Neuroscience and Neuroimmunology Solutions for Osteoarthritis Pain: Biological Drugs, Growth Factors, Peptides and Monoclonal Antibodies Targeting Peripheral Nerves
Abstract
1. Introduction
2. Osteoarthritis (OA)
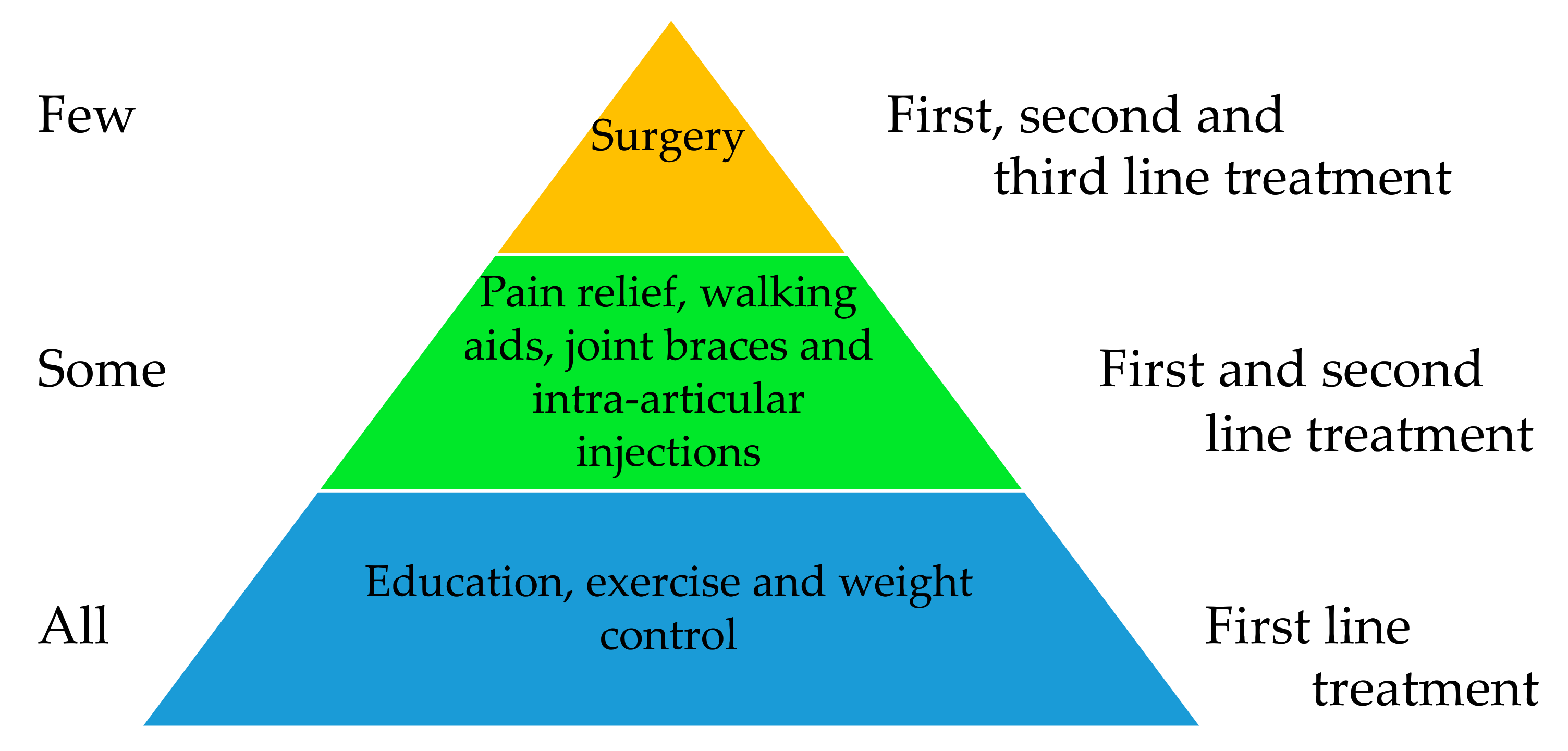
3. Existing Recommended Treatments for OA
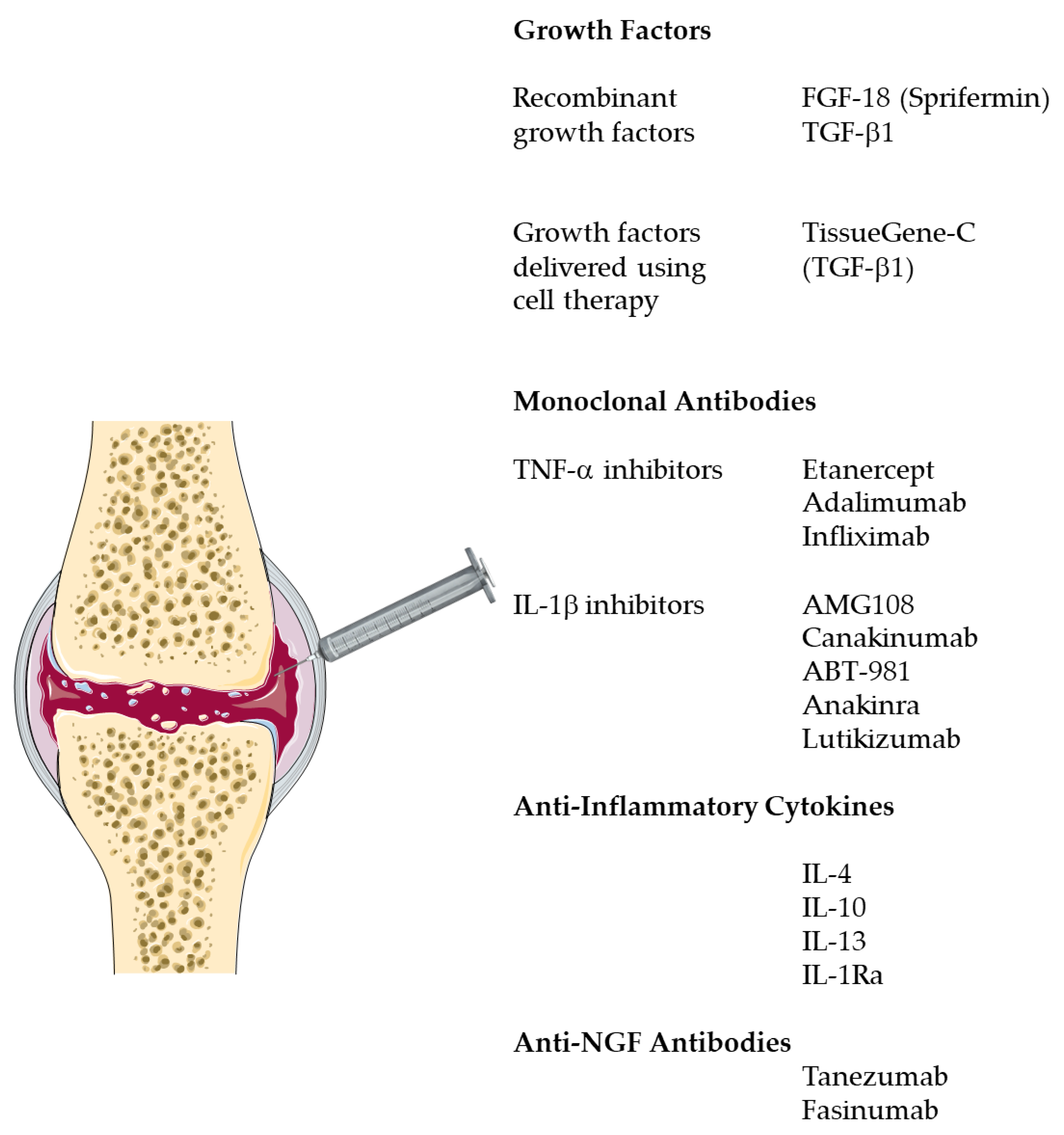
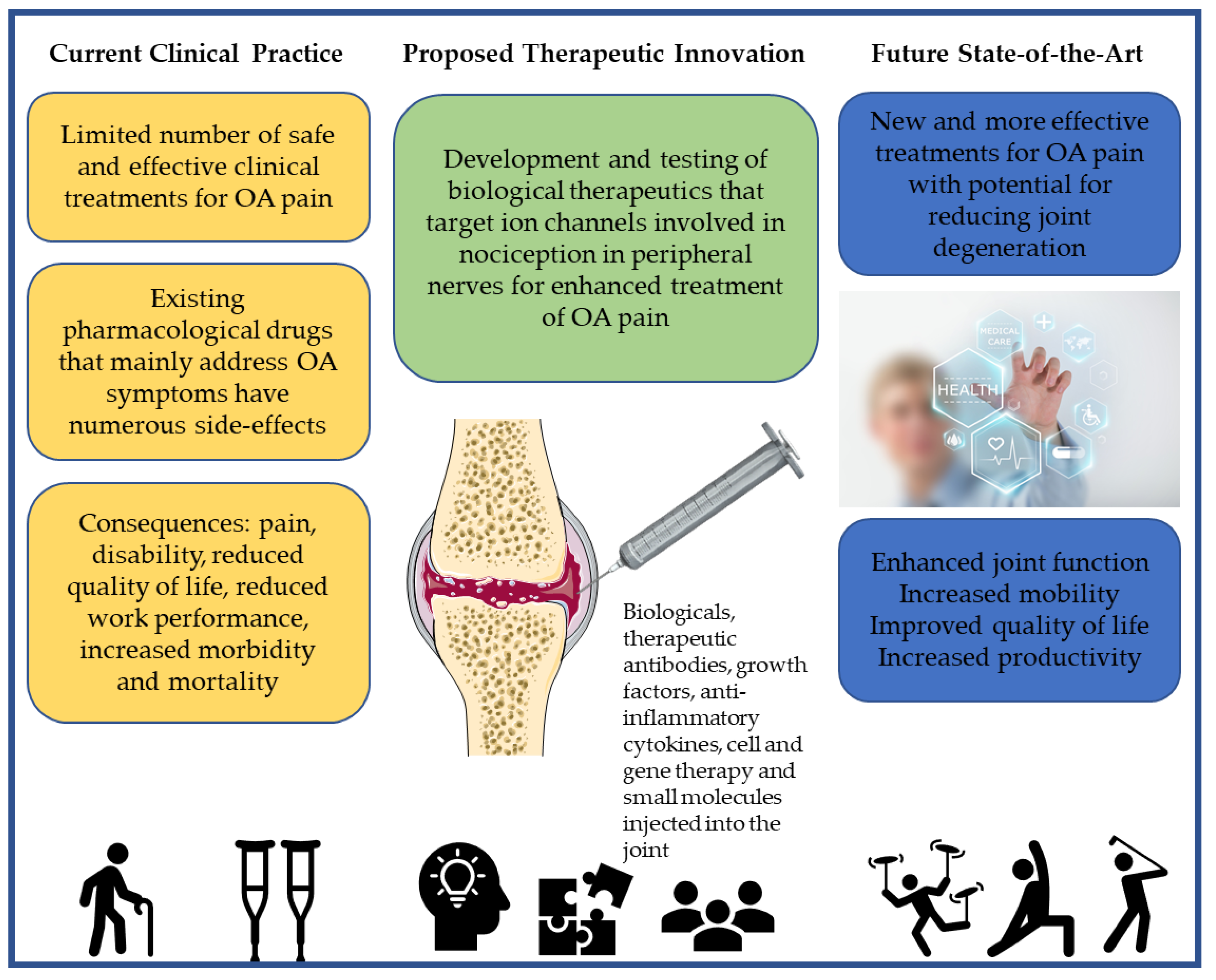
4. Emerging Biological Treatments for OA
5. Ion Channels and Pain
6. Drug Pipelines with Putative Ion Channels Targets
7. mAbs Targeting Ion Channels
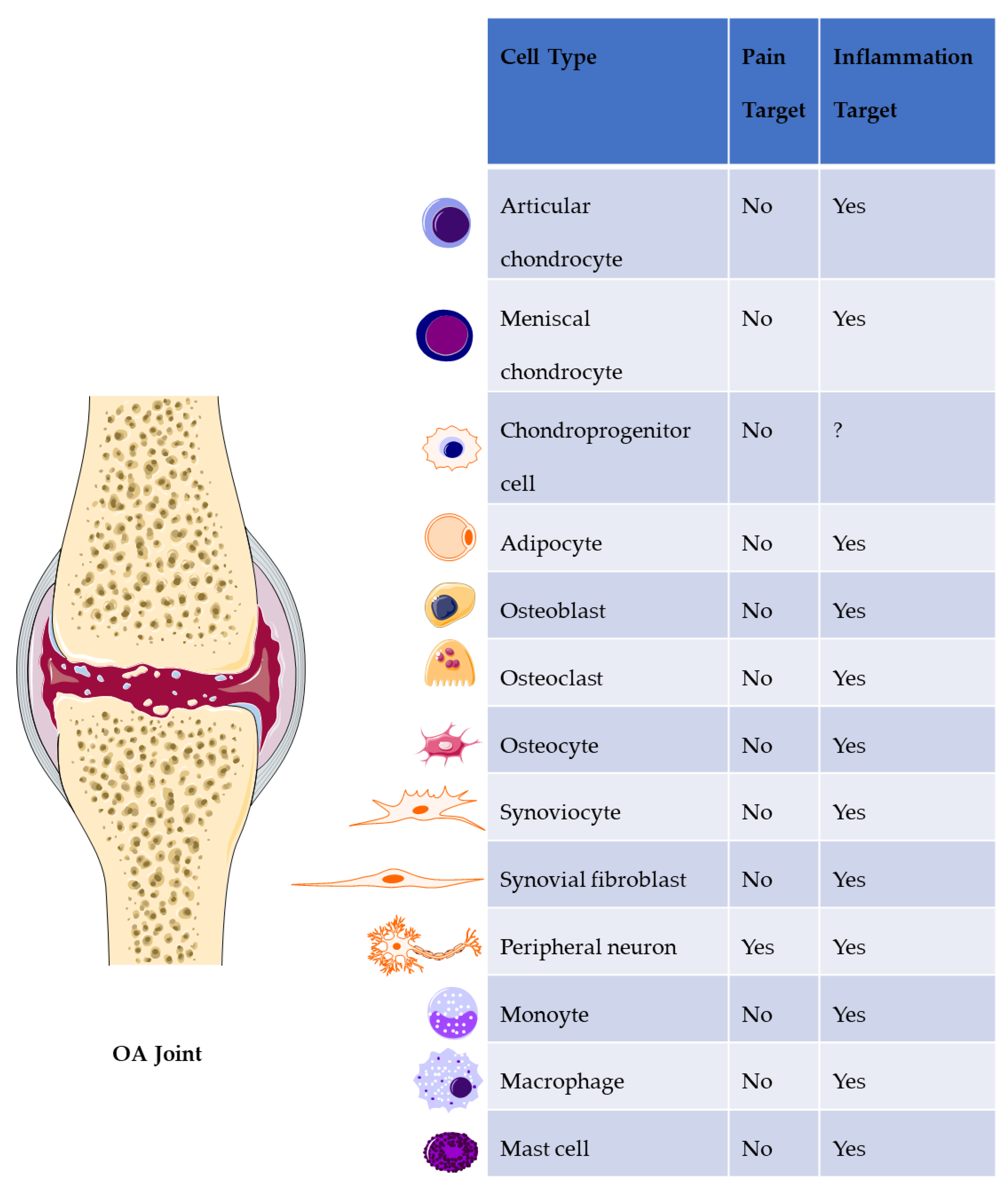
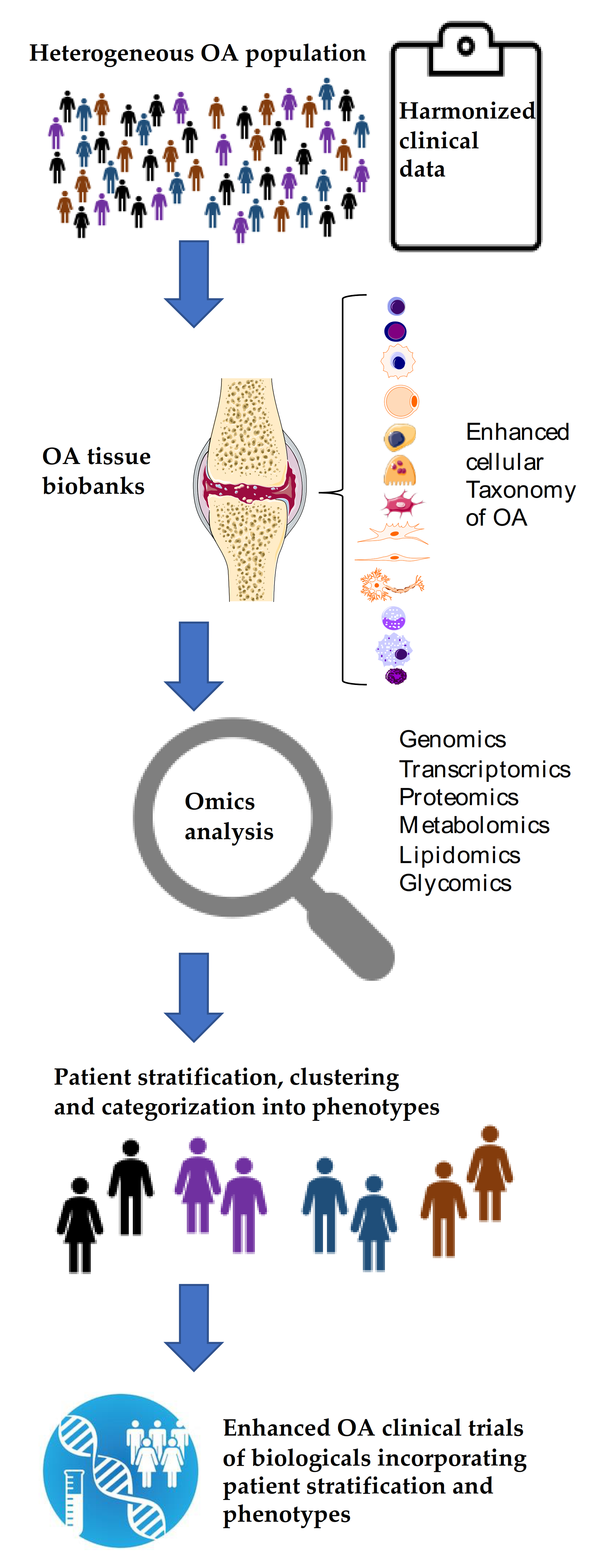
8. Discussion
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pavlov, V.A.; Chavan, S.S.; Tracey, K.J. Molecular and functional neuroscience in immunity. Annu. Rev. Immunol. 2018, 36, 783–812. [Google Scholar] [CrossRef] [PubMed]
- Leibold, A.T.; Monaco, G.N.; Dey, M. The role of the immune system in brain metastasis. Curr. Neurobiol. 2019, 10, 33–48. [Google Scholar] [PubMed]
- Vincent, A. Autoimmune channelopathies: Well-established and emerging immunotherapy-responsive diseases of the peripheral and central nervous systems. J. Clin. Immunol. 2010, 30 (Suppl. 1), S97–S102. [Google Scholar] [CrossRef]
- Madry, H. Translating orthopaedic basic science into clinical relevance. J. Exp. Ortop. 2014, 1, 5. [Google Scholar] [CrossRef]
- Restrepo, H.E.; Rozental, M. The social impact of aging populations: Some major issues. Soc. Sci. Med. 1994, 39, 1323–1338. [Google Scholar] [CrossRef]
- Bongaarts, J. Human population growth and the demographic transition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2985–2990. [Google Scholar] [CrossRef] [PubMed]
- Diczfalusy, E. The demographic revolution and our common future. Maturitas 2001, 38, 5–14, discussion 14. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Hawker, G.A. Osteoarthritis is a serious disease. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 120), 3–6. [Google Scholar]
- Hunter, D.J.; March, L.; Chew, M. Osteoarthritis in 2020 and beyond: A Lancet Commission. Lancet 2020, 396, 1711–1712. [Google Scholar] [CrossRef]
- Hanna, F.S.; Wluka, A.E.; Bell, R.J.; Davis, S.R.; Cicuttini, F.M. Osteoarthritis and the postmenopausal woman: Epidemiological, magnetic resonance imaging, and radiological findings. Semin. Arthritis Rheum. 2004, 34, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.A.; Bay-Jensen, A.C.; Henriksen, K.; Christiansen, C. The pathogenesis of osteoarthritis involves bone, cartilage and synovial inflammation: May estrogen be a magic bullet? Menopause Int. 2012, 18, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.C. A woman’s journey through the reproductive, transitional and postmenopausal periods of life: Impact on cardiovascular and musculo-skeletal risk and the role of estrogen replacement. Maturitas 2011, 70, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Felson, D.T. Weight and osteoarthritis. Am. J. Clin. Nutr. 1996, 63, 430S–432S. [Google Scholar] [CrossRef]
- Zhang, Y.; Jordan, J.M. Epidemiology of osteoarthritis. Clin. Geriatr Med. 2010, 26, 355–369. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2012, 2, 1143–1211. [Google Scholar]
- Courties, A.; Berenbaum, F.; Sellam, J. The Phenotypic Approach to Osteoarthritis: A Look at Metabolic Syndrome-Associated Osteoarthritis. Joint Bone Spine 2019, 86, 725–730. [Google Scholar] [CrossRef]
- Felson, D.T. Risk factors for osteoarthritis: Understanding joint vulnerability. Clin. Orthop. Relat. Res. 2004, 427, S16–S21. [Google Scholar] [CrossRef]
