The Same Magnocellular Neurons Send Axon Collaterals to the Posterior Pituitary and Retina or to the Posterior Pituitary and Autonomic Preganglionic Centers of the Eye in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Viruses Used for Inoculation
2.3. Inoculation of the Pituitary Gland
2.4. Sympathectomy
2.5. Optic Nerve Cut
2.6. Experimental Groups
- Group 1: Ka-gEI-memGFP-RV injection into the pituitary of intact rats (No = 2).
- Group 2: Ka-gEI-memGFP-RV injection into the pituitary of sympathectomized rats (No = 2).
- Group 3: Ka-Vhs-memCherry-A-RV or Ka-Vhs-CHR-memTomato-A-RV injection into the pituitary of intact rats (No = 2).
- Group 4: Ka-Vhs-memCherry-A-RV or Ka-Vhs-CHR-memTomato-A-RV injection into the pituitary of sympathectomized rats (No = 2).
- Group 5: Ka-gEI-memGFP-RV injection into the vitreous body of the eye, inserting the needle of the Hamilton syringe at the corneoscleral junction at the temporal side of intact rats and Ka-Vhs-memCherry-A-RV or Ka-Vhs-CHR-memTomato-A-RV injection into the pituitary (No = 7; sympathetic, parasympathetic, and centrifugal visual fibers remain intact).
- Group 6: Ka-gEI-memGFP-RV injection into the vitreous body of the eye of sympathectomized rats and Ka-Vhs-CHR-memTomato-A-RV injection into the pituitary (No = 7; parasympathetic and centrifugal visual fibers remain intact).
- Group 7: Ka-gEI-memGFP-RV injection into the vitreous body of eye of rats bearing the right optic nerve cut (No = 3; all autonomic fibers remain intact).
2.7. Preparing Tissues for Investigations
2.8. Immunohistochemistry
2.9. Specificity of Labeling
2.10. Evaluation of the Number of PVN and SON Neurons Labeled by Virus from the Eye
3. Results
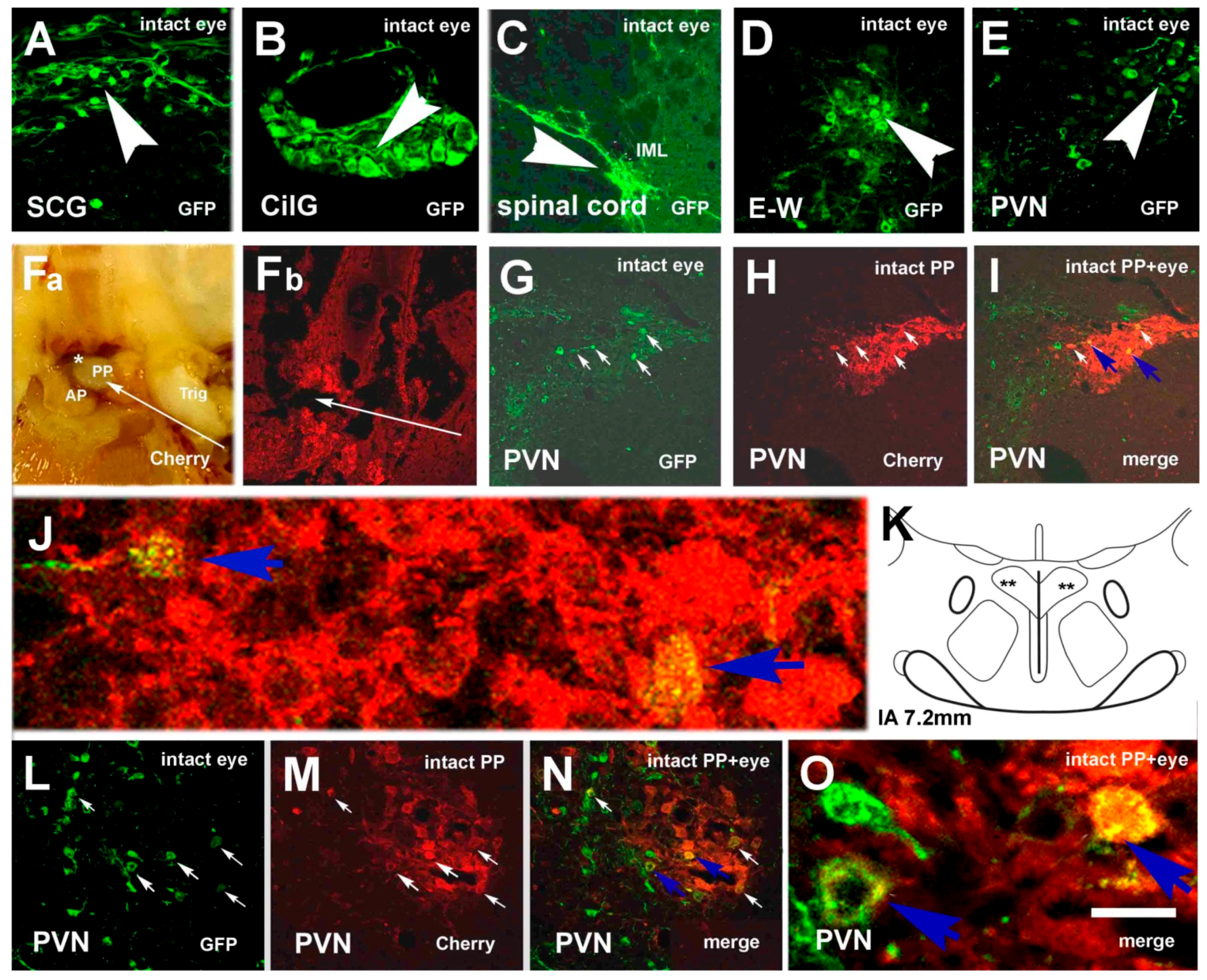
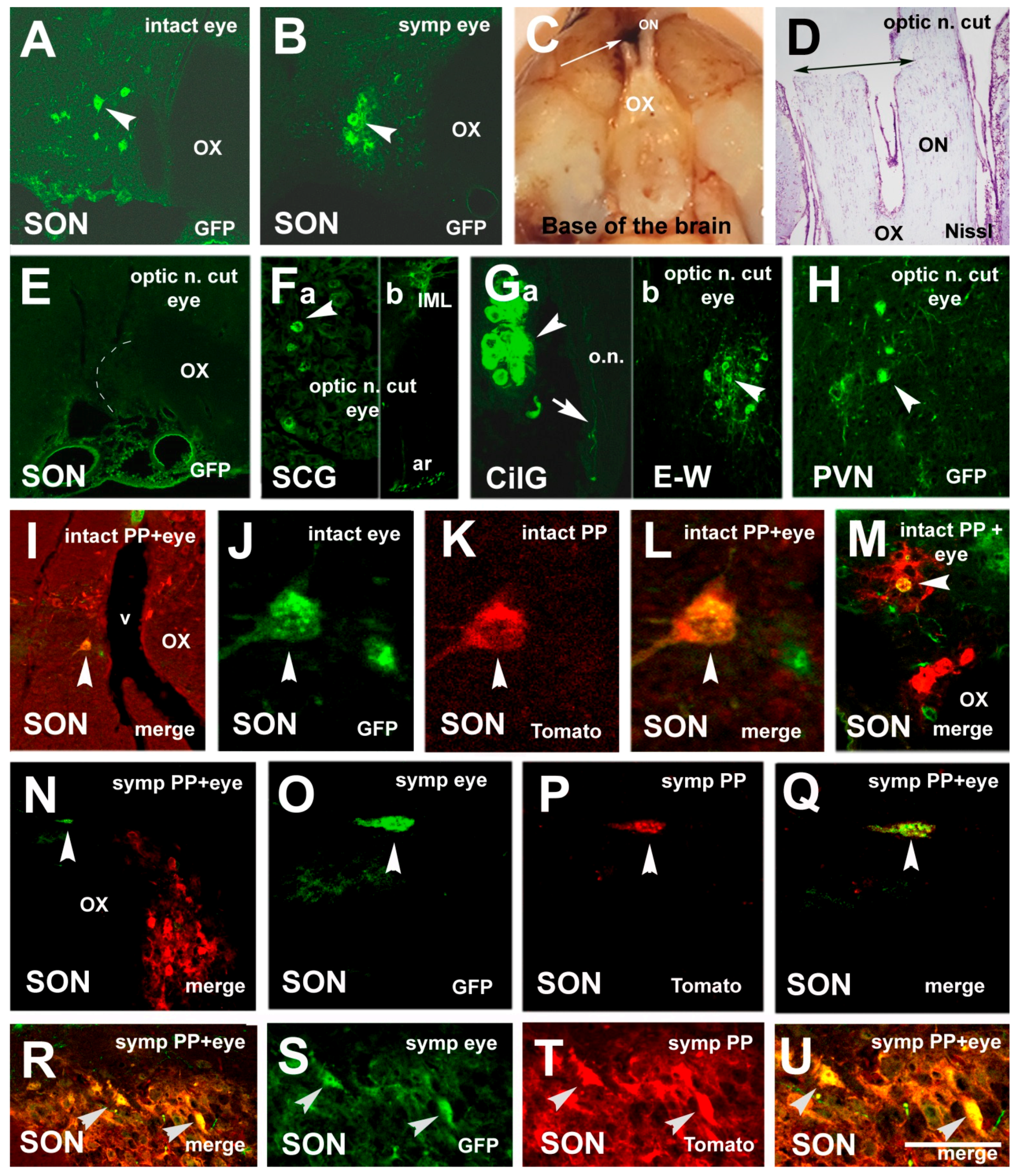
3.1. Labeling Induced by Pituitary Inoculation
