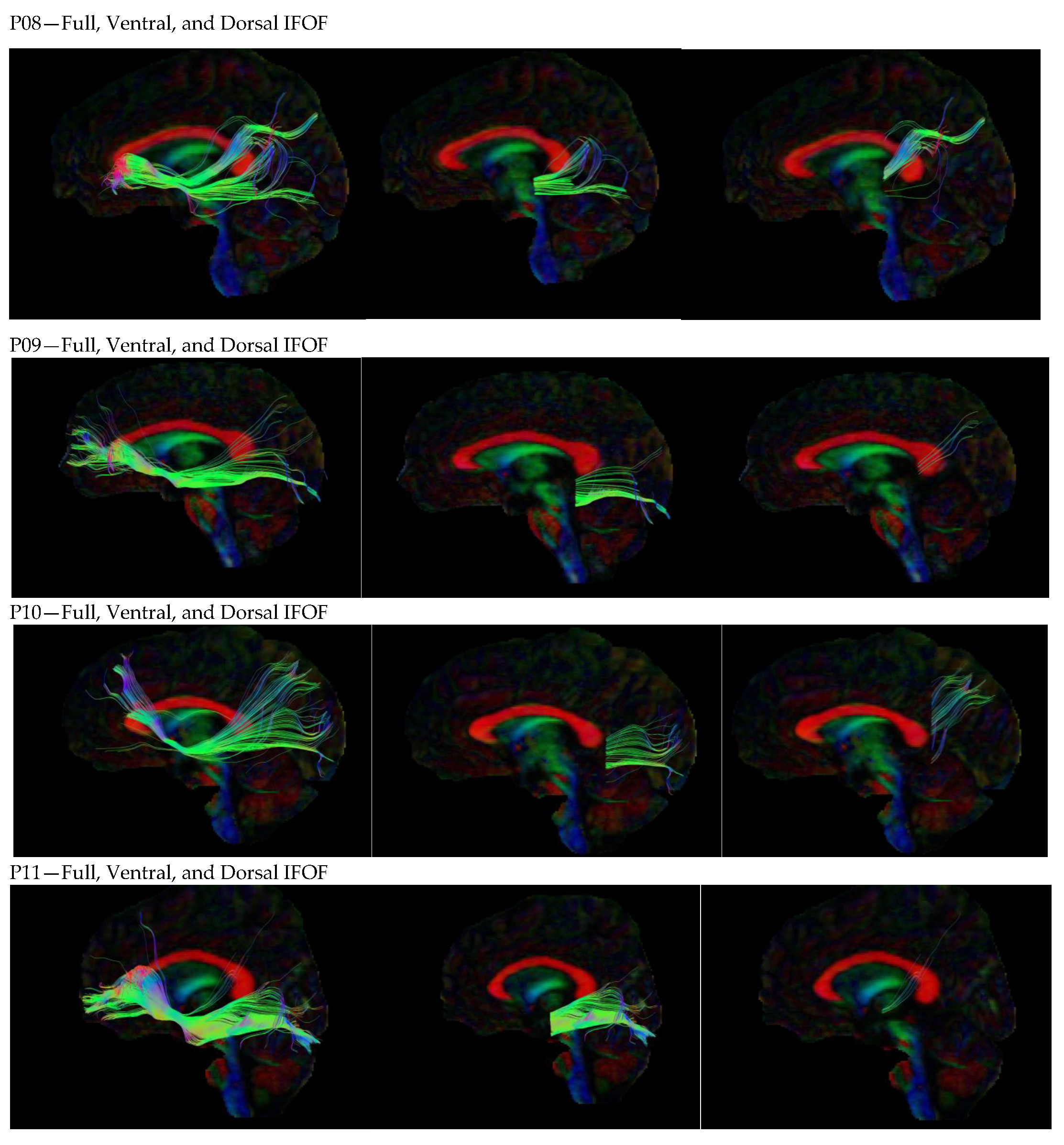
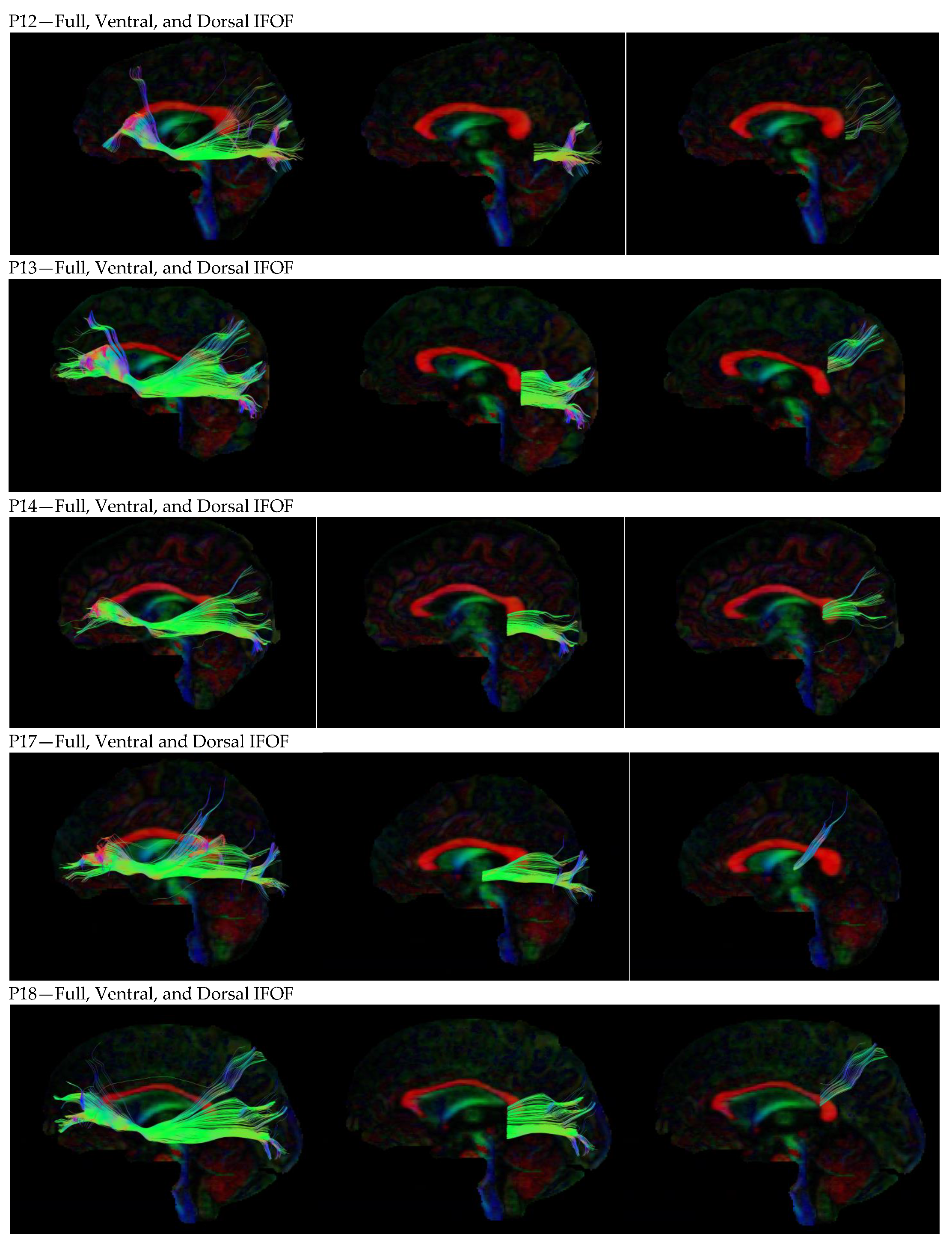
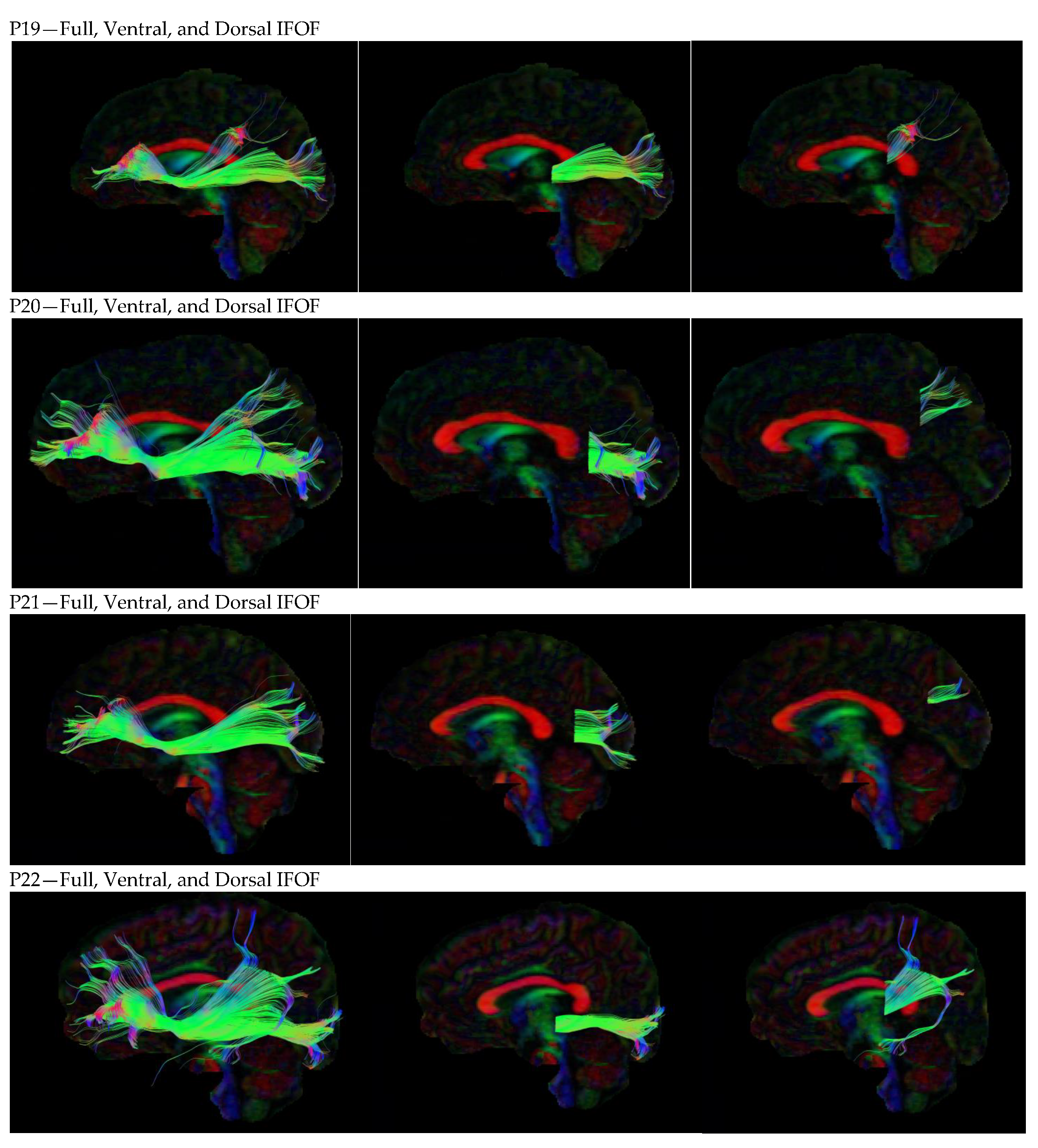
3. Results
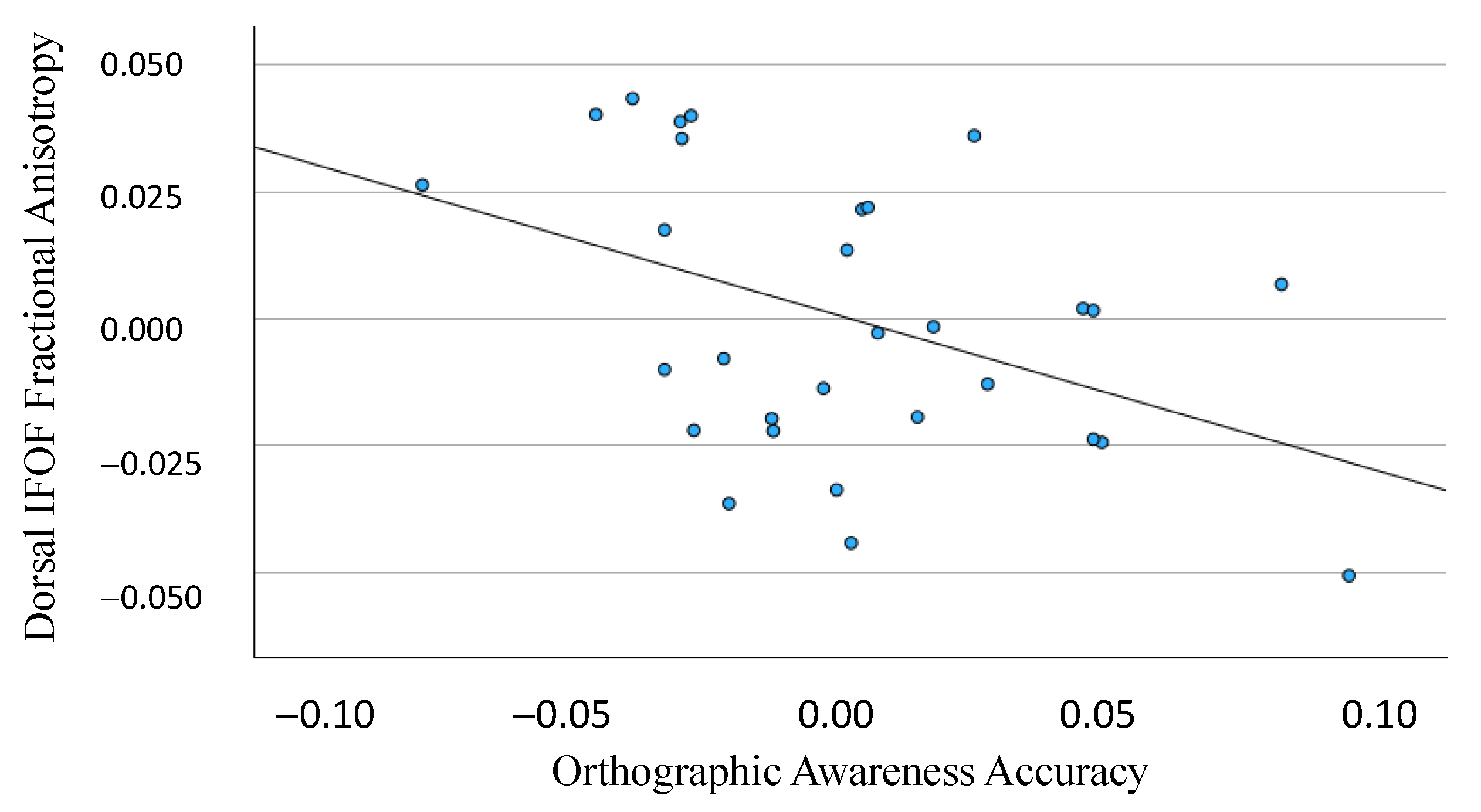
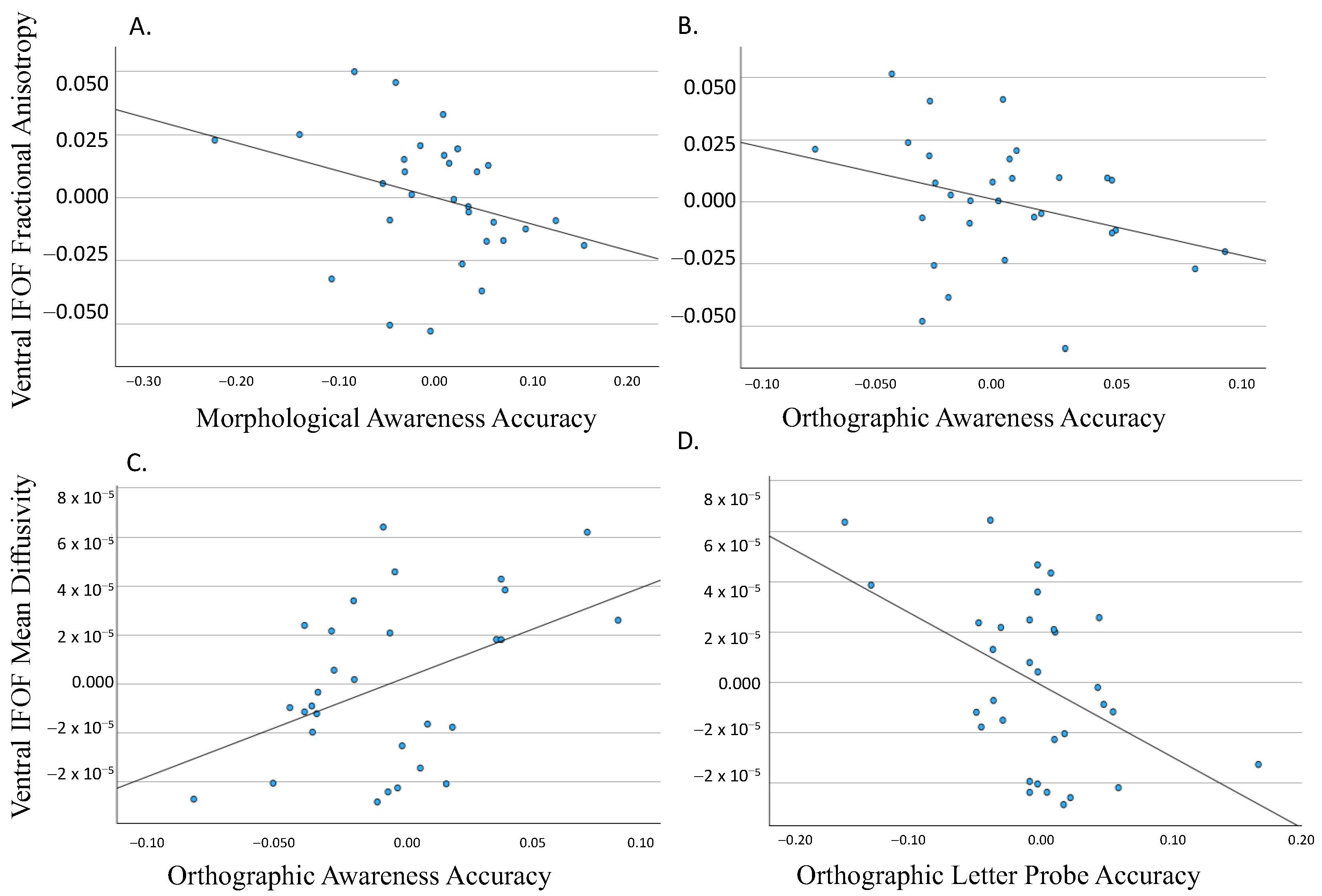
3.1. Dorsal IFOF
3.2. Ventral IFOF
4. Discussion
4.1. Functional Specificity of IFOF Segments
4.2. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
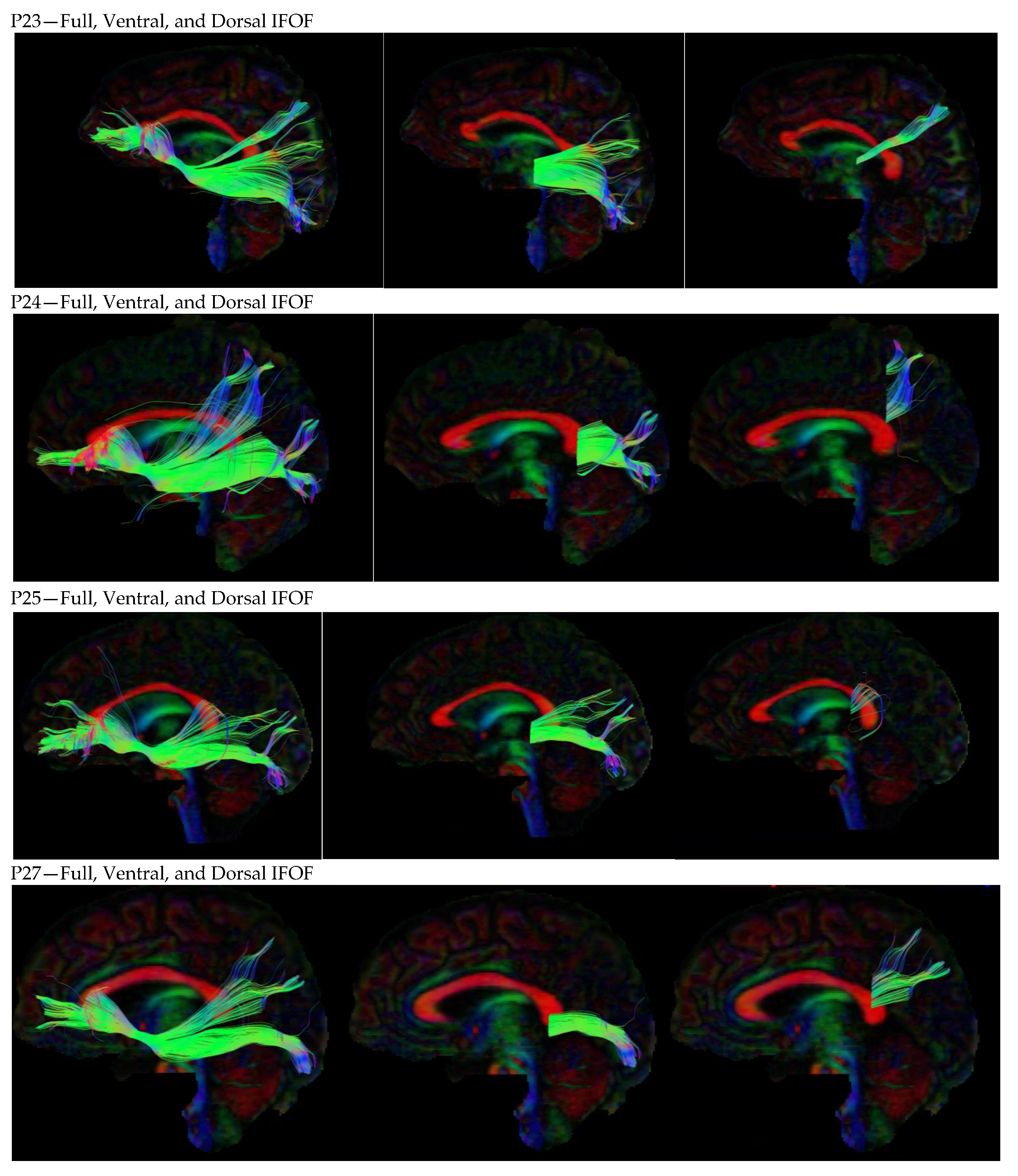
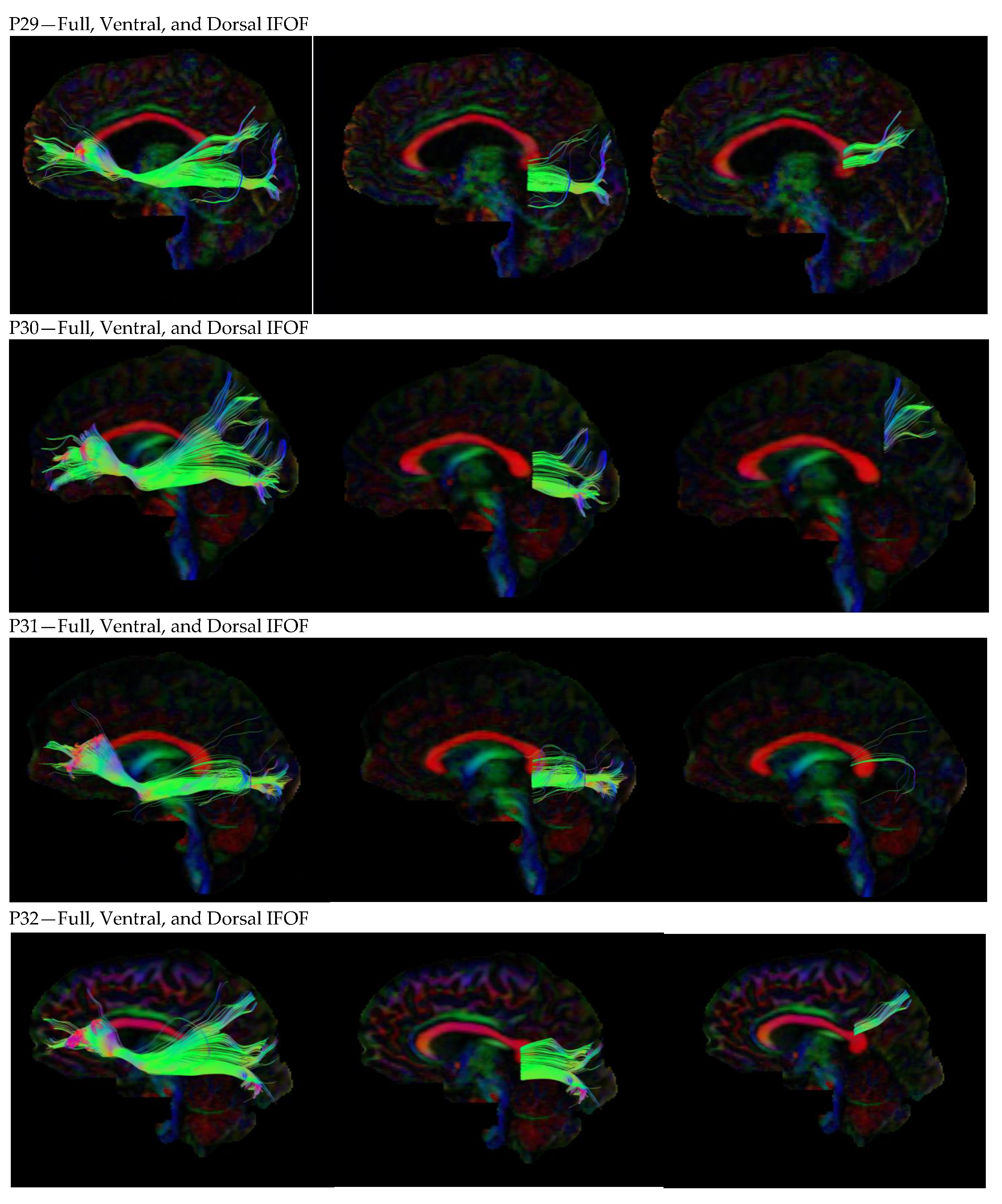
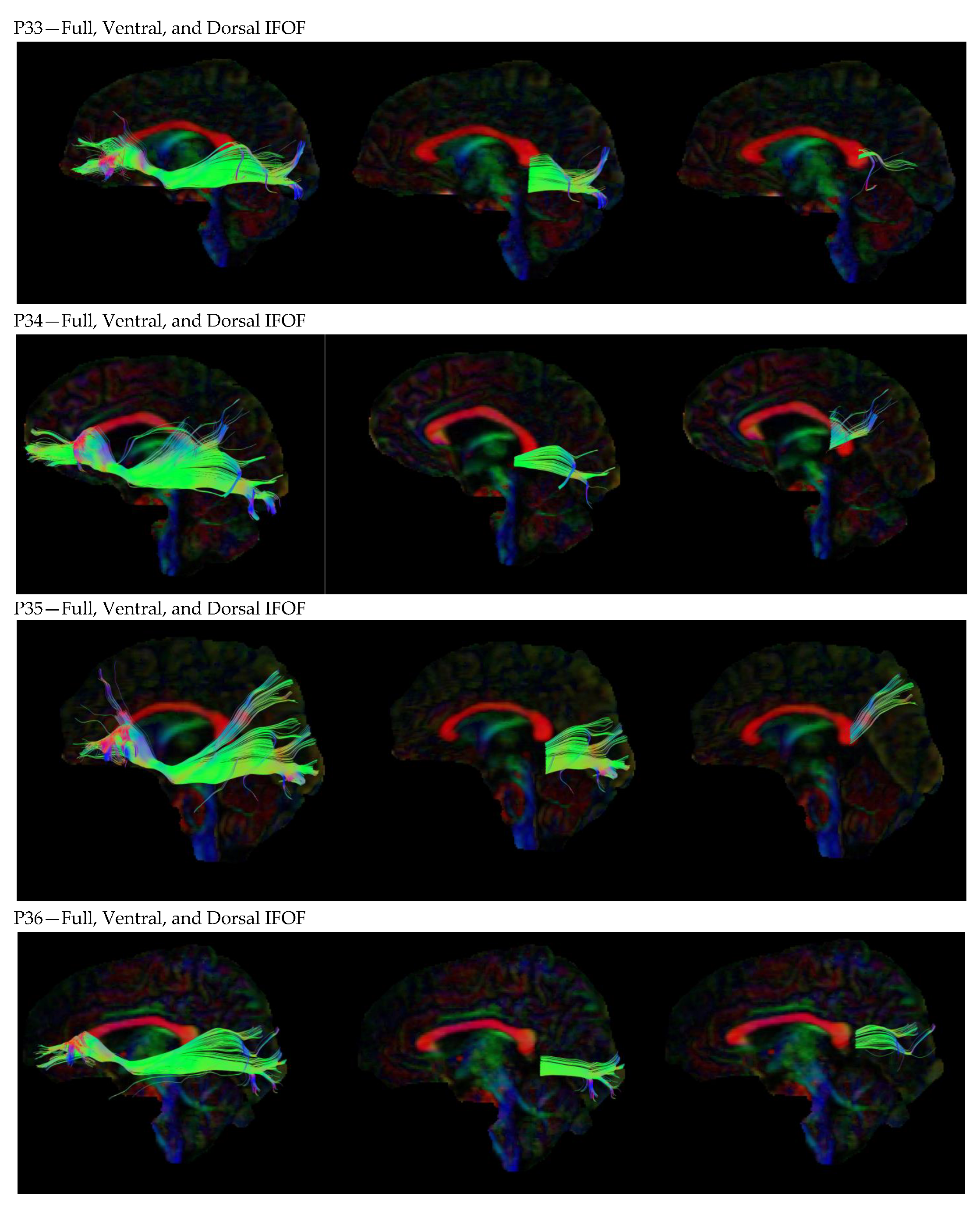
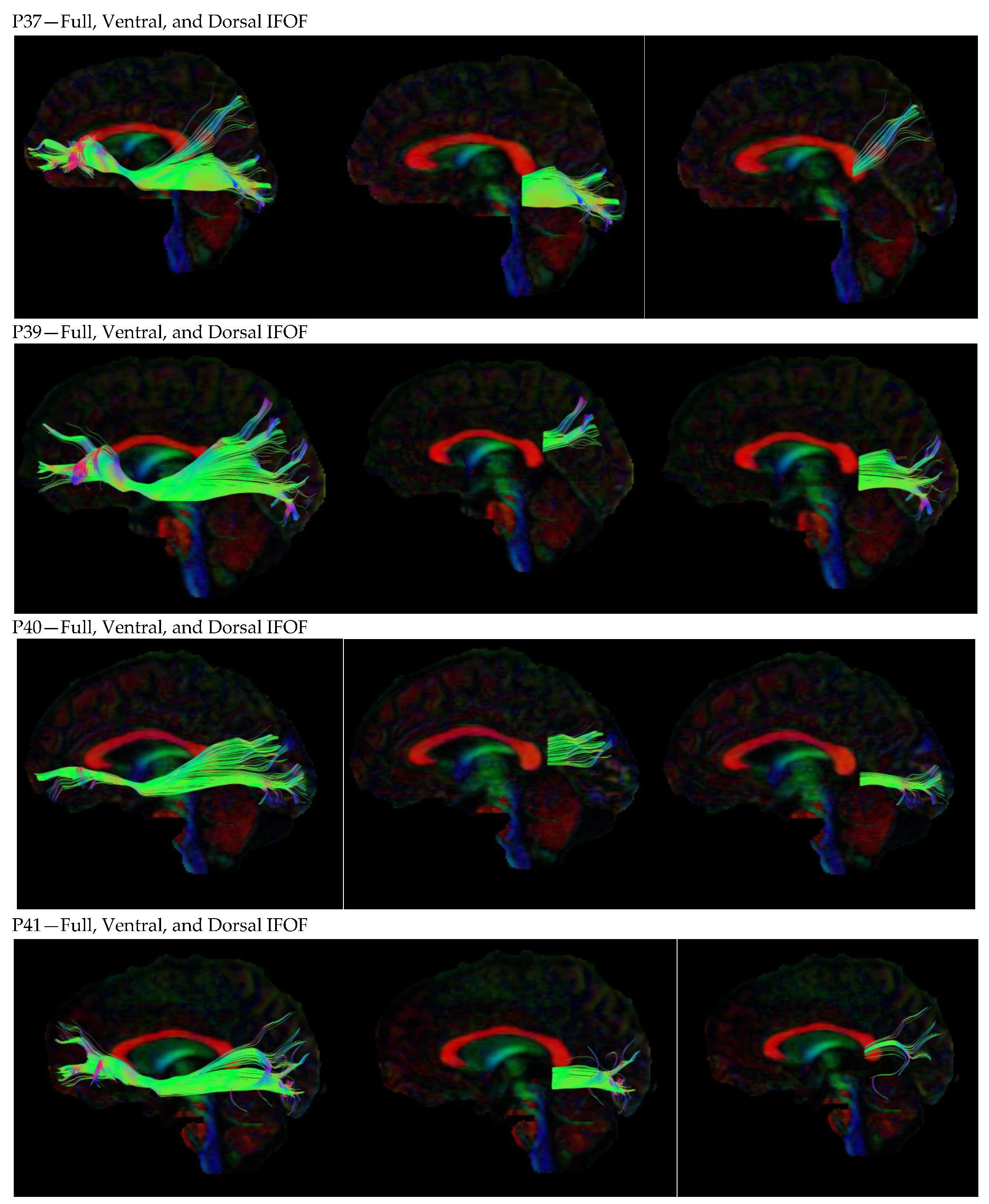
References
- Fields, R.D. White Matter Matters. Sci. Am. 2008, 298, 54–61. Available online: https://www.jstor.org/stable/pdf/26000517.pdf (accessed on 5 January 2022). [CrossRef]
- Beaulieu, C.; Plewes, C.; Paulson, L.A.; Roy, D.; Snook, L.; Concha, L.; Phillips, L. Imaging brain connectivity in children with diverse reading ability. NeuroImage 2005, 25, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Cheema, K.; Lantz, N.; Cummine, J. Exploring the role of subcortical structures in developmental reading impairments. NeuroReport 2018, 29, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Cheema, K.; Ostevik, A.V.; Westover, L.; Hodgetts, W.E.; Cummine, J. Resting-state networks and reading in adults with and without reading impairments. J. Neurolinguist. 2021, 60, 101016. [Google Scholar] [CrossRef]
