The Effect of Doxapram on Proprioceptive Neurons: Invertebrate Model
Abstract
1. Introduction
2. Materials and methods
2.1. Animals
2.2. Chemicals and Dissection
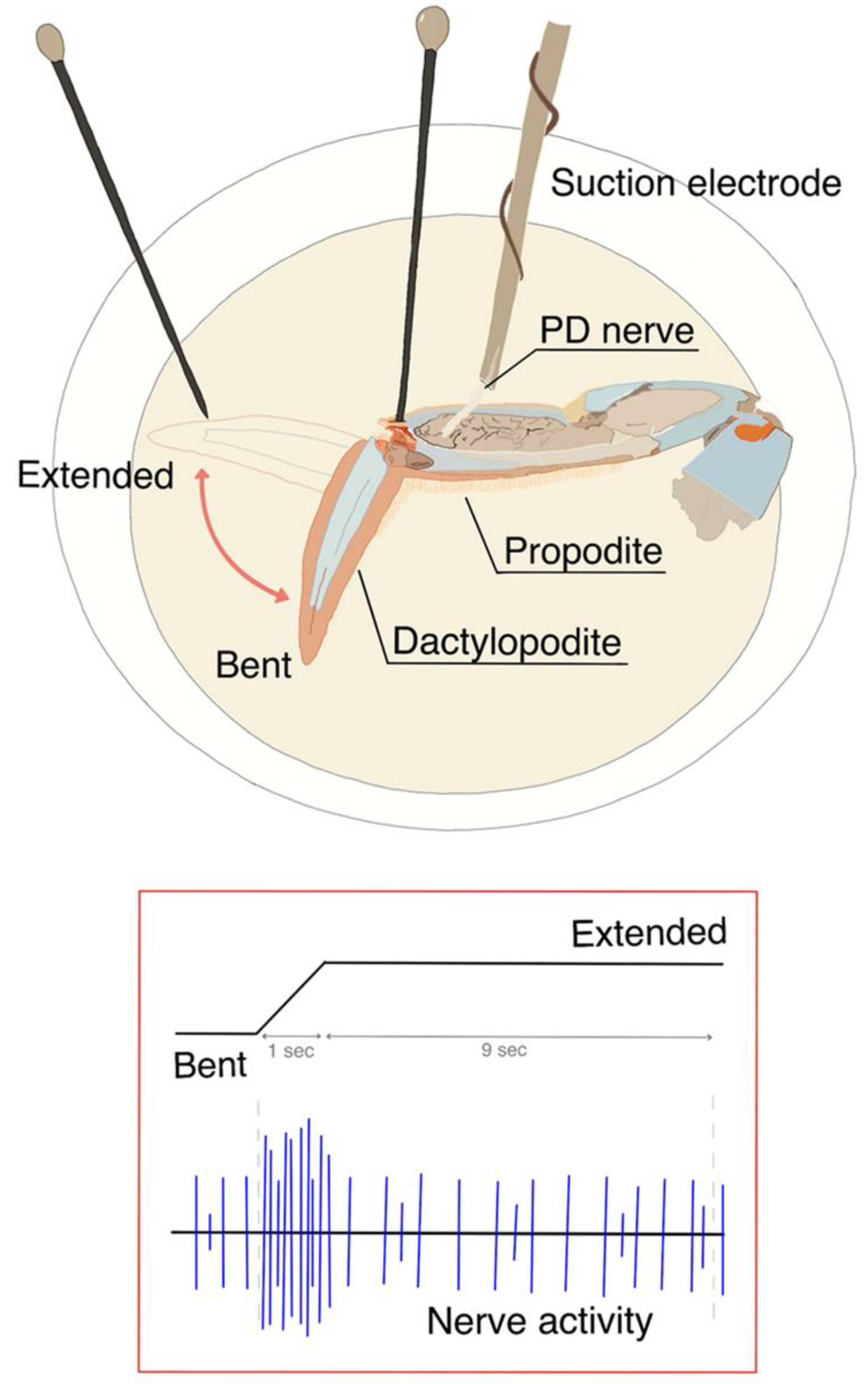
2.3. Data Collection
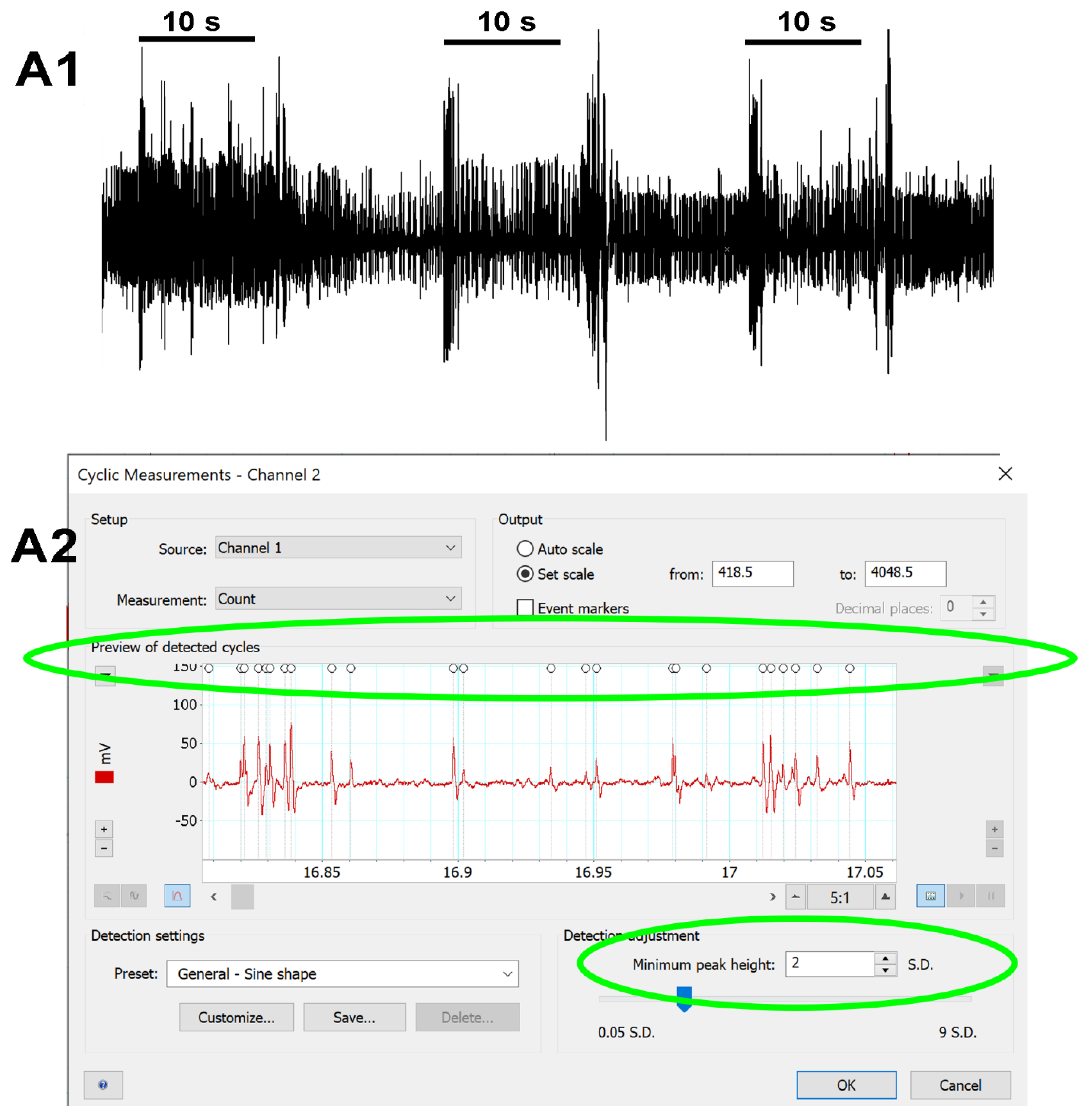
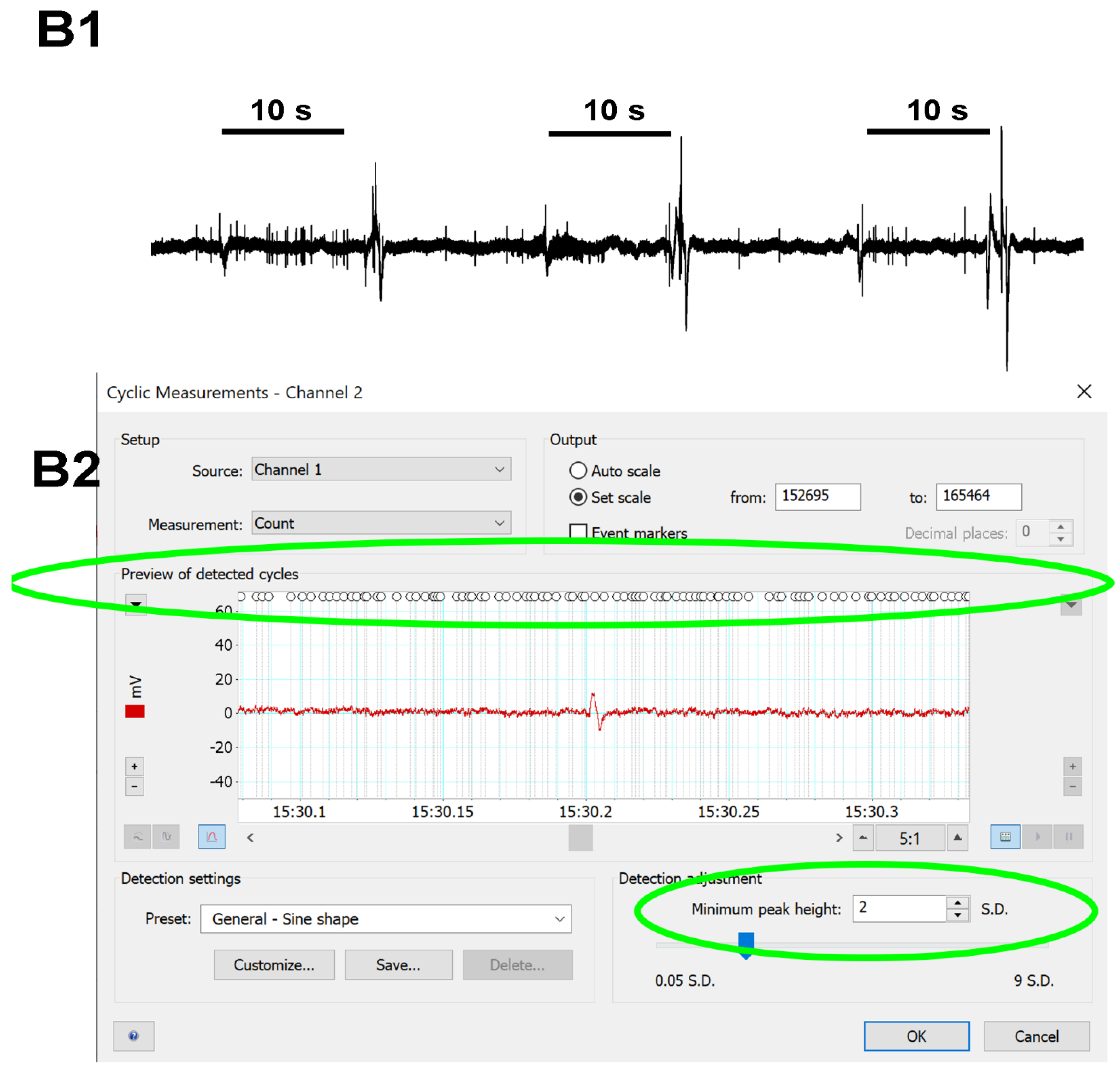
2.4. Analysis of the Neural Activity
2.5. Statistical Methods
3. Results
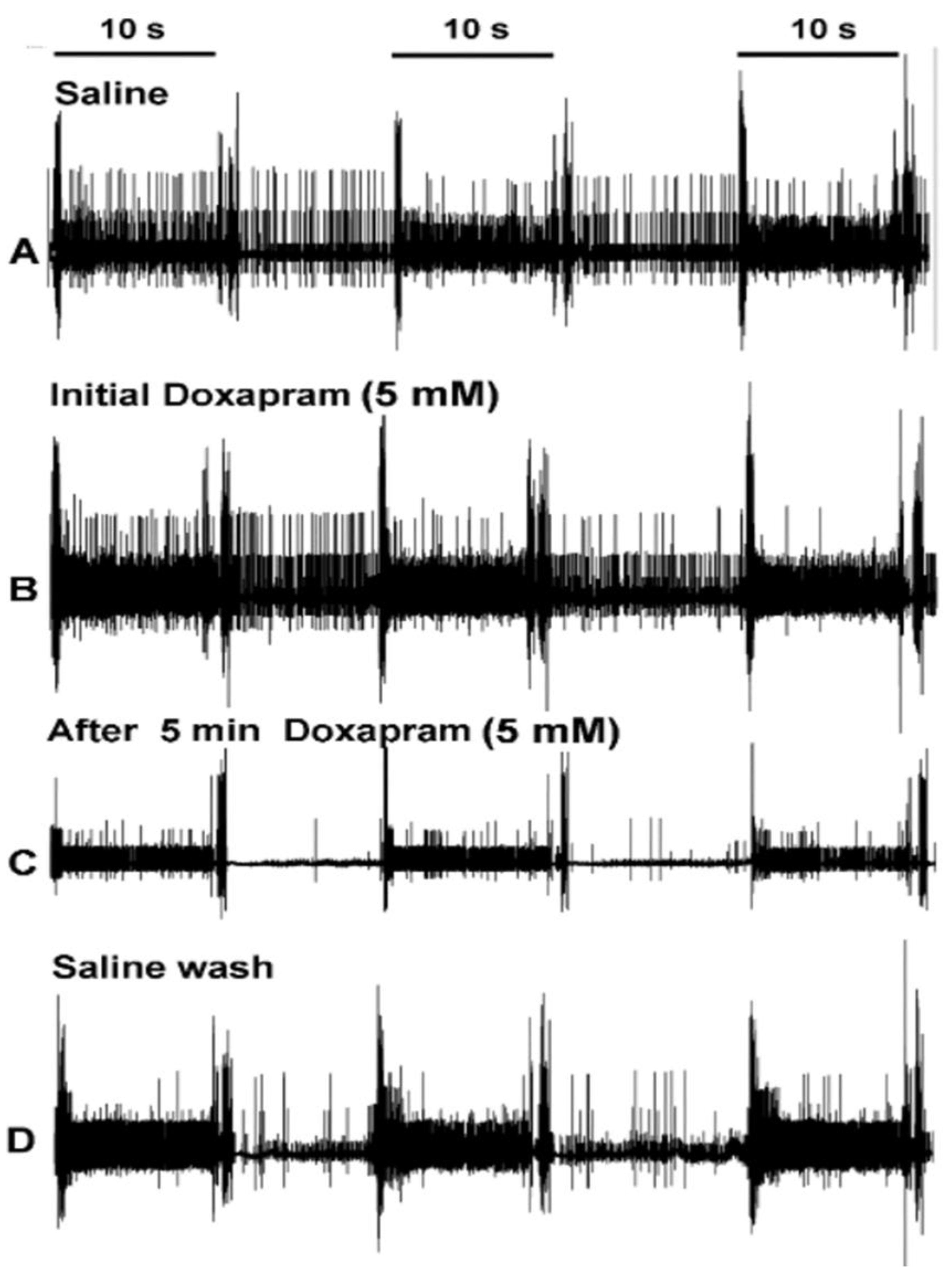
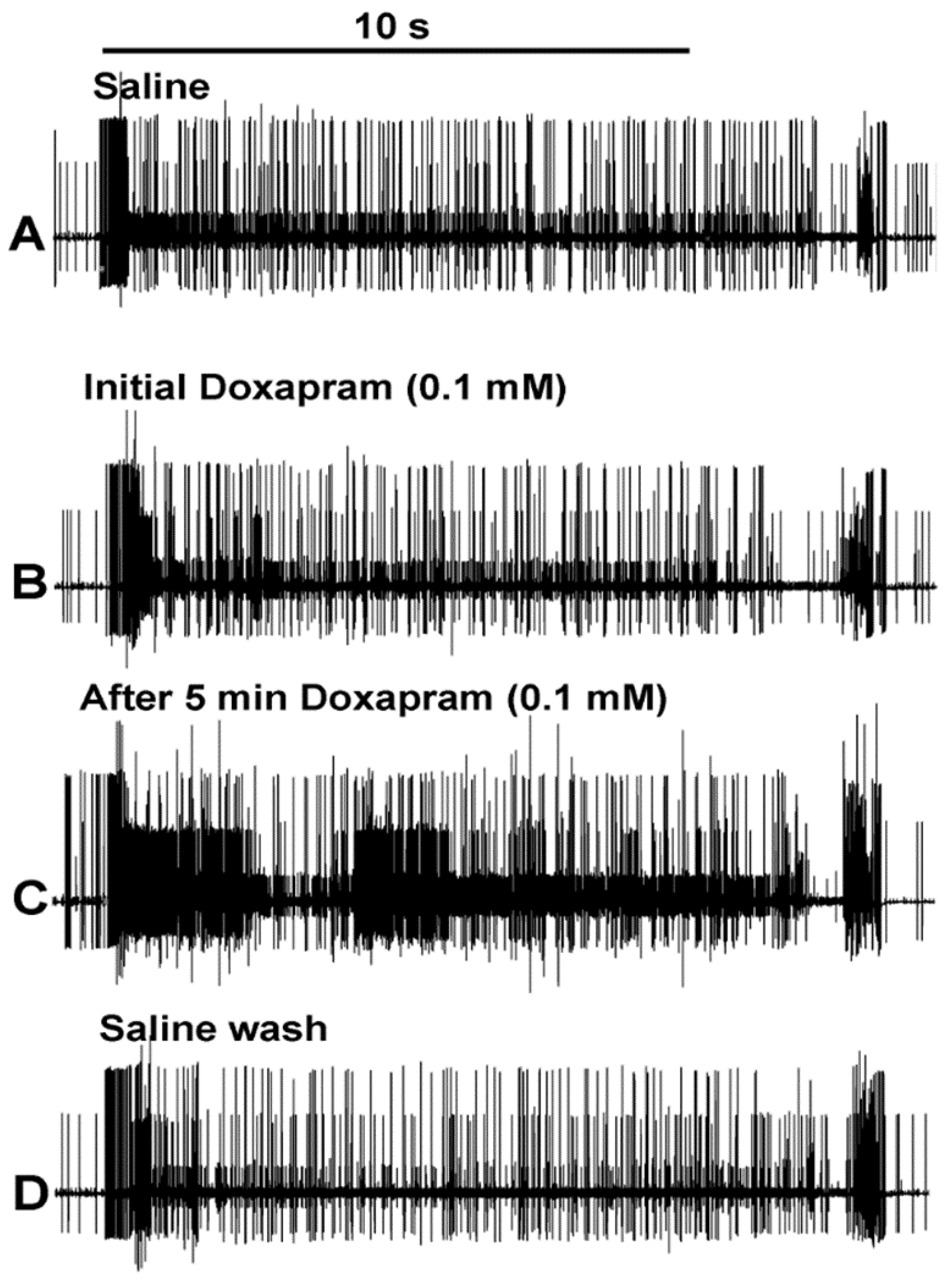
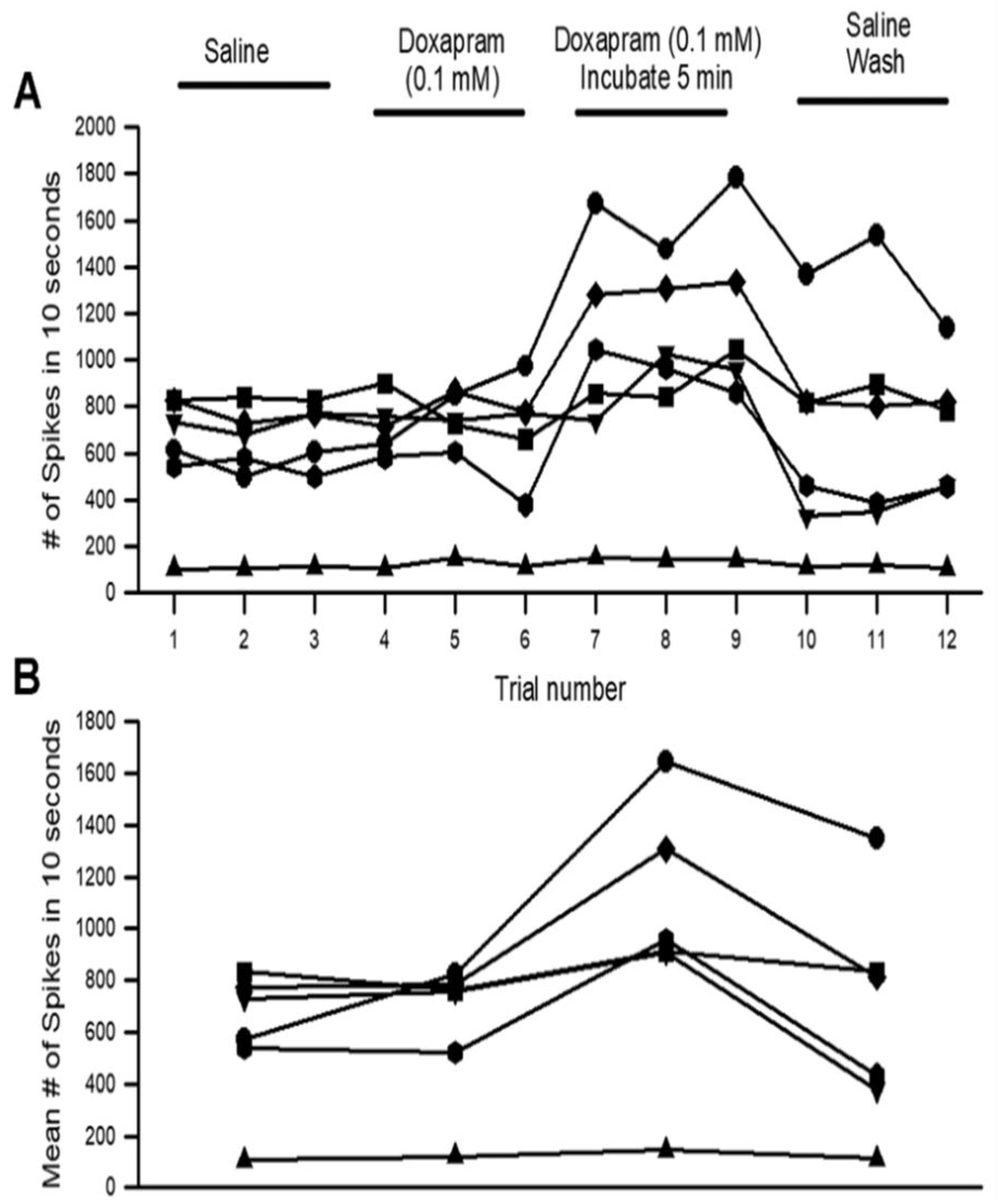
3.1. Effects of Doxapram on Neural Activity
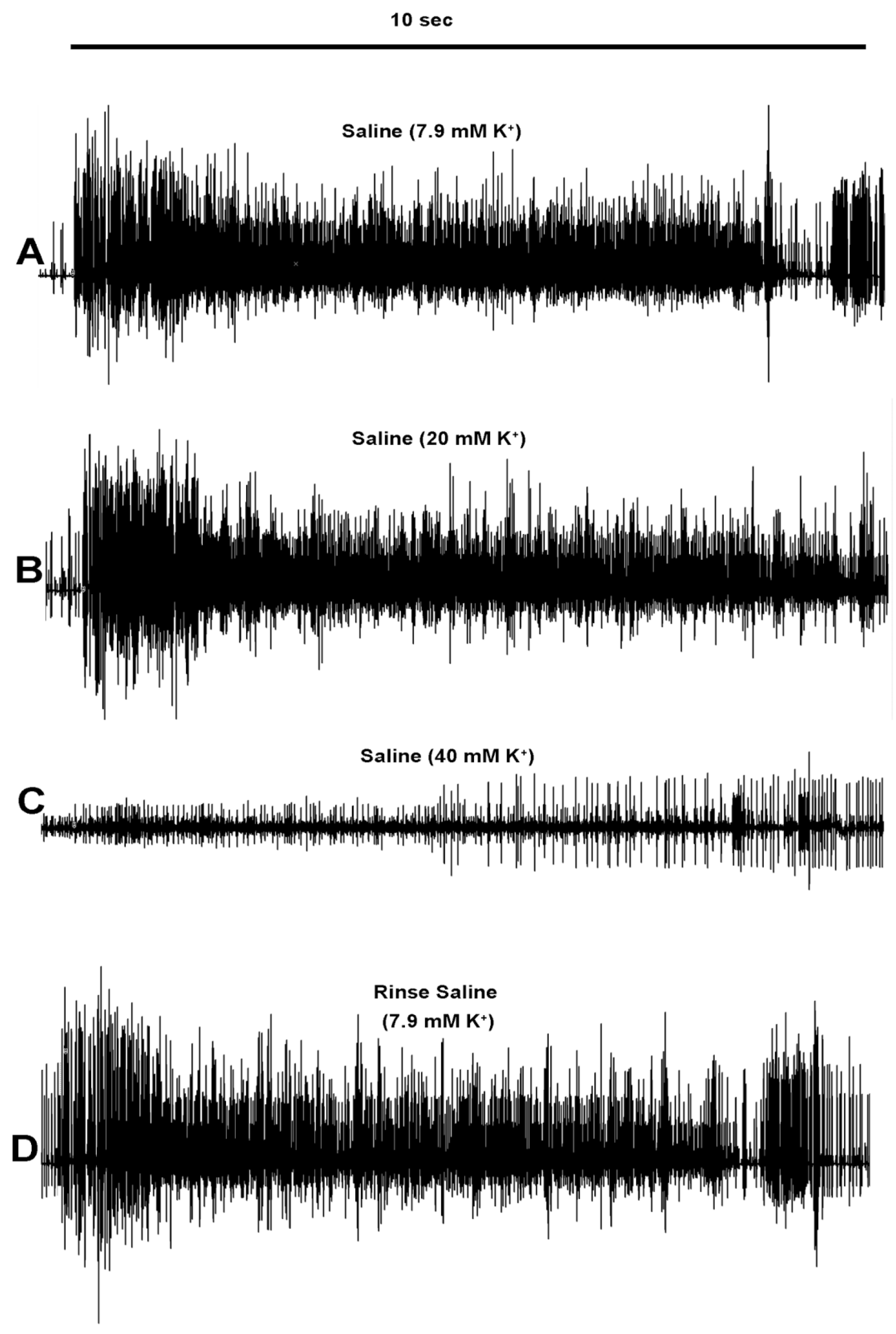
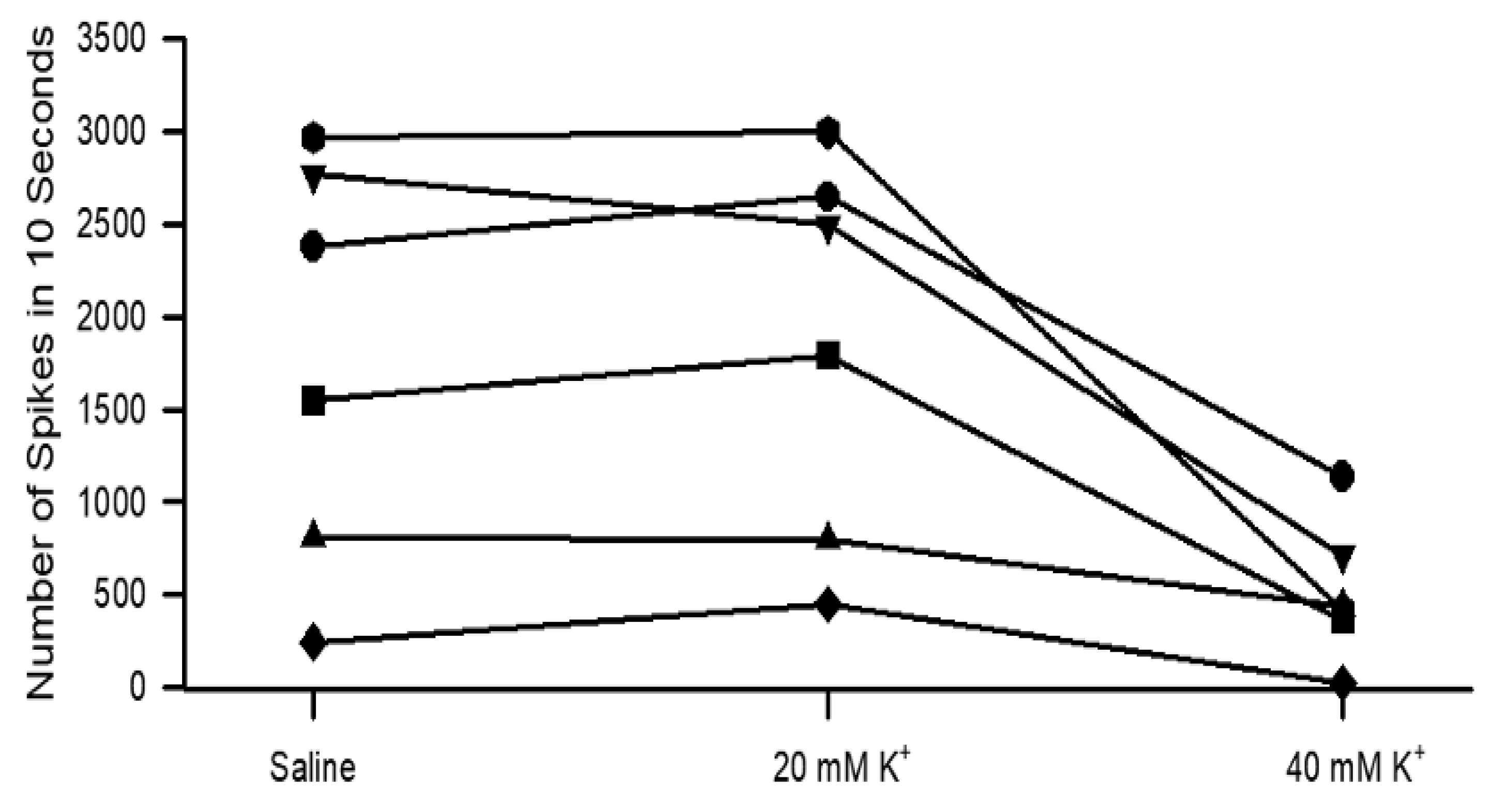
3.2. Effects of Raised Extracellular K+ on Neural Activity
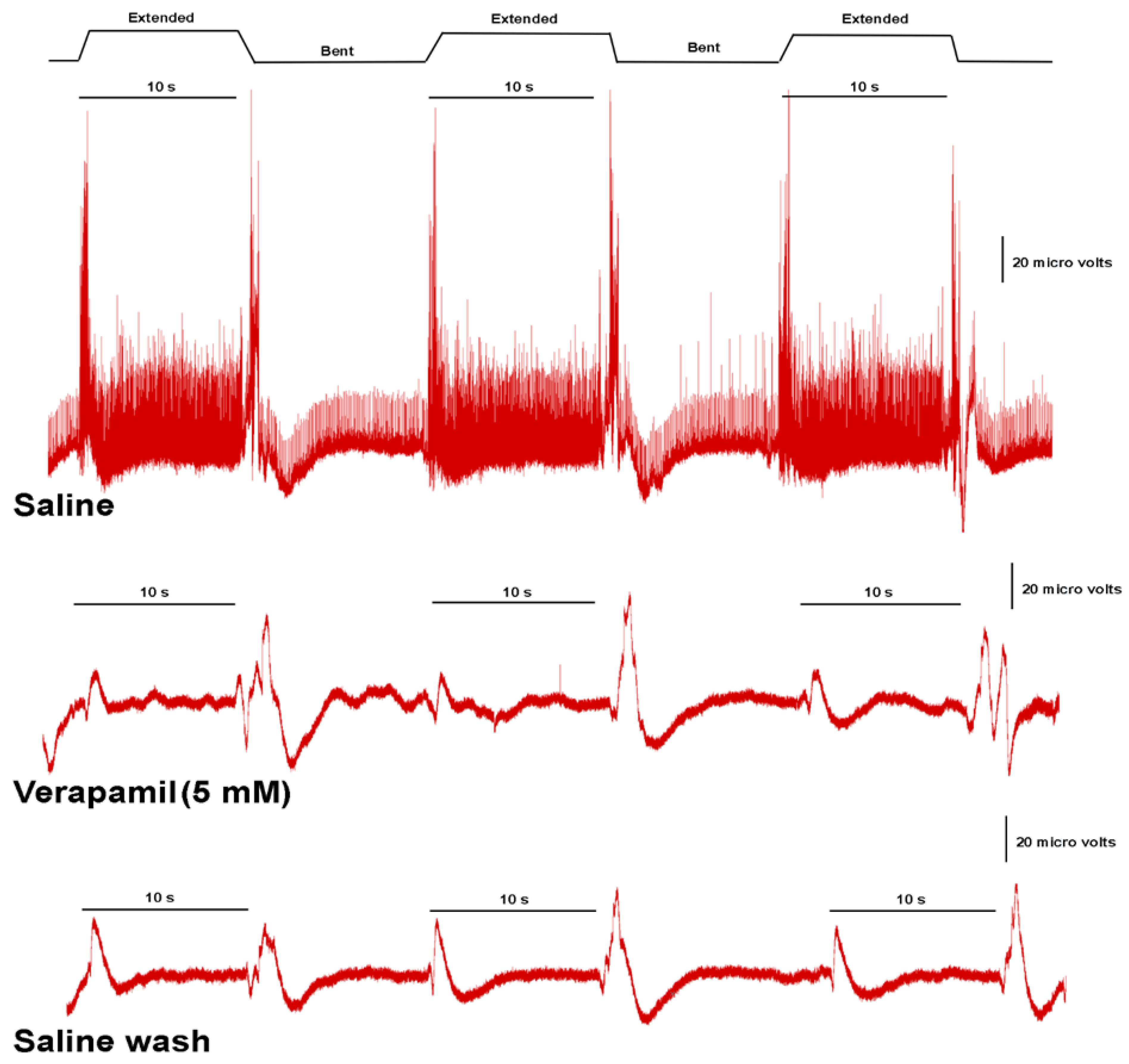
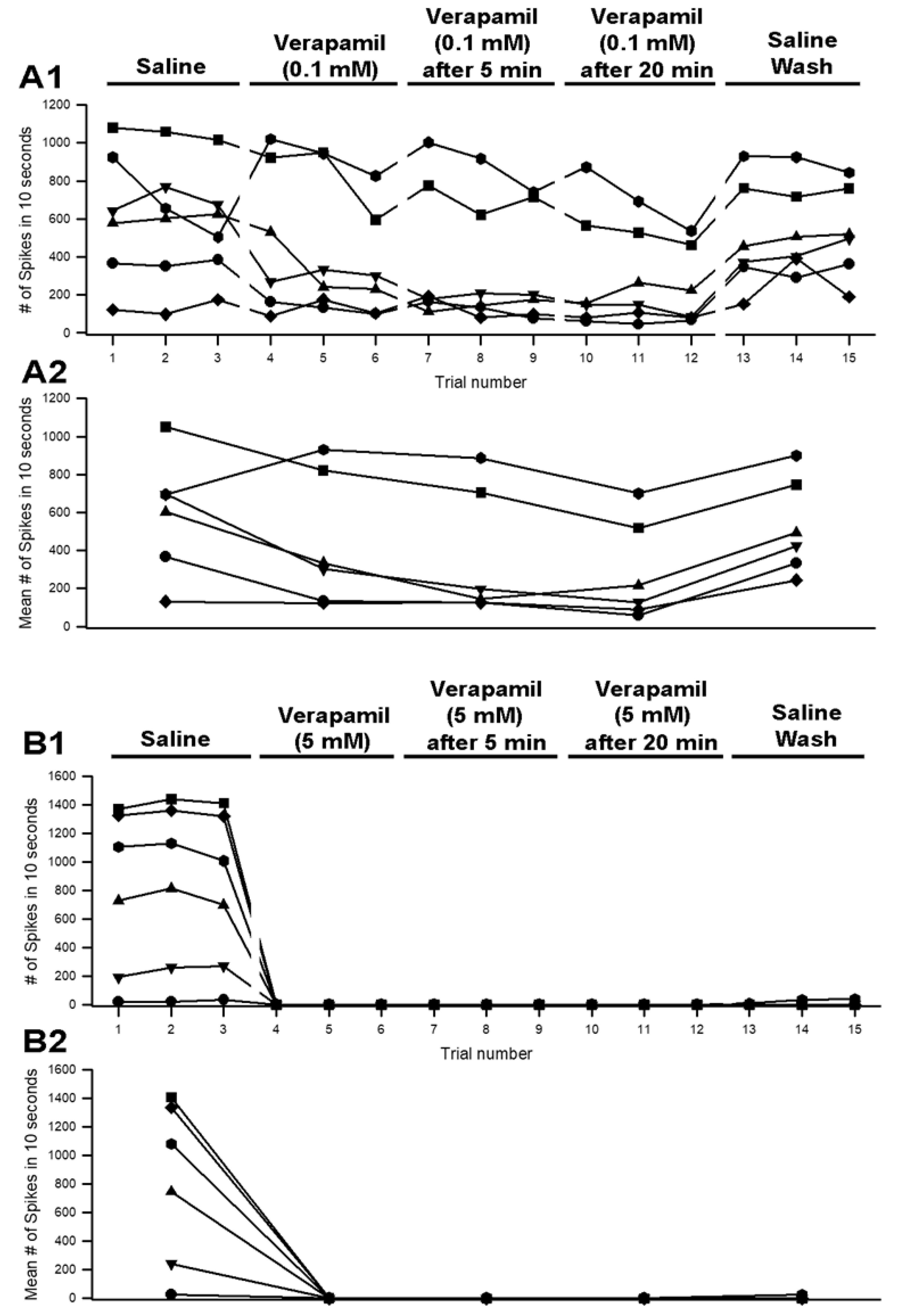
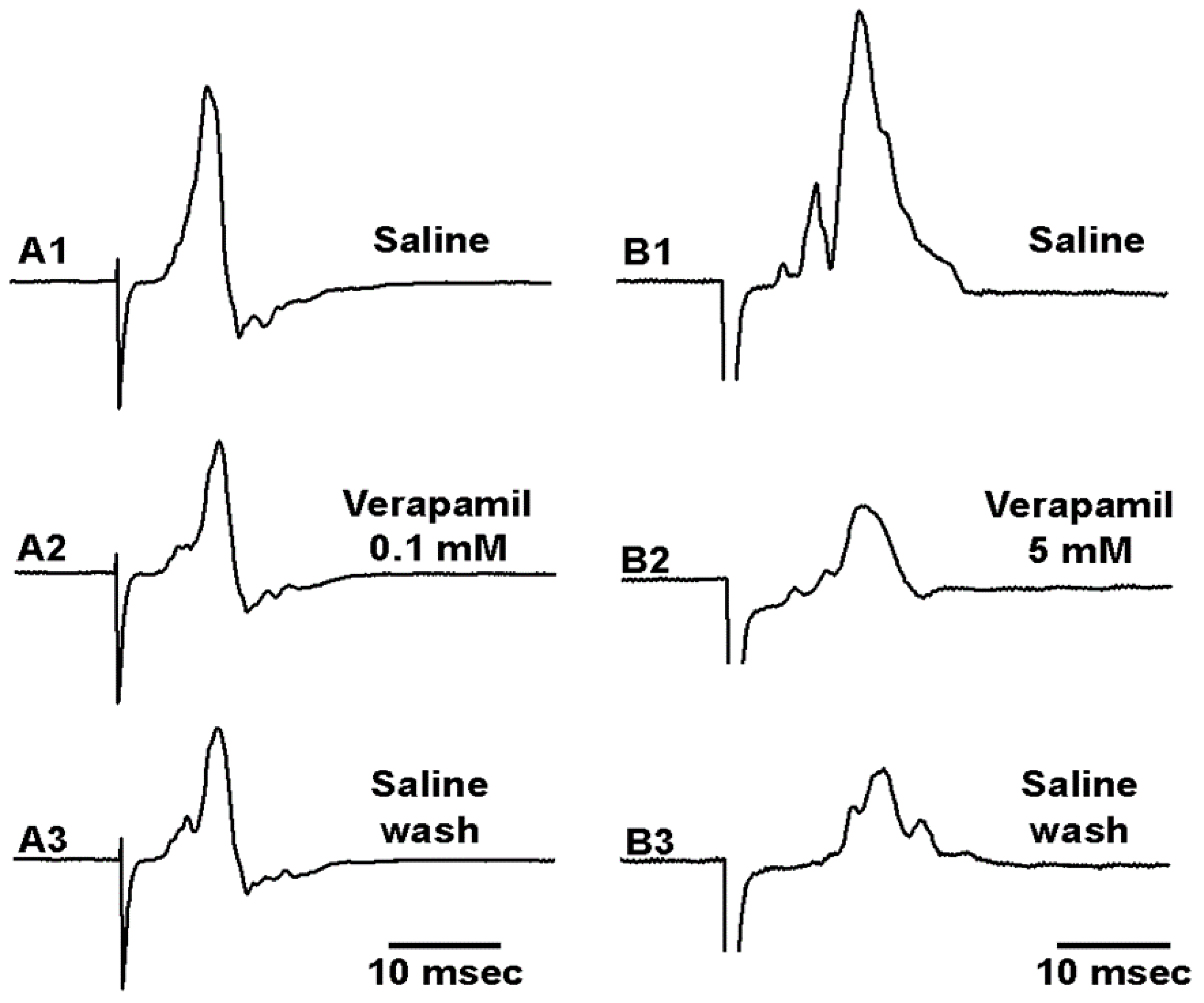
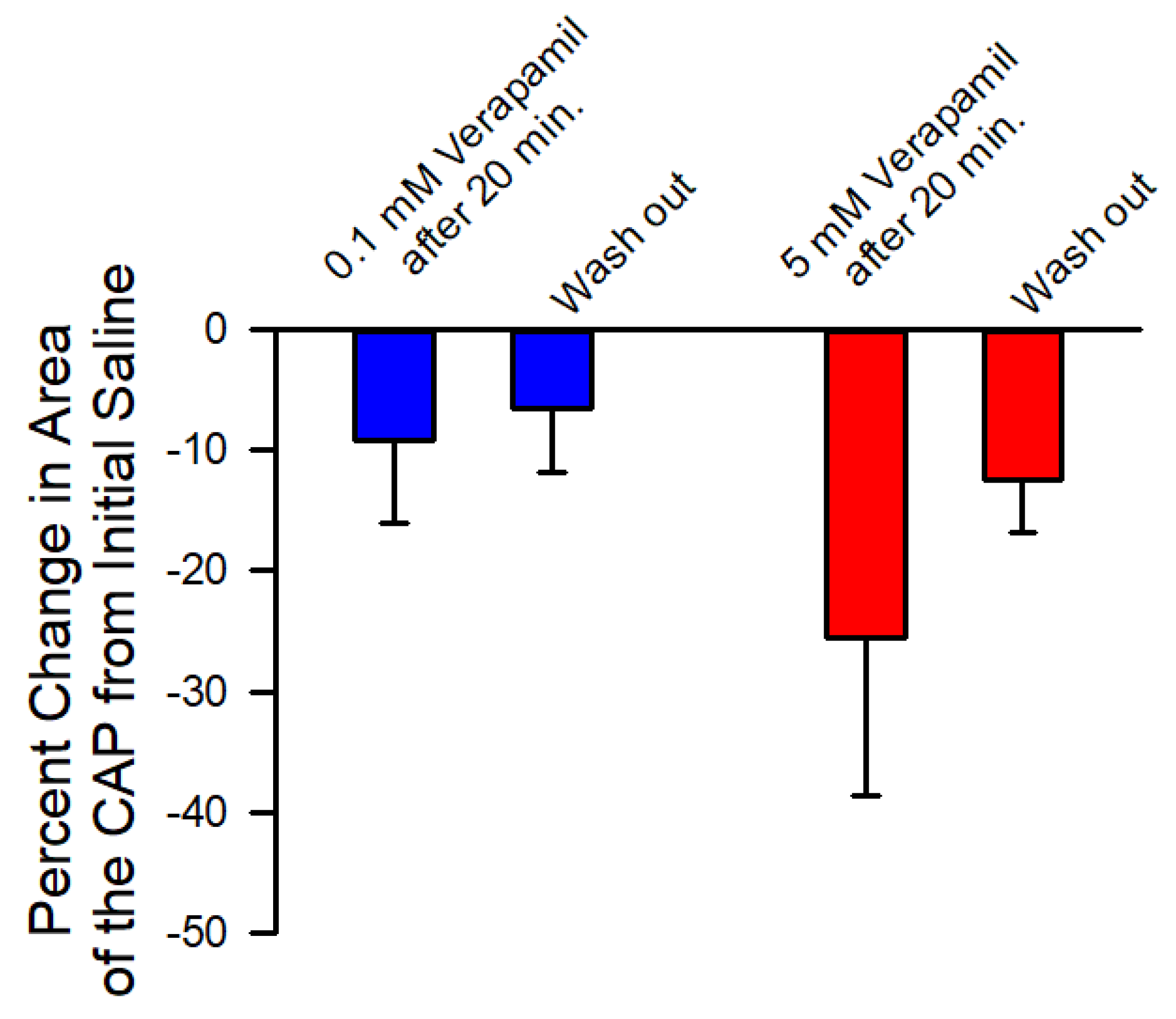
3.3. Effects of Verapamil on Neural Activity
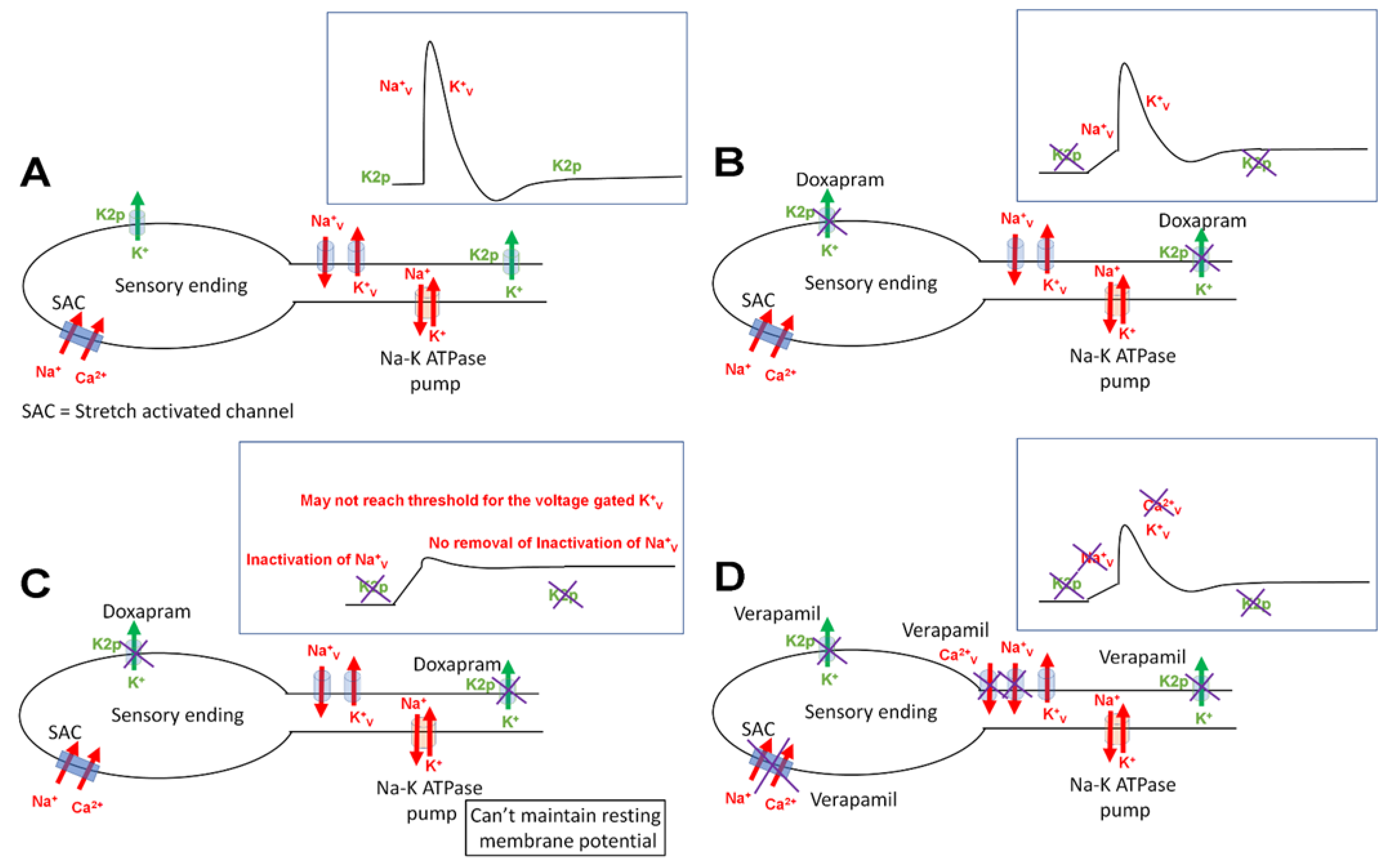
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Reproducibility in the Effects of Doxapram with a University Course
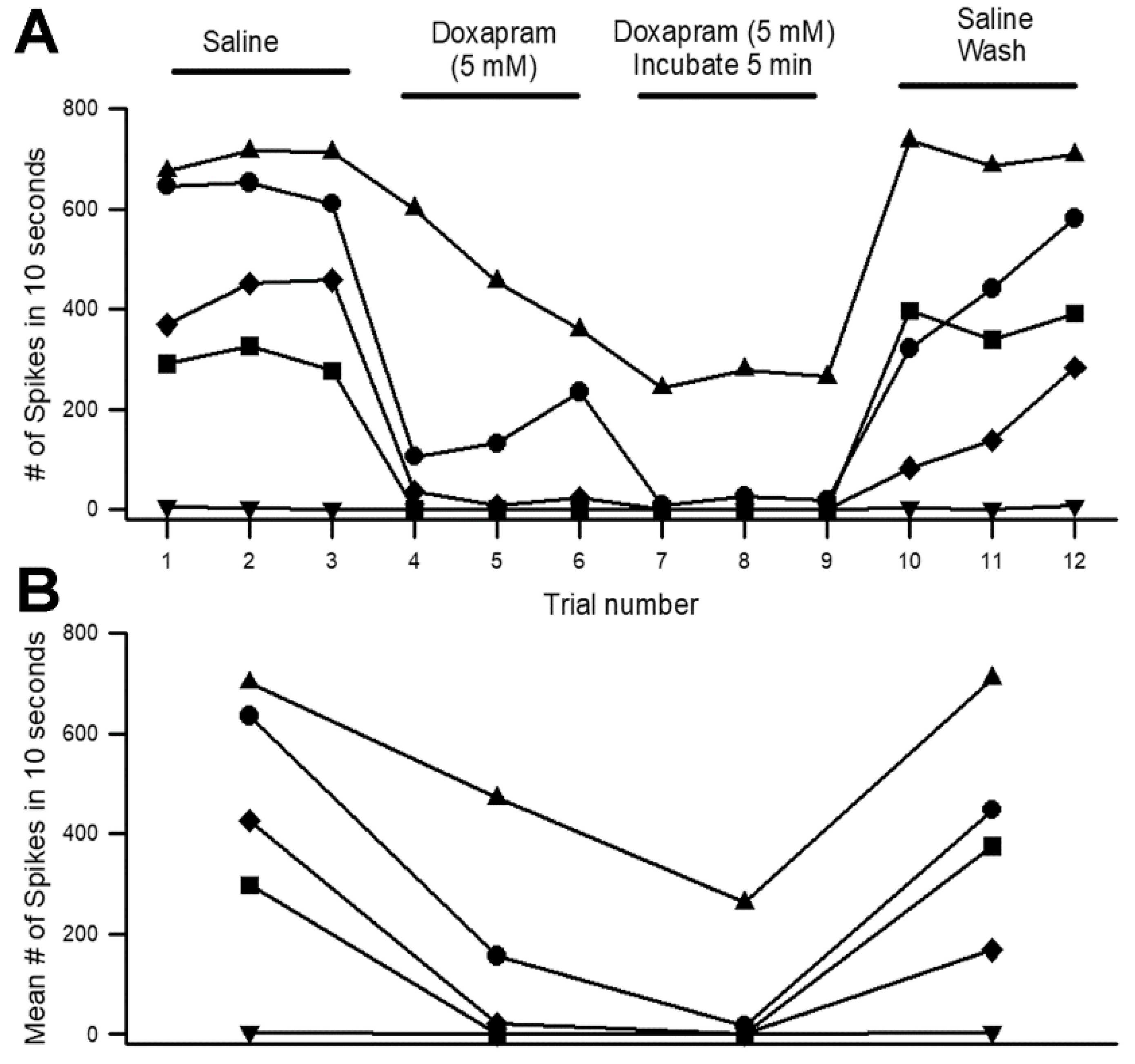
Appendix A.2. Reproducibility in Analysis of Given Data Sets
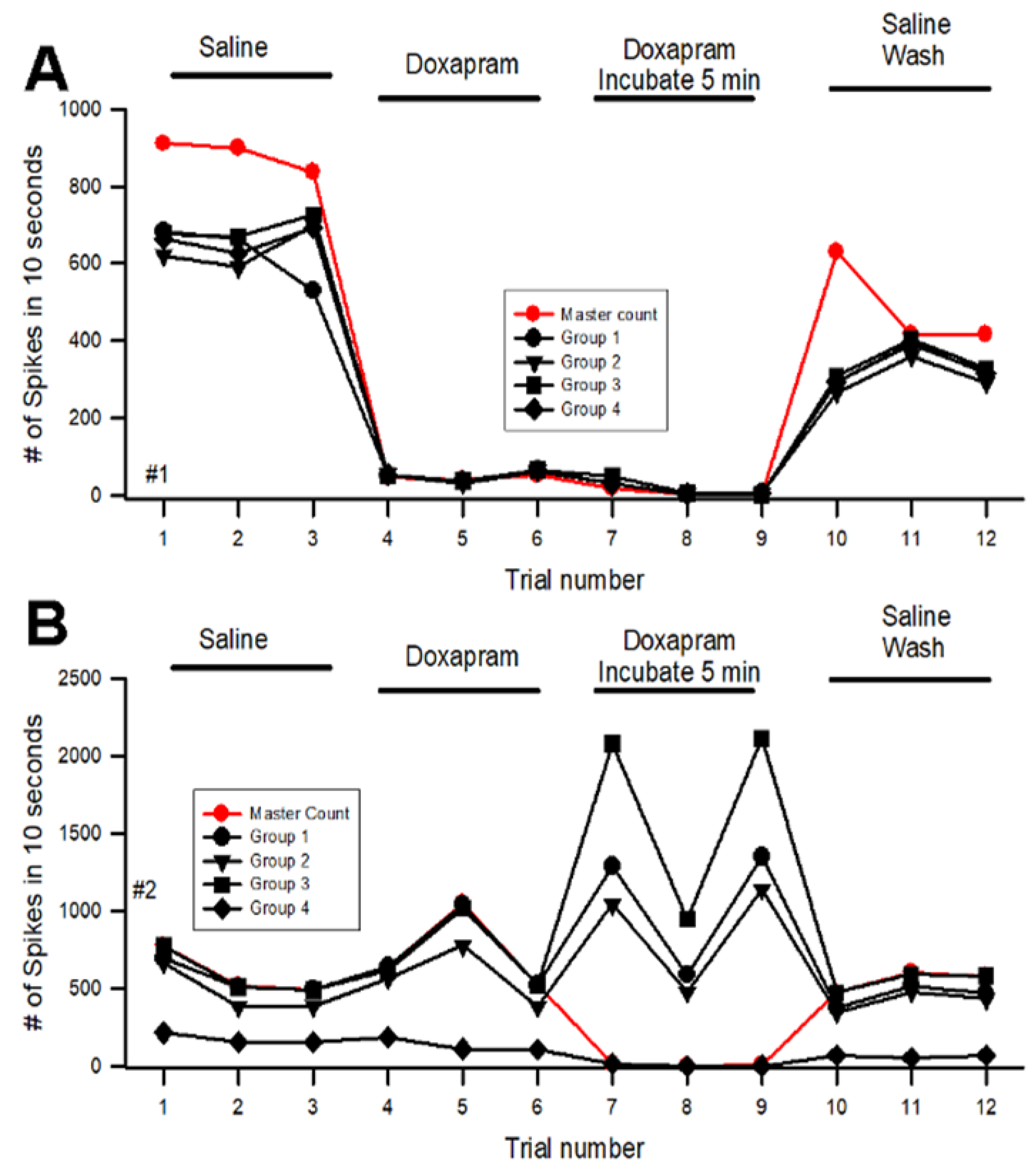
References
