Abstract
α-Synuclein is a key driver of the pathogenesis of Parkinson disease (PD). Heme oxygenase-1 (HO-1), a stress protein that catalyzes the conversion of heme to biliverdin, carbon monoxide and free ferrous iron, is elevated in PD-affected neural tissues and promotes iron deposition and mitochondrial dysfunction in models of the disease, pathways also impacted by α-synuclein. Elevated expression of human HO-1 in astrocytes of GFAP.HMOX1 transgenic mice between 8.5 and 19 months of age elicits a parkinsonian phenotype characterized by nigrostriatal hypodopaminergia, locomotor incoordination and overproduction of neurotoxic native S129-phospho-α-synuclein. Two microRNAs (miRNA) known to regulate α-synuclein, miR-153 and miR-223, are significantly decreased in the basal ganglia of GFAP.HMOX1 mice. Serum concentrations of both miRNAs progressively decline in wild-type (WT) and GFAP.HMOX1 mice between 11 and 18 months of age. Moreover, circulating levels of miR-153 and miR-223 are significantly lower, and erythrocyte α-synuclein concentrations are increased, in GFAP.HMOX1 mice relative to WT values. MiR-153 and miR-223 are similarly decreased in the saliva of PD patients compared to healthy controls. Upregulation of glial HO-1 may promote parkinsonism by suppressing miR-153 and miR-223, which, in turn, enhance production of neurotoxic α-synuclein. The aim of the current review is to explore the link between HO-1, α-synuclein and PD, evaluating evidence derived from our laboratory and others. HO-1, miR-153 and miR-223 and α-synuclein may serve as potential biomarkers and targets for disease-modifying therapy in idiopathic PD.
1. Introduction
The neurodegenerative movement disorder, Parkinson disease (PD) is the most prevalent of several human synucleinopathies. Shortly after the discovery of SNCA mutations causing a rare monogenic form of PD in 1997 [1], α-synuclein protein aggregates were identified as a major component of hallmark Lewy bodies and a key player in PD pathology [2]. As with other synucleinopathies, including multiple system atrophy and Lewy body dementia, cardinal symptoms of PD include bradykinesia, rigidity, rest tremor and postural instability. Non-motor symptoms, such as cognitive decline, hyposmia (loss of sense of smell), rapid eye movement (REM) sleep behavior disorder (RBD), constipation and other autonomic dysfunctions and depression and anxiety, complete the clinical picture. Although symptomatic pharmacotherapy is available, there currently exists no treatment that unequivocally mitigates neuronal attrition and clinical decline in this condition.
2. α-Synuclein: A Key Player in Parkinson Disease
α-Synuclein has long been considered a major component of PD pathology. While precise mechanisms of abnormal α-synuclein aggregation remain disputed, there is fair consensus implicating oxidative reactions in this process [3,4]. Dimerization of Tyr125 is the initial and rate-limiting step that ultimately leads to a greater potential for self-interaction of this protein [5]. The dimerized α-synuclein serves as the template for native α-synuclein monomers to refold into oligomers, protofibrils and fibrils rich in β-pleated sheets. α-Synuclein oligomers, protofibrils and fibrils contribute to neuronal death via oxidative stress, energy failure, excitotoxicity and neuroinflammation [6,7]. Phosphorylation, oxidation, nitration and glycation are post-translational modifications of α-synuclein that enhance its propensity for aggregation [8]. Of particular importance, phosphorylation is a necessary event during the formation of Lewy bodies, as de-phosphorylation, specifically at Ser129, ameliorates the phenotype [9]. These are important considerations when evaluating α-synuclein as a potential biomarker for neurodegenerative synucleinopathies, such as PD.
α-Synuclein has been the primary focus of many PD biomarker studies. Altered α-synuclein levels (soluble, aggregated and post-translationally modified forms) in cerebrospinal fluid (CSF), blood and saliva have been reported, with general consensus describing reduced total α-synuclein, higher oligomeric α-synuclein and higher oligomeric-to-total-α-synuclein ratios in PD patients compared to controls [10,11,12,13,14,15,16,17,18]. Relating to α-synuclein but acting upstream, we have also reported alterations in circulating levels of potential α-synuclein regulators, including heme oxygenase-1 (HO-1), microRNA (miR)-153 and miR-223 [19,20,21].
The current review is centered on the link between α-synuclein, HO-1 and key miRNAs as they relate to PD.
3. Involvement of Heme Oxygenase-1 in Parkinson Disease Pathology
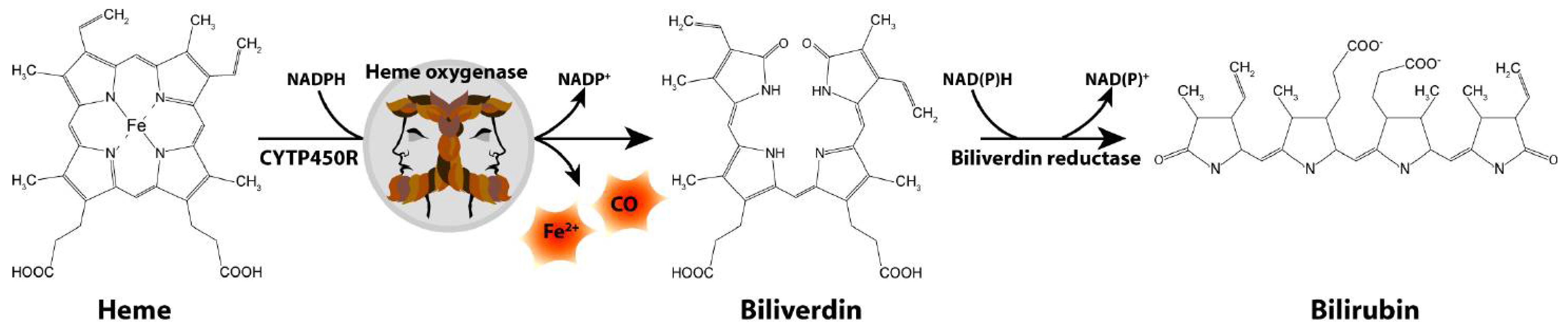
The Schipper laboratory has been studying neurodegenerative disorders, such as PD, for over two decades through the lens of a highly inducible stress protein, HO-1 [22]. HO-1 catalyzes the conversion of heme into biliverdin, carbon monoxide and free ferrous iron in brain and other tissues (Figure 1). The HMOX1 promoter contains numerous response elements which render the gene exquisitely sensitive to induction by heme, β-amyloid, lipopolysaccharide, hyperoxia, ultraviolet light, IL-1β, TNF-α, hydrogen peroxide and heavy metals, among many others [23]. Though typically assuming a protective role via the antioxidant properties of biliverdin and bilirubin [24,25,26,27], our laboratory and others have shown that chronic overexposure of HO-1 may exacerbate intracellular oxidative damage in mitochondria and other subcellular compartments via heme-derived iron and carbon monoxide [28,29,30,31]. The latter may prevail in PD and other chronic neurodegenerative conditions.
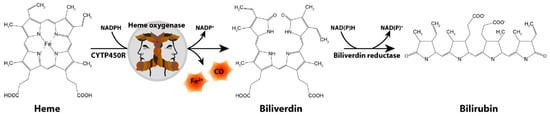
Figure 1.
Heme degradation pathway. CO, carbon monoxide; CYTP450R, cytochrome P450 reductase; Fe2+, ferrous iron.
Astrocytes and microglia in PD basal ganglia, although not neurons, exhibit significant elevations of HO-1 protein [32,33]. HMOX1 induction in dopaminergic neurons is commensurate with the fact that, during normal aging, nigral dopaminergic neurons are subjected to elevated levels of reactive oxygen species (ROS) arising from the iron-catalyzed oxidation and enzymatic deamination of dopamine [34]. Further, cytoplasmic Lewy bodies within affected dopaminergic neurons of the PD substantia nigra exhibited prominent HO-1 immunoreactivity [32,35]. Some studies have linked polymorphisms in HMOX1 to the disease. One such study disclosed a strong association between rs2071746 (A/T), a single-nucleotide polymorphism that enhances HMOX1 promoter activity/transcription and clinical idiopathic PD [36]. Another group found a synergistic association of HMOX1 rs2077146TT polymorphism and pesticide exposure, specifically increasing the risk for PD [37]. Finally, several polymorphisms in the promotor region of NRF2 and the Nrf2 binding region of the microtubule-associated protein tau (MAPT) promoter, a known transcriptional activator of HMOX1, have been associated with PD [38,39].
Astrocyte activation, particularly in the substantia nigra, has been documented in patients with PD and animal models of PD [40,41]. Astrocyte activation can produce TNF-α, IL-1β, nitric oxide and other inflammatory factors, all of which may lead to the induction of HMOX1. In addition to pro-inflammatory cytokines, other plausible inducers of HMOX1 in astrocytes of the PD substantia nigra include environmental or endogenous MPTP-like neurotoxins and dopamine-derived ROS [42,43]. The local uptick in glial HO-1 activity seen in PD may accelerate the deposition of non-transferrin iron and mitochondrial complex I deficits documented in PD-affected neural tissues [44]. This glial mitochondrial iron mediates the oxidation of dopamine to neurotoxic o-quinone radicals [44] and oxidizes the pro-neurotoxin MPTP to the dopamine neurotoxin MPP+ [45], which inhibits mitochondrial complex 1 and leads to oxidative stress, the loss of adenosine triphosphate, protein nitration and apoptosis of dopaminergic neurons [41,46]. Autophagy in astrocytes is considered to have a vital role in the pathogenesis of aging and neurodegenerative disease, particularly for the removal of pro-oxidant species [47]. Furthermore, brain astrocytes exposed to high glucose concentration undergo apoptosis through the activation of HO-1 via NF-kB and AP-1, providing evidence for Nrf2-independent induction of neurotoxic HO-1 [48]. In addition to the above reports, extensive in vitro and in vivo evidence from our laboratory and others has directly implicated HO-1 in PD pathogenesis [22].
HMOX1 transfection of human M17 neuroblastoma was shown to stimulate proteasomal catabolism of WT α-synuclein [49] but had no effect on proteasomal degradation of A30P α-synuclein, a mutant protein implicated in familial autosomal-dominant PD [1]. Insofar as non-digestible α-synuclein aggregates are cytotoxic [50], the imperviousness of A30P α-synuclein protein to HO-1-directed proteolysis may contribute to Lewy body formation and parkinsonism in people with this mutation [49]. Iron is also believed to serve as a major contributor by inducing oxidative stress and α-synuclein aggregation, and changes in Nrf2 and HO-1 have been shown contribute to iron-induced α-synuclein aggregation [51]. Xu et al. showed a significant positive correlation between elevated serum HO-1 concentrations and iron deposition within the substantia nigra and an inverse correlation between elevated serum HO-1 and reduced hemoglobin levels in PD patients relative to controls [52]. This increase in HO-1 may be a common mechanism underlying the iron deposition and low hemoglobin documented in PD. Another study demonstrated a time-dependent HO-1 upregulation in primary cultured ventral mesencephalon astrocytes treated with MPP+ or recombinant α-synuclein [53]. Attesting to the dual nature of HO-1, treatment with cobalt protoporphyein IX, an HO-1 activator, exerted protective effects against MPP+ or α-synuclein during moderate HO-1 upregulation, but it aggravated damage at the peak of the HO-1 response [53]. Importantly, core cytopathology implicated in PD (oxidative stress, mitochondrial damage and macroautophagy) is abrogated in HMOX1-transfected primary astrocytes treated with the competitive HO inhibitor, tin mesoporphyrin [54].