- Cheema, K.; Sweneya, S.; Craig, J.; Huynh, T.; Ostevik, A.V.; Reed, A.; Cummine, J. An investigation of white matter properties as they relate to spelling behaviour in skilled and impaired readers. Neuropsychol. Rehabil. 2023, 33, 989–1017. [Google Scholar] [CrossRef] [PubMed]
- Cummine, J.; Dai, W.; Borowsky, R.; Gould, L.; Rollans, C.; Boliek, C. Investigating the ventral-lexical, dorsal-sublexical model of basic reading processes using diffusion tensor imaging. Brain Struct. Funct. 2013, 220, 445–455. [Google Scholar] [CrossRef]
- Rollans, C.; Cheema, K.; Georgiou, G.K.; Cummine, J. Pathways of the inferior frontal occipital fasciculus in overt speech and reading. Neuroscience 2017, 364, 93–106. [Google Scholar] [CrossRef]
- Rollans, C.; Cummine, J. One tract, two tract, old tract, new tract: A pilot study of the structural and functional differentiation of the inferior fronto-occipital fasciculus. J. Neurolinguist. 2018, 46, 122–137. [Google Scholar] [CrossRef]
- Beaulieu, C. The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed. 2002, 15, 435–455. [Google Scholar] [CrossRef]
- Hunt, R.H.; Thomas, K.M. Magnetic resonance imaging methods in developmental science: A primer. Dev. Psychopathol. 2008, 20, 1029–1051. [Google Scholar] [CrossRef]
- Deprez, S.; Billiet, T.; Sunaert, S.; Leemans, A. Diffusion tensor MRI of chemotherapy-induced cognitive impairment in non-CNS cancer patients: A review. Brain Imaging Behav. 2013, 7, 409–435. [Google Scholar] [CrossRef] [PubMed]