- Guilak, F. Biomechanical factors in osteoarthritis. Best Pract Res. Clin. Rheumatol 2011, 25, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Englund, M. The role of biomechanics in the initiation and progression of OA of the knee. Best Pract Res. Clin. Rheumatol 2010, 24, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Rayman, M.P.; Gualillo, O.; Sellam, J.; van der Kraan, P.; Fearon, U. The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2017, 13, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Cooper, C.; Reginster, J.-Y.; Hochberg, M.; Branco, J.; Bruyère, O.; Chapurlat, R.; Al-Daghri, N.; Dennison, E.; Herrero-Beaumont, G.; et al. Type 2 diabetes mellitus and osteoarthritis. Semin. Arthritis Rheum. 2019, 49, 9–19. [Google Scholar] [CrossRef]
- Felson, D.T. Does excess weight cause osteoarthritis and, if so, why? Ann. Rheum. Dis. 1996, 55, 668–670. [Google Scholar] [CrossRef]
- Katon, W.J. The comorbidity of diabetes mellitus and depression. Am. J. Med. 2008, 121, S8–S15. [Google Scholar] [CrossRef]
- De la Cruz-Cano, E.; Tovilla-Zarate, C.A.; Reyes-Ramos, E.; Gonzalez-Castro, T.B.; Juarez-Castro, I.; López-Narváez, M.L.; Fresan, A. Association between obesity and depression in patients with diabetes mellitus type 2; a study protocol. [version 1; peer review: 2 approved]. F1000Research 2015, 4, 7. [Google Scholar] [CrossRef]
- Kim, K.W.; Han, J.W.; Cho, H.J.; Chang, C.B.; Park, J.H.; Lee, J.J.; Lee, S.B.; Seong, S.C.; Kim, T.K. Association between comorbid depression and osteoarthritis symptom severity in patients with knee osteoarthritis. J. Bone Joint Surg. Am. 2011, 93, 556–563. [Google Scholar] [CrossRef]
- Carstensen, J.; Andersson, D.; André, M.; Engström, S.; Magnusson, H.; Borgquist, L.A. How does comorbidity influence healthcare costs? A population-based cross-sectional study of depression, back pain and osteoarthritis. BMJ Open 2012, 2, e000809. [Google Scholar] [CrossRef]
- Zheng, S.; Tu, L.; Cicuttini, F.; Zhu, Z.; Han, W.; Antony, B.; Wluka, A.E.; Winzenberg, T.; Aitken, D.; Blizzard, L.; et al. Depression in patients with knee osteoarthritis: Risk factors and associations with joint symptoms. BMC Musculoskelet. Disord. 2021, 22, 40. [Google Scholar] [CrossRef] [PubMed]
- Grässel, S.G. The role of peripheral nerve fibers and their neurotransmitters in cartilage and bone physiology and pathophysiology. Arthritis Res. Ther. 2014, 16, 485. [Google Scholar] [CrossRef] [PubMed]
- Grässel, S.; Muschter, D. Peripheral nerve fibers and their neurotransmitters in osteoarthritis pathology. Int. J. Mol. Sci. 2017, 18, 931. [Google Scholar] [CrossRef] [PubMed]
- Rainov, N.G.; Heidecke, V. Targeted biological therapies for pain. Expert Opin. Biol. Ther. 2011, 11, 1315–1326. [Google Scholar] [CrossRef]
- Syx, D.; Tran, P.B.; Miller, R.E.; Malfait, A.-M. Peripheral mechanisms contributing to osteoarthritis pain. Curr Rheumatol. Rep. 2018, 20, 9. [Google Scholar] [CrossRef]
- Sagar, D.R.; Nwosu, L.; Walsh, D.A.; Chapman, V. Dissecting the contribution of knee joint NGF to spinal nociceptive sensitization in a model of OA pain in the rat. Osteoarthr. Cartil. 2015, 23, 906–913. [Google Scholar] [CrossRef]
- He, B.H.; Christin, M.; Mouchbahani-Constance, S.; Davidova, A.; Sharif-Naeini, R. Mechanosensitive ion channels in articular nociceptors drive mechanical allodynia in osteoarthritis. Osteoarthr. Cartil. 2017, 25, 2091–2099. [Google Scholar] [CrossRef]
- Malfait, A.-M.; Miller, R.J. Emerging targets for the management of osteoarthritis pain. Curr. Osteoporos. Rep. 2016, 14, 260–268. [Google Scholar] [CrossRef]
- Rosenbaum, M.I.; Clemmensen, L.S.; Bredt, D.S.; Bettler, B.; Strømgaard, K. Targeting receptor complexes: A new dimension in drug discovery. Nat. Rev. Drug Discov. 2020, 19, 884–901. [Google Scholar] [CrossRef]