- Buckingham, S.D.; Kidd, J.F.; Law, R.J.; Franks, C.J.; Sattelle, D.B. Structure and function of two-pore-domain K+ channels: Contributions from genetic model organisms. Trends Pharmacol. Sci. 2005, 26, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.A.; Price, L.A.; Rosenthal, D.N.; Pausch, M.H. ORK1, a potassium-selective leak channel with two pore domains cloned from Drosophila melanogaster by expression in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1996, 93, 13256–13261. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.A.; Wang, K.W.; Ilan, N.; Pausch, M.H. Sequence and function of the two P domain potassium channels: Implications of an emerging superfamily. J. Mol. Med. 1998, 76, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Plant, L.D.; Goldstein, S.A.N. Two-Pore Domain Potassium Channels. In Handbook of Ion Channels, 1st ed.; Zheng, J., Trudeau, M.C., Eds.; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9780429193965. [Google Scholar]
- Enyedi, P.; Czirják, G. Molecular background of leak K+ currents: Two-pore domain potassium channels. Physiol. Rev. 2010, 90, 559–605. [Google Scholar] [CrossRef] [PubMed]
- Kim, D. Physiology and pharmacology of two-pore domain potassium channels. Curr. Pharm. Des. 2005, 11, 2717–2736. [Google Scholar] [CrossRef]
- Kamuene, J.M.; Xu, Y.; Plant, L.D. The pharmacology of two-pore domain potassium channels. Handb. Exp. Pharmacol. 2021, 267, 417–443. [Google Scholar] [PubMed]
