The Computer Simulation for Triggering Anxiety in Panic Disorder Patients Modulates the EEG Alpha Power during an Oddball Task
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
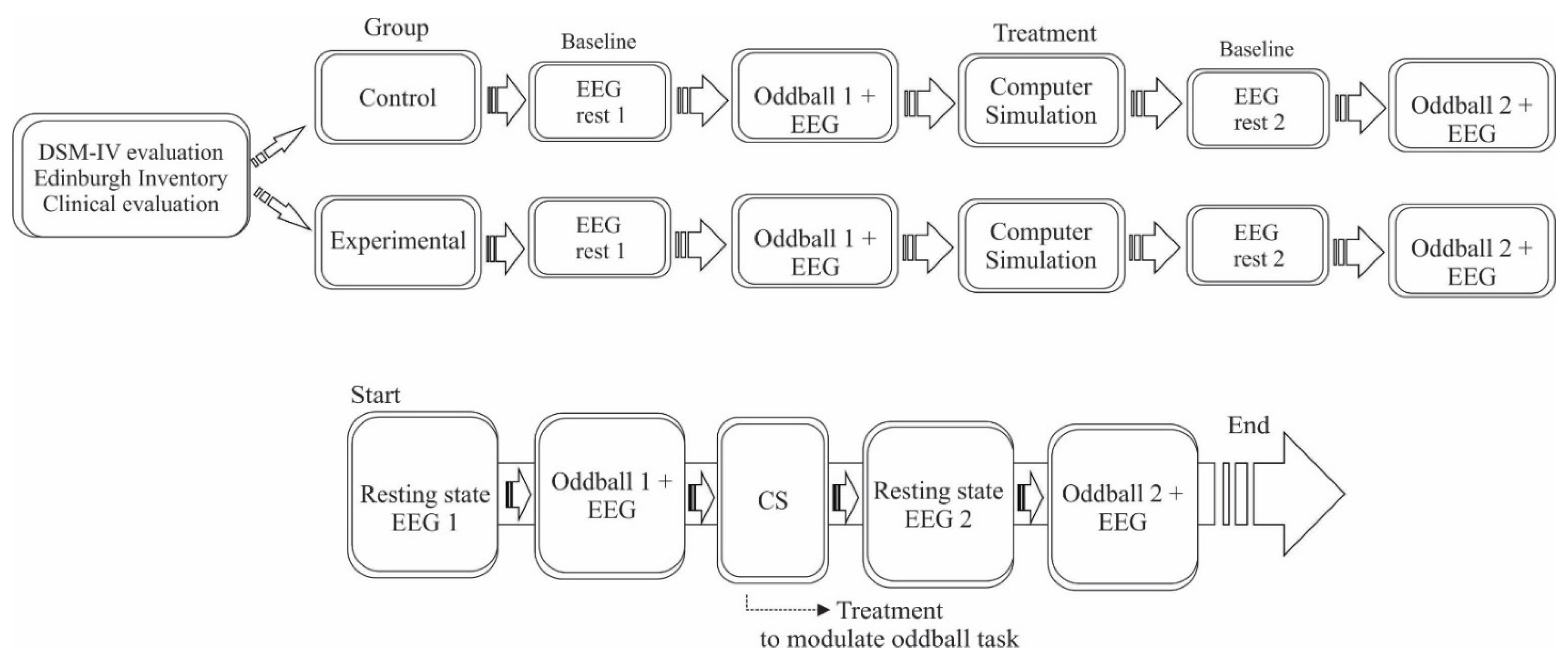
2.2. Experimental Procedure
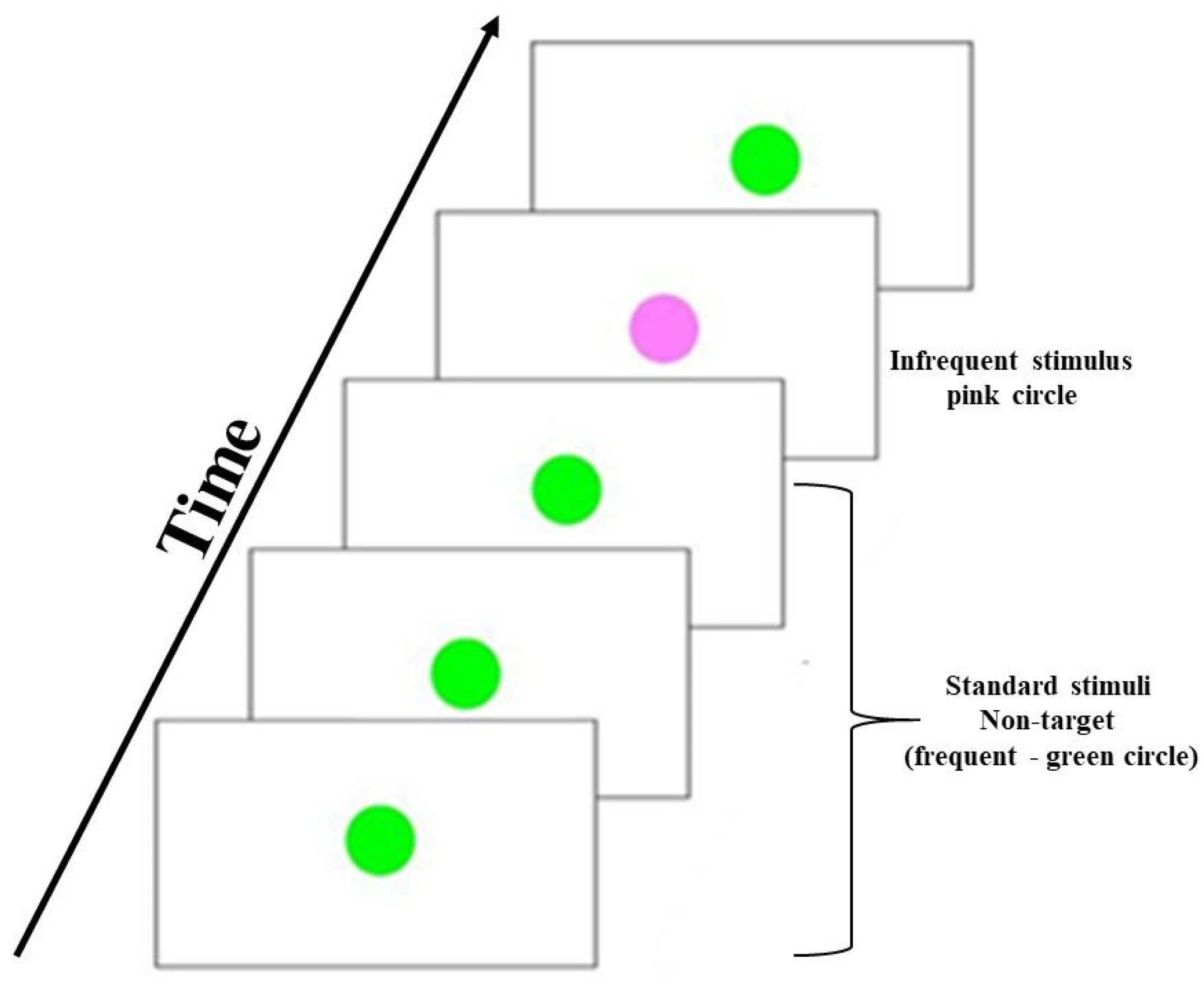
2.3. Visual Oddball Task
2.4. Computer Simulation
2.5. EEG Recording
Electrodes of Interest
2.6. Data Processing
2.7. Statistical Analysis
3. Results
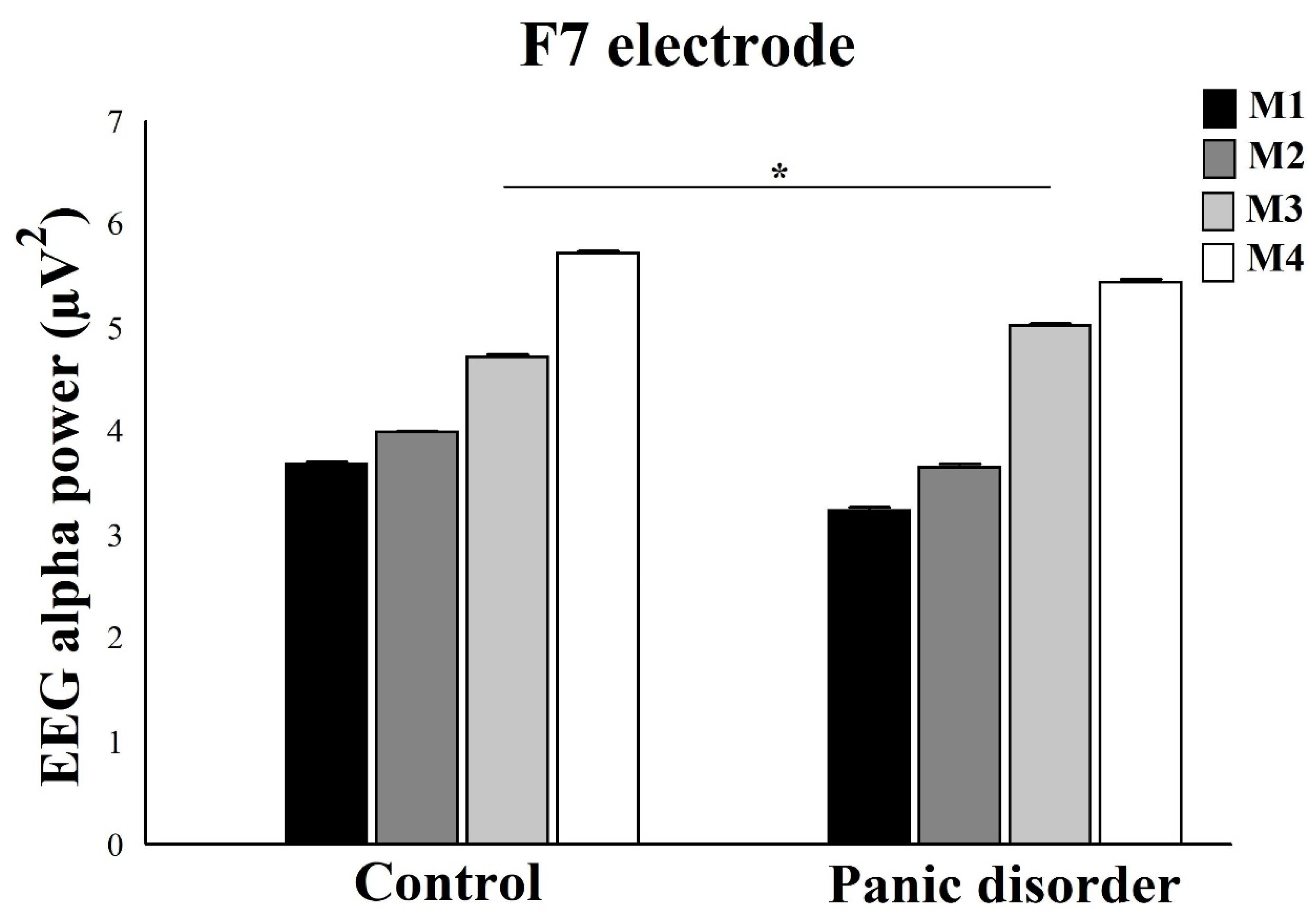
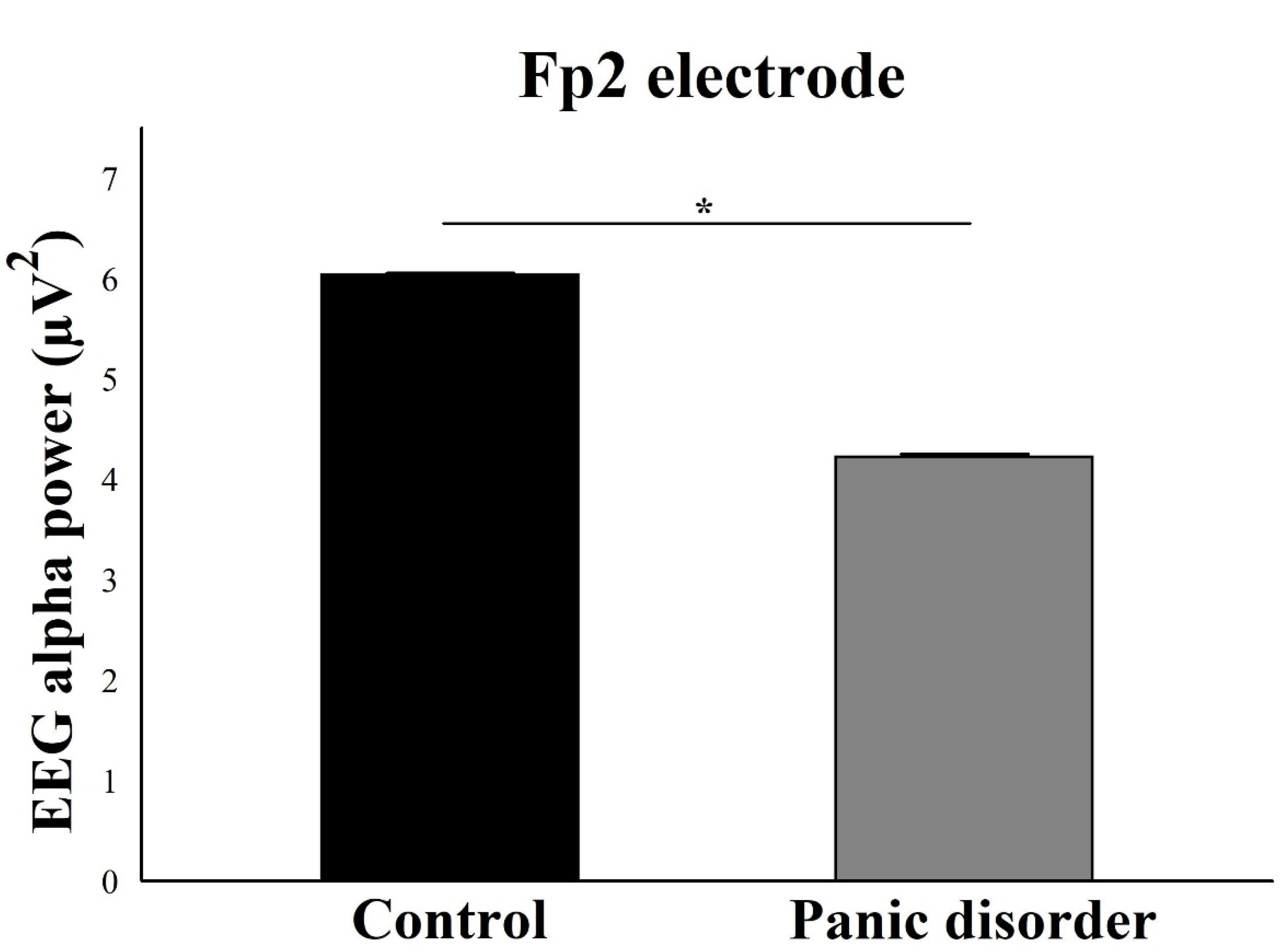
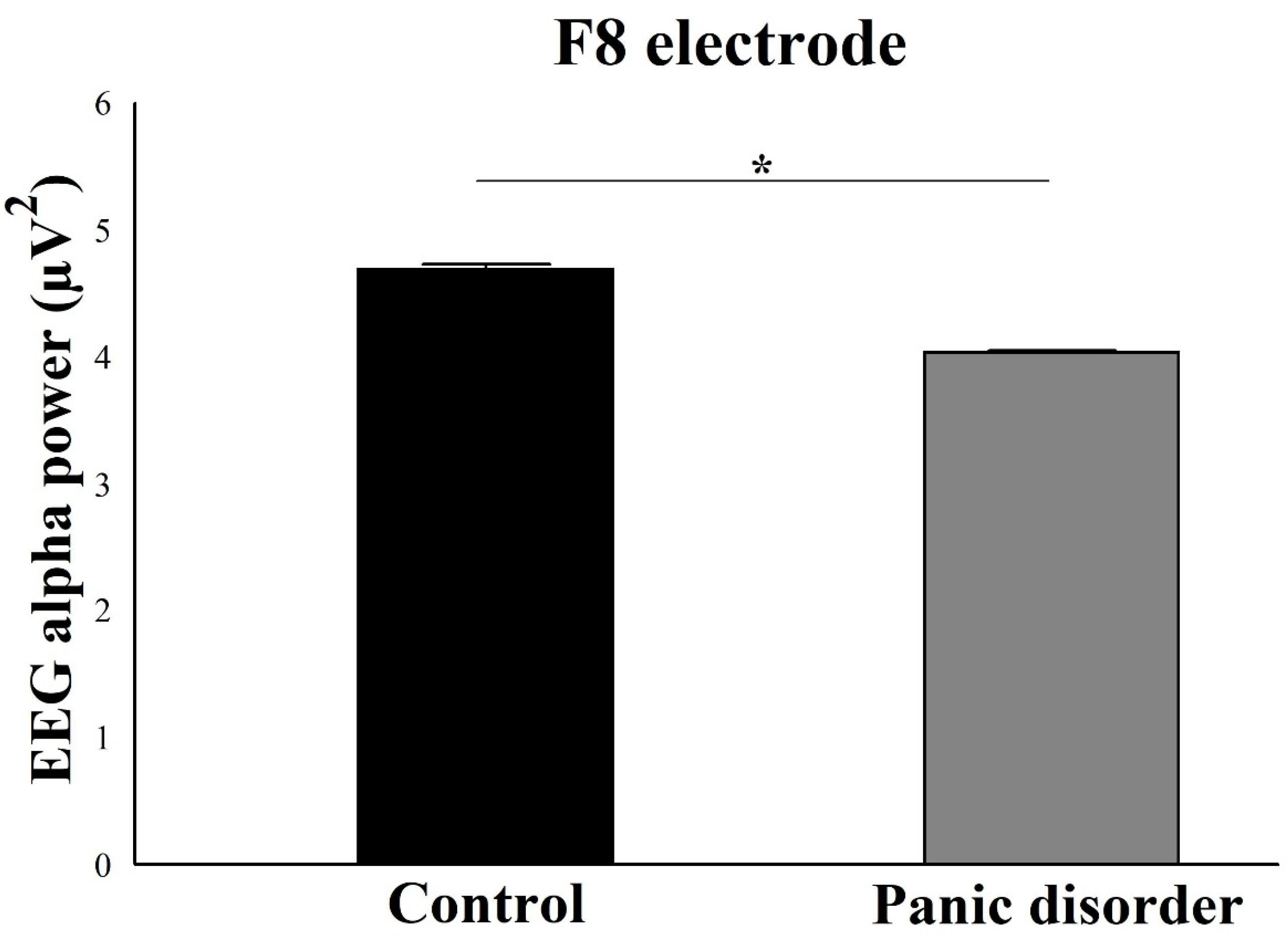
3.1. EEG Alpha Power Analysis
3.2. Reaction Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub: Washington, DC, USA, 2013. [Google Scholar]
- Clark, D.M. A cognitive approach to panic. Behav. Res. Ther. 1986, 24, 461–470. [Google Scholar] [CrossRef]
- Pauli, P.; Dengler, W.; Wiedemann, G.; Montoya, P.; Flor, H.; Birbaumer, N.; Buchkremer, G. Behavioral and neurophysiological evidence for altered processing of anxiety-related words in panic disorder. J. Abnorm. Psychol. 1997, 106, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Teachman, B.A.; Marker, C.D.; Smith-Janik, S.B. Automatic associations and panic disorder: Trajectories of change over the course of treatment. J. Consult. Clin. Psychol. 2008, 76, 988–1002. [Google Scholar] [CrossRef] [PubMed]
- Bouton, M.E.; Mineka, S.; Barlow, D.H. A modern learning theory perspective on the etiology of panic disorder. Psychol. Rev. 2001, 108, 4–32. [Google Scholar] [CrossRef] [PubMed]
- Gorman, J.M.; Kent, J.M.; Sullivan, G.M.; Coplan, J.D. Neuroanatomical hypothesis of panic disorder, revised. Am. J. Psychiatry 2000, 157, 493–505. [Google Scholar] [CrossRef]
- Wise, V.; McFarlane, A.C.; Clark, C.R.; Battersby, M. Event-related potential and autonomic signs of maladaptive information processing during an auditory oddball task in panic disorder. Int. J. Psychophysiol. 2009, 74, 34–44. [Google Scholar] [CrossRef]