- Gough, N.R. Channeling pain through GPCRs. Science 2017, 355, 143–144. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hamers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Riechmann, L.; Muyldermans, S. Single domain antibodies: Comparison of camel VH and camelised human VH domains. J. Immunol. Methods 1999, 231, 25–38. [Google Scholar] [CrossRef]
- Muyldermans, S. Single domain camel antibodies: Current status. J. Biotechnol. 2001, 74, 277–302. [Google Scholar] [CrossRef]
- Els Conrath, K.; Lauwereys, M.; Wyns, L.; Muyldermans, S. Camel single-domain antibodies as modular building units in bispecific and bivalent antibody constructs. J. Biol. Chem. 2001, 276, 7346–7350. [Google Scholar] [CrossRef]
- Zhao, Q. Bispecific antibodies for autoimmune and inflammatory diseases: Clinical progress to date. BioDrugs 2020, 34, 111–119. [Google Scholar] [CrossRef]
- Grässel, S.; Muschter, D. Recent advances in the treatment of osteoarthritis. F1000Research 2020, 9. [Google Scholar] [CrossRef]
- Fogel, D.B. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review. Contemp. Clin. Trials Commun. 2018, 11, 156–164. [Google Scholar] [CrossRef]
- Latourte, A.; Kloppenburg, M.; Richette, P. Emerging pharmaceutical therapies for osteoarthritis. Nat. Rev. Rheumatol. 2020, 16, 673–688. [Google Scholar] [CrossRef]
- Sabha, M.; Siaton, B.C.; Hochberg, M.C. Lorecivivint, an intra-articular potential disease-modifying osteoarthritis drug. Expert Opin. Investig. Drugs 2020, 29, 1339–1346. [Google Scholar] [CrossRef]
- Ghouri, A.; Conaghan, P.G. Prospects for therapies in osteoarthritis. Calcif. Tissue Int. 2020. [Google Scholar] [CrossRef]
- Kraus, V.B.; Simon, L.S.; Katz, J.N.; Neogi, T.; Hunter, D.; Guermazi, A.; Karsdal, M.A. Proposed study designs for approval based on a surrogate endpoint and a post-marketing confirmatory study under FDA’s accelerated approval regulations for disease modifying osteoarthritis drugs. Osteoarthr. Cartil. 2019, 27, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Lohmander, L.S.; Hellot, S.; Dreher, D.; Krantz, E.F.W.; Kruger, D.S.; Guermazi, A.; Eckstein, F. Intraarticular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: A randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2014, 66, 1820–1831. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-M.; Chen, Y.-H.; Sun, H.S.; Tsai, S.-J. Fibroblast growth factors: Potential novel targets for regenerative therapy of osteoarthritis. Chin. J. Physiol. 2019, 62, 2–10. [Google Scholar] [PubMed]
- Xie, Y.; Zinkle, A.; Chen, L.; Mohammadi, M. Fibroblast growth factor signalling in osteoarthritis and cartilage repair. Nat. Rev. Rheumatol. 2020, 16, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Badlani, N.; Oshima, Y.; Healey, R.; Coutts, R.; Amiel, D. Use of bone morphogenic protein-7 as a treatment for osteoarthritis. Clin. Orthop. Relat. Res. 2009, 467, 3221–3229. [Google Scholar] [CrossRef]
- Hunter, D.J.; Pike, M.C.; Jonas, B.L.; Kissin, E.; Krop, J.; McAlindon, T. Phase 1 safety and tolerability study of BMP-7 in symptomatic knee osteoarthritis. BMC Musculoskelet. Disord. 2010, 11, 232. [Google Scholar] [CrossRef]
- Tao, K.; Rey-Rico, A.; Frisch, J.; Venkatesan, J.K.; Schmitt, G.; Madry, H.; Lin, J.; Cucchiarini, M. rAAV-mediated combined gene transfer and overexpression of TGF-β and SOX9 remodels human osteoarthritic articular cartilage. J. Orthop. Res. 2016, 34, 2181–2190. [Google Scholar] [CrossRef]