- Patel, A.J.; Honore, E.; Lesage, F.; Fink, M.; Romey, G.; Lazdunski, M. Inhalational anesthetics activate two-pore-domain background K channels. Nat. Neurosci. 1999, 2, 422–426. [Google Scholar] [CrossRef]
- Rajan, S.; Wischmeyer, E.; Karschin, C.; Preisig-Muller, R.; Grzeschik, K.H.; Daut, J.; Karschin, A.; Derst, C. THIK-1 and THIK-2, a novel subfamily of tandem pore domain K+ channels. J. Biol. Chem. 2001, 276, 7302–7311. [Google Scholar] [CrossRef]
- Cotten, J.F.; Keshavaprasad, B.; Laster, M.J.; Eger, E.I., 2nd; Yost, C.S. The ventilatory stimulant doxapram inhibits TASK tandem pore (K2P) potassium channel function but does not affect minimum alveolar anesthetic concentration. Anesth. Analg. 2006, 102, 779–785. [Google Scholar] [CrossRef]
- Komatsu, R.; Sengupta, P.; Cherynak, G.; Wadhwa, A.; Sessler, D.I.; Liu, J.; Hurst, H.E.; Lenhardt, R. Doxapram only slightly reduces the shivering threshold in healthy volunteers. Anesth. Analg. 2005, 101, 1368–1373. [Google Scholar] [CrossRef]
- Yost, C.S. A new look at the respiratory stimulant doxapram. CNS Drug Rev. 2006, 12, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Song, S.S.; Lyden, P.D. Overview of therapeutic hypothermia. Curr. Treat. Options. Neurol. 2012, 14, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.P.; MacIntyre, D.E.; Mathie, A.; Veale, E.L. Effects of the ventilatory stimulant, doxapram on human TASK-3 (KCNK9, K2P9.1) channels and TASK-1 (KCNK3, K2P3.1) channels. Acta Physiol. 2020, 228, e13361. [Google Scholar] [CrossRef] [PubMed]