- Howe, A.S.; Pinto, A.; De Luca, V. Meta-analysis of P300 waveform in panic disorder. Exp. Brain Res. 2014, 232, 3221–3232. [Google Scholar] [CrossRef]
- Carvalho, M.R.; Velasques, B.B.; Cagy, M.; Marques, J.B.; Teixeira, S.; Nardi, A.E.; Piedade, R.; Ribeiro, P. Electroencephalographic findings in panic disorder. Trends Psychiatry Psychother. 2013, 35, 238–251. [Google Scholar] [CrossRef]
- Silva, L.W.D.G.; Aprigio, D.; Di Giacomo, J.; Gongora, M.; Budde, H.; Bittencourt, J.; Cagy, M.; Teixeira, S.; Ribeiro, P.; de Carvalho, M.R.; et al. How high level of anxiety in Panic Disorder can interfere in working memory—A computer simulation and electrophysiological investigation. J. Psychiatr. Res. 2017, 95, 238–246. [Google Scholar] [CrossRef]
- de Carvalho, M.R.; Dias, G.P.; Cosci, F.; de-Melo-Neto, V.L.; Bevilaqua, M.C.D.N.; Gardino, P.F.; Nardi, A.E. Current findings of fMRI in panic disorder: Contributions for the fear neurocircuitry and CBT effects. Expert Rev. Neurother. 2010, 10, 291–303. [Google Scholar] [CrossRef]
- Windmann, S.; Sakhavat, Z.; Kutas, M. Electrophysiological evidence reveals affective evaluation deficits early in stimulus processing in patients with panic disorder. J. Abnorm. Psychol. 2002, 111, 357–369. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, K.; Newman, E.F. Neuropsychological impairments in panic disorder: A systematic review. J. Affect. Disord. 2014, 167, 268–284. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M.; delPace, L.; Placidi, G.; Keilp, J. Selective cognitive deficits in obsessive-compulsive disorder compared to panic disorder with agoraphobia. Acta Psychiatr. Scand. 