This in vitro work led to the development of a novel transgenic mouse model, the GFAP.HMOX18.5−19 m mouse, in which human HMOX1 was selectively overexpressed in astrocytes between 8.5 to 19 months of age [55]. The transgene cascade of the GFAP.HMOX1 mouse leads to activation of the human HO-1 coding sequence through the upstream promoter drive of glial fibrillary acidic protein (GFAP) and the “valve-controller” of tetracycline activator. This design confers two advantages: (1) The GFAP promoter selectively targets HMOX1 gene expression to the astrocytes, and (2) the Tetracycline (Tet)-Off system permits temporal control of the transgene expression [55]. The doxycycline diet, which inhibits transgene expression, was replaced with a regular rodent diet between 8.5 and 19 months of age to permit HMOX1 expression in astrocytes [55]. Our glia-centric focus on HO-1 expression is pivotal to our group’s longstanding perspective on mechanisms of neurodegeneration in the parkinsonian brain. This approach is based on the following considerations: (1) The excess iron reported in the aging and PD brain largely implicates glial and other non-neuronal cells [23]. (2) HO-1 is significantly upregulated in the astrocytes, not neurons, of the PD substantia nigra relative to normal age-matched control values [32]. Further, astrocytes and microglia have a greater propensity to mount a strong HO-1 (as well as other stress protein) response compared to neurons and oligodendrocytes [56,57,58]. (3) Hmox1 induction by stressors implicated in PD (e.g., hydrogen peroxide, heavy metals, TNF-α, etc.) is a common pathway leading to mitochondrial iron deposition, oxidative mitochondrial damage and macroautophagy in aging subcortical astroglia [23,31,32,59]. (4) The ability of astrocytes to effectively revert to anaerobic metabolism for their energy needs, also known as the Warburg effect, may permit astroglia (but not neurons) to sacrifice a considerable fraction of their mitochondria with minimal consequence [43]. (5) Finally, the progressive accumulation of glial-mitochondrial iron within subcortical brain regions enhances the vulnerability of nearby dopaminergic neurons to oxidative injury and may thereby render the senescent central nervous system (CNS) more prone to PD [44,59]. For these reasons, HMOX1 was selectively overexpressed in astrocytes of this novel transgenic mouse model. Adding further predictive validity to our model, inhibition of HO-1 activity in vitro or in vivo rescues the resulting phenotype [54,60]. The following section outlines evidence accrued from parkinsonian GFAP.HMOX18.5−19 m mice.
4. GFAP.HMOX18.5−19 m Transgenic Mice
At 19 months of age, GFAP.HMOX18.5−19 m mice recapitulate key features of PD relative to age-matched wild-type (WT) controls, as evidenced by the following: (i) impaired locomotion (rotarod), circling behavior, motor incoordination (pole test), altered ambulation (gait test) and reduced olfaction (buried pellet test); (ii) dopaminergic neuron degeneration in the substantia nigra, with substantial vacuolation manifesting in the remaining neurons and decreased dopamine levels in the striatum; (iii) upregulated pituitary homeobox 3 (Pitx3)– and dopamine transporter (DAT)–targeting miR-133b and nuclear receptor related-1 protein (Nurr1)–targeting miR-145 in the nigrostriatum; (iv) significant elevation of gamma amino butyric acid (GABA) in the substantia nigra [61,62,63]; (v) increased iron deposition and improved rotarod performance after treatment with the iron chelator deferiprone; (vi) enhanced protein carbonylation, a marker of oxidative stress, in the striatum; (vii) reduced and disorganized mitochondrial cristae and fragmented mitochondrial membranes within the striatum [55,64,65]; and (viii) dysregulated autophagy and accumulation of striatal osmiophilic inclusions, indices of autophagosome formation and mitophagy. Notably, this phenotype was not observed in mice expressing the HMOX1 transgene for an identical duration between 1.5 and 12 months of age [55], underscoring the importance of brain aging for symptom manifestation in both human and experimental parkinsonism.
The upregulation of brain HO-1 is similarly observed in other animal models of PD, including MPTP- and rotenone-treated mice [66,67,68]. HO-1 upregulation was also associated with loss of dopaminergic neurons in the MPP+-induced parkinsonian rat model [69]. Furthermore, the significant increases in serum HO-1 levels and iron deposition in the substantia nigra observed in the MPTP mouse model of PD were abrogated by treatment with the HO inhibitor tin mesoporphyrin [52]. HO-1 is involved in a number of other disease pathways, primarily due to the high inducibility and ubiquity of HMOX1. Complete Hmox1 knockout results in a general pro-inflammatory phenotype in mice [70,71], attesting to the protective nature of heme degradation products, biliverdin and bilirubin. The cellular localization of HO-1 also seems to be important. While we and others report a behavioral, pathological and biochemical phenotype consistent with PD when HMOX1 overexpression is restricted to astrocytes, overexpression of HO-1 in neurons in mice protects against oxidative insult [72]. Transgenic mice overexpressing HO-1 in neurons also exhibit low levels of lipid peroxidation end-products and enhanced expression of the anti-apoptotic protein bcl-2 after cerebral ischemia [73]. Similarly, upregulation of HO-1 in microglia confers a strong anti-inflammatory effect and reduction in oxidative damage [74,75]. The chronicity of HO-1 action appears to be a crucial factor in determining outcome as HMOX1 in the GFAP.HMOX1 mouse model confers significant neuroprotection in the face of acute intracerebral hemorrhage [76,77].
4.1. GFAP.HMOX18.5–19 m Mice and α-Synuclein
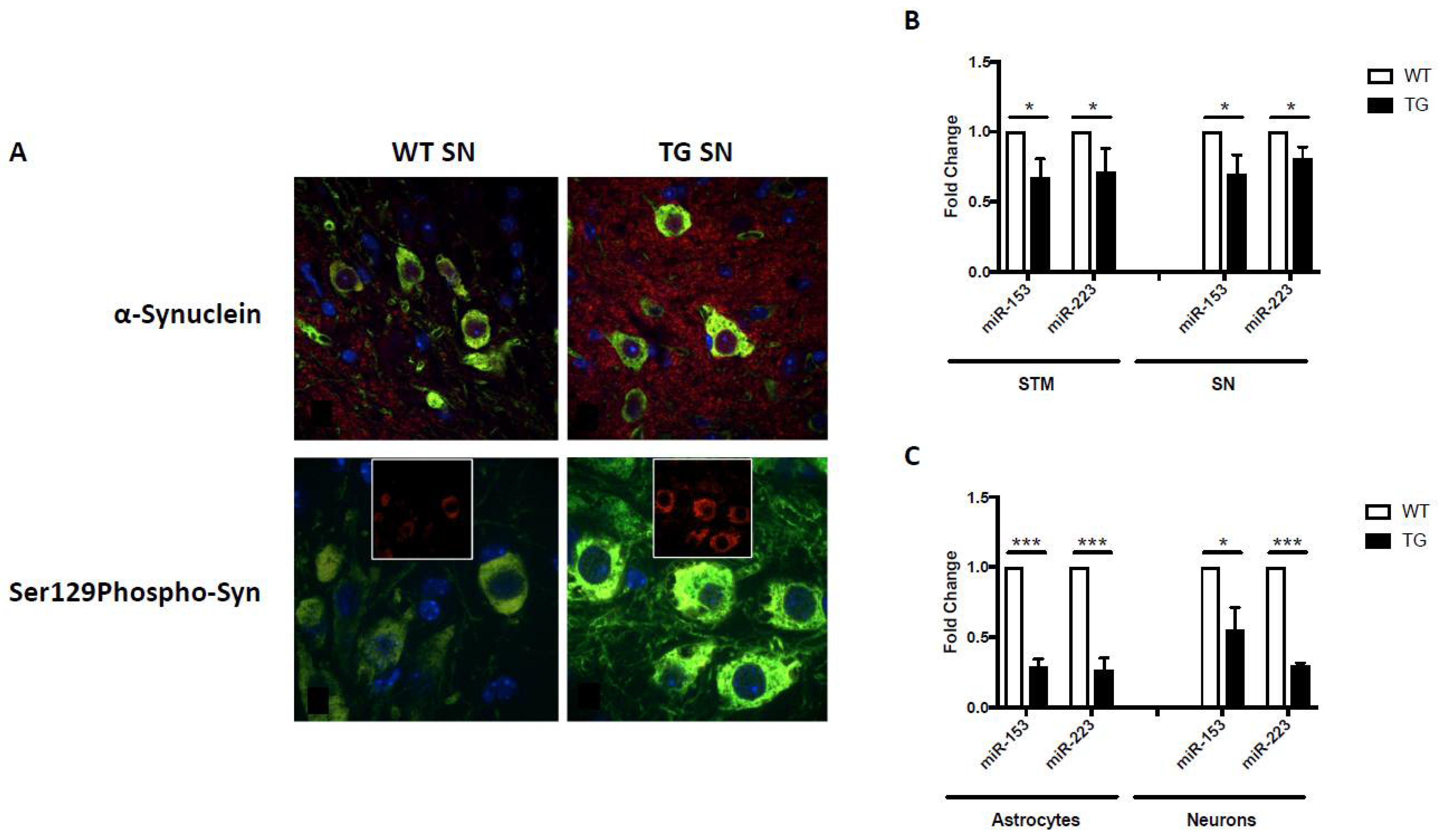
In addition to the aforementioned behavioral, pathological and biochemical alterations, GFAP.HMOX18.5−19 m mice also exhibited significant α-synuclein-related pathologies. Notably, α-synuclein and pathological Ser129-phosphorylated α-synuclein were significantly increased within and surrounding the dopaminergic neurons of the substantia nigra of 19-month-old GFAP.HMOX18.5−19 m mice compared to age-matched WT controls (Figure 2A) [55,64]. Significant elevations in ubiquitin, ubiquitin-binding protein p62 (p62) and HO-1, previously associated with Lewy body inclusions [32,78,79], were also observed in our transgenic mice, though no overt Lewy pathology was noted [55]. The lack of Lewy body inclusions observed in 19-month-old GFAP.HMOX18.5−19 m mice, a common criticism of parkinsonian animal models, may be rationalized as follows: (i) GFAP.HMOX18.5−19m mice may represent a mid-stage model of PD, considering the other early-to-mid-stage features of parkinsonism in this model, such as reduced olfaction and only 47% loss of nigral dopaminergic neurons [55,65]; whether GFAP.HMOX18.5−19 m mice would display Lewy body inclusions later in life (e.g., at 24 months of age) remains to be determined; and (ii) HMOX1 overexpression may not be sufficient to elicit Lewy body inclusion formation. To interrogate the latter, offspring of GFAP.HMOX1 mice crossed with an α-synuclein overexpressing model could be assayed for augmented Lewy body inclusion formation. Enhanced α-synuclein production and aggregation has been linked to increased levels of iron, and induction of HMOX1 was noted to result in greater α-synuclein protein deposition in vitro [64]. Further supporting the link between HO-1 and α-synuclein, HMOX1 overexpression from embryogenesis until 12 months of age, as seen in the GFAP.HMOX10–12 m mouse model of schizophrenia, similarly resulted in significant elevation of α-synuclein mRNA and protein [80].