- Vliegenthart, R.J.; Ten Hove, C.H.; Onland, W.; van Kaam, A.H. Doxapram treatment for apnea of prematurity: A systematic review. Neonatology 2017, 111, 162–171. [Google Scholar] [CrossRef]
- Baxter, A.D. Side effects of doxapram infusion. Eur. J. Intensive Care Med. 1976, 2, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Massoudi, N.; Nooraee, N.; Beheshti Monfared, R. The effects of doxapram on time to tracheal extubation and early recovery in young morbidly obese patients scheduled for bariatric surgery: A randomised controlled trial. Eur. J. Anaesthesiol. 2020, 37, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.M.; Müntefering, T.; Budde, T.; Meuth, S.G.; Ruck, T. Pathophysiological role of K2P channels in human diseases. Cell Physiol. Biochem. 2021, 55, 65–86. [Google Scholar]
- Holter, J.; Carter, D.; Leresche, N.; Crunelli, V.; Vincent, P. A TASK3 channel (KCNK9) mutation in a genetic model of absence epilepsy. J. Mol. Neurosci. 2005, 25, 37–51. [Google Scholar] [CrossRef]
- DeGiorgis, J.A.; Jang, M.; Bearer, E.L. The giant axon of the squid: A simple system for axonal transport studies. Meth. Mol. Biol. 2022, 2431, 3–22. [Google Scholar]
- Dow, J.A.T.; Simons, M.; Romero, M.F. Drosophila melanogaster: A simple genetic model of kidney structure, function and disease. Nat. Rev. Nephrol. 2022, 18, 417–434. [Google Scholar] [CrossRef]