2005, 111, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Deckersbach, T.; Moshier, S.J.; Tuschen-Caffier, B.; Otto, M.W. Memory dysfunction in panic disorder: An investigation of the role of chronic benzodiazepine use. Depress Anxiety 2011, 28, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Dratcu, L.; Bond, A. Panic patients in the non-panic state: Physiological and cognitive dysfunction. Eur. Psychiatry 1998, 13, 18–25. [Google Scholar] [CrossRef]
- Riva, G. Virtual reality in psychotherapy: Review. Cyberpsychol. Behav. 2005, 8, 220–230. [Google Scholar] [CrossRef] [PubMed]
- de Tommaso, M.; Ricci, K.; Delussi, M.; Montemurno, A.; Vecchio, E.; Brunetti, A.; Bevilacqua, V. Testing a novel method for improving wayfinding by means of a P3b Virtual Reality Visual Paradigm in normal aging. Springerplus 2016, 5, 1297. [Google Scholar] [CrossRef]
- De Carvalho, M.R.; Freire, R.C.; Nardi, A.E. Virtual reality as a mechanism for exposure therapy. World J. Biol. Psychiatry 2010, 11, 220–230. [Google Scholar] [CrossRef]
- Boschin, E.A.; Buckley, M.J. Differential contributions of dorsolateral and frontopolar cortices to working memory processes in the primate. Front. Syst. Neurosci. 2015, 9, 144. [Google Scholar] [CrossRef]
- Reinecke, A.; Thilo, K.V.; Croft, A.; Harmer, C.J. Early effects of exposure-based cognitive behaviour therapy on the neural correlates of anxiety. Transl. Psychiatry 2018, 8, 225. [Google Scholar] [CrossRef]
- Ball, T.M.; Ramsawh, H.J.; Campbell-Sills, L.; Paulus, M.P.; Stein, M.B. Prefrontal dysfunction during emotion regulation in generalized anxiety and panic disorders. Psychol. Med. 2013, 43, 1475–1486. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, A.; Thilo, K.; Filippini, N.; Croft, A.; Harmer, C.J. Predicting rapid response to cognitive-behavioural treatment for panic disorder: The role of hippocampus, insula, and dorsolateral prefrontal cortex. Behav. Res. Ther. 2014, 62, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhong, Y.; Ma, Z.; Lu, X.; Zhang, N.; Fox, P.T.; Wang, C. Gray matter changes in panic disorder: A voxel-based meta-analysis and meta-analytic connectivity modeling. Psychiatry Res. Neuroimaging 2018, 282, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Gonsalvez, C.J.; Johnstone, S.J. Neural time course of threat-related attentional bias and interference in panic and obsessive-compulsive disorders. Biol. Psychol. 2013, 94, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Farias, T.L.; Marinho, V.; Carvalho, V.; Rocha, K.; da Silva, P.R.A.; Silva, F.; Teles, A.S.; Gupta, D.; Ribeiro, P.; Velasques, B.; et al. Methylphenidate modifies activity in the prefrontal and parietal cortex accelerating the time judgment. Neurol. Sci. 2019, 40, 829–837. [Google Scholar] [CrossRef]
- Fontes, R.M.; Marinho, V.; Carvalho, V.; Rocha, K.; Magalhães, F.; Moura, I.; Ribeiro, P.; Velasques, B.; Cagy, M.; Gupta, D.S.; et al. Time estimation exposure modifies cognitive aspects and cortical activity of attention deficit hyperactivity disorder adults. Int. J. Neurosci. 2020, 130, 999–1014. [Google Scholar] [CrossRef]
- Pavone, E.F.; Tieri, G.; Rizza, G.; Tidoni, E.; Grisoni, L.; Aglioti, S.M. Embodying Others in Immersive Virtual Reality: Electro-Cortical Signatures of Monitoring the Errors in the Actions of an Avatar Seen from a First-Person Perspective. J. Neurosci. 2016, 36, 268–279. [Google Scholar] [CrossRef]
- Vourvopoulos, A.; Bermúdez, I.; Badia, S. Motor priming in virtual reality can augment motor-imagery training efficacy in restorative brain-computer interaction: A within-subject analysis. J. Neuroeng. Rehabil. 2016, 13, 69. [Google Scholar] [CrossRef]