- Venkatesan, J.K.; Rey-Rico, A.; Schmitt, G.; Wezel, A.; Madry, H.; Cucchiarini, M. rAAV-mediated overexpression of TGF-β stably restructures human osteoarthritic articular cartilage in situ. J. Transl. Med. 2013, 11, 211. [Google Scholar] [CrossRef]
- Blaney Davidson, E.N.; Vitters, E.L.; van den Berg, W.B.; van der Kraan, P.M. TGF beta-induced cartilage repair is maintained but fibrosis is blocked in the presence of Smad7. Arthritis Res. Ther. 2006, 8, R65. [Google Scholar] [CrossRef]
- Yu, S.P.; Hunter, D.J. Intra-articular therapies for osteoarthritis. Expert Opin. Pharmacother. 2016, 17, 2057–2071. [Google Scholar] [CrossRef]
- Sutton, S.; Clutterbuck, A.; Harris, P.; Gent, T.; Freeman, S.; Foster, N.; Barrett-Jolley, R.; Mobasheri, A. The contribution of the synovium, synovial derived inflammatory cytokines and neuropeptides to the pathogenesis of osteoarthritis. Vet. J. 2009, 179, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Hunter, D.J.; Xu, J.; Ding, C. Monoclonal antibodies for the treatment of osteoarthritis. Expert Opin. Biol. Ther. 2016, 16, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Smelter, E.; Hochberg, M.C. New treatments for osteoarthritis. Curr. Opin. Rheumatol. 2013, 25, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, T.J.; Easton, R.; Pang, S.; Levinson, D.J.; Pixton, G.; Viktrup, L.; Davignon, I.; Brown, M.T.; West, C.R.; Verburg, K.M. Effect of tanezumab on joint pain, physical function, and patient global assessment of osteoarthritis among patients with osteoarthritis of the hip or knee: A randomized clinical trial. JAMA 2019, 322, 37–48. [Google Scholar] [CrossRef]
- Schnitzer, T.J.; Khan, A.; Bessette, L.; Davignon, I.; Brown, M.T.; Pixton, G.; Prucka, W.R.; Tive, L.; Viktrup, L.; West, C.R. Onset and maintenance of efficacy of subcutaneous tanezumab in patients with moderate to severe osteoarthritis of the knee or hip: A 16-week dose-titration study. Semin. Arthritis Rheum. 2020, 50, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F.; Blanco, F.J.; Guermazi, A.; Miki, K.; Yamabe, T.; Viktrup, L.; Junor, R.; Carey, W.; Brown, M.T.; West, C.R.; et al. Subcutaneous tanezumab for osteoarthritis of the hip or knee: Efficacy and safety results from a 24-week randomised phase III study with a 24-week follow-up period. Ann. Rheum. Dis. 2020, 79, 800–810. [Google Scholar] [CrossRef]
- Mobasheri, A. Future Cell and Gene Therapy for Osteoarthritis (OA): Potential for Using Mammalian Protein Production Platforms, Irradiated and Transfected Protein Packaging Cell Lines for Over-Production of Therapeutic Proteins and Growth Factors. Adv. Exp. Med. Biol. 2020, 1247, 17–31. [Google Scholar] [PubMed]
- Mobasheri, A.; Richardson, S.M. Cell and gene therapy for spine regeneration: Mammalian protein production platforms for overproduction of therapeutic proteins and growth factors. Neurosurg. Clin. N. Am. 2020, 31, 131–139. [Google Scholar] [CrossRef]
- Mobasheri, A.; Choi, H.; Martín-Vasallo, P. Over-Production of Therapeutic Growth Factors for Articular Cartilage Regeneration by Protein Production Platforms and Protein Packaging Cell Lines. Biology 2020, 9, 330. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.; Seo, J.; Choi, K.; Lee, Y.; Park, K.; Kim, S.; Mobasheri, A.; Choi, H. TissueGene-C promotes an anti-inflammatory micro-environment in a rat monoiodoacetate model of osteoarthritis via polarization of M2 macrophages leading to pain relief and structural improvement. Inflammopharmacology 2020, 28, 1237–1252. [Google Scholar] [CrossRef]