- Ecovoiu, A.A.; Ratiu, A.C.; Micheu, M.M.; Chifiriuc, M.C. Inter-species rescue of mutant phenotype-the standard for genetic analysis of human genetic disorders in Drosophila melanogaster model. Int. J. Mol. Sci. 2022, 23, 2613. [Google Scholar] [CrossRef] [PubMed]
- Haley, J.A.; Hampton, D.; Marder, E. Two central pattern generators from the crab, Cancer borealis, respond robustly and differentially to extreme extracellular pH. Elife 2018, 7, e41877. [Google Scholar] [CrossRef] [PubMed]
- Otopalik, A.G.; Pipkin, J.; Marder, E. Neuronal morphologies built for reliable physiology in a rhythmic motor circuit. Elife 2019, 8, e41728. [Google Scholar] [CrossRef] [PubMed]
- Ugur, B.; Chen, K.; Bellen, H.J. Drosophila tools and assays for the study of human diseases. Dis. Model. Mech. 2016, 9, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Dayaram, V.; Malloy, C.A.; Martha, S.; Alvarez, B.; Chukwudolue, I.; Dabbain, N.; Goleva, S.; Hickey, T.; Ho, A.; King, M.; et al. The effect of CO2, intracellular pH and extracellular pH on mechanosensory proprioceptor responses in crayfish and crab. Am. J. Undergrad. Res. 2017, 14, 85–99. [Google Scholar]
- Pankau, C.; Nadolski, J.; Tanner, H.; Cryer, C.; Di Girolamo, J.; Haddad, C.; Lanning, M.; Miller, M.; Neely, D.; Wilson, R.; et al. Effects of manganese on physiological processes in Drosophila, crab and crayfish: Cardiac, neural and behavioral assays. Comp. Biochem. Physiol. C 2022, 251, 109209. [Google Scholar]
