Long COVID and the Autonomic Nervous System: The Journey from Dysautonomia to Therapeutic Neuro-Modulation through the Retrospective Analysis of 152 Patients
Abstract
:1. Introduction
2. Methods
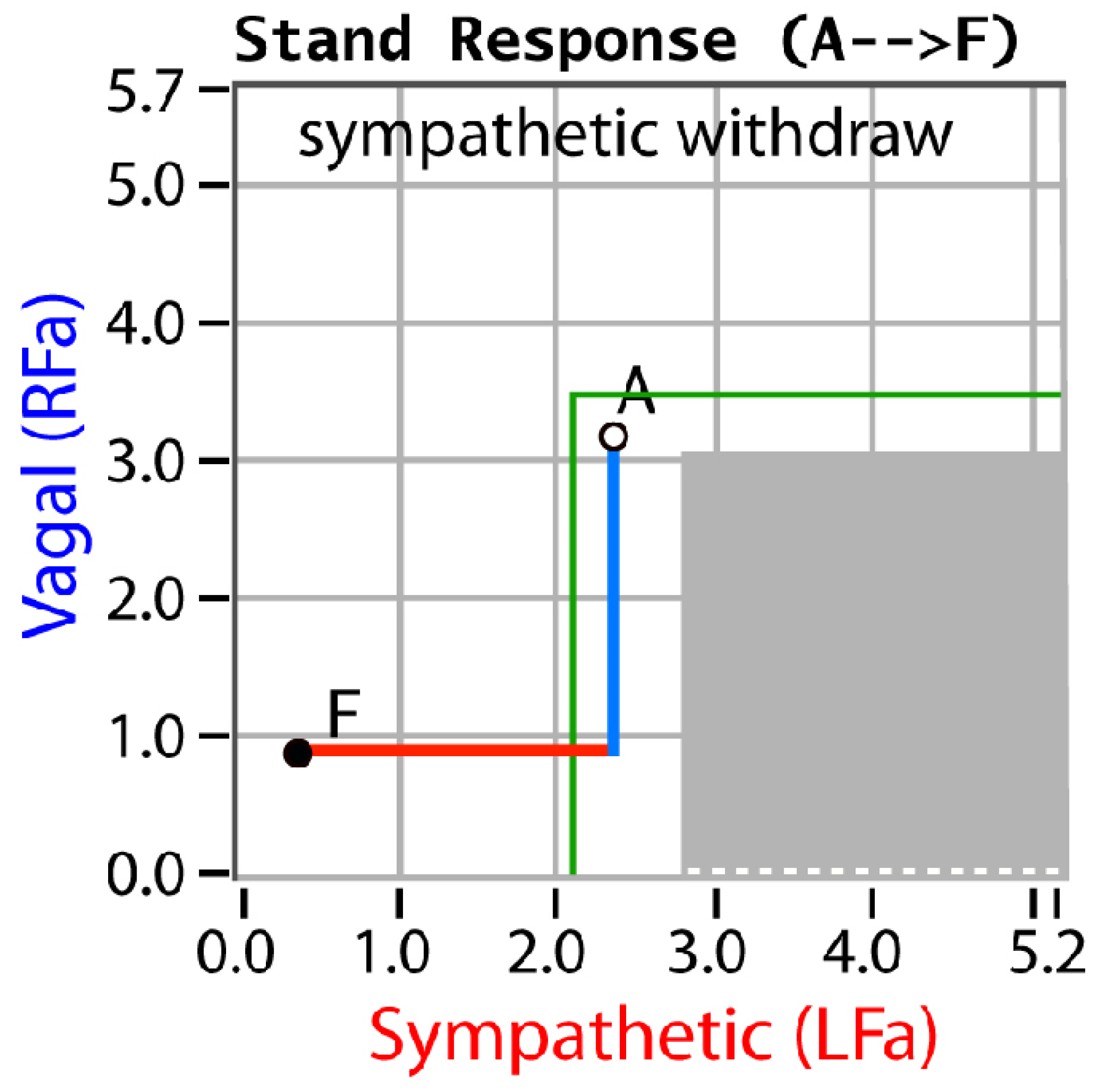
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DePace, N.L.; Colombo, J. Long-covid syndrome: A multi-organ disorder. Cardio. Open 2022, 7, 212–223. [Google Scholar]
- Alwan, N.A.; Johnson, L. Defining long COVID: Going back to the start. Med 2021, 2, 501–504. [Google Scholar] [CrossRef] [PubMed]
- DePace, N.L.; Colombo, J. Long-COVID syndrome: A review of what we have learned clinically to date. 2022; Submitted. [Google Scholar]
- DePace, N.L.; Colombo, J. Autonomic and Mitochondrial Dysfunction in Clinical Diseases: Diagnostic, Prevention, and Therapy; Springer Science + Business Media: New York, NY, USA, 2019. [Google Scholar]
- Colombo, J.; Arora, R.R.; DePace, N.L.; Vinik, A.I. Clinical Autonomic Dysfunction: Measurement, Indications, Therapies, and Outcomes; Springer Science + Business Media: New York, NY, USA, 2014. [Google Scholar]
- Vinik, A.I.; Maser, R.E.; Nakave, A.A. Diabetic cardiovascular autonomic nerve dysfunction. US Endocr. Dis. 2007, 2, 2–9. [Google Scholar] [CrossRef]
- Felton, D.L. Neural influence on immune responses: Underlying suppositions and basic principles of neural-immune signaling. Prog. Brain Res. 2000, 122, 381–389. [Google Scholar]
- Dixit, N.M.; Churchill, A.; Nsair, A.; Hsu, J.J. Post-acute COVID-19 syndrome and the cardiovascular system: What is known? Am. Heart J. Plus. 2021, 5, 100025. [Google Scholar] [CrossRef] [PubMed]
- Baig, A.M. Computing the effects of SARS-CoV-2 on respiration regulatory mechanisms in COVID-19. ACS Chem. Neurosci. 2020, 11, 2416–2421. [Google Scholar] [CrossRef]
- Li, Z.; Liu, T.; Yang, N.; Han, D.; Mi, X.; Li, Y.; Liu, K.; Vuylsteke, A.; Xiang, H.; Guo, X. Neurological manifestations of patients with COVID-19: Potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain. Front Med. 2020, 14, 533–541. [Google Scholar] [CrossRef]
- Román, G.C.; Spencer, P.S.; Reis, J.; Buguet, A.; Faris, M.E.A.; Katrak, S.M.; Láinez, M.; Medina, M.T.; Meshram, C.; Mizusawa, H.; et al. The neurology of COVID-19 revisited: A proposal from the environmental neurology specialty group of the world federation of neurology to implement international neurological registries. J. Neurol. Sci. 2020, 414, 116884. [Google Scholar] [CrossRef]
- Baig, A.M.; Khaleeq, A.; Ali, U.; Syeda, H. Evidence of the COVID-19 virus targeting the CNS: Tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020, 11, 995–998. [Google Scholar] [CrossRef] [Green Version]