- Martino, J.; Brogna, C.; Robles, S.G.; Vergani, F.; Duffau, H. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex 2010, 46, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Hau, J.; Sarubbo, S.; Perchey, G.; Crivello, F.; Zago, L.; Mellet, E.; Jobard, G.; Joliot, M.; Mazoyer, B.M.; Tzourio-Mazoyer, N.; et al. Cortical Terminations of the Inferior Fronto-Occipital and Uncinate Fasciculi: Anatomical Stem-Based Virtual Dissection. Front. Neuroanat. 2016, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Sun, D.; Wang, Y.; Wang, Y. Subcomponents and Connectivity of the Inferior Fronto-Occipital Fasciculus Revealed by Diffusion Spectrum Imaging Fiber Tracking. Front. Neuroanat. 2016, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Caverzasi, E.; Papinutto, N.; Amirbekian, B.; Berger, M.S.; Henry, R.G. Q-Ball of Inferior Fronto-Occipital Fasciculus and Beyond. PLoS ONE 2014, 9, e100274. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, S.; De Benedictis, A.; Maldonado, I.L.; Basso, G.; Duffau, H. Frontal terminations for the inferior fronto-occipital fascicle: Anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain Struct. Funct. 2011, 218, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Conner, A.K.; Briggs, R.G.; Sali, G.; Rahimi, M.; Baker, C.M.; Burks, J.D.; Glenn, C.A.; Battiste, J.D.; Sughrue, M.E. A connectomic atlas of the human cerebrum—Chapter 13: Tractographic description of the inferior fronto-occipital fasciculus. Oper. Neurosurg. 2018, 15 (Suppl. S1), S436. [Google Scholar] [CrossRef]