- Majeed, Z.R.; Titlow, J.; Hartman, H.B.; Cooper, R.L. Proprioception and tension receptors in crab limbs: Student laboratory exercises. J. Vis. Exp. 2013, 80, e51050. [Google Scholar] [CrossRef]
- Tanner, H.N.; Atkins, D.E.; Bosh, K.L.; Breakfield, G.W.; Daniels, S.E.; Devore, M.J.; Fite, H.E.; Guo, L.Z.; Henry, D.K.J.; Kaffenberger, A.K.; et al. Effect of TEA and 4-AP on primary sensory neurons in a crustacean model. J. Pharmacol. Toxicol. 2022, 17, 14–27. [Google Scholar] [CrossRef]
- Triggle, D.J. Calcium channel antagonists: Clinical uses--past, present and future. Biochem. Pharmacol. 2007, 74, 1–9. [Google Scholar] [CrossRef]
- Reuter, H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature 1983, 301, 569–574. [Google Scholar] [CrossRef]
- Adams, M.D.; Celniker, S.E.; Holt, R.A.; Evans, C.A.; Gocayne, J.D.; Amanatides, P.G.; Scherer, S.E.; Li, P.W.; Hoskins, R.A.; Galle, R.F.; et al. The genome sequence of Drosophila melanogaster. Science 2000, 287, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Littleton, J.T.; Ganetzky, B. Ion channels and synaptic organization: Analysis of the Drosophila genome. Neuron 2000, 26, 35–43. [Google Scholar] [CrossRef]
