Hematological Alterations Related to Treatment with Teriflunomide and Dimethyl Fumarate in Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Lymphopenia
2.3. Statistics
2.4. Ethical Issues
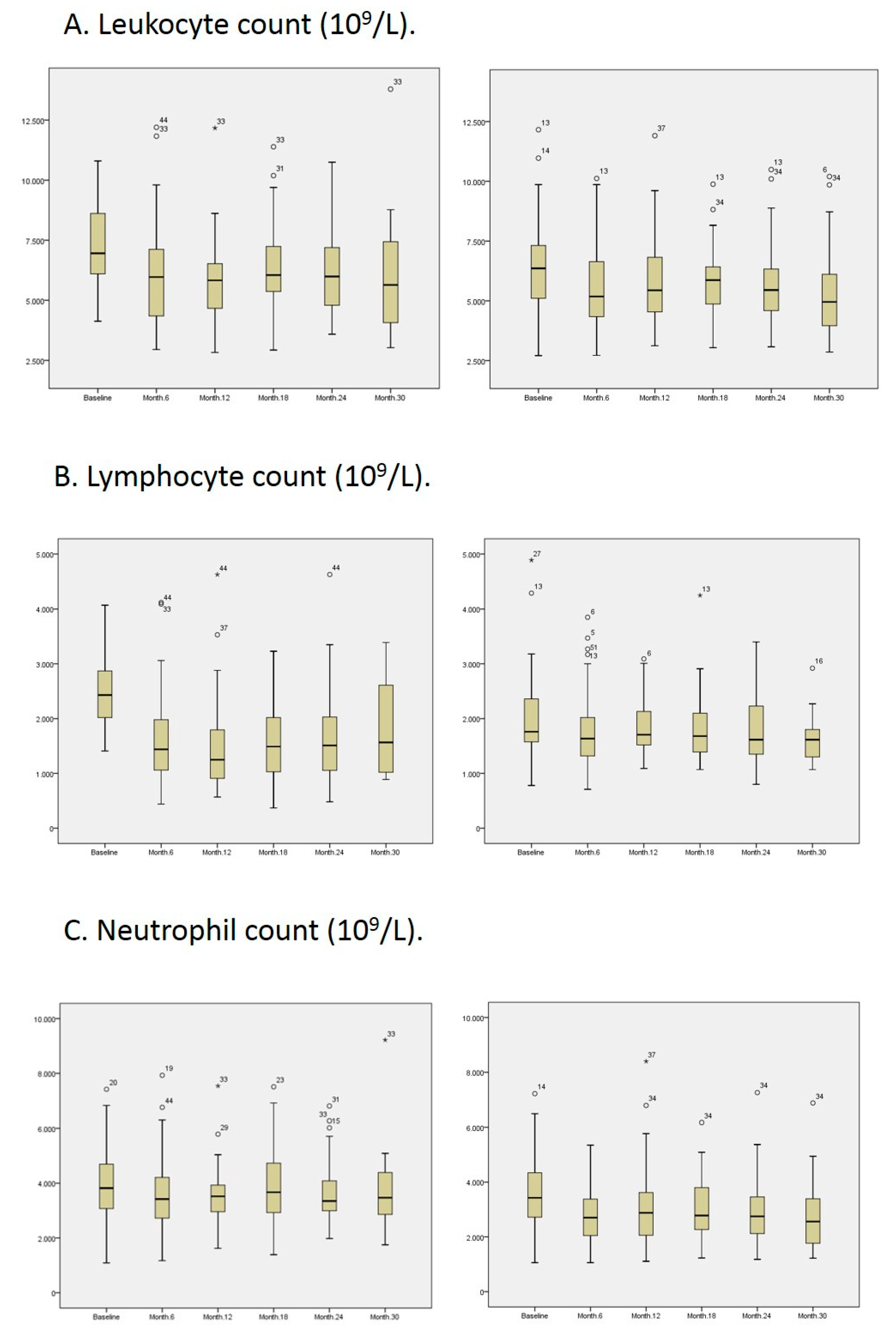
3. Results
4. Discussion
Funding
Conflicts of Interest
References
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple Sclerosis: Mechanisms and Immunotherapy. Neuron 2018, 97, 742–768. [Google Scholar] [CrossRef]
- Racke, M.K. The role of B cells in multiple sclerosis: Rationale for B-cell-targeted therapies. Curr. Opin. Neurol. 2008, 21, S9–S18. [Google Scholar] [CrossRef]
- Constantinescu, C.S.; Gran, B. The essential role of t cells in multiple sclerosis: A reappraisal. Biomed. J. 2014, 37, 34. [Google Scholar] [CrossRef]
- Fox, E.J.; Buckle, G.J.; Singer, B.; Singh, V.; Boster, A. Lymphopenia and DMTs for relapsing forms of MS. Neurol. Clin. Pr. 2019, 9, 53–63. [Google Scholar] [CrossRef]
- Rommer, P.S.; Milo, R.; Han, M.H.; Satyanarayan, S.; Sellner, J.; Hauer, L.; Illes, Z.; Warnke, C.; Laurent, S.; Weber, M.S.; et al. Immunological Aspects of Approved MS Therapeutics. Front. Immunol. 2019, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Comi, G.; Miller, A.E.; Benamor, M.; Truffinet, P.; Poole, E.M.; Freedman, M.S. Characterizing lymphocyte counts and infection rates with long-term teriflunomide treatment: Pooled analysis of clinical trials. Mult. Scler. J. 2019, 26, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Gold, R.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Giovannoni, G.; Selmaj, K.; Tornatore, C.; Sweetser, M.T.; Yang, M.; Sheikh, S.I.; et al. Placebo-Controlled Phase 3 Study of Oral BG-12 for Relapsing Multiple Sclerosis. New Engl. J. Med. 2012, 367, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.J.; Miller, D.H.; Phillips, J.T.; Hutchinson, M.; Havrdova, E.K.; Kita, M.; Yang, M.; Raghupathi, K.; Novas, M.; Sweetser, M.T.; et al. Placebo-Controlled Phase 3 Study of Oral BG-12 or Glatiramer in Multiple Sclerosis. New Engl. J. Med. 2012, 367, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Gold, R.; Arnold, D.L.; Bar-Or, A.; Hutchinson, M.; Kappos, L.; Havrdova, E.K.; MacManus, D.G.; Yousry, T.A.; Pozzilli, C.; Selmaj, K.; et al. Long-term effects of delayed-release dimethyl fumarate in multiple sclerosis: Interim analysis of ENDORSE, a randomized extension study. Mult. Scler. J. 2016, 23, 253–265. [Google Scholar] [CrossRef]