- Dan, A.; Reiner, M. EEG-based cognitive load of processing events in 3D virtual worlds is lower than processing events in 2D displays. Int. J. Psychophysiol. 2017, 122, 75–84. [Google Scholar] [CrossRef]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG alpha oscillations: The inhibition-timing hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef]
- Wiedemann, G.; Stevens, A.; Pauli, P.; Dengler, W. Decreased duration and altered topography of electroencephalographic microstates in patients with panic disorder. Psychiatry Res. 1998, 84, 37–48. [Google Scholar] [CrossRef]
- Gordeev, S.A. Clinical-psychophysiological studies of patients with panic attacks with and without agoraphobic disorders. Neurosci. Behav. Physiol. 2008, 38, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Wise, V.; McFarlane, A.C.; Clark, C.R.; Battersby, M. An integrative assessment of brain and body function ‘at rest’ in panic disorder: A combined quantitative EEG/autonomic function study. Int. J. Psychophysiol. 2011, 79, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Propping, P.; Kruger, J.; Janah, A. Effect of alcohol on genetically determined variants of the normal electroencephalogram. Psychiatry Res. 1980, 2, 85–98. [Google Scholar] [CrossRef]
- Metzger, L.J.; Paige, S.R.; Carson, M.A.; Lasko, N.B.; Paulus, L.A.; Pitman, R.K.; Orr, S.P. PTSD arousal and depression symptoms associated with increased right-sided parietal EEG asymmetry. J. Abnorm. Psychol. 2004, 113, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Freire, R.C.; De Carvalho, M.R.; Joffily, M.; Zin, W.A.; Nardi, A.E. Anxiogenic properties of a computer simulation for panic disorder with agoraphobia. J. Affect. Disord. 2010, 125, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Makino, M.; Hashizume, M.; Nakano, K.; Tsuboi, K. Electroencephalogram abnormalities in panic disorder patients: A study of symptom characteristics and pathology. Biopsychosoc. Med. 2010, 4, 9. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Daltrozzo, J.; Conway, C.M. Neurocognitive mechanisms of statistical-sequential learning: What do event-related potentials tell us? Front. Hum. Neurosci. 2014, 8, 437. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Boudreau, M.; Bacon, S.L.; Paine, N.J.; Cartier, A.; Trutschnigg, B.; Morizio, A.; Lavoie, K.L. Impact of Panic Attacks on Bronchoconstriction and Subjective Distress in Asthma Patients with and Without Panic Disorder. Psychosom. Med. 2017, 79, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Minc, D.; Machado, S.; Bastos, V.H.; Machado, D.; Cunha, M.; Cagy, M.; Budde, H.; Basile, L.; Piedade, R.; Ribeiro, P. Gamma band oscillations under influence of bromazepam during a sensorimotor integration task: An EEG coherence study. Neurosci. Lett. 2010, 469, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Rocha, K.; Marinho, V.; Magalhães, F.; Carvalho, V.; Fernandes, T.; Ayres, M.; Crespo, E.; Velasques, B.; Ribeiro, P.; Cagy, M.; et al. Unskilled shooters improve both accuracy and grouping shot having as reference skilled shooters cortical area: An EEG and tDCS study. Physiol. Behav. 2020, 224, 113036. [Google Scholar] [CrossRef] [PubMed]
- Brunner, C.; Delorme, A.; Makeig, S. EEGlab—An open source Matlab toolbox for electrophysiological research. Biomed. Tech. Berl. 2013, 58 (Suppl. 1). [Google Scholar] [CrossRef] [PubMed]
- Siciliani, O.; Schiavon, M.; Tansella, M. Anxiety and EEG alpha activity in neurotic patients. Acta Psychiatr. Scand. 1975, 52, 116–131. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge Academic: New York, NY, USA, 1988. [Google Scholar]