- Park, H.; Kim, E.J.; Ryu, J.H.; Lee, D.K.; Hong, S.G.; Han, J.; Han, J.; Kang, D. Verapamil inhibits TRESK (K2P18.1) current in trigeminal ganglion neurons independently of the blockade of Ca2+ influx. Int. J. Mol. Sci. 2018, 19, 1961. [Google Scholar] [CrossRef] [PubMed]
- Dayaram, V.; Malloy, C.A.; Martha, S.; Alvarez, B.; Chukwudolue, I.; Dabbain, N.; Goleva, S.; Hickey, T.; Ho, A.; King, M.; et al. Stretch activated channels in proprioceptive chordotonal organs of crab and crayfish are sensitive to Gd3+ but not amiloride, ruthenium red or low pH. IMPULSE 2017, 14. Available online: https://impulse.pubpub.org/pub/8xdynrg8 (accessed on 20 August 2022).
- McCubbin, S.; Jeoung, A.; Waterbury, C.; Cooper, R.L. Pharmacological profiling of stretch activated channels in proprioceptive neuron. Comp. Biochem. Physiol. C 2020, 233, 108765. [Google Scholar] [CrossRef]
- Wojtowicz, J.M.; Atwood, H.L. Presynaptic membrane potential and transmitter release at the crayfish neuromuscular junction. J. Neurophysiol. 1984, 52, 99–113. [Google Scholar] [CrossRef]
- Sivaramakrishnan, S.; Brodwick, M.S.; Bittner, G.D. Presynaptic facilitation at the crayfish neuromuscular junction. Role of calcium-activated potassium conductance. J. Gen. Physiol. 1991, 98, 1181–1196. [Google Scholar] [CrossRef]