- Jobard, G.; Crivello, F.; Tzourio-Mazoyer, N. Evaluation of the dual route theory of reading: A metanalysis of 35 neuroimaging studies. NeuroImage 2003, 20, 693–712. [Google Scholar] [CrossRef]
- Klingberg, T.; Hedehus, M.; Temple, E.; Salz, T.; Gabrieli, J.D.E.; Moseley, M.E.; Poldrack, R.A. Microstructure of Temporo-Parietal White Matter as a Basis for Reading Ability. Neuron 2000, 25, 493–500. [Google Scholar] [CrossRef]
- Steinbrink, C.; Vogt, K.; Kastrup, A.; Müller, H.-P.; Juengling, F.D.; Kassubek, J.; Riecker, A. The contribution of white and gray matter differences to developmental dyslexia: Insights from DTI and VBM at 3.0T. Neuropsychologia 2008, 46, 3170–3178. [Google Scholar] [CrossRef]
- Saur, D.; Kreher, B.W.; Schnell, S.; Kummerer, D.; Kellmeyer, P.; Vry, M.-S.; Umarova, R.; Musso, M.; Glauche, V.; Abel, S.; et al. Ventral and dorsal pathways for language. Proc. Natl. Acad. Sci. USA 2008, 105, 18035–18040. [Google Scholar] [CrossRef] [PubMed]
- Dick, A.S.; Tremblay, P. Beyond the arcuate fasciculus: Consensus and controversy in the connectional anatomy of language. Brain 2012, 135, 3529–3550. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Barroso, D.; de Diego-Balaguer, R.; Cunillera, T.; Camara, E.; Münte, T.F.; Rodriguez-Fornells, A. Language Learning under Working Memory Constraints Correlates with Microstructural Differences in the Ventral Language Pathway. Cereb. Cortex 2011, 21, 2742–2750. [Google Scholar] [CrossRef] [PubMed]
