Mechanochemical Solvent-Free Synthesis and Biological Profiling of Novel 2-Hydrazone-Bridged Benzothiazoles as Potent Anticancer Agents
Abstract
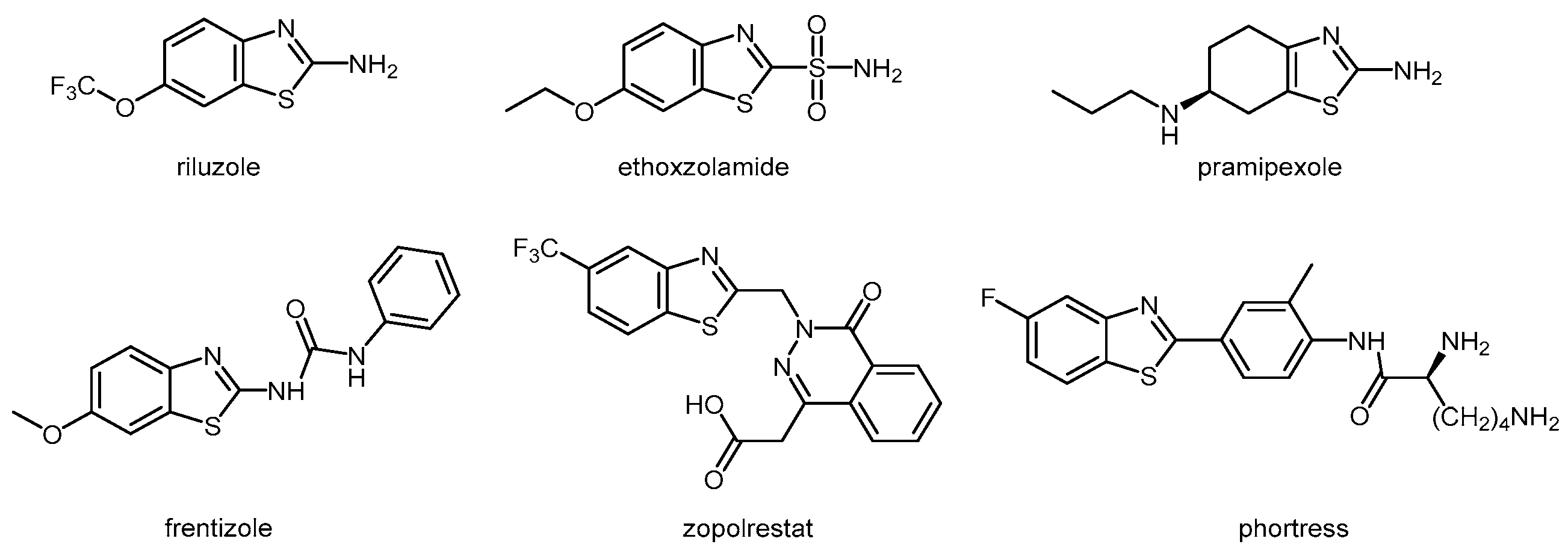
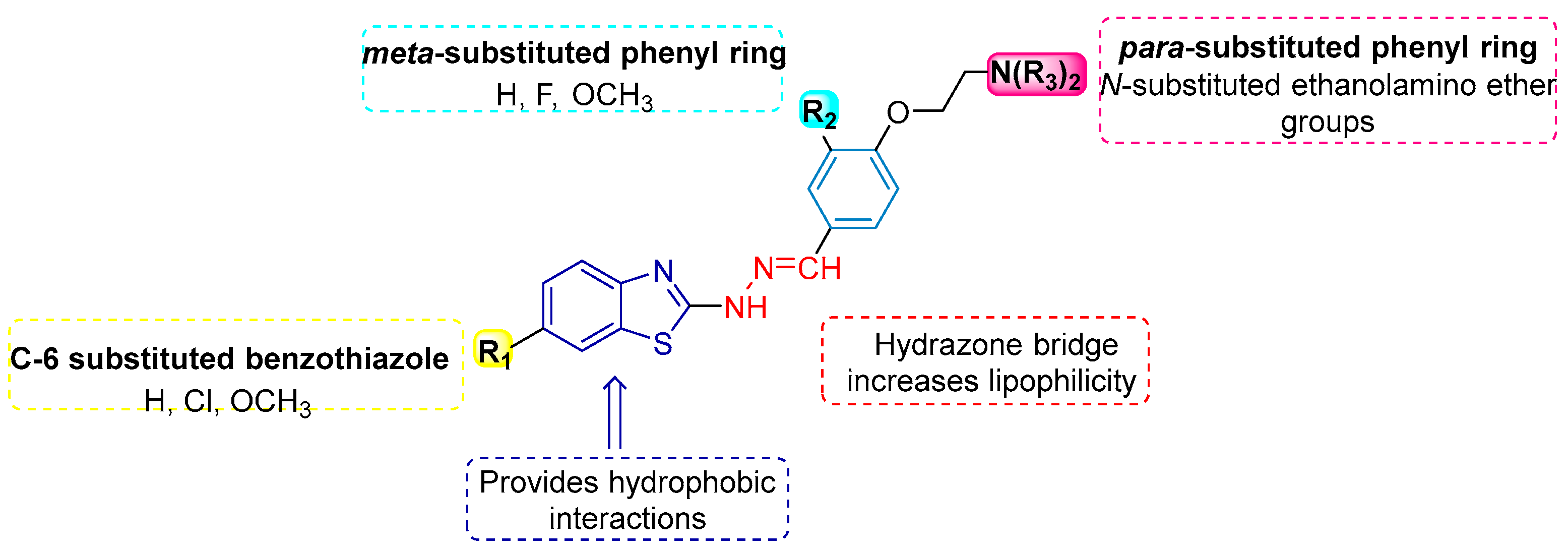
1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthetic Procedures
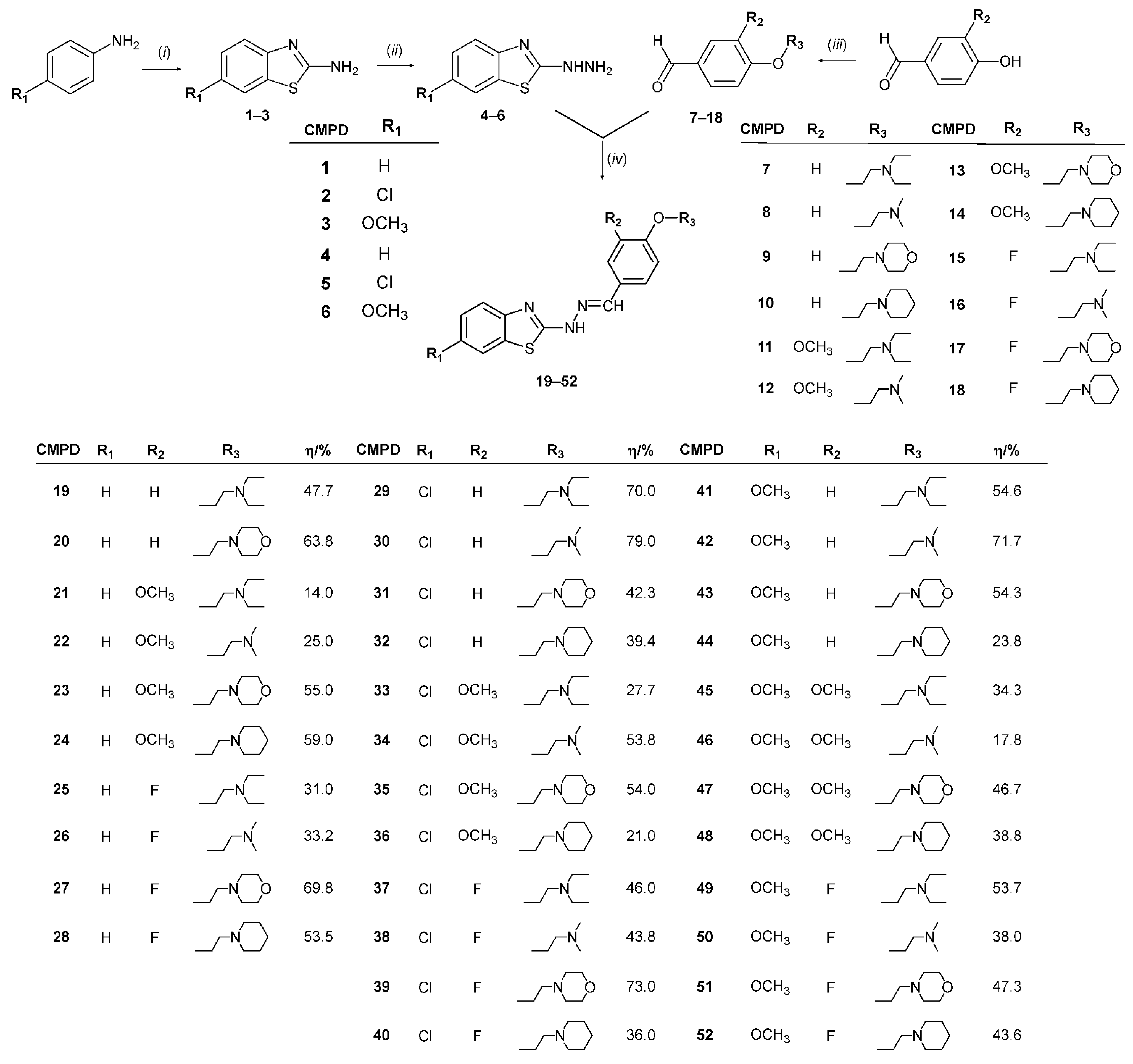
2.2.1. General Procedure for the Preparation of 2-hydrazinylbenzo[d]thiazoles 4–6 [65]
2.2.2. General Procedure for the Preparation of O-alkylated Benzaldehydes 7–18 [65]
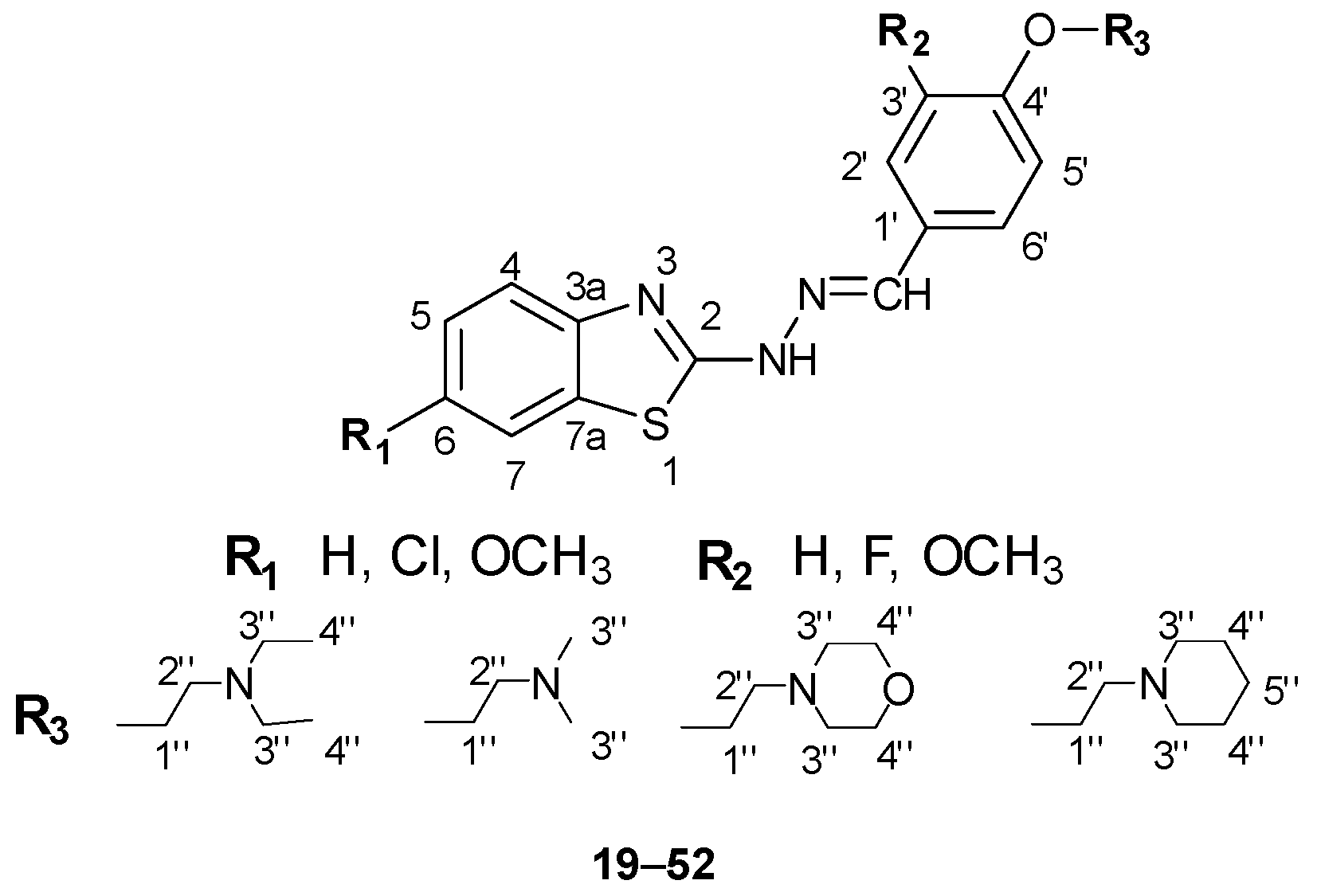
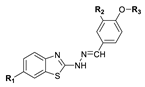
2.2.3. General Procedure for the Preparation of 2-hydrazonylbenzo[d]thiazole Derivatives 19–52