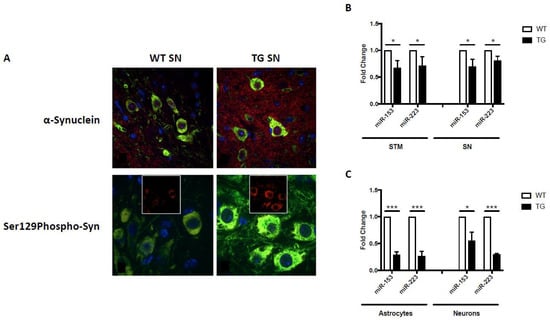
Figure 2.
α-Synuclein expression and miRNA regulation in GFAP.HMOX1 mice. (A) Confocal imaging of intra- and extraperikaryal α-synuclein (red) and Ser129-phosphorylated (S129Phospho) α-synuclein (red) immunofluorescence in dopaminergic neurons of the substantia nigra (SN). TH (green) and DAPI (blue) in 19-month-old WT and transgenic substantia nigra. (B) Downregulation of miR-153 and miR-223 in transgenic substantia nigra (SN) and striatum (STM) in vivo and (C) transgenic primary astrocyte–neuronal co-cultures. All data were obtained via RT-qPCR and analyzed using the ΔΔCt method relative to internal and endogenous controls. n = 5; * p < 0.05; *** p < 0.001. (From [64], with permission).
4.2. GFAP.HMOX1 Primary Cultures and α-Synuclein
Targeting α-synuclein is an important therapeutic consideration in PD, highlighted by the fact that siRNA inhibition of the gene encoding this protein, Snca, in primary GFAP.HMOX1 astrocytes attenuated oxidative stress to levels observed in negative control and WT preparations [64]. Moreover, when co-culturing primary GFAP.HMOX1 astrocytes with WT neurons, followed by treatment with siRNA against Snca, WT neurons displayed normalization of mRNAs involved in oxidative stress (MnSOD), dopaminergic neuron development and maintenance (Nurr1, Pitx3), dopamine metabolism (TH, DAT), mitophagy (DJ-1), mitochondrial fission (Drp1) and mitochondrial fusion (Mfn2, Mfn1) [64]. These observations underscore the importance of α-synuclein suppression in mitigating key pathomechanisms in PD, possibly in combination with upstream HO-1 inhibition. Potentially viable targets of α-synuclein mitigation include miRNAs, miR-153 and miR-223, which were found to negatively regulate α-synuclein mRNA and protein [64].
4.3. MicroRNAs In Vitro and In Vivo
MiRNAs have gained immense traction as key regulators in development, normal aging and disease, including PD [81,82,83]. In 2015, we published a study looking at miRNA profiles in HMOX1-transfected primary rat astrocytes compared to sham-transfected controls and identified three significantly upregulated miRNAs (miR-140*, miR-17 and miR-16) and six significantly downregulated miRNAs (miR-297, miR-206, miR-187, miR-181a, miR-138 and miR-29c) in the HMOX1-transfected cells [84]. Moreover, the effects of HO-1 induction on glial miRNA profiles were abrogated by a competitive HO inhibitor (tin mesoporphyrin), an iron chelator (deferoxamine) and a CO antagonist (methylene blue), directly implicating HO-1, iron and CO, respectively, in the aberrant miRNA expression profiles [84]. On the other hand, the addition of bilirubin, the final product of heme catabolism, had little effect on these miRNA levels in cultured astrocytes [84]. These salient miRNA profiles were recapitulated in WT cells treated with the iron donor Fe(NO3)3 [84]. Taken together, these results lend support to the canonical nature of HO-1 activity in our system and its role in the perturbed expression of PD-relevant miRNAs.
Akin to miRNA changes in HMOX1-transfected primary rat astrocytes, GFAP.HMOX18.5−19 m mice and primary GFAP.HMOX1 astrocyte–neuron co-cultures display altered miRNA expression profiles. In addition to changes in Pitx3- and DAT-targeting miR-133b and Nurr1-targeting miR-145 described above [55,64], changes in miRNAs targeting α-synuclein were also observed. Conserved binding sites for miR-153 and miR-223 were predicted to lie within the 3′-untranslated region (UTR) of SNCA with partial complementarity (TargetScan software), and miR-153 was confirmed to bind the 3′-UTR of SNCA and suppress α-synuclein mRNA and protein [85,86,87]. This partial complementarity suggests regulation at the level of both SNCA mRNA via targeted mRNA degradation and α-synuclein protein via translation repression, a notion that is supported by our mimic and inhibitor experiments [64]. M17 human neuroblastoma cells transfected for 12 h with either a miR-153 or miR-223 mimic resulted in significant downregulation of α-synuclein mRNA and protein relative to controls [64]. Conversely, M17 human neuroblastoma cells transfected for 12 h with either a miR-153 or miR-223 inhibitor resulted in significant upregulation of α-synuclein mRNA and protein relative to controls [64]. In the earlier HMOX1-transfected primary rat astrocyte experiments, miR-153 expression trended downwards while miR-223 showed no significant change, though these results were not further validated by RT-qPCR [84]. In GFAP.HMOX18.5−19 m mice; on the other hand, α-synuclein-targeting miR-153 and miR-223 were significantly downregulated in the substantia nigra and striatum, as well as in primary astrocyte and neuron co-cultures generated from transgenic mouse brain, compared to WT control preparations (Figure 2B,C) [64]. This falls in line with the apparent upregulation of α-synuclein protein observed in GFAP.HMOX18.5−19 m mouse brains [64] and further attests to the link between HO-1, α-synuclein and miR-153/miR-223.
Other targets of miR-153 include β-amyloid and nuclear factor erythroid 2-related factor 2 (Nrf2). Similar to α-synuclein deposition in the PD brain, suppression of miR-153 has been correlated with high levels of amyloid precursor protein and β-amyloid in the Alzheimer disease (AD) brain [88,89]. Additionally, inhibition of miR-153 promotes the expression of Nrf2 and HO-1 [90], and this miRNA has been reported to be dysregulated in the APPswe/PS1ΔE9 mouse model of AD [91] as well as the MPTP mouse model of PD [92]. Other targets of miR-223 include NF-kB and IL-1. Notably, β-amyloid, amyloid precursor protein, Nrf2, NF-kB and IL-1 have all been identified as inducers of HMOX1 [22].
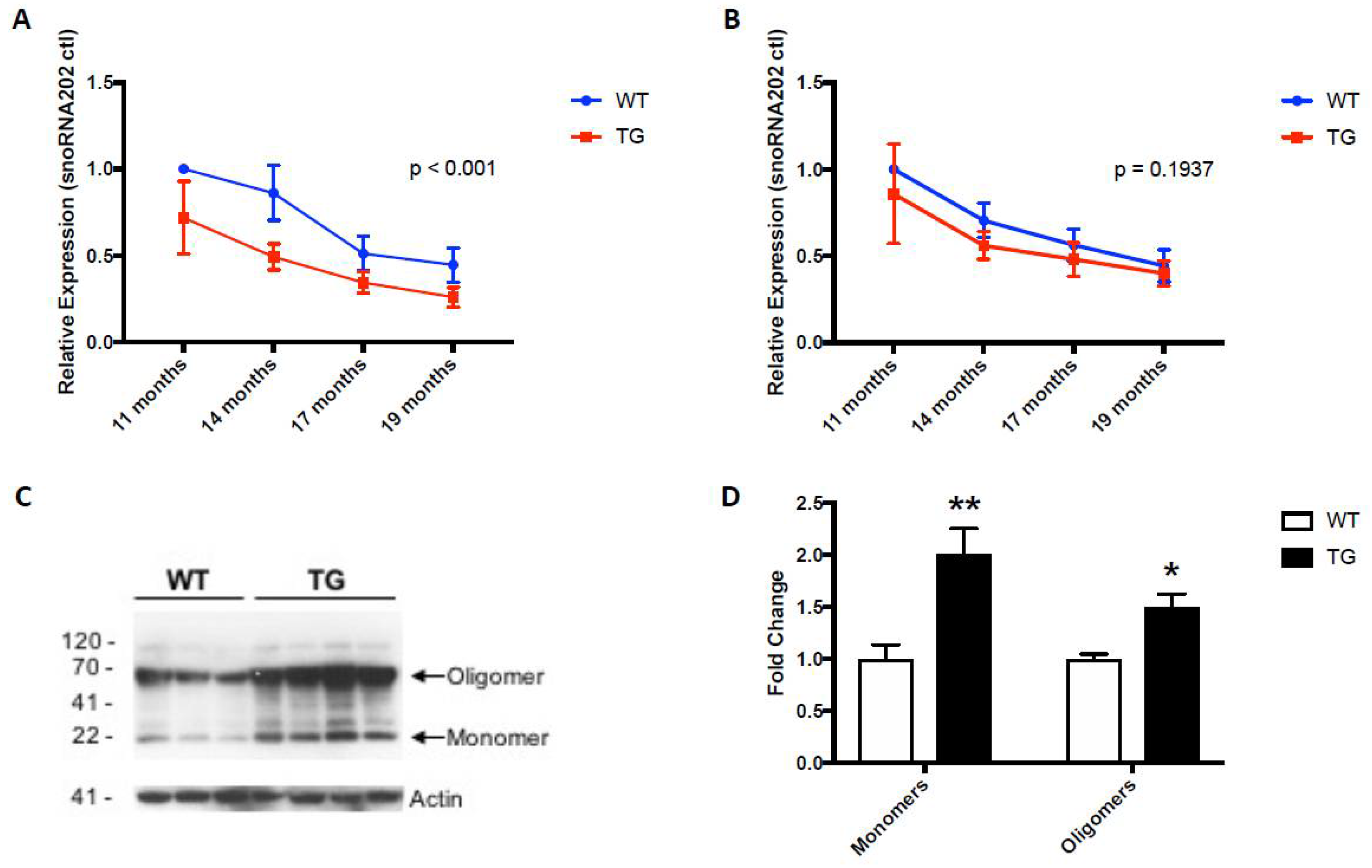
Intriguingly, circulating levels of miR-153 and miR-223 were similarly lower in the 19-month-old GFAP.HMOX18.5−19 m mice compared to age-matched WT controls (Figure 3A,B) [64], commensurate with the systemic alterations in α-synuclein gene and protein expression profiles in GFAP.HMOX18.5−19 m mouse erythrocytes (Figure 3C,D), as well as in idiopathic human PD [15,18,55,64,93]. The peripheral changes in miR-153 and miR-223 levels in GFAP.HMOX18.5−19 m mice are what led to the study of these key miRNAs in human PD saliva as potential biomarkers of the disease.