- Lee, B.; Parvizi, J.; Bramlet, D.; Romness, D.W.; Guermazi, A.; Noh, M.; Sodhi, N.; Khlopas, A.; Mont, M.A. Results of a Phase II Study to Determine the Efficacy and Safety of Genetically Engineered Allogeneic Human Chondrocytes Expressing TGF-β1. J. Knee Surg. 2020, 33, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Wenham, C.Y.J.; McDermott, M.; Conaghan, P.G. Biological therapies in osteoarthritis. Curr. Pharm. Des. 2015, 21, 2206–2215. [Google Scholar] [CrossRef] [PubMed]
- Migliore, A.; Paoletta, M.; Moretti, A.; Liguori, S.; Iolascon, G. The perspectives of intra-articular therapy in the management of osteoarthritis. Expert Opin Drug Deliv 2020, 17, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.S.; Parvizi, J.; Ehiorobo, J.O.; Mont, M.A. The Safety and Efficacy of a Novel Cell-Based Gene Therapy for Knee Osteoarthritis. Surg Technol. Int 2019, 35, 370–376. [Google Scholar]
- Madry, H.; Cucchiarini, M. Gene therapy for human osteoarthritis: Principles and clinical translation. Expert Opin. Biol. Ther. 2016, 16, 331–346. [Google Scholar] [CrossRef]
- Miller, R.E.; Malfait, A.-M.; Block, J.A. Current status of nerve growth factor antibodies for the treatment of osteoarthritis pain. Clin. Exp. Rheumatol. 2017, 35 (Suppl. 107), 85–87. [Google Scholar]
- Bannwarth, B.; Kostine, M. Targeting nerve growth factor (NGF) for pain management: What does the future hold for NGF antagonists? Drugs 2014, 74, 619–626. [Google Scholar] [CrossRef]
- McMahon, S.B. NGF as a mediator of inflammatory pain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996, 351, 431–440. [Google Scholar]
- Sommer, C.; Petrausch, S.; Lindenlaub, T.; Toyka, K.V. Neutralizing antibodies to interleukin 1-receptor reduce pain associated behavior in mice with experimental neuropathy. Neurosci. Lett. 1999, 270, 25–28. [Google Scholar] [CrossRef]
- Eitner, A.; Hofmann, G.O.; Schaible, H.-G. Mechanisms of osteoarthritic pain. studies in humans and experimental models. Front. Mol. Neurosci. 2017, 10, 349. [Google Scholar] [CrossRef]
- Chevalier, X.; Goupille, P.; Beaulieu, A.D.; Burch, F.X.; Bensen, W.G.; Conrozier, T.; Loeuille, D.; Kivitz, A.J.; Silver, D.; Appleton, B.E. Intraarticular injection of anakinra in osteoarthritis of the knee: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009, 61, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.B.; Proudman, S.; Kivitz, A.J.; Burch, F.X.; Donohue, J.P.; Burstein, D.; Sun, Y.-N.; Banfield, C.; Vincent, M.S.; Ni, L.; et al. A randomized, double-blind study of AMG 108 (a fully human monoclonal antibody to IL-1R1) in patients with osteoarthritis of the knee. Arthritis Res. Ther. 2011, 13, R125. [Google Scholar] [CrossRef] [PubMed]
- Kloppenburg, M.; Peterfy, C.; Haugen, I.K.; Kroon, F.; Chen, S.; Wang, L.; Liu, W.; Levy, G.; Fleischmann, R.M.; Berenbaum, F.; et al. Phase IIa, placebo-controlled, randomised study of lutikizumab, an anti-interleukin-1α and anti-interleukin-1β dual variable domain immunoglobulin, in patients with erosive hand osteoarthritis. Ann. Rheum. Dis. 2019, 78, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, R.M.; Bliddal, H.; Blanco, F.J.; Schnitzer, T.J.; Peterfy, C.; Chen, S.; Wang, L.; Feng, S.; Conaghan, P.G.; Berenbaum, F.; et al. A Phase II Trial of Lutikizumab, an Anti-Interleukin-1α/β Dual Variable Domain Immunoglobulin, in Knee Osteoarthritis Patients With Synovitis. Arthritis Rheumatol. 2019, 71, 1056–1069. [Google Scholar] [CrossRef] [PubMed]
- McCleskey, E.W.; Gold, M.S. Ion channels of nociception. Annu. Rev. Physiol. 1999, 61, 835–856. [Google Scholar] [CrossRef]
- Levinson, S.R.; Luo, S.; Henry, M.A. The role of sodium channels in chronic pain. Muscle Nerve 2012, 46, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Oortgiesen, M.; Li, L.; Simon, S.A. Capsaicin inhibits activation of voltage-gated sodium currents in capsaicin-sensitive trigeminal ganglion neurons. J. Neurophysiol. 2001, 85, 745–758. [Google Scholar] [CrossRef]