2.3. Biological Assays
2.3.1. In Vitro Antiproliferative Activity
2.3.2. Cytotoxicity Test Against Peripheral Blood Mononuclear Cells (PBMCs)
2.3.3. In Vitro Antibacterial Activity [68]
3. Results and Discussion
3.1. Chemistry
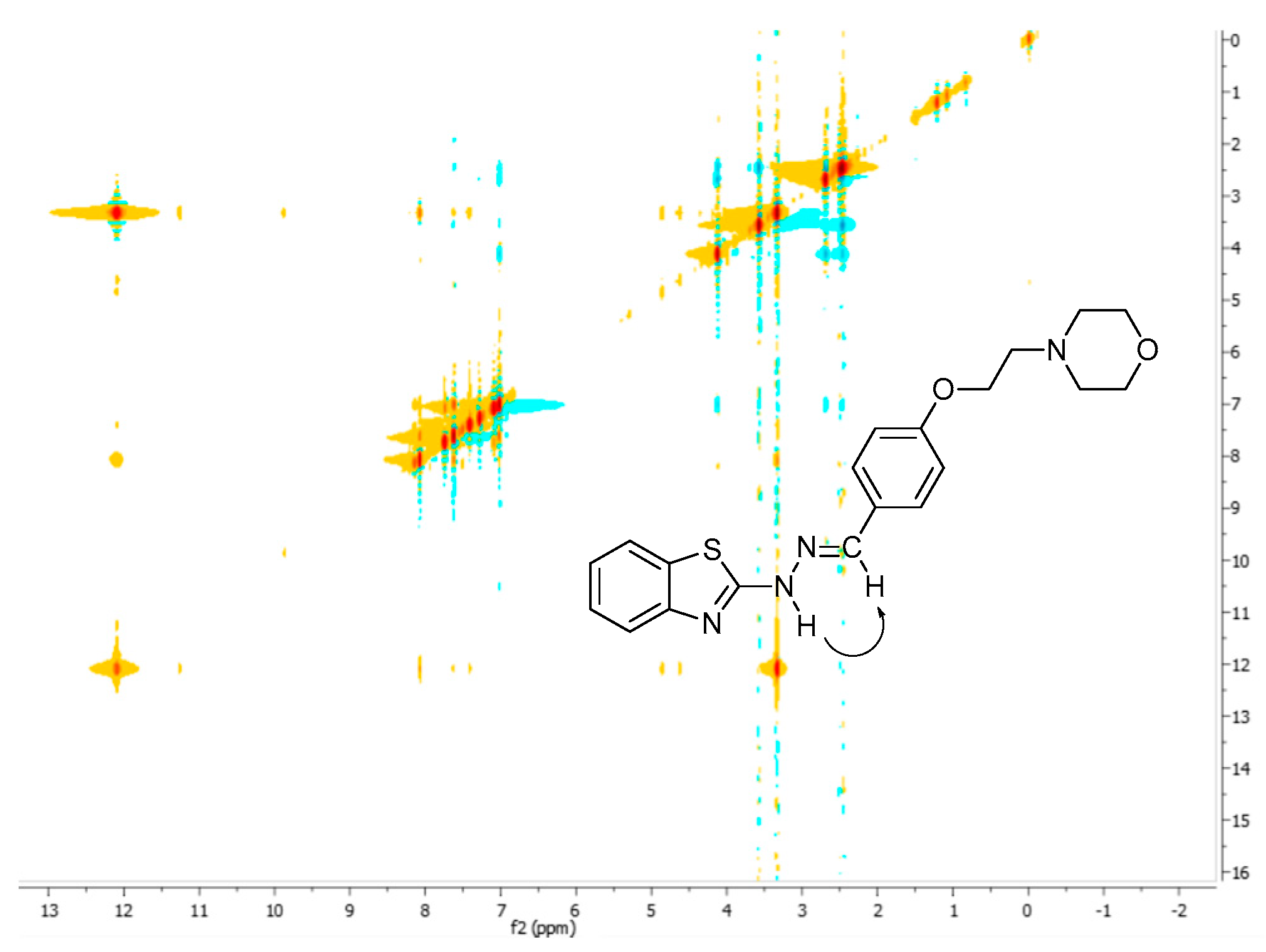
3.2. 1H-, 13C-NMR and NOESY Spectra Analysis
3.3. Biological Activity
3.3.1. In Vitro Antiproliferative Activity Evaluation
3.3.2. In Vitro Antibacterial Activity Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| MDR | Multidrug-resistance |
| EGFR | Epidermal growth factor receptor |
| LAG | Liquid-assisted grinding |
| TLC | Thin-layer chromatography |