- Brauer, J.; Anwander, A.; Perani, D.; Friederici, A.D. Dorsal and ventral pathways in language development. Brain Lang. 2013, 127, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Brauer, J.; Anwander, A.; Friederici, A.D. Neuroanatomical prerequisites for language functions in the maturing brain. Cereb. Cortex 2011, 21, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Pustina, D.; Doucet, G.; Evans, J.; Sharan, A.; Sperling, M.; Skidmore, C.; Tracy, J. Distinct Types of White Matter Changes Are Observed after Anterior Temporal Lobectomy in Epilepsy. PLoS ONE 2014, 9, e104211. [Google Scholar] [CrossRef] [PubMed]
- Yogarajah, M.; Focke, N.K.; Bonelli, S.B.; Thompson, P.; Vollmar, C.; McEvoy, A.W.; Alexander, D.C.; Symms, M.R.; Koepp, M.J.; Duncan, J.S. The structural plasticity of white matter networks following anterior temporal lobe resection. Brain 2010, 133, 2348–2364. [Google Scholar] [CrossRef]
- Dávolos, J.M.; Arias, J.C.; Jefferies, E. Linking individual differences in semantic cognition to white matter microstructure. Neuropsychologia 2020, 141, 107438. [Google Scholar] [CrossRef]
- Byrd, C.T.; McGill, M.; Usler, E. Nonword repetition and phoneme elision in adults who do and do not stutter: Vocal versus nonvocal performance differences. J. Fluen. Disord. 2015, 44, 17–31. [Google Scholar] [CrossRef]
- Siegel, L.S.; Share, D.; Geva, E. Evidence for superior orthographic skills in dyslexics. Psychol. Sci. 1995, 6, 250–254. [Google Scholar] [CrossRef]