- Enoch, M.A.; Rohrbaugh, J.W.; Davis, E.Z.; Harris, C.R.; Ellingson, R.J.; Andreason, P.; Moore, V.; Varner, J.L.; Brown, G.L.; Eckardt, M.J.; et al. Relationship of genetically transmitted alpha EEG traits to anxiety disorders and alcoholism. Am. J. Med. Genet. 1995, 60, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.G.; Stanley, M.A.; Averill, P.M.; Baldwin, L.E.; Deagle, E.A., 3rd. Attention and memory for threat in panic disorder. Behav. Res. Ther. 1992, 30, 619–629. [Google Scholar] [CrossRef]
- Pavlenko, V.B.; Chernyi, S.V.; Goubkina, D.G. EEG correlates of anxiety and emotional stability in adult healthy subjects. Neurophysiology 2009, 41, 337–345. [Google Scholar] [CrossRef]
- Palomares, C.E.; Campos, C.P.E.; Ostrosky, S.F.; Tirado, D.E.; Mendieta, C.D. Evaluación de funciones cognitivas: Atención y memoria en pacientes con trastorno de pánico. Salud Ment. 2010, 33, 481–488. [Google Scholar]
- Airaksinen, E.; Larsson, M.; Forsell, Y. Neuropsychological functions in anxiety disorders in population-based samples: Evidence of episodic memory dysfunction. J. Psychiatr. Res. 2005, 39, 207–214. [Google Scholar] [CrossRef]
- Di Giorgio, L.M.; Velasques, B.B.; Ribeiro, P.; Nardi, A.E.; de Carvalho, M.R. Evoked Potential in Panic Disorder Patients: A Systematic Review. CNS Neurol. Disord. Drug Targets 2015, 14, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Cahn, B.R.; Polich, J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol. Bull. 2006, 132, 180–211. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.J.; Ekman, P.; Saron, C.D.; Senulis, J.A.; Friesen, W.V. Approach-withdrawal and cerebral asymmetry: Emotional expression and brain physiology. I. J. Pers. Soc. Psychol. 1990, 58, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi, J.; Hieda, K.; Aoki, Y.; Nagayama, H. Frontal brain hypoactivity as a biological substrate of anxiety in patients with panic disorders. Neuropsychobiology 2003, 47, 165–170. [Google Scholar] [CrossRef]
- Goldstein, K. The Organism; Academic Book: New York, NY, USA, 1939. [Google Scholar]