- Liu, L.; Yang, T.; Bruno, M.J.; Andersen, O.S.; Simon, S.A. Voltage-gated ion channels in nociceptors: Modulation by cGMP. J. Neurophysiol. 2004, 92, 2323–2332. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Wickenden, A.D.; Chaplan, S.R. Sodium channel blockers for the treatment of neuropathic pain. Neurotherapeutics 2009, 6, 663–678. [Google Scholar] [CrossRef]
- Fertleman, C.R.; Baker, M.D.; Parker, K.A.; Moffatt, S.; Elmslie, F.V.; Abrahamsen, B.; Ostman, J.; Klugbauer, N.; Wood, J.N.; Gardiner, R.M.; et al. SCN9A mutations in paroxysmal extreme pain disorder: Allelic variants underlie distinct channel defects and phenotypes. Neuron 2006, 52, 767–774. [Google Scholar] [CrossRef]
- Waxman, S.G. A channel sets the gain on pain. Nature 2006, 444, 831–832. [Google Scholar] [CrossRef] [PubMed]
- King, G.F.; Vetter, I. No gain, no pain: NaV1.7 as an analgesic target. ACS Chem. Neurosci. 2014, 5, 749–751. [Google Scholar] [CrossRef] [PubMed]
- Eagles, D.A.; Chow, C.Y.; King, G.F. Fifteen years of NaV 1.7 channels as an analgesic target: Why has excellent in vitro pharmacology not translated into in vivo analgesic efficacy? Br. J. Pharmacol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, S. Translational research challenges: Finding the right animal models. J. Investig. Med. 2012, 60, 1141–1146. [Google Scholar] [CrossRef]
- Deuis, J.R.; Dekan, Z.; Wingerd, J.S.; Smith, J.J.; Munasinghe, N.R.; Bhola, R.F.; Imlach, W.L.; Herzig, V.; Armstrong, D.A.; Rosengren, K.J.; et al. Pharmacological characterisation of the highly NaV1.7 selective spider venom peptide Pn3a. Sci. Rep. 2017, 7, 40883. [Google Scholar] [CrossRef]
- Haustrate, A.; Hantute-Ghesquier, A.; Prevarskaya, N.; Lehen’kyi, V. Monoclonal antibodies targeting ion channels and their therapeutic potential. Front. Pharmacol. 2019, 10, 606. [Google Scholar] [CrossRef]
- Durán-Lobato, M.; Niu, Z.; Alonso, M.J. Oral delivery of biologics for precision medicine. Adv. Mater. Weinheim 2020, 32, e1901935. [Google Scholar] [CrossRef]
- Jullien, M.; Touzeau, C.; Moreau, P. Monoclonal antibodies as an addition to current myeloma therapy strategies. Expert Rev. Anticancer Ther. 2020, 1–11. [Google Scholar] [CrossRef]
- Lee, J.K.; Priceman, S.J. Precision Medicine-Enabled Cancer Immunotherapy. Cancer Treat. Res. 2019, 178, 189–205. [Google Scholar]
- Matta, C.; Boocock, D.J.; Fellows, C.R.; Miosge, N.; Dixon, J.E.; Liddell, S.; Smith, J.; Mobasheri, A. Molecular phenotyping of the surfaceome of migratory chondroprogenitors and mesenchymal stem cells using biotinylation, glycocapture and quantitative LC-MS/MS proteomic analysis. Sci. Rep. 2019, 9, 9018. [Google Scholar] [CrossRef]
- Jeremiasse, B.; Matta, C.; Fellows, C.R.; Boocock, D.J.; Smith, J.R.; Liddell, S.; Lafeber, F.; van Spil, W.E.; Mobasheri, A. Alterations in the chondrocyte surfaceome in response to pro-inflammatory cytokines. BMC Mol. and Cell Biol 2020, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Bausch-Fluck, D.; Goldmann, U.; Müller, S.; van Oostrum, M.; Müller, M.; Schubert, O.T.; Wollscheid, B. The in silico human surfaceome. Proc. Natl. Acad. Sci. USA 2018, 115, E10988–E10997. [Google Scholar] [CrossRef] [PubMed]
- Bates, E. Ion channels in development and cancer. Annu. Rev. Cell Dev. Biol. 2015, 31, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Bagal, S.K.; Brown, A.D.; Cox, P.J.; Omoto, K.; Owen, R.M.; Pryde, D.C.; Sidders, B.; Skerratt, S.E.; Stevens, E.B.; Storer, R.I.; et al. Ion channels as therapeutic targets: A drug discovery perspective. J. Med. Chem. 2013, 56, 593–624. [Google Scholar] [CrossRef]