- Apel, K.; Henbest, V.S.; Masterson, J. Orthographic knowledge: Clarifications, challenges, and future directions. Read. Writ. 2019, 32, 873–889. [Google Scholar] [CrossRef]
- Hsieh, L.; Rapp, B. Functional magnetic resonance imaging of the cognitive components of the spelling process. Brain Lang. 2004, 91, 40–41. [Google Scholar] [CrossRef]
- Cheema, K.; Fleming, C.; Craig, J.; Hodgetts, W.E.; Cummine, J. Reading and spelling profiles of adult poor readers: Phonological, orthographic and morphological considerations. Dyslexia 2023, 29, 58–77. [Google Scholar] [CrossRef] [PubMed]
- Cheema, K.; Hodgetts, W.E.; Cummine, J. Is the letter ‘t’in the word ‘gourmet’? Disruption in task-evoked connectivity networks in adults with impaired literacy skills. NeuroSci 2021, 2, 75–94. [Google Scholar] [CrossRef]
- Torgeson, J.K.; Wagner, R.K.; Rashotte, C.A. Test of Word Reading Efficiency; Pro-Ed: Austin, TX, USA, 1999. [Google Scholar]
- Balota, D.A.; Yap, M.J.; Cortese, M.J.; Hutchison, K.A.; Kessler, B.; Loftis, B.; Neely, J.H.; Nelson, D.L.; Simpson, G.B.; Treiman, R. The English Lexicon Project. Behav. Res. Methods 2007, 39, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Leemans, A.; Jeurissen, B.; Sijbers, J.; Jones, D.K. ExploreDTI: A graphical toolbox for processing, analyzing, and visualizing diffusion MR data. Proc. Intl. Soc. Mag. Reason. Med. 2009, 17, 3537. [Google Scholar]
- Wakana, S.; Caprihan, A.; Panzenboeck, M.M.; Fallon, J.H.; Perry, M.; Gollub, R.L.; Hua, K.; Zhang, J.; Jiang, H.; Dubey, P.; et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage 2007, 36, 630–644. [Google Scholar] [CrossRef] [PubMed]