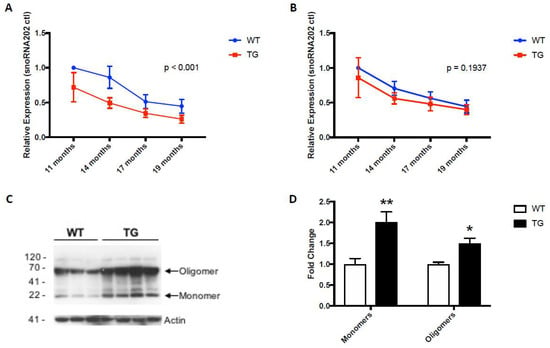
Figure 3.
Expression patterns of miR-153 (A), miR-223 (B) and α-synuclein (C,D) in WT and GFAP.HMOX1 mouse blood. Blood serum was procured from WT and transgenic mice at 11, 14, 17 and 19 months of age, while RBCs were isolated from WT and transgenic mice at 19 months of age only. MiRNA expression levels were determined by RT-qPCR and analyzed using the ΔΔCt method relative to internal and endogenous controls. Western blot was used to measure α-synuclein protein. For (A,B), n = 7–13; for (C,D), n = 7–8; * p < 0.05; ** p < 0.01. (From [64], with permission).
5. miR-153, miR-223 and Heme Oxygenase-1 as Biomarkers of Parkinson Disease
Prior to evaluating miR-153 and miR-223 levels in human PD, we assessed the potential of HO-1 protein to serve as a biomarker for this condition. In 2018, we published a study reporting increased concentrations of salivary HO-1 in early stage (Hoehn and Yahr [H&Y] stage 1) PD patients compared to non-neurological (healthy) controls [21]. This corroborated previous findings of elevated HO-1 levels in the serum and plasma of PD patients [52,94,95]. Saliva offers advantages over other biofluids as its acquisition is non-invasive, inexpensive and requires minimal training of personnel [96,97,98]. In 2021, we published a study corroborating the significant elevation of salivary HO-1 in PD subjects compared to non-neurological control subjects and non-degenerative neurological controls (multiple sclerosis, epilepsy, essential tremor, stroke, nerve pain) though not compared to subjects with other neurodegenerative conditions (AD, mild cognitive impairment) [20]. Importantly, overexpression of HO-1 protein and mRNA has also been observed in AD and mild cognitive impairment [99,100,101]. Considering that HO-1 has also been linked to a variety of neurodegenerative conditions, including AD and mild cognitive impairment, the lack of specificity of high salivary HO-1 levels in PD is not surprising. Receiver operating characteristic (ROC) curves using HO-1 and covariates yielded areas under the curve above 85% in models for PD or neurodegenerative conditions relative to controls [20], suggesting HO-1 may be a reliable biomarker that distinguishes patients with PD from non-neurological and non-degenerative controls. Additional secondary analyses from the Galindez et al. study revealed a significant difference between non-PD controls and H&Y stage 2 PD participants [20]. More likely, HO-1 induction may be involved in the pathophysiology of neurodegeneration in general, rather than in one specific neurodegenerative condition. This may prove valuable in a clinical setting, as movement disorder specialists are often at a loss to differentiate idiopathic PD from neuroleptic-induced extrapyramidal disorders or vascular parkinsonism. Similarly, non-degenerative causes of mild cognitive impairment and dementia, such as toxic-metabolic encephalopathies or the psychomotor retardation complications of severe depression, may confound the diagnosis of AD. The ability to accurately distinguish between the degenerative and non-degenerative etiologies responsible for these clinical presentations by means of a salivary HO-1 protein or other simple biochemical assay, including miRNA analysis, would be a welcome development [20].
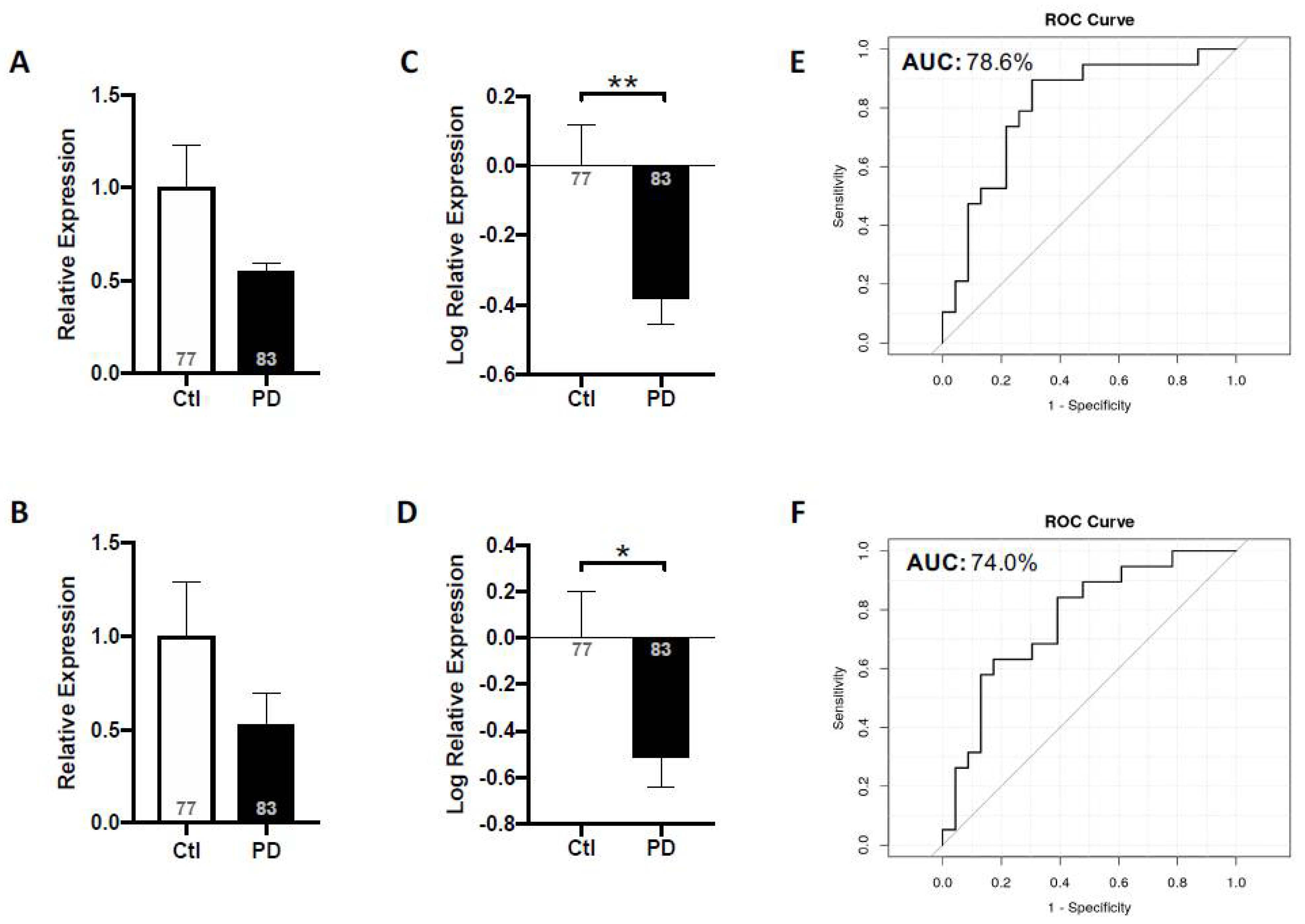
The results of our 2018 and 2021 studies on salivary HO-1 prompted us to measure miR-153 and miR-223, acting downstream of HO-1 and targeting α-synuclein, in PD saliva [19]. We observed that the expression levels of miR-153 and miR-223 were significantly reduced in PD saliva relative to that of non-neurological controls, and these expression patterns were unaffected by age, sex, various comorbidities, disease duration or medication exposure (Figure 4A–D) [19]. The area under the ROC curve separating controls from PD patients was 79% (confidence interval: 64–99%) for miR-153 and 74% (confidence interval: 60–93%) for miR-223 (Figure 4E,F) [19], suggesting moderately good biological markers of PD. Another miRNA that has been linked to α-synuclein regulation, miR-7 [85,102], was also assayed in saliva, though we did not observe significant alterations in miR-7a or miR-7b in PD patients relative to controls [19]. Additionally, the measurement of the ratios of key proteins acting upstream (HO-1) or downstream (oligomeric or total α-synuclein) of miR-153 or miR-223 to these miRNA expression levels did not improve the accuracy of the test as a PD neurodiagnostic relative to the ascertainment of miR-153 or miR-223 alone [19]. The alterations in salivary miRNAs described herein are consistent with our earlier findings in the brain and serum of parkinsonian GFAP.HMOX18.5−19 m mice [64].
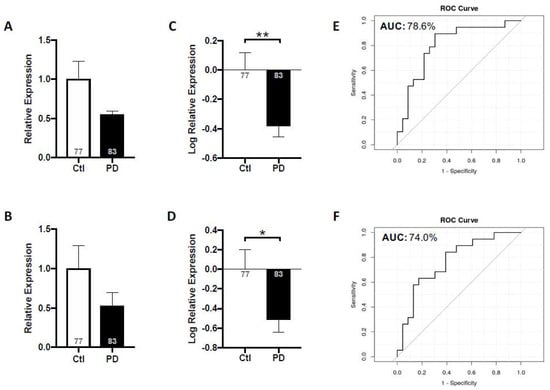
Figure 4.
Salivary miR-153 and miR-223 expression levels in idiopathic PD subjects relative to non-neurological controls. Mean expression levels of miR-153 (A) and miR-223 (B) were determined by RT-qPCR and analyzed using the ΔΔCt method relative to internal and endogenous controls. Data was normalized via log-transformation and reported for miR-153 (C) and miR-223 (D). ROC curves were estimated for miR-153 (E) and miR-223 (F), with area under the curve indicated. Values shown within bars denote number of subjects. N = 77–83; * p < 0.05; ** p < 0.01. (From [19], with permission).
MiR-153, miR-223 and SNCA expression levels in the human brain are highest in the midbrain, an important region at the epicenter of PD pathophysiology [85]. They have also been detected in extracellular compartments, including blood [93,103,104], CSF [15,105] and saliva [17,18,106], with their levels fluctuating in response to disease state. Several groups, in addition to our own, have linked miR-153 and, to a lesser extent, miR-223 to PD pathology [64,85,87,107]. Alterations in miR-153 levels have been documented in PD CSF [105] and plasma [108], while changes in miR-223 have been reported in PD serum [107]. To our knowledge, ours is the first study to document aberrant expression levels of miR-153 and miR-223 in the saliva of PD patients. However, considering other CNS conditions similarly involve differential expression of miR-153 and miR-223, it is critical to assay these miRNA levels in PD patients relative to neurological controls (e.g., other neurodegenerative conditions, other forms of parkinsonism, other synucleinopathies), using methodology akin to that of our 2021 salivary HO-1 study.