3.2. Labeling, Focused on PVN, after Pituitary and Eye Inoculations (Groups 5 and 6)
3.3. Labeling Focused on the SON in the Case of the PP and Eye Inoculation of Intact, Sympathectomized Rats (Groups 5 and 6) and of Those Bearing Optic Nerve Cuts (Group 7)
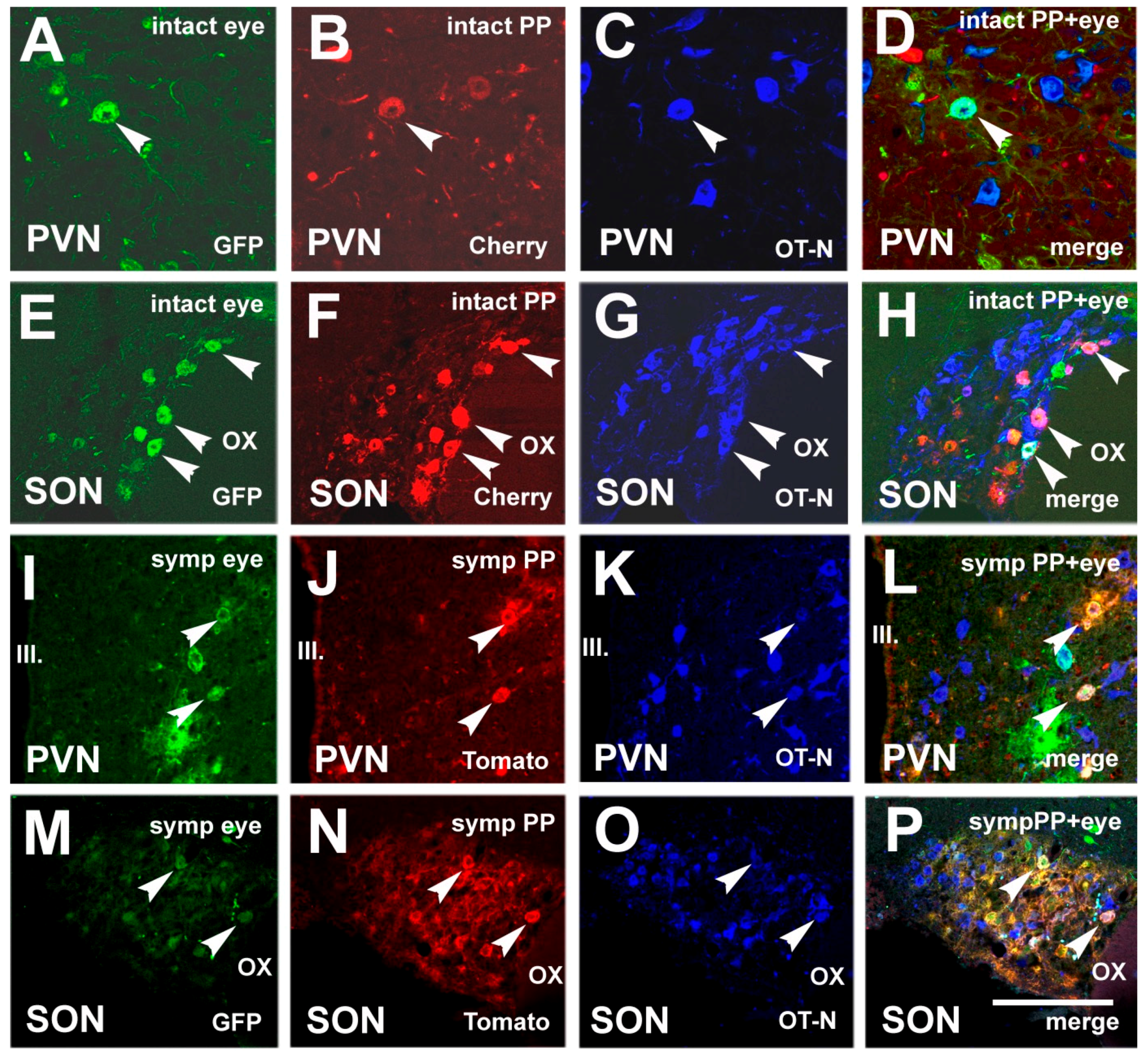
3.4. Chemical Characterization of Double-Labeled Neurons in the mPVN and SON
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMCA | Aminomethylcoumarin Acetate |
| ANOVA | one-way analysis of variance |
| ARC | arcuate nucleus |
| AVP | arginin vasopressin |
| BDA | biotinylated dextran amine |
| CilG | ciliary ganglion |
| DR | Dextran tetramethyl Rhodamine |
| DVC | dorsal vagal complex |
| E-W | Edinger-Westfal nucleus |
| FB | FastBlue |
| FG | FluoroGold |
| IA | interaural line |
| IML | intermediolateral cell column |
| Intact | not sympathectomized |
| KPB | potassium phosphate buffer |
| ME | median eminence |
| OT | oxytocin |
| OT-N | OT-neurophysin |
| Pf | perifornical area |
| PFA | paraformaldehyde |
| PP | posterior pituitary |
| PPPN | posterior pituitary projecting neurons |
| PVN | paraventricular nucleus |
| mPVN | magnocellular subdivision of PVN |
| pPVN | parvocellular subdivision of PVN |
| rVLM | rostral ventrolateral medulla |
| SCG | superior cervical ganglion |
| SON | supraoptic nucleus |
| SPAN | spinal projecting autonomic neurons |
| T2 | second thoracic segment |
References
- Wright, C.B.; Redmond, T.M.; Nickerson, J.M. A History of the Classical Visual Cycle. Prog. Mol. Biol. Transl. Sci. 2015, 134, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.Y.; Lenn, N.J. A retinohypothalamic projection in the rat. J. Comp. Neurol. 1972, 146, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, A.E.; Wagoner, N.; Cowan, W.M. An autoradiographic and electron microscopic study of retino-hypothalamic connections. Z. Zellforsch. Mikrosk. Anat. 1972, 135, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Ramon y Cajal, S. La retine des Vertebres. Cellule 1893, 9, 17–257. [Google Scholar]