- Davidson, R.J. Anterior cerebral asymmetry and the nature of emotion. Brain Cogn. 1992, 20, 125–151. [Google Scholar] [CrossRef]
- Demaree, H.A.; Everhart, D.E.; Youngstrom, E.A.; Harrison, D.W. Brain lateralization of emotional processing: Historical roots and a future incorporating “dominance”. Behav. Cogn. Neurosci. Rev. 2005, 4, 3–20. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, L.D.G.; Aprigio, D.; Marinho, V.; Teixeira, S.; Di Giacomo, J.; Gongora, M.; Budde, H.; Nardi, A.E.; Bittencourt, J.; Cagy, M.; et al. The Computer Simulation for Triggering Anxiety in Panic Disorder Patients Modulates the EEG Alpha Power during an Oddball Task. NeuroSci 2022, 3, 332-346. https://doi.org/10.3390/neurosci3020024
Silva LDG, Aprigio D, Marinho V, Teixeira S, Di Giacomo J, Gongora M, Budde H, Nardi AE, Bittencourt J, Cagy M, et al. The Computer Simulation for Triggering Anxiety in Panic Disorder Patients Modulates the EEG Alpha Power during an Oddball Task. NeuroSci. 2022; 3(2):332-346. https://doi.org/10.3390/neurosci3020024
Chicago/Turabian StyleSilva, Luiza Di Giorgio, Danielle Aprigio, Victor Marinho, Silmar Teixeira, Jesse Di Giacomo, Mariana Gongora, Henning Budde, Antonio E. Nardi, Juliana Bittencourt, Mauricio Cagy, and et al. 2022. "The Computer Simulation for Triggering Anxiety in Panic Disorder Patients Modulates the EEG Alpha Power during an Oddball Task" NeuroSci 3, no. 2: 332-346. https://doi.org/10.3390/neurosci3020024
APA StyleSilva, L. D. G., Aprigio, D., Marinho, V., Teixeira, S., Di Giacomo, J., Gongora, M., Budde, H., Nardi, A. E., Bittencourt, J., Cagy, M., Basile, L. F., Orsini, M., Ribeiro, P., & Velasques, B. (2022). The Computer Simulation for Triggering Anxiety in Panic Disorder Patients Modulates the EEG Alpha Power during an Oddball Task. NeuroSci, 3(2), 332-346. https://doi.org/10.3390/neurosci3020024