- Selvaraj, C.; Selvaraj, G.; Kaliamurthi, S.; Cho, W.C.; Wei, D.-Q.; Singh, S.K. Ion channels as therapeutic targets for type 1 diabetes mellitus. Curr Drug Targets 2020, 21, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, C.J.; Colussi, P.; Clark, T.G. Ion channels as therapeutic antibody targets. MAbs 2019, 11, 265–296. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, K. Exploiting the diversity of ion channels: Modulation of ion channels for therapeutic indications. Handb Exp. Pharmacol 2019, 260, 187–205. [Google Scholar]
- Mathie, A. Ion channels as novel therapeutic targets in the treatment of pain. J. Pharm. Pharmacol. 2010, 62, 1089–1095. [Google Scholar] [CrossRef]
- Vasconcelos, L.H.C.; Souza, I.L.L.; Pinheiro, L.S.; Silva, B.A. Ion channels in obesity: Pathophysiology and potential therapeutic targets. Front. Pharmacol. 2016, 7, 58. [Google Scholar] [CrossRef]
- Sun, H.; Li, M. Antibody therapeutics targeting ion channels: Are we there yet? Acta Pharmacol. Sin. 2013, 34, 199–204. [Google Scholar] [CrossRef]
- Pless, S.A.; Ahern, C.A. Introduction: Applying chemical biology to ion channels. Adv. Exp. Med. Biol. 2015, 869, 1–4. [Google Scholar] [PubMed]
- Mobasheri, A.; Matta, C.; Uzielienè, I.; Budd, E.; Martín-Vasallo, P.; Bernotiene, E. The chondrocyte channelome: A narrative review. Joint Bone Spine 2019, 86, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Matta, C.; Zákány, R.; Mobasheri, A. Voltage-dependent calcium channels in chondrocytes: Roles in health and disease. Curr Rheumatol. Rep. 2015, 17, 43. [Google Scholar] [CrossRef]
- Mobasheri, A.; Lewis, R.; Ferreira-Mendes, A.; Rufino, A.; Dart, C.; Barrett-Jolley, R. Potassium channels in articular chondrocytes. Channels 2012, 6, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Barrett-Jolley, R.; Lewis, R.; Fallman, R.; Mobasheri, A. The emerging chondrocyte channelome. Front. Physiol. 2010, 1, 135. [Google Scholar] [CrossRef]
- Naylor, J.; Beech, D.J. Generation of antibodies that are externally acting isoform-specific inhibitors of ion channels. Methods Mol. Biol. 2013, 998, 245–256. [Google Scholar]
- Gerlach, A.C.; Antonio, B.M. Validation of ion channel targets. Channels 2015, 9, 376–379. [Google Scholar] [CrossRef][Green Version]
- Mohammadinejad, R.; Ashrafizadeh, M.; Pardakhty, A.; Uzieliene, I.; Denkovskij, J.; Bernotiene, E.; Janssen, L.; Lorite, G.S.; Saarakkala, S.; Mobasheri, A. Nanotechnological strategies for osteoarthritis diagnosis, monitoring, clinical management, and regenerative medicine: Recent advances and future opportunities. Curr Rheumatol. Rep. 2020, 22, 12. [Google Scholar] [CrossRef]
- Kalamegam, G.; Memic, A.; Budd, E.; Abbas, M.; Mobasheri, A. A comprehensive review of stem cells for cartilage regeneration in osteoarthritis. Adv. Exp. Med. Biol. 2018, 1089, 23–36. [Google Scholar]
- Bernotiene, E.; Bagdonas, E.; Kirdaite, G.; Bernotas, P.; Kalvaityte, U.; Uzieliene, I.; Thudium, C.S.; Hannula, H.; Lorite, G.S.; Dvir-Ginzberg, M.; et al. Emerging technologies and platforms for the immunodetection of multiple biochemical markers in osteoarthritis research and therapy. Front. Med. 2020, 7, 572977. [Google Scholar] [CrossRef]
- Rahmati, M.; Pennisi, C.P.; Budd, E.; Mobasheri, A.; Mozafari, M. Biomaterials for regenerative medicine: Historical perspectives and current trends. Adv. Exp. Med. Biol. 2018, 1119, 1–19. [Google Scholar] [PubMed]