- Repérant, J.; Miceli, D.; Vesselkin, N.P.; Molotchnikoff, S. The centrifugal visual system of vertebrates: A century-old search reviewed. Int. Rev. Cytol. 1989, 118, 115–171. [Google Scholar] [CrossRef]
- Vereczki, V.; Köves, K.; Csáki, Á.; Grosz, K.; Hoffman, G.E.; Fiskum, G. Distribution of hypothalamic, hippocampal and other limbic peptidergic neuronal cell bodies giving rise to retinopetal fibers: Anterograde and retrograde tracing and neuropeptide immunohistochemical studies. Neuroscience 2006, 140, 1089–1100. [Google Scholar] [CrossRef]
- Hosoya, Y.; Matsushita, M. Identification and distribution of the spinal and hypophyseal projection neurons in the paraventricular nucleus of the rat. A light and electron microscopic study with the horseradish peroxidase method. Exp. Brain. Res. 1979, 35, 315–331. [Google Scholar] [CrossRef]
- Swanson, L.W.; Kuypers, H.G. The paraventricular nucleus of the hypothalamus: Cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J. Comp. Neurol. 1980, 194, 555–570. [Google Scholar] [CrossRef]
- Hallbeck, M.; Larhammar, D.; Blomqvist, A. Neuropeptide expression in rat paraventricular hypothalamic neurons that project to the spinal cord. J. Comp. Neurol. 2001, 433, 222–238. [Google Scholar] [CrossRef]
- Pyner, S.; Coote, J.H. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience 2000, 100, 549–556. [Google Scholar] [CrossRef]
- Maejima, Y.; Kumamoto, K.; Takenoshita, S.; Shimomura, K. Projections from a single NUCB2/nesfatin-1 neuron in the paraventricular nucleus to different brain regions involved in feeding. Brain Struct. Funct. 2016, 221, 4723–4731. [Google Scholar] [CrossRef]