- Baharnoori, M.; Gonzalez, C.; Chua, A.S.; Diaz-Cruz, C.; Healy, B.; Stankiewicz, J.; Weiner, H.; Chitnis, T. Predictors of hematological abnormalities in multiple sclerosis patients treated with fingolimod and dimethyl fumarate and impact of treatment switch on lymphocyte and leukocyte count. Mult. Scler. Relat. Disord. 2018, 20, 51–57. [Google Scholar] [CrossRef]
- Longbrake, E.E.; Naismith, R.T.; Parks, B.J.; Wu, G.F.; Cross, A.H. Dimethyl fumarate-associated lymphopenia: Risk factors and clinical significance. Mult. Scler. J. Exp. Transl. Clin. 2015, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Briner, M.; Bagnoud, M.; Miclea, A.; Friedli, C.; Diem, L.; Chan, A.; Hoepner, R.; Salmen, A. Time course of lymphocyte repopulation after dimethyl fumarate-induced grade 3 lymphopenia: Contribution of patient age. Ther. Adv. Neurol. Disord. 2019, 12, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Rose, J.; Alvarez, E.; Bar-Or, A.; Butzkueven, H.; Fox, R.J.; Gold, R.; Gudesblatt, M.; Haartsen, J.; Spelman, T.; et al. Lymphocyte reconstitution after DMF discontinuation in clinical trial and real-world patients with MS. Neurol. Clin. Pract. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- De La Maza, S.S.; Medina, S.; Villarrubia, N.; Costa-Frossard, L.; Monreal, E.; Tejeda-Velarde, A.; Rodríguez-Martín, E.; Roldán, E.; Álvarez-Cermeño, J.C.; Villar, L.M. Factors associated with dimethyl fumarate-induced lymphopenia. J. Neurol. Sci. 2019, 398, 4–8. [Google Scholar] [CrossRef]
- Fox, R.J.; Chan, A.; Gold, R.; Phillips, J.T.; Selmaj, K.; Chang, I.; Novas, M.; Rana, J.; Marantz, J.L. Characterizing absolute lymphocyte count profiles in dimethyl fumarate–treated patients with MS. Neurol. Clin. Pr. 2016, 6, 220–229. [Google Scholar] [CrossRef]
- Warnke, C.; Hartung, H.-P. Challenging a concept: Pulsed treatment regimen—No risk of PML? Mult. Scler. J. 2019, 25, 1076–1078. [Google Scholar] [CrossRef] [PubMed]
- Braune, S.; NTD Study Group; Grimm, S.; Van Hövell, P.; Freudensprung, U.; Pellegrini, F.; Hyde, R.; Bergmann, A. Comparative effectiveness of delayed-release dimethyl fumarate versus interferon, glatiramer acetate, teriflunomide, or fingolimod: Results from the German NeuroTransData registry. J. Neurol. 2018, 265, 2980–2992. [Google Scholar] [CrossRef]
- Condé, S.; Moisset, X.; Pereira, B.; Zuel, M.; Colamarino, R.; Maillet-Vioud, M.; Lauxerois, M.; Taithe, F.; Clavelou, P.; Auvergne, T.R.N.; et al. Dimethyl fumarate and teriflunomide for multiple sclerosis in a real-life setting: A French retrospective cohort study. Eur. J. Neurol. 2018, 26, 460–467. [Google Scholar] [CrossRef]