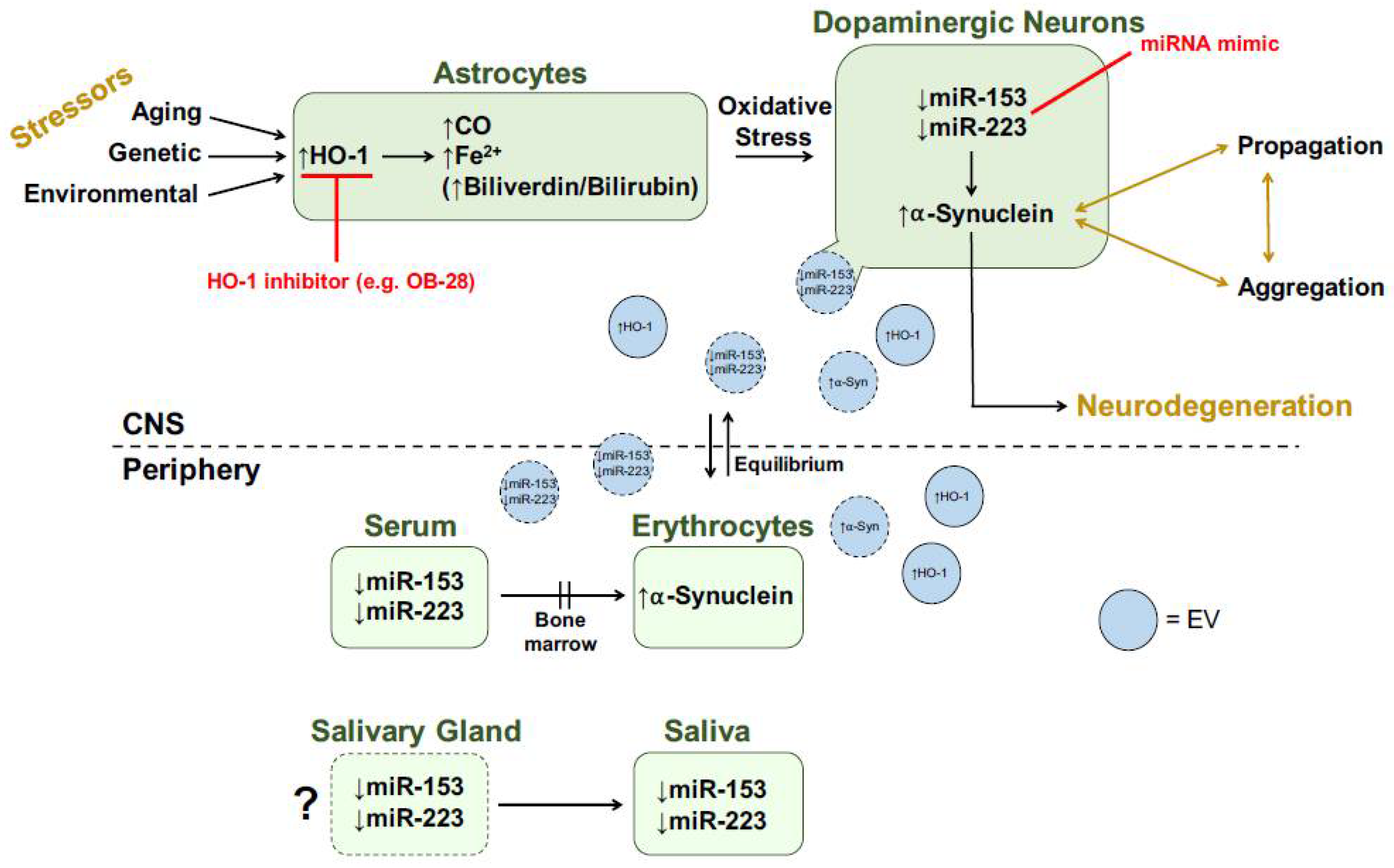
It is unclear whether salivary HO-1, miR-153 and miR-223 originate as a transudate from plasma or are actively secreted by the salivary glands. In support of the latter, immunoreactive HO-1 has been demonstrated in normal salivary gland acini and duct epithelia and in benign salivary gland pleomorphic adenomas [109]. Salivary changes in HO-1 concentrations may also be associated mechanistically with the prominent autonomic innervation of the salivary glands and the relatively early appearance of Lewy pathology in the PD autonomic nervous system [14,110,111]. One possible mechanism of action for CNS changes being reflected in peripheral biofluids is the transfer of specific cargo across the blood–brain barrier (BBB) via extracellular vesicles (EVs). In fact, HO-1 protein, acting upstream of miR-153 and miR-223, was predominantly found in CNS-derived and non-CNS-derived EV fractions across five different human biofluids: saliva, plasma, serum, urine and CSF [112]. We hypothesize that CNS changes in key proteins (e.g., HO-1, α-synuclein) and miRNAs (e.g., miR-153, miR-223) are being reflected in the periphery via centrifugal EV transport (Figure 5).
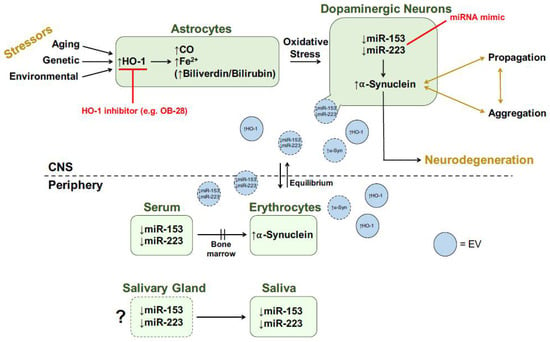
Figure 5.
Transducer model for role of HO-1 in PD. Red text denotes potential therapeutic strategies. Shapes with dotted borders represent speculative hypotheses. See text for more details. CNS, central nervous system; CO, carbon monoxide; EV, extracellular vesicle; Fe2+, ferrous iron; HO-1, heme oxygenase-1; miRNA, microRNA; α-Syn, α-synuclein. Modified from [64].
The latter has important therapeutic and diagnostic implications for PD research. For instance, systemic exosomal siRNA delivery in transgenic mice expressing the human phosphorylation-mimic S129D α-synuclein (which exhibit aggregation pathology) significantly reduced levels of α-synuclein mRNA and protein as well as intracellular protein aggregates within dopaminergic neurons of the substantia nigra relative to WT controls [113]. While the therapeutic potential of EVs has not been fully elucidated in humans, their use holds promise for the treatment of different disorders of the CNS, where many drugs are of limited benefit due to their inability to penetrate the BBB [114]. The ability of EVs to cross the BBB via transcytosis has been experimentally confirmed [115,116,117]. Furthermore, EVs have been extensively studied in recent years as a potential source of biomarkers for PD, particularly neural-derived EVs, which offer a ‘window’ into the CNS. We are currently using nano-flow liquid-chromatography mass spectrometry to analyze differences, if any exist, between salivary EV and whole saliva proteomic profiles (Cressatti and Schipper, unpublished results). This line of inquiry should reveal whether EVs portray a more precise snapshot of PD pathophysiology than whole biofluids.
6. Concluding Remarks: HO-1, miR-153, miR-223 and α-Synuclein
In contrast to existing toxin and genetic animal models of PD, the GFAP.HMOX18.5−19 m mouse model of PD unveils a third category of animal model, namely the transducer model. GFAP.HMOX18.5−19 m mice recapitulate key features of the human disorder, such as non-regulated brain iron trapping, oxidative stress, mitochondrial damage and macroautophagy. These core pathological features common to neurodegeneration may represent a single lesion devolving from the sustained induction of HMOX1 within astrocytes. This formulation strengthens the conceptual link between normal brain aging, often characterized by a milder version of this core pathology, and the major senescence-associated neurodegenerative disorders, such as PD [22]. For instance, when present in abundance, certain pathological features are considered characteristic of PD. This includes gliosis, increased α-synuclein protein, brain iron deposition and mitochondrial insufficiency [118]. However, when present at low densities, these are instead construed as features of normal brain aging [119,120]. Recognition that this core cytopathology drives the neurodegenerative process may shift the design of disease-modifying therapeutics towards the disruption of molecular pathways which act to integrate the constituents of this multifaceted lesion [22]. This highlights the astroglial HO-1 transducer as a potential therapeutic target to simultaneously mitigate several core pathological features of the disease.
Acting downstream of HO-1, miR-153 and miR-223 were identified as negative regulators of α-synuclein and moderately good diagnostic biomarkers of idiopathic PD [19,64]. The presence of these miRNA alterations in saliva is particularly relevant considering saliva acquisition is a minimally invasive and user-friendly protocol for the elderly PD population. Furthermore, peripheral changes in miR-153 and miR-223 were similarly observed in GFAP.HMOX18.5−19 m mouse and human PD brains, and EVs may offer a suitable mechanism of action to explain this phenomenon. It is important to note that this is not only relevant to miR-153 and miR-223 but also to other key players involved in PD neuropathology, including native and oligomeric α-synuclein, DJ-1, tau, other salient miRNAs and HO-1. Understanding how peripheral pathologies reflect CNS afflictions may accelerate our knowledge relating to CNS physiology in health and disease.
Most importantly, contemporary pharmacotherapy for PD is almost exclusively symptomatic in nature and effective neuroprotection would be a welcome development. Several metalloporphyrin inhibitors of HO activity are already in clinical use for the control of neonatal hyperbilirubinemia (jaundice) and certain adult liver conditions and, if secondary (‘bystander’) effects are successfully mitigated, could be adapted for the treatment of PD. The small-molecule inhibitor OB-28 may confer additional advantages for human use in light of its selectivity for HO-1 over HO-2, BBB permeability and favorable toxicity profile [60]. After many years of exploring the fundamentals of glial HO-1 behavior in CNS senescence and disease, we may now be at the cusp of translating this experience into the development of definitive, disease-modifying approaches to the management of PD and related neurodegenerative disorders—an unmet clinical need that heavily impacts the health and wellbeing of our aging population.
Author Contributions
Conceptualization, M.C. and H.M.S.; writing—original draft preparation, M.C.; writing, review and editing, H.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Canadian Institutes of Health Research (CIHR), grant number MOP-68887; Parkinson Canada; Fonds de recherche du Québec-Santé, grant number 257822; MITACS; and the Mary Katz Claman Foundation.
Institutional Review Board Statement
The animal and human studies reported herein were conducted in accordance with the Declaration of Helsinki, and approved by the Research Ethics Committee of the Jewish General Hospital (Protocol Number: 2001-2739).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the studies described herein (Protocol Number: 2019-1220).
Conflicts of Interest
HMS served as an officer of HemOx Biotechnologies and as a consultant to Osta Biotechnologies, Molecular Biometrics Inc., TEVA Neurosciences and Caprion Pharmaceuticals. MC has no conflict of interest to declare.