- Strack, A.M.; Sawyer, W.B.; Hughes, J.H.; Platt, K.B.; Loewy, A.D. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res. 1989, 491, 156–162. [Google Scholar] [CrossRef]
- Köves, K.; Györgyi, Z.; Szabó, F.K.; Boldogkői, Z. Characterization of the autonomic innervation of mammary gland in lactating rats studied by retrograde transynaptic virus labeling and immunohistochemistry. Acta Physiol. Hung. 2012, 99, 148–158. [Google Scholar] [CrossRef]
- Szabó, E.; Boldogkői, Z.; Csáki, Á.; Tóth, Z.; Köves, K. Autonomic neuronal chains innervating lower incisive gingiva and lip in rat. Trans-synaptic tracing study. Auton. Neurosci. Basic Clin. 2015, 190, 10–19. [Google Scholar] [CrossRef]
- Gerendai, I.; Tóth, I.E.; Boldogkői, Z.; Halász, B. Recent findings on the organization of central nervous system structures involved in the innervation of endocrine glands and other organs; observations obtained by the transneuronal viral double-labeling technique. Endocrine 2009, 36, 179–188, Review. [Google Scholar] [CrossRef]
- Hettigoda, N.S.; Fong, A.Y.; Badoer, E.; McKinley, M.J.; Oldfield, B.J.; Allen, A.M. Identification of CNS neurons with polysynaptic connections to both the sympathetic and parasympathetic innervation of the submandibular gland. Brain Struct. Funct. 2015, 220, 2103–2120. [Google Scholar] [CrossRef]
- Althammer, F.; Grinevich, V. Diversity of oxytocin neurons: Beyond magno- and parvocellular cell types? J. Neuroendocrinol. 2017, 29, e12549. [Google Scholar] [CrossRef]
- Knobloch, H.S.; Charlet, A.; Hoffmann, L.C.; Eliava, M.; Khrulev, S.; Cetin, A.H.; Osten, P.; Schwarz, M.K.; Seeburg, P.H.; Stoop, R.; et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 2012, 73, 553–566. [Google Scholar] [CrossRef]