- IBM Corp. Released IBM SPSS Statistics for MacOS, Version 27; IBM Corp.: Armonk, NY, USA, 2020. [Google Scholar]
- Vandermosten, M.; Boets, B.; Poelmans, H.; Sunaert, S.; Wouters, J.; Ghesquiere, P. A tractography study in dyslexia: Neuroanatomic correlates of orthographic, phonological and speech processing. Brain 2012, 135, 935–948. [Google Scholar] [CrossRef]
- Yeatman, J.D.; Dougherty, R.F.; Rykhlevskaia, E.; Sherbondy, A.J.; Deutsch, G.K.; Wandell, B.A.; Ben-Shachar, M. Anatomical Properties of the Arcuate Fasciculus Predict Phonological and Reading Skills in Children. J. Cogn. Neurosci. 2011, 23, 3304–3317. [Google Scholar] [CrossRef]
- Sierpowska, J.; Gabarrós, A.; Fernandez-Coello, A.; Camins, À.; Castañer, S.; Juncadella, M.; Morís, J.; Rodríguez-Fornells, A. Words are not enough: Nonword repetition as an indicator of arcuate fasciculus integrity during brain tumor resection. J. Neurosurg. 2017, 126, 435–445. [Google Scholar] [CrossRef]
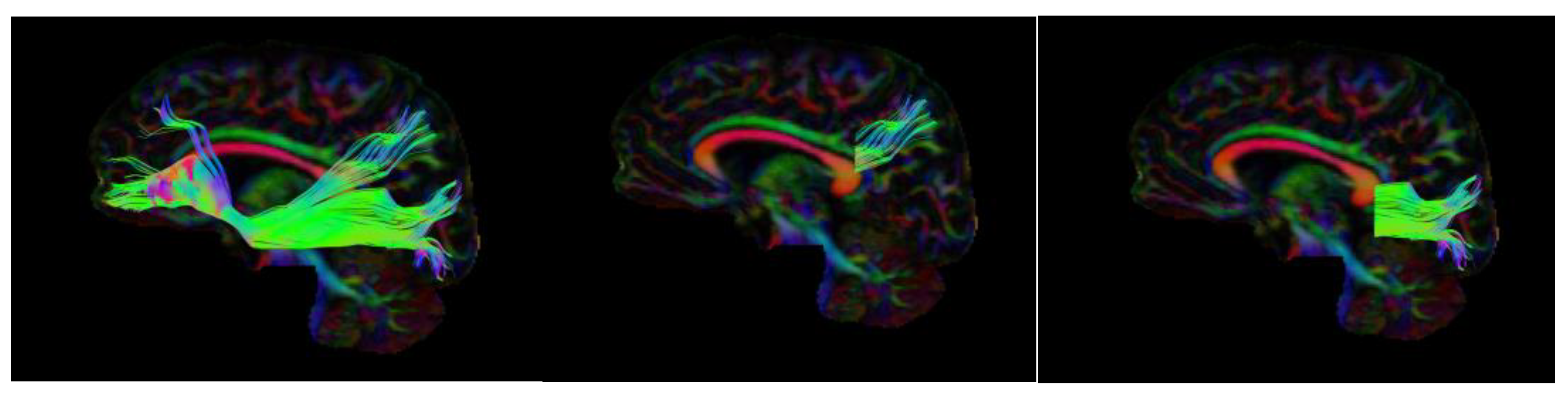
| Dorsal-IFOF | Ventral-IFOF | |
|---|---|---|
| Phonological-semantic hypothesis | P, OP, PA | O, OA, MA |
| Difficulty hypothesis | P, MA | O, OP, PA, OA |
| Automaticity hypothesis | P, OA, PA, MA | O, OP |
| Awareness Tasks | Letter Probe Tasks | ||||
|---|---|---|---|---|---|
| Phonological | Orthographic | Morphological | Phonological | Phonological-Orthographic | Orthographic |