- Laplaud, D.-A.; Casey, R.; Barbin, L.; Debouverie, M.; De Sèze, J.; Brassat, D.; Wiertlewski, S.; Brochet, B.; Pelletier, J.; Vermersch, P.; et al. Comparative effectiveness of teriflunomide vs dimethyl fumarate in multiple sclerosis. Neurology 2019, 93, e635–e646. [Google Scholar] [CrossRef]
- Buard, G.; Giovannelli, J.; Outteryck, O.; Hadhoum, N.; Lannoy, J.; Vermersch, P.; Zephir, H. Switching for convenience from first-line injectable treatments to oral treatments in multiple sclerosis: Data from a retrospective cohort study. Mult. Scler. Relat. Disord. 2019, 33, 39–43. [Google Scholar] [CrossRef]
- Coyle, P.K.; Khatri, B.; Edwards, K.R.; Meca-Lallana, J.; Cavalier, S.; Rufi, P.; Benamor, M.; Poole, E.M.; Robinson, M.; Gold, R. Teriflunomide real-world evidence: Global differences in the phase 4 Teri-PRO study. Mult. Scler. Relat. Disord. 2019, 31, 157–164. [Google Scholar] [CrossRef] [PubMed]
| TERIFLUNOMIDE (n = 55) | DIMETHYL FUMARATE (n = 44) | |
|---|---|---|
| SEX (M/F) | 9 M/46 F | 12 M/32 F |
| AGE (years) | ||
| Mean ± SD * | 49.4 ± 8.6 | 38.1 ± 8.5 |
| Median (range) * | 48 (32–73) | 35.5 (25–55) |
| 75th percentile * | 56 | 45 |
| PREVIOUS TREATMENTS | ||
| Naïve | 14 | 20 |
| Glatiramer acetate | 11 | 10 |
| Interferon-β 1b | 2 | 4 |
| Interferon-β 1a sc | 12 | 7 |
| Interferon-β 1a im | 7 | 1 |
| Azathioprine | 1 | |
| Natalizumab | 1 | |
| Teriflunomide | 2 | |
| Dimethyl fumarate | 7 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Estévez, D.A. Hematological Alterations Related to Treatment with Teriflunomide and Dimethyl Fumarate in Multiple Sclerosis. NeuroSci 2020, 1, 17-23. https://doi.org/10.3390/neurosci1010003
García-Estévez DA. Hematological Alterations Related to Treatment with Teriflunomide and Dimethyl Fumarate in Multiple Sclerosis. NeuroSci. 2020; 1(1):17-23. https://doi.org/10.3390/neurosci1010003
Chicago/Turabian StyleGarcía-Estévez, Daniel Apolinar. 2020. "Hematological Alterations Related to Treatment with Teriflunomide and Dimethyl Fumarate in Multiple Sclerosis" NeuroSci 1, no. 1: 17-23. https://doi.org/10.3390/neurosci1010003
APA StyleGarcía-Estévez, D. A. (2020). Hematological Alterations Related to Treatment with Teriflunomide and Dimethyl Fumarate in Multiple Sclerosis. NeuroSci, 1(1), 17-23. https://doi.org/10.3390/neurosci1010003