- Althammer, F.; Jirikowski, G.; Grinevich, V. The oxytocin system of mice and men—Similarities and discrepancies of oxytocinergic modulation in rodents and primates. Peptides 2018, 109, 1–8. [Google Scholar] [CrossRef]
- Freeman, S.M.; Young, L.J. Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: Translational implications. J. Neuroendocrinol. 2016, 28. [Google Scholar] [CrossRef]
- Halbach, P.; De-Ann, M.P.; York, N.; Asuma, M.P.; Chiu, M.A.; Luo, W.; Tokarz, S.; Bird, I.M.; Pattnaik, B.R. Oxytocin and Function in the Posterior Retina: A Novel Signaling Pathway. Investig. Ophthalmol. Vis. Sci. 2015, 6, 751–760. [Google Scholar] [CrossRef]
- York, N.; Halbach, P.; Chiu, M.A.; Bird, I.M.; De-Ann, M.P.; Pattnaik, B.R. (OXT)-stimulated inhibition of Kir7. 1 activity is through PIP2-dependent Ca2+ response of the oxytocin receptor in the retinal pigment epithelium in vitro. Cell Signal. 2017, 37, 93–102. [Google Scholar] [CrossRef]
- Moritoh, S.; Sato, K.; Okada, Y.; Koizumi, A. Endogenous arginine vasopressin-positive retinal cells in arginine vasopressin-eGFP transgenic rats identified by immunohistochemistry and reverse transcriptase-polymerase chain reaction. Mol. Vis. 2011, 17, 3254–3261. [Google Scholar]
- Csáki, Á.; Vígh, B.; Boldogkői, Z.; Szél, Á.; Köves, K. Is a neuronal chain between the pineal body and the retina in rats and hamsters? Transneuronal tracing studies. Neurosci. Lett. 2015, 588, 1–6. [Google Scholar] [CrossRef]
- Dandy, W.E. The nerve supply to the pituitary body. Am. J. Anat. 1913, 15, 333–343. [Google Scholar] [CrossRef]
- Saavedra, J.M. Central and peripheral catecholamine innervation of the rat intermediate and posterior pituitary lobes. Neuroendocrinology 1985, 40, 281–284. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press Inc.: San Diego, CA, USA, 1986. [Google Scholar]