| 73.0 (18.1) | 89.5 (4.5) | 90.7 (13.4) | 85.9 (4.5) | 84.9 (6.0) | 76.0 (8.1) |
| Total IFOF FA | Total IFOF MD | Dorsal FA | Dorsal MD | Ventral FA | Ventral MD |
|---|---|---|---|---|---|
| 0.48 (0.02) | 0.0008 (0.00002) | 0.45 (0.03) | 0.0008 (0.00003) | 0.51 (0.03) | 0.0008 (0.00003) |
| Dorsal-IFOF | Ventral-IFOF | |
|---|---|---|
| Phonological-semantic hypothesis | O *, OA *, MA + | |
| Difficulty hypothesis | O *, OA * | |
| Automaticity hypothesis | OA * | O * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eze, P.; Omorotionmwan, E.; Cummine, J. Moving towards an Understanding of the Role of the Inferior Fronto-Occipital Fasciculus in Language Processing. NeuroSci 2024, 5, 39-58. https://doi.org/10.3390/neurosci5010003
Eze P, Omorotionmwan E, Cummine J. Moving towards an Understanding of the Role of the Inferior Fronto-Occipital Fasciculus in Language Processing. NeuroSci. 2024; 5(1):39-58. https://doi.org/10.3390/neurosci5010003
Chicago/Turabian StyleEze, Princess, Efrem Omorotionmwan, and Jacqueline Cummine. 2024. "Moving towards an Understanding of the Role of the Inferior Fronto-Occipital Fasciculus in Language Processing" NeuroSci 5, no. 1: 39-58. https://doi.org/10.3390/neurosci5010003
APA StyleEze, P., Omorotionmwan, E., & Cummine, J. (2024). Moving towards an Understanding of the Role of the Inferior Fronto-Occipital Fasciculus in Language Processing. NeuroSci, 5(1), 39-58. https://doi.org/10.3390/neurosci5010003