- Pergolizzi, J.V., Jr.; Raffa, R.B.; Varrassi, G.; Magnusson, P.; LeQuang, J.A.; Paladini, A.; Taylor, R.; Wollmuth, C.; Breve, F.; Chopra, M.; et al. Potential neurological manifestations of COVID-19: A narra-tive review. Postgrad. Med. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Fischer, L.; Barop, H.; Ludin, S.M.; Schaible, H.G. Regulation of acute reflectory hyperinflammation in viral and other diseases by means of stellate ganglion block. A conceptual view with a focus on COVID-19. Auton. Neurosci. 2022, 237, 102903. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.M.; Shah, M.; Shah, S.; Li, A.; Jauhar, S. Takotsubo syndrome and COVID-19: Associations and implications. Curr. Probl. Cardiol. 2021, 46, 100763. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Qusti, S.; Alshammari, E.M.; Gyebi, G.A.; Batiha, G.E. COVID-19-induced dysautonomia: A menace of sympathetic storm. ASN Neuro. 2021, 13, 17590914211057635. [Google Scholar] [CrossRef] [PubMed]
- Woiciechowsky, C.; Asadullah, K.; Nestler, D.; Eberhardt, B.; Platzer, C.; Schöning, B.; Glöckner, F.; Lanksch, W.R.; Volk, H.D.; Döcke, W.D. Sympathetic activation triggers systemic interleukin-10 release in immunode-pression induced by brain injury. Nat. Med. 1998, 4, 808–813. [Google Scholar] [CrossRef]
- Miller, A.J.; Arnold, A.C. The renin-angiotensin system in cardiovascular autonomic control: Recent developments and clinical implications. Clin. Auton. Res. 2019, 29, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Emmi, A.; Barbon, S.; Boscolo-Berto, R.; Stecco, C.; Stocco, E.; Macchi, V.; De Caro, R. Sympathetic activation: A potential link between comorbidities and COVID-19. FEBS J. 2020, 287, 3681–3688. [Google Scholar] [CrossRef]
- Forsythe, P. The nervous system as a critical regulator of immune responses underlying allergy. Curr. Pharm. Des. 2012, 18, 2290–2304. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Tracey, K.J. The vagus nerve and the inflammatory reflex—Linking immunity and metabolism. Nat. Rev. Endocrinol. 2012, 8, 743–754. [Google Scholar] [CrossRef]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front Psychiatry. 2018, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Lambert, N.J. ; Survivor Corps. COVID-19 “Long Hauler” Symptoms Survey Report; Indiana University School of Medicine: Indianapolis, IN, USA, 2020; Available online: https://dig.abclocal.go.com/wls/documents/2020/072720-wls-covid-symptom-study-doc.pdf (accessed on 20 August 2021).