- Kuroda, T. The effects of D600 and verapamil on action potential in the X-organ neuron of the crayfish. Jpn. J. Physiol. 1976, 26, 189–202. [Google Scholar] [CrossRef][Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ison, B.J.; Abul-Khoudoud, M.O.; Ahmed, S.; Alhamdani, A.W.; Ashley, C.; Bidros, P.C.; Bledsoe, C.O.; Bolton, K.E.; Capili, J.G.; Henning, J.N.; et al. The Effect of Doxapram on Proprioceptive Neurons: Invertebrate Model. NeuroSci 2022, 3, 566-588. https://doi.org/10.3390/neurosci3040041
Ison BJ, Abul-Khoudoud MO, Ahmed S, Alhamdani AW, Ashley C, Bidros PC, Bledsoe CO, Bolton KE, Capili JG, Henning JN, et al. The Effect of Doxapram on Proprioceptive Neurons: Invertebrate Model. NeuroSci. 2022; 3(4):566-588. https://doi.org/10.3390/neurosci3040041
Chicago/Turabian StyleIson, Bethany J., Maya O. Abul-Khoudoud, Sufia Ahmed, Abraham W. Alhamdani, Clair Ashley, Patrick C. Bidros, Constance O. Bledsoe, Kayli E. Bolton, Jerone G. Capili, Jamie N. Henning, and et al. 2022. "The Effect of Doxapram on Proprioceptive Neurons: Invertebrate Model" NeuroSci 3, no. 4: 566-588. https://doi.org/10.3390/neurosci3040041
APA StyleIson, B. J., Abul-Khoudoud, M. O., Ahmed, S., Alhamdani, A. W., Ashley, C., Bidros, P. C., Bledsoe, C. O., Bolton, K. E., Capili, J. G., Henning, J. N., Moon, M., Phe, P., Stonecipher, S. B., Tanner, H. N., Turner, L. T., Taylor, I. N., Wagers, M. L., West, A. K., & Cooper, R. L. (2022). The Effect of Doxapram on Proprioceptive Neurons: Invertebrate Model. NeuroSci, 3(4), 566-588. https://doi.org/10.3390/neurosci3040041