- Rahmati, M.; Pennisi, C.P.; Mobasheri, A.; Mozafari, M. Bioengineered scaffolds for stem cell applications in tissue engineering and regenerative medicine. Adv. Exp. Med. Biol. 2018, 1107, 73–89. [Google Scholar] [PubMed]
- Maini, R.N.; Brennan, F.M.; Williams, R.; Chu, C.Q.; Cope, A.P.; Gibbons, D.; Elliott, M.; Feldmann, M. TNF-alpha in rheumatoid arthritis and prospects of anti-TNF therapy. Clin. Exp. Rheumatol. 1993, 11 (Suppl. 8), S173–S175. [Google Scholar]
- Maini, R.N.; Elliott, M.; Brennan, F.M.; Williams, R.O.; Feldmann, M. Targeting TNF alpha for the therapy of rheumatoid arthritis. Clin. Exp. Rheumatol. 1994, 12 Suppl 11, S63-6. [Google Scholar]
- Feldmann, M.; Brennan, F.M.; Williams, R.O.; Elliott, M.J.; Maini, R.N. Cytokine expression and networks in rheumatoid arthritis: Rationale for anti-TNF alpha antibody therapy and its mechanism of action. J. Inflamm. 1995, 47, 90–96. [Google Scholar] [PubMed]
- Qvist, P.; Bay-Jensen, A.-C.; Christiansen, C.; Dam, E.B.; Pastoureau, P.; Karsdal, M.A. The disease modifying osteoarthritis drug (DMOAD): Is it in the horizon? Pharmacol. Res. 2008, 58, 1–7. [Google Scholar] [CrossRef]
- Roemer, F.W.; Kwoh, C.K.; Hayashi, D.; Felson, D.T.; Guermazi, A. The role of radiography and MRI for eligibility assessment in DMOAD trials of knee OA. Nat. Rev. Rheumatol. 2018, 14, 372–380. [Google Scholar] [CrossRef]
- Kraus, V.B.; Burnett, B.; Coindreau, J.; Cottrell, S.; Eyre, D.; Gendreau, M.; Gardiner, J.; Garnero, P.; Hardin, J.; Henrotin, Y.; et al. Application of biomarkers in the development of drugs intended for the treatment of osteoarthritis. Osteoarthr. Cartil. 2011, 19, 515–542. [Google Scholar] [CrossRef]
- Mobasheri, A.; Henrotin, Y. Biomarkers of (osteo)arthritis. Biomarkers 2015, 20, 513–518. [Google Scholar] [CrossRef]
- Bay-Jensen, A.C.; Thudium, C.S.; Mobasheri, A. Development and use of biochemical markers in osteoarthritis: Current update. Curr. Opin. Rheumatol. 2018, 30, 121–128. [Google Scholar] [CrossRef]
- Wyman, B.; Perl, A. Metabolic pathways mediate pathogenesis and offer targets for treatment in rheumatic diseases. Curr. Opin. Rheumatol. 2020, 32, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Matta, C.; Zákány, R.; Musumeci, G. Chondrosenescence: Definition, hallmarks and potential role in the pathogenesis of osteoarthritis. Maturitas 2015, 80, 237–244. [Google Scholar] [CrossRef] [PubMed]
- van der Kraan, P.; Matta, C.; Mobasheri, A. Age-Related Alterations in Signaling Pathways in Articular Chondrocytes: Implications for the Pathogenesis and Progression of Osteoarthritis—A Mini-Review. Gerontology 2017, 63, 29–35. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mobasheri, A. Neuroscience and Neuroimmunology Solutions for Osteoarthritis Pain: Biological Drugs, Growth Factors, Peptides and Monoclonal Antibodies Targeting Peripheral Nerves. NeuroSci 2021, 2, 45-58. https://doi.org/10.3390/neurosci2010003
Mobasheri A. Neuroscience and Neuroimmunology Solutions for Osteoarthritis Pain: Biological Drugs, Growth Factors, Peptides and Monoclonal Antibodies Targeting Peripheral Nerves. NeuroSci. 2021; 2(1):45-58. https://doi.org/10.3390/neurosci2010003
Chicago/Turabian StyleMobasheri, Ali. 2021. "Neuroscience and Neuroimmunology Solutions for Osteoarthritis Pain: Biological Drugs, Growth Factors, Peptides and Monoclonal Antibodies Targeting Peripheral Nerves" NeuroSci 2, no. 1: 45-58. https://doi.org/10.3390/neurosci2010003
APA StyleMobasheri, A. (2021). Neuroscience and Neuroimmunology Solutions for Osteoarthritis Pain: Biological Drugs, Growth Factors, Peptides and Monoclonal Antibodies Targeting Peripheral Nerves. NeuroSci, 2(1), 45-58. https://doi.org/10.3390/neurosci2010003