References
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Deas, E.; Cremades, N.; Angelova, P.R.; Ludtmann, M.H.R.; Yao, Z.; Chen, S.; Horrocks, M.H.; Banushi, B.; Little, D.; Devine, M.J.; et al. Alpha-Synuclein Oligomers Interact with Metal Ions to Induce Oxidative Stress and Neuronal Death in Parkinson’s Disease. Antioxid. Redox Signal. 2016, 24, 376–391. [Google Scholar] [CrossRef] [Green Version]
- Goedert, M. Alpha-synuclein and neurodegenerative diseases. Nat. Rev. Neurosci. 2001, 2, 492–501. [Google Scholar] [CrossRef]
- Takahashi, T.; Yamashita, H.; Nakamura, T.; Nagano, Y.; Nakamura, S. Tyrosine 125 of alpha-synuclein plays a critical role for dimerization following nitrative stress. Brain Res. 2002, 938, 73–80. [Google Scholar] [CrossRef]
- Kim, W.S.; Kagedal, K.; Halliday, G.M. Alpha-synuclein biology in Lewy body diseases. Alzheimer’s Res. Ther. 2014, 6, 73. [Google Scholar] [CrossRef] [Green Version]
- Marques, O.; Outeiro, T.F. Alpha-synuclein: From secretion to dysfunction and death. Cell Death Dis. 2012, 3, e350. [Google Scholar] [CrossRef]
- Miranda, H.V.; Cassio, R.; Correia-Guedes, L.; Gomes, M.A.; Chegao, A.; Miranda, E.; Soares, T.; Coelho, M.; Rosa, M.M.; Ferreira, J.J.; et al. Posttranslational modifications of blood-derived alpha-synuclein as biochemical markers for Parkinson’s disease. Sci. Rep. 2017, 7, 13713. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.P.; Walker, D.E.; Goldstein, J.M.; de Laat, R.; Banducci, K.; Caccavello, R.J.; Barbour, R.; Huang, J.; Kling, K.; Lee, M.; et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J. Biol. Chem. 2006, 281, 29739–29752. [Google Scholar] [CrossRef] [Green Version]
- Al-Nimer, M.S.; Mshatat, S.F.; Abdulla, H.I. Saliva alpha-Synuclein and A High Extinction Coefficient Protein: A Novel Approach in Assessment Biomarkers of Parkinson’s Disease. N. Am. J. Med. Sci. 2014, 6, 633–637. [Google Scholar] [CrossRef] [Green Version]
- Bougea, A.; Stefanis, L.; Paraskevas, G.P.; Emmanouilidou, E.; Vekrelis, K.; Kapaki, E. Plasma alpha-synuclein levels in patients with Parkinson’s disease: A systematic review and meta-analysis. Neurol. Sci. 2019, 40, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Daniele, S.; Costa, B.; Pietrobono, D.; Giacomelli, C.; Iofrida, C.; Trincavelli, M.L.; Fusi, J.; Franzoni, F.; Martini, C. Epigenetic Modifications of the alpha-Synuclein Gene and Relative Protein Content Are Affected by Ageing and Physical Exercise in Blood from Healthy Subjects. Oxidative Med. Cell. Longev. 2018, 2018, 3740345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniele, S.; Frosini, D.; Pietrobono, D.; Petrozzi, L.; Lo Gerfo, A.; Baldacci, F.; Fusi, J.; Giacomelli, C.; Siciliano, G.; Trincavelli, M.L.; et al. alpha-Synuclein Heterocomplexes with beta-Amyloid Are Increased in Red Blood Cells of Parkinson’s Disease Patients and Correlate with Disease Severity. Front. Mol. Neurosci. 2018, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Devic, I.; Hwang, H.; Edgar, J.S.; Izutsu, K.; Presland, R.; Pan, C.; Goodlett, D.R.; Wang, Y.; Armaly, J.; Tumas, V.; et al. Salivary alpha-synuclein and DJ-1, potential biomarkers for Parkinson’s disease. Brain 2011, 134, e178. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Tang, H.; Nie, K.; Wang, L.; Zhao, J.; Gan, R.; Huang, J.; Zhu, R.; Feng, S.; Duan, Z.; et al. Cerebrospinal fluid alpha-synuclein as a biomarker for Parkinson’s disease diagnosis: A systematic review and meta-analysis. Int. J. Neurosci. 2015, 125, 645–654. [Google Scholar] [CrossRef]
- Kang, W.; Chen, W.; Yang, Q.; Zhang, L.; Zhang, L.; Wang, X.; Dong, F.; Zhao, Y.; Chen, S.; Quinn, T.J.; et al. Salivary total alpha-synuclein, oligomeric alpha-synuclein and SNCA variants in Parkinson’s disease patients. Sci. Rep. 2016, 6, 28143. [Google Scholar] [CrossRef] [Green Version]
- Vivacqua, G.; Latorre, A.; Suppa, A.; Nardi, M.; Pietracupa, S.; Mancinelli, R.; Fabbrini, G.; Colosimo, C.; Gaudio, E.; Berardelli, A. Abnormal Salivary Total and Oligomeric Alpha-Synuclein in Parkinson’s Disease. PLoS ONE 2016, 11, e0151156. [Google Scholar] [CrossRef] [Green Version]
- Vivacqua, G.; Suppa, A.; Mancinelli, R.; Belvisi, D.; Fabbrini, A.; Costanzo, M.; Formica, A.; Onori, P.; Fabbrini, G.; Berardelli, A. Salivary alpha-synuclein in the diagnosis of Parkinson’s disease and Progressive Supranuclear Palsy. Parkinsonism Relat. Disord. 2019, 63, 143–148. [Google Scholar] [CrossRef]
- Cressatti, M.; Juwara, L.; Galindez, J.M.; Velly, A.M.; Nkurunziza, E.S.; Marier, S.; Canie, O.; Gornistky, M.; Schipper, H.M. Salivary microR-153 and microR-223 Levels as Potential Diagnostic Biomarkers of Idiopathic Parkinson’s Disease. Mov. Disord. 2019, 35, 468–477. [Google Scholar] [CrossRef]
- Galindez, J.M.; Juwara, L.; Cressatti, M.; Gornitsky, M.; Velly, A.M.; Schipper, H.M. Salivary heme oxygenase-1, a potential biomarker for central neurodegeneration. J. Cent. Nerv. Syst. Dis. 2021, 13, 11795735211029114. [Google Scholar] [CrossRef]
- Song, W.; Kothari, V.; Velly, A.M.; Cressatti, M.; Liberman, A.; Gornitsky, M.; Schipper, H.M. Evaluation of salivary heme oxygenase-1 as a potential biomarker of early Parkinson’s disease. Mov. Disord. 2018, 33, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Schipper, H.M.; Song, W.; Tavitian, A.; Cressatti, M. The sinister face of heme oxygenase-1 in brain aging and disease. Prog. Neurobiol. 2019, 172, 40–70. [Google Scholar] [CrossRef] [PubMed]
- Schipper, H.M.; Song, W. A heme oxygenase-1 transducer model of degenerative and developmental brain disorders. Int. J. Mol. Sci. 2015, 16, 5400–5419. [Google Scholar] [CrossRef] [Green Version]
- Dore, S.; Takahashi, M.; Ferris, C.D.; Zakhary, R.; Hester, L.D.; Guastella, D.; Snyder, S.H. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc. Natl. Acad. Sci. USA 1999, 96, 2445–2450. [Google Scholar] [CrossRef] [Green Version]
- Llesuy, S.F.; Tomaro, M.L. Heme oxygenase and oxidative stress. Evidence of involvement of bilirubin as physiological protector against oxidative damage. Biochim. Biophys. Acta 1994, 1223, 9–14. [Google Scholar] [CrossRef]
- Nakagami, T.; Toyomura, K.; Kinoshita, T.; Morisawa, S. A beneficial role of bile pigments as an endogenous tissue protector: Anti-complement effects of biliverdin and conjugated bilirubin. Biochim. Biophys. Acta 1993, 1158, 189–193. [Google Scholar] [CrossRef]
- Stocker, R.; Yamamoto, Y.; McDonagh, A.F.; Glazer, A.N.; Ames, B.N. Bilirubin is an antioxidant of possible physiological importance. Science 1987, 235, 1043–1046. [Google Scholar] [CrossRef]
- Frankel, D.; Mehindate, K.; Schipper, H.M. Role of heme oxygenase-1 in the regulation of manganese superoxide dismutase gene expression in oxidatively-challenged astroglia. J. Cell. Physiol. 2000, 185, 80–86. [Google Scholar] [CrossRef]
- Piantadosi, C.A.; Carraway, M.S.; Suliman, H.B. Carbon monoxide, oxidative stress, and mitochondrial permeability pore transition. Free Radic. Biol. Med. 2006, 40, 1332–1339. [Google Scholar] [CrossRef]
- Ryter, S.W.; Tyrrell, R.M. The heme synthesis and degradation pathways: Role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic. Biol. Med. 2000, 28, 289–309. [Google Scholar] [CrossRef]
- Schipper, H.M.; Song, W.; Zukor, H.; Hascalovici, J.R.; Zeligman, D. Heme oxygenase-1 and neurodegeneration: Expanding frontiers of engagement. J. Neurochem. 2009, 110, 469–485. [Google Scholar] [CrossRef] [PubMed]
- Schipper, H.M.; Liberman, A.; Stopa, E.G. Neural heme oxygenase-1 expression in idiopathic Parkinson’s disease. Exp. Neurol. 1998, 150, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Béraud, D.; Hathaway, H.A.; Trecki, J.; Chasovskikh, S.; Johnson, D.A.; Johnson, J.A.; Federoff, H.J.; Shimoji, M.; Mhyre, T.R.; Maguire-Zeiss, K.A. Microglial activation and antioxidant responses induced by the Parkinson’s disease protein α-synuclein. J. Neuroimmune Pharmacol. 2013, 8, 94–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stokes, A.H.; Hastings, T.G.; Vrana, K.E. Cytotoxic and genotoxic potential of dopamine. J. Neurosci. Res. 1999, 55, 659–665. [Google Scholar] [CrossRef]
- Castellani, R.; Smith, M.A.; Richey, P.L.; Perry, G. Glycoxidation and oxidative stress in Parkinson disease and diffuse Lewy body disease. Brain Res. 1996, 737, 195–200. [Google Scholar] [CrossRef]
- Ayuso, P.; Martinez, C.; Pastor, P.; Lorenzo-Betancor, O.; Luengo, A.; Jimenez-Jimenez, F.J.; Alonso-Navarro, H.; Agundez, J.A.; Garcia-Martin, E. An association study between Heme oxygenase-1 genetic variants and Parkinson’s disease. Front. Cell. Neurosci. 2014, 8, 298. [Google Scholar] [CrossRef] [Green Version]
- Infante, J.; Sierra, M.; Sanchez-Juan, P.; Garcia-Gorostiaga, I.; Gonzalez-Aramburu, I.; Fernandez-Viadero, C.; Berciano, J.; Combarros, O. Interaction between heme oxygenase-1 genotypes and exposure to pesticides in Parkinson’s disease. Mov. Disord. 2011, 26, 916–917. [Google Scholar] [CrossRef]
- Ran, C.; Wirdefeldt, K.; Brodin, L.; Ramezani, M.; Westerlund, M.; Xiang, F.; Anvret, A.; Willows, T.; Sydow, O.; Johansson, A.; et al. Genetic Variations and mRNA Expression of NRF2 in Parkinson’s Disease. Parkinsons Dis. 2017, 2017, 4020198. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Campbell, M.R.; Lacher, S.E.; Cho, H.Y.; Wan, M.; Crowl, C.L.; Chorley, B.N.; Bond, G.L.; Kleeberger, S.R.; Slattery, M.; et al. A Polymorphic Antioxidant Response Element Links NRF2/sMAF Binding to Enhanced MAPT Expression and Reduced Risk of Parkinsonian Disorders. Cell Rep. 2016, 15, 830–842. [Google Scholar] [CrossRef] [Green Version]