- Ben-Barak, J.Y.; Russel, T.; Whitnall, M.H.; Ozato, K.; Gainer, H. Neurophysin in the hypothalamo-hypophysial system, I. Production and characterization of monoclonal antibodies. J. Neurosci. 1985, 5, 81–97. [Google Scholar] [CrossRef]
- Nunn, N.; Womack, M.; Dart, C.; Barrett-Jolley, R. Function and pharmacology of spinally-projecting sympathetic pre-autonomic neurones in the paraventricular nucleus of the hypothalamus. Curr. Neuropharmacol. 2011, 9, 262–277. [Google Scholar] [CrossRef]
- Boll, S.; Almeida de Minas, A.C.; Raftogianni, A.; Herpertz, S.C.; Grinevich, V. Oxytocin and Pain Perception: From Animal Models to Human Research. Neuroscience 2018, 387, 149–161. [Google Scholar] [CrossRef]
- Daniel, S.; Quintana, D.S.; Kemp, A.H.; Alvares, G.A.; Guastella, A.J. A role for autonomic cardiac control in the effects of oxytocin on social behavior and psychiatric illness. Front Neurosci. 2013, 7, 48. [Google Scholar] [CrossRef]
- Dos Santos Junior, E.D.; Da Silva, A.V.; Da, K.R.T.; Haemmerle, C.A.S.; Batagello, D.S.; Da Silva, J.M.; Lima, L.B.; Da Silva, R.J.; Diniz, G.B.; Sita, L.V.; et al. The centrally projecting Edinger–Westphal nucleus—I: Efferents in the rat brain. J. Chem. Neuroanat. 2015, 68, 22–38. [Google Scholar] [CrossRef]
- Buijs, R.M. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Pathways to the limbic system, medulla oblongata and spinal cord. Cell Tissue Res. 1978, 192, 423–435. [Google Scholar] [CrossRef]
- Eliava, M.; Melchior, M.; Knobloch-Bollmann, H.S.; Wahis, J.; da Silva Gouveia, M.; Tang, Y.; Ciobanu, A.C.; del Rio, R.T.; Roth, L.C.; Althammer, F.; et al. A New Population of Parvocellular Oxytocin Neurons Controlling Magnocellular Neuron Activity and Inflammatory Pain Processing. Neuron 2016, 89, 1291–1304. [Google Scholar] [CrossRef]