- Bisaccia, G.; Ricci, F.; Recce, V.; Serio, A.; Iannetti, G.; Chahal, A.A.; Ståhlberg, M.; Khanji, M.Y.; Fedorowski, A.; Gallina, S. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: What do we know? J. Cardiovasc. Dev. Dis. 2021, 8, 156. [Google Scholar] [CrossRef]
- Bloomfield, D.M.; Kaufman, E.S.; Bigger, J.T., Jr.; Fleiss, J.; Rolnitzky, L.; Steinman, R. Passive head-up tilt and actively standing up produce similar overall changes in autonomic balance. Am. Heart J. 1997, 134, 316–320. [Google Scholar] [CrossRef]
- Akselrod, S. Spectral analysis of fluctuations in cardiovascular parameters: A quantitative tool for the investigation of autonomic control. Trends Pharmacol. Sci. 1988, 9, 6–9. [Google Scholar] [CrossRef]
- Arora, R.R.; Bulgarelli, R.J.; Ghosh-Dastidar, S.; Colombo, J. Autonomic mechanisms and therapeutic implications of postural diabetic cardiovascular abnormalities. J. Diabetes Sci. Technol. 2008, 2, 568–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobias, H.; Vinitsky, A.; Bulgarelli, R.J.; Ghosh-Dastidar, S.; Colombo, J. Autonomic nervous system monitoring of patients with excess parasympathetic responses to sympathetic challenges—Clinical observations. US Neurol. 2010, 5, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Prendergast, J.J. Diabetic autonomic neuropathy: Part 2. Treatment. Pract. Diabetol. 2001, 18, 30–36. [Google Scholar]
- Ziegler, D.; Low, P.A.; Litchy, W.J.; Boulton, A.J.M.; Vinik, A.I.; Freeman, R.; The NATHAN 1 Trial Group. Efficacy and safety of antioxidant treatment with α-lipoic acid over 4 years in diabetic polyneuropathy. Diabetes Care 2011, 34, 2054–2060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Q.; Levine, B.D. Exercise and the autonomic nervous system. Handb. Clin. Neurol. 2013, 117, 147–160. [Google Scholar] [CrossRef]
- Goldstein, D.S. The extended autonomic system, dyshomeostasis, and COVID-19. Clin. Auton. Res. 2020, 30, 299–315. [Google Scholar] [CrossRef]
- Larsen, N.W.; Stiles, L.E.; Miglis, M.G. Preparing for the long-haul: Autonomic complications of COVID-19. Auton. Neurosci. 2021, 235, 102841. [Google Scholar] [CrossRef]
- Dani, M.; Dirksen, A.; Taraborrelli, P.; Torocastro, M.; Panagopoulos, D.; Sutton, R.; Lim, P.B. Autonomic dys-function in ‘long COVID’: Rationale, physiology and management strategies. Clin. Med. 2021, 21, e63–e67. [Google Scholar] [CrossRef]
- Raj, S.R.; Arnold, A.C.; Barboi, A.; Claydon, V.E.; Limberg, J.K.; Lucci, V.M.; Numan, M.; Peltier, A.; Snapper, H.; Vernino, S.; et al. Long-COVID postural tachycardia syndrome: An American Auto-nomic Society statement. Clin. Auton. Res. 2021, 31, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Shouman, K.; Vanichkachorn, G.; Cheshire, W.P.; Suarez, M.D.; Shelly, S.; Lamotte, G.J.; Sandroni, P.; Benarroch, E.E.; Berini, S.E.; Cutsforth-Gregory, J.K.; et al. Autonomic dysfunction following COVID-19 infection: An early experience. Clin. Auton. Res. 2021, 31, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Poloni, T.E.; Medici, V.; Moretti, M.; Visonà, S.D.; Cirrincione, A.; Carlos, A.F.; Davin, A.; Gagliardi, S.; Pansarasa, O.; Cereda, C.; et al. COVID-19-related neuropathology and microglial activation in elderly with and without dementia. Brain Pathol. 2021, 31, e12997. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, M.R.; Moekotte, J.; Balvers, K.; Admiraal, M.M.; Pittet, J.F.; Colombo, J.; Wagener, B.M.; Goslings, J.C.; Juffermans, N. Autonomic nervous system activity and the risk of nosocomial infection in critically ill patients with brain injury. Intensive Care Med. Exp. 2020, 8, 69. [Google Scholar] [CrossRef]