- Kuter, K.; Olech, Ł.; Głowacka, U. Prolonged dysfunction of astrocytes and activation of microglia accelerate degeneration of dopaminergic neurons in the rat substantia nigra and block compensation of early motor dysfunction induced by 6-OHDA. Mol. Neurobiol. 2018, 55, 3049–3066. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Su, X.; Piao, L.; Jin, Z.; Jin, R. Involvement of astrocytes and microRNA dysregulation in neurodegenerative diseases: From pathogenesis to therapeutic potential. Front. Mol. Neurosci. 2021, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Mehindate, K.; Sahlas, D.J.; Frankel, D.; Mawal, Y.; Liberman, A.; Corcos, J.; Dion, S.; Schipper, H.M. Proinflammatory cytokines promote glial heme oxygenase-1 expression and mitochondrial iron deposition: Implications for multiple sclerosis. J. Neurochem. 2001, 77, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Schipper, H.M. Glial HO-1 expression, iron deposition and oxidative stress in neurodegenerative diseases. Neurotox. Res. 1999, 1, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Schipper, H.M. Mitochondrial Iron Deposition in Aging Astroglia: Mechanisms and Disease Implications. In Mitochondrial Ubiquinone (Coenzyme Q): Biochemical, Functional, Medical, and Therapeutic Aspects in Human Health and Disease; Ebadi, M.C.R.K., Ed.; Prominent Press: Shepparton, Australia, 2001; pp. 267–280. [Google Scholar]
- Di Monte, D.A.; Schipper, H.M.; Hetts, S.; Langston, J.W. Iron-mediated bioactivation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in glial cultures. Glia 1995, 15, 203–206. [Google Scholar] [CrossRef]
- Morale, M.C.; Serra, P.A.; L’Episcopo, F.; Tirolo, C.; Caniglia, S.; Testa, N.; Gennuso, F.; Giaquinta, G.; Rocchitta, G.; Desole, M.S.; et al. Estrogen, neuroinflammation and neuroprotection in Parkinson’s disease: Glia dictates resistance versus vulnerability to neurodegeneration. Neuroscience 2006, 138, 869–878. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, C.J. Astrocytes autophagy in aging and neurodegenerative disorders. Biomed. Pharmacother. 2020, 122, 109691. [Google Scholar] [CrossRef]
- Yang, C.M.; Lin, C.C.; Hsieh, H.L. High-glucose-derived oxidative stress-dependent heme oxygenase-1 expression from astrocytes contributes to the neuronal apoptosis. Mol. Neurobiol. 2017, 54, 470–483. [Google Scholar] [CrossRef]
- Song, W.; Patel, A.; Qureshi, H.Y.; Han, D.; Schipper, H.M.; Paudel, H.K. The Parkinson disease-associated A30P mutation stabilizes alpha-synuclein against proteasomal degradation triggered by heme oxygenase-1 over-expression in human neuroblastoma cells. J. Neurochem. 2009, 110, 719–733. [Google Scholar] [CrossRef]
- Volles, M.J.; Lansbury, P.T., Jr. Vesicle permeabilization by protofibrillar alpha-synuclein is sensitive to Parkinson’s disease-linked mutations and occurs by a pore-like mechanism. Biochemistry 2002, 41, 4595–4602. [Google Scholar] [CrossRef]
- He, Q.; Song, N.; Jia, F.; Xu, H.; Yu, X.; Xie, J.; Jiang, H. Role of α-synuclein aggregation and the nuclear factor E2-related factor 2/heme oxygenase-1 pathway in iron-induced neurotoxicity. Int. J. Biochem. Cell Biol. 2013, 45, 1019–1030. [Google Scholar] [CrossRef]
- Xu, J.; Xiao, C.; Song, W.; Cui, X.; Pan, M.; Wang, Q.; Feng, Y.; Xu, Y. Elevated heme oxygenase-1 correlates with increased brain iron deposition measured by quantitative susceptibility mapping and decreased hemoglobin in patients with Parkinson’s disease. Front. Aging Neurosci. 2021, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Song, N.; Guo, X.; Jiang, H.; Zhang, H.; Xie, J. Differences in vulnerability of neurons and astrocytes to heme oxygenase-1 modulation: Implications for mitochondrial ferritin. Sci. Rep. 2016, 6, 24200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zukor, H.; Song, W.; Liberman, A.; Mui, J.; Vali, H.; Fillebeen, C.; Pantopoulos, K.; Wu, T.D.; Guerquin-Kern, J.L.; Schipper, H.M. HO-1-mediated macroautophagy: A mechanism for unregulated iron deposition in aging and degenerating neural tissues. J. Neurochem. 2009, 109, 776–791. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Cressatti, M.; Zukor, H.; Liberman, A.; Galindez, C.; Schipper, H.M. Parkinsonian features in aging GFAP.HMOX1 transgenic mice overexpressing human HO-1 in the astroglial compartment. Neurobiol. Aging 2017, 58, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, B.E.; Nishimura, R.N.; Lu, S.Y. Differential expression of heme oxygenase-1 in cultured cortical neurons and astrocytes determined by the aid of a new heme oxygenase antibody. Response to oxidative stress. Mol. Brain Res. 1995, 30, 37–47. [Google Scholar] [CrossRef]
- Manganaro, F.; Chopra, V.S.; Mydlarski, M.B.; Bernatchez, G.; Schipper, H.M. Redox perturbations in cysteamine-stressed astroglia: Implications for inclusion formation and gliosis in the aging brain. Free Radic. Biol. Med. 1995, 19, 823–835. [Google Scholar] [CrossRef]
- Snyder, S.H.; Jaffrey, S.R.; Zakhary, R. Nitric oxide and carbon monoxide: Parallel roles as neural messengers. Brain Res. Rev. 1998, 26, 167–175. [Google Scholar] [CrossRef]
- Schipper, H.M. Brain iron deposition and the free radical-mitochondrial theory of ageing. Ageing Res. Rev. 2004, 3, 265–301. [Google Scholar] [CrossRef]
- Gupta, A.; Lacoste, B.; Pistell, P.J.; Ingram, D.K.; Hamel, E.; Alaoui-Jamali, M.A.; Szarek, W.A.; Vlahakis, J.Z.; Jie, S.; Song, W.; et al. Neurotherapeutic effects of novel HO-1 inhibitors in vitro and in a transgenic mouse model of Alzheimer’s disease. J. Neurochem. 2014, 131, 778–790. [Google Scholar] [CrossRef]
- Emir, U.E.; Tuite, P.J.; Oz, G. Elevated pontine and putamenal GABA levels in mild-moderate Parkinson disease detected by 7 tesla proton MRS. PLoS ONE 2012, 7, e30918. [Google Scholar] [CrossRef] [Green Version]
- Kish, S.J.; Rajput, A.; Gilbert, J.; Rozdilsky, B.; Chang, L.J.; Shannak, K.; Hornykiewicz, O. Elevated gamma-aminobutyric acid level in striatal but not extrastriatal brain regions in Parkinson’s disease: Correlation with striatal dopamine loss. Ann. Neurol. 1986, 20, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Oz, G.; Terpstra, M.; Tkac, I.; Aia, P.; Lowary, J.; Tuite, P.J.; Gruetter, R. Proton MRS of the unilateral substantia nigra in the human brain at 4 tesla: Detection of high GABA concentrations. Magn. Reson. Med. 2006, 55, 296–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cressatti, M.; Song, W.; Turk, A.Z.; Garabed, L.R.; Benchaya, J.A.; Galindez, C.; Liberman, A.; Schipper, H.M. Glial HMOX1 expression promotes central and peripheral alpha-synuclein dysregulation and pathogenicity in parkinsonian mice. Glia 2019, 67, 1730–1744. [Google Scholar] [PubMed]
- Tavitian, A.; Cressatti, M.; Song, W.; Turk, A.Z.; Galindez, C.; Smart, A.; Liberman, A.; Schipper, H.M. Strategic timing of glial HMOX1 expression results in either schizophrenia-like or parkinsonian behavior in mice. Antioxid. Redox Signal. 2019, 32, 1259–1272. [Google Scholar] [CrossRef]
- Chen, P.C.; Vargas, M.R.; Pani, A.K.; Smeyne, R.J.; Johnson, D.A.; Kan, Y.W.; Johnson, J.A. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: Critical role for the astrocyte. Proc. Natl. Acad. Sci. USA 2009, 106, 2933–2938. [Google Scholar] [CrossRef] [Green Version]
- Innamorato, N.G.; Jazwa, A.; Rojo, A.I.; Garcia, C.; Fernandez-Ruiz, J.; Grochot-Przeczek, A.; Stachurska, A.; Jozkowicz, A.; Dulak, J.; Cuadrado, A. Different susceptibility to the Parkinson’s toxin MPTP in mice lacking the redox master regulator Nrf2 or its target gene heme oxygenase-1. PLoS ONE 2010, 5, e11838. [Google Scholar] [CrossRef] [Green Version]
- Pan, P.K.; Qiao, L.Y.; Wen, X.N. Safranal prevents rotenone-induced oxidative stress and apoptosis in an in vitro model of Parkinson’s disease through regulating Keap1/Nrf2 signaling pathway. Cell. Mol. Biol. 2016, 62, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Tsou, Y.H.; Shih, C.T.; Ching, C.H.; Huang, J.Y.; Jen, C.J.; Yu, L.; Kuo, Y.M.; Wu, F.S.; Chuang, J.I. Treadmill exercise activates Nrf2 antioxidant system to protect the nigrostriatal dopaminergic neurons from MPP+ toxicity. Exp. Neurol. 2015, 263, 50–62. [Google Scholar] [CrossRef]
- Kapturczak, M.H.; Wasserfall, C.; Brusko, T.; Campbell-Thompson, M.; Ellis, T.M.; Atkinson, M.A.; Agarwal, A. Heme oxygenase-1 modulates early inflammatory responses: Evidence from the heme oxygenase-1-deficient mouse. Am. J. Pathol. 2004, 165, 1045–1053. [Google Scholar] [CrossRef]
- Paine, A.; Eiz-Vesper, B.; Blasczyk, R.; Immenschuh, S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010, 80, 1895–1903. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Gunter, K.; Maines, M.D. Neurons overexpressing heme oxygenase-1 resist oxidative stress-mediated cell death. J. Neurochem. 2000, 75, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Panahian, N.; Yoshiura, M.; Maines, M.D. Overexpression of heme oxygenase-1 is neuroprotective in a model of permanent middle cerebral artery occlusion in transgenic mice. J. Neurochem. 1999, 72, 1187–1203. [Google Scholar] [CrossRef] [PubMed]
- More, S.; Choi, D.K. Neuroprotective role of atractylenolide-I in an in vitro and in vivo model of Parkinson’s disease. Nutrients 2017, 9, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.A.; Kim, J.H.; Woo, S.Y.; Son, H.J.; Han, S.H.; Jang, B.K.; Choi, J.W.; Kim, D.J.; Park, K.D.; Hwang, O. A novel compound VSC 2 has anti-inflammatory and antioxidant properties in microglia and in Parkinson’s disease animal model. Br. J. Pharmacol. 2015, 172, 1087–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen-Roetling, J.; Song, W.; Schipper, H.M.; Regan, C.S.; Regan, R.F. Astrocyte overexpression of heme oxygenase-1 improves outcome after intracerebral hemorrhage. Stroke 2015, 46, 1093–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen-Roetling, J.; Kamalapathy, P.; Cao, Y.; Song, W.; Schipper, H.M.; Regan, R.F. Astrocyte heme oxygenase-1 reduces mortality and improves outcome after collagenase-induced intracerebral hemorrhage. Neurobiol. Dis. 2017, 102, 140–146. [Google Scholar] [CrossRef]