- Wrobel, L.; Schorscher-Peten, A.; Dupré, A.; Yoshida, M.; Nishimori, K.; Tribollet, E. Distribution and identity of neurons expressing the oxytocin-receptor in the mouse spinal cord. Neurosci. Lett. 2011, 495, 49–54. [Google Scholar] [CrossRef]
- Kret, M.E.; De Dreu, C.K.W. Pupil-mimicry condition trust in partners: Modration by oxytocin and group membership. Proc. R. Soc. B Biol. Sci. 2017. [Google Scholar] [CrossRef]
- Horowitz, J.; Sears, M. Neurohypophyseal peptides and the eye: Use of synthetic analogs in analyzing effects on the pupil. Graefe’s Arch. Clin. Exp. Ophthalmol. 1983, 220, 253–256. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csáki, Á.; Köves, K.; Boldogkői, Z.; Tombácz, D.; Tóth, Z.E. The Same Magnocellular Neurons Send Axon Collaterals to the Posterior Pituitary and Retina or to the Posterior Pituitary and Autonomic Preganglionic Centers of the Eye in Rats. NeuroSci 2021, 2, 27-44. https://doi.org/10.3390/neurosci2010002
Csáki Á, Köves K, Boldogkői Z, Tombácz D, Tóth ZE. The Same Magnocellular Neurons Send Axon Collaterals to the Posterior Pituitary and Retina or to the Posterior Pituitary and Autonomic Preganglionic Centers of the Eye in Rats. NeuroSci. 2021; 2(1):27-44. https://doi.org/10.3390/neurosci2010002
Chicago/Turabian StyleCsáki, Ágnes, Katalin Köves, Zsolt Boldogkői, Dóra Tombácz, and Zsuzsanna E. Tóth. 2021. "The Same Magnocellular Neurons Send Axon Collaterals to the Posterior Pituitary and Retina or to the Posterior Pituitary and Autonomic Preganglionic Centers of the Eye in Rats" NeuroSci 2, no. 1: 27-44. https://doi.org/10.3390/neurosci2010002
APA StyleCsáki, Á., Köves, K., Boldogkői, Z., Tombácz, D., & Tóth, Z. E. (2021). The Same Magnocellular Neurons Send Axon Collaterals to the Posterior Pituitary and Retina or to the Posterior Pituitary and Autonomic Preganglionic Centers of the Eye in Rats. NeuroSci, 2(1), 27-44. https://doi.org/10.3390/neurosci2010002