- DePace, N.L.; Vinik, A.I.; Acosta, C.R.; Eisen, H.J.; Colombo, J. Oral vasoactive medications: A summary of midodrine and droxidopa as applied to orthostatic dysfunction. Cardio. Open 2022, 7, 224–240. [Google Scholar]
- Abboud, H.; Abboud, F.Z.; Kharbouch, H.; Arkha, Y.; El Abbadi, N.; El Ouahabi, A. COVID-19 and SARS-COV-2 infection: Pathophysiology and clinical effects on the nervous system. World Neurosurg. 2020, 140, 49–53. [Google Scholar] [CrossRef]




| 1. Lightheaded | 2. Fatigue | 3.Chest Pain, Palpitations | 4. Short of Breath | 5. Fainting and Near Fainting | 6. Difficulty Standing | 7. Sweat Too Much, Too Little |
| 8. Brain fog or mental cloudiness | 9. Difficulty finding words | 10. Short-term memory loss | 11. Insomnia, sleep difficulty | 12. Depression, anxiety | 13. Tension headaches | 14. Migraine, headache |
| 15. Chronic pain | 16. Coat hanger pain in neck and shoulders | 17. Pins and needs in arms/legs | 18. Numbness in hands and feet | 19. Hypermobility of joints, joints pop out | 20. Nausea, vomiting | 21. Diarrhea, constipation |
| 22. Sensory: hypersensitive to light, sound, motion, touch | 23. Sensory deficits: vision, hearing, taste, smell | 24. Cold hands or feet | 25. Ringing in ears | 26. Does hot or cold weather bother you? | 27. Hands or feet turn different colors (red, white, or blue) in cold temperatures | 28. Salivate too little, dry mouth |
| Cohort | No. | No. Female | Ave. Age | Ave. BMI | LH | Fatigue | Anxiety | Headache, Migraine | EDSh |
|---|---|---|---|---|---|---|---|---|---|
| # (%) | 152 | 88 (57.8) | 47.0 yrs | 26.9 lbs/ft2 | 152 (100) | 56 (36.9) | 39 (25.7) | 71 (46.9) | 39 (25.7) |
| N = 152 # (%) | SW | PE | SE | Low SB | Hi SB | AAD | CAN | Ave. Sx | Ave. ADs |
|---|---|---|---|---|---|---|---|---|---|
| Pre-COVID-19 | 69 (45.4) | 41 (27.0) | 19 (12.5) | 23 (15.1) | 41 (27.0) | 26 (17.1) | 8 (5.3) | 9.74 | 2.34 |
| Pre-COVID-19 Follow-up | 41 (27.2) | 25 (16.2) | 11 (7.5) | 14 (9.1) | 25 (16.2) | 6 (3.9) | 0 (0) | 6.25 | 0.95 |
| p-value | 0.023 | 0.011 | 0.009 | 0.001 | 0.002 | <0.001 | <0.001 | 0.009 | <0.001 |
| Post-COVID-19 | 55 (36.2) | 71 (46.7) | 44 (28.9) | 58 (38.2) | 69 (45.4) | 31 (20.4) | 10 (6.6) | 14.6 | 3.67 |
| Post-COVID-19 Follow-up | 33 (21.7) | 43 (28.0) | 26 (17.4) | 29 (19.1) | 31 (20.4) | 6 (3.9) | 2 (1.3) | 7.44 | 1.63 |
| p-value | 0.041 | 0.024 | 0.016 | 0.010 | 0.002 | <0.001 | 0.001 | 0.004 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, J.; Weintraub, M.I.; Munoz, R.; Verma, A.; Ahmad, G.; Kaczmarski, K.; Santos, L.; DePace, N.L. Long COVID and the Autonomic Nervous System: The Journey from Dysautonomia to Therapeutic Neuro-Modulation through the Retrospective Analysis of 152 Patients. NeuroSci 2022, 3, 300-310. https://doi.org/10.3390/neurosci3020021
Colombo J, Weintraub MI, Munoz R, Verma A, Ahmad G, Kaczmarski K, Santos L, DePace NL. Long COVID and the Autonomic Nervous System: The Journey from Dysautonomia to Therapeutic Neuro-Modulation through the Retrospective Analysis of 152 Patients. NeuroSci. 2022; 3(2):300-310. https://doi.org/10.3390/neurosci3020021
Chicago/Turabian StyleColombo, Joseph, Michael I. Weintraub, Ramona Munoz, Ashish Verma, Ghufran Ahmad, Karolina Kaczmarski, Luis Santos, and Nicholas L. DePace. 2022. "Long COVID and the Autonomic Nervous System: The Journey from Dysautonomia to Therapeutic Neuro-Modulation through the Retrospective Analysis of 152 Patients" NeuroSci 3, no. 2: 300-310. https://doi.org/10.3390/neurosci3020021