- Gai, W.P.; Yuan, H.X.; Li, X.Q.; Power, J.T.; Blumbergs, P.C.; Jensen, P.H. In situ and in vitro study of colocalization and segregation of alpha-synuclein, ubiquitin, and lipids in Lewy bodies. Exp. Neurol. 2000, 166, 324–333. [Google Scholar] [CrossRef]
- Mahul-Mellier, A.L.; Burtscher, J.; Maharjan, N.; Weerens, L.; Croisier, M.; Kuttler, F.; Leleu, M.; Knott, G.W.; Lashuel, H.A. The process of Lewy body formation, rather than simply alpha-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc. Natl. Acad. Sci. USA 2020, 117, 4971–4982. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Zukor, H.; Lin, S.H.; Hascalovici, J.; Liberman, A.; Tavitian, A.; Mui, J.; Vali, H.; Tong, X.K.; Bhardwaj, S.K.; et al. Schizophrenia-like features in transgenic mice overexpressing human HO-1 in the astrocytic compartment. J. Neurosci. 2012, 32, 10841–10853. [Google Scholar] [CrossRef] [Green Version]
- Basak, I.; Patil, K.S.; Alves, G.; Larsen, J.P.; Moller, S.G. microRNAs as neuroregulators, biomarkers and therapeutic agents in neurodegenerative diseases. Cell. Mol. Life Sci. 2016, 73, 811–827. [Google Scholar] [CrossRef]
- Lukiw, W.J. Micro-RNA speciation in fetal, adult and Alzheimer’s disease hippocampus. Neuroreport 2007, 18, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Martinez, B.; Peplow, P.V. MicroRNAs in Parkinson’s disease and emerging therapeutic targets. Neural Regen. Res. 2017, 12, 1945. [Google Scholar] [PubMed]
- Lin, S.H.; Song, W.; Cressatti, M.; Zukor, H.; Wang, E.; Schipper, H.M. Heme oxygenase-1 modulates microRNA expression in cultured astroglia: Implications for chronic brain disorders. Glia 2015, 63, 1270–1284. [Google Scholar] [CrossRef] [PubMed]
- Doxakis, E. Post-transcriptional regulation of alpha-synuclein expression by mir-7 and mir-153. J. Biol. Chem. 2010, 285, 12726–12734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fragkouli, A.; Doxakis, E. miR-7 and miR-153 protect neurons against MPP(+)-induced cell death via upregulation of mTOR pathway. Front. Cell. Neurosci. 2014, 8, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Je, G.; Kim, Y.S. Mitochondrial ROS-mediated post-transcriptional regulation of alpha-synuclein through miR-7 and miR-153. Neurosci. Lett. 2017, 661, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ridzon, D.; Wong, L.; Chen, C. Characterization of microRNA expression profiles in normal human tissues. BMC Genom. 2007, 8, 166. [Google Scholar] [CrossRef] [Green Version]
- Long, J.M.; Ray, B.; Lahiri, D.K. MicroRNA-153 physiologically inhibits expression of amyloid-beta precursor protein in cultured human fetal brain cells and is dysregulated in a subset of Alzheimer disease patients. J. Biol. Chem. 2012, 287, 31298–31310. [Google Scholar] [CrossRef] [Green Version]
- Ji, Q.; Gao, J.; Zheng, Y.; Liu, X.; Zhou, Q.; Shi, C.; Yao, M.; Chen, X. Inhibition of microRNA-153 protects neurons against ischemia/reperfusion injury in an oxygen-glucose deprivation and reoxygenation cellular model by regulating Nrf2/HO-1 signaling. J. Biochem. Mol. Toxicol. 2017, 31, e21905. [Google Scholar] [CrossRef]
- Liang, C.; Zhu, H.; Xu, Y.; Huang, L.; Ma, C.; Deng, W.; Liu, Y.; Qin, C. MicroRNA-153 negatively regulates the expression of amyloid precursor protein and amyloid precursor-like protein 2. Brain Res. 2012, 1455, 103–113. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, S.; Qi, W.; Xu, X.; Liang, Y. Overexpression of miR-153 promotes oxidative stress in MPP(+)-induced PD model by negatively regulating the Nrf2/HO-1 signaling pathway. Int. J. Clin. Exp. Pathol. 2018, 11, 4179–4187. [Google Scholar] [PubMed]
- Matsumoto, J.; Stewart, T.; Sheng, L.; Li, N.; Bullock, K.; Song, N.; Shi, M.; Banks, W.A.; Zhang, J. Transmission of alpha-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: Another mechanism for initiation and progression of Parkinson’s disease? Acta Neuropathol. Commun. 2017, 5, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateo, I.; Infante, J.; Sanchez-Juan, P.; Garcia-Gorostiaga, I.; Rodriguez-Rodriguez, E.; Vazquez-Higuera, J.L.; Berciano, J.; Combarros, O. Serum heme oxygenase-1 levels are increased in Parkinson’s disease but not in Alzheimer’s disease. Acta Neurol. Scand. 2010, 121, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zheng, J.; Ma, J.; Wang, Z.; Shi, X.; Li, M.; Huang, S.; Hu, S.; Zhao, Z.; Li, D. Increased Plasma Heme Oxygenase-1 Levels in Patients with Early-Stage Parkinson’s Disease. Front. Aging Neurosci. 2021, 39. [Google Scholar] [CrossRef]
- Roi, A.; Rusu, L.C.; Roi, C.I.; Luca, R.E.; Boia, S.; Munteanu, R.I. A new approach for the diagnosis of systemic and oral diseases based on salivary biomolecules. Dis. Markers 2019, 2019, 8761860. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Schipper, H.M.; Velly, A.M.; Mohit, S.; Gornitsky, M. Salivary biomarkers of oxidative stress: A critical review. Free Radic. Biol. Med. 2015, 85, 95–104. [Google Scholar] [CrossRef]
- Orive, G.; Lopera, F.; Carro, E. Saliva is a Good Candidate to be the New Gold-Standard Sample for Neurodegenerative Diseases. J. Alzheimer’s Dis. JAD 2022. [Google Scholar] [CrossRef]
- Schipper, H.M. Heme oxygenase-1, role in brain aging and neurodegeneration. Exp. Gerontol. 2000, 35, 821–830. [Google Scholar] [CrossRef]
- Schipper, H.M.; Cisse, S.; Stopa, E.G. Expression of heme oxygenase-1 in the senescent and Alzheimer-diseased brain. Ann. Neurol. 1995, 37, 758–768. [Google Scholar] [CrossRef]
- Smith, M.A.; Kutty, R.K.; Richey, P.L.; Yan, S.D.; Stern, D.; Chader, G.J.; Wiggert, B.; Petersen, R.B.; Perry, G. Heme oxygenase-1 is associated with the neurofibrillary pathology of Alzheimer’s disease. Am. J. Pathol. 1994, 145, 42–47. [Google Scholar]
- Junn, E.; Lee, K.W.; Jeong, B.S.; Chan, T.W.; Im, J.Y.; Mouradian, M.M. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc. Natl. Acad. Sci. USA 2009, 106, 13052–13057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farh, K.K.; Grimson, A.; Jan, C.; Lewis, B.P.; Johnston, W.K.; Lim, L.P.; Burge, C.B.; Bartel, D.P. The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science 2005, 310, 1817–1821. [Google Scholar] [CrossRef] [Green Version]
- Tagliafierro, L.; Glenn, O.C.; Zamora, M.E.; Beach, T.G.; Woltjer, R.L.; Lutz, M.W.; Chiba-Falek, O. Genetic analysis of alpha-synuclein 3′ untranslated region and its corresponding microRNAs in relation to Parkinson’s disease compared to dementia with Lewy bodies. Alzheimer’s Dement. 2017, 13, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.; Liu, H.; Zhang, L.; Lv, W.; Hu, X. Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget 2015, 6, 37043–37053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, R.S.; Jakymiw, A.; Yao, B.; Pauley, B.A.; Carcamo, W.C.; Katz, J.; Cheng, J.Q.; Chan, E.K. High resolution of microRNA signatures in human whole saliva. Arch. Oral Biol. 2011, 56, 1506–1513. [Google Scholar] [CrossRef] [Green Version]
- Vallelunga, A.; Ragusa, M.; Di Mauro, S.; Iannitti, T.; Pilleri, M.; Biundo, R.; Weis, L.; Di Pietro, C.; De Iuliis, A.; Nicoletti, A.; et al. Identification of circulating microRNAs for the differential diagnosis of Parkinson’s disease and Multiple System Atrophy. Front. Cell. Neurosci. 2014, 8, 156. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yang, R.; Hu, B.L.; Lu, P.; Zhou, L.L.; He, Z.Y.; Wu, H.M.; Zhu, J.H. Reduced Circulating Levels of miR-433 and miR-133b Are Potential Biomarkers for Parkinson’s Disease. Front. Cell. Neurosci. 2017, 11, 170. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Huang, F.; Zhu, X.; Li, D.; Fu, S.; He, H. The heme oxygenase system and oral diseases. Oral Dis. 2011, 17, 252–257. [Google Scholar] [CrossRef]
- Del Tredici, K.; Hawkes, C.H.; Ghebremedhin, E.; Braak, H. Lewy pathology in the submandibular gland of individuals with incidental Lewy body disease and sporadic Parkinson’s disease. Acta Neuropathol. 2010, 119, 703–713. [Google Scholar] [CrossRef]
- Roberts, H.L.; Brown, D.R. Seeking a mechanism for the toxicity of oligomeric alpha-synuclein. Biomolecules 2015, 5, 282–305. [Google Scholar] [CrossRef] [Green Version]
- Cressatti, M.; Galindez, J.M.; Juwara, L.; Orlovetskie, N.; Velly, A.M.; Eintracht, S.; Liberman, A.; Gornitsky, M.; Schipper, H.M. Characterization and heme oxygenase-1 content of extracellular vesicles in human biofluids. J. Neurochem. 2021, 157, 2195–2209. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.M.; Wiklander, P.B.; Nordin, J.Z.; Al-Shawi, R.; Wood, M.J.; Vithlani, M.; Schapira, A.H.; Simons, J.P.; El-Andaloussi, S.; Alvarez-Erviti, L. Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov. Disord. 2014, 29, 1476–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galieva, L.R.; James, V.; Mukhamedshina, Y.O.; Rizvanov, A.A. Therapeutic Potential of Extracellular Vesicles for the Treatment of Nerve Disorders. Front. Neurosci. 2019, 13, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Chu, Y.; Kordower, J.H. Age-associated increases of alpha-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: Is this the target for Parkinson’s disease? Neurobiol. Dis. 2007, 25, 134–149. [Google Scholar] [CrossRef]
- Harada, C.N.; Natelson Love, M.C.; Triebel, K.L. Normal cognitive aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).