| 1H-NMR | Proton nuclear magnetic resonance |
| 13C-NMR | Carbon-13 nuclear magnetic resonance |
| DMSO | Dimethyl sulfoxide |
| TMS | Tetramethylsilane |
| IR | Infrared |
| ATR | Attenuated total reflection |
| KSCN | Potassium thiocyanate |
| K2CO3 | Potassium Carbonate |
| CH2Cl2 | Dichloromethane |
| MeOH | Methanol |
| NH4OH | Ammonium hydroxide |
| FBS | Fetal bovine serum |
| IC50 | A concentration that causes a 50% inhibition of cell growth |
| PBMC | Peripheral blood mononuclear cells |
| MIC | Minimum inhibitory concentration |
| NOESY | Nuclear Overhauser Effect Spectroscopy |
| SAR | Structure–activity relationship |
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The Ever-Increasing Importance of Cancer as a Leading Cause of Premature Death Worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes. Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the Definition of Cancer. Mol. Cancer Res. 2023, 21, 1142–1147. [Google Scholar] [CrossRef]
- McKenna, M. Antibiotic resistance: The last resort. Nature 2013, 499, 394–396. [Google Scholar] [CrossRef]
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.S.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic Resistance: The Challenges and Some Emerging Strategies for Tackling a Global Menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef]
- Read, A.F.; Woods, R.J. Antibiotic Resistance Management. Evol. Med. Public Health 2014, 2014, 147. [Google Scholar] [CrossRef] [PubMed]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic Resistance in Microbes: History, Mechanisms, Therapeutic Strategies and Future Prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef]
- Ho, C.S.; Wong, C.T.H.; Aung, T.T.; Lakshminarayanan, R.; Mehta, J.S.; Rauz, S.; Mcnally, A.; Kintses, B.; Peacock, S.J.; de la Fuente-Nunez, C.; et al. Review Antimicrobial Resistance: A Concise Update. Lancet Microbe. 2025, 6, 100947. [Google Scholar] [CrossRef]
- Parmanik, A.; Das, S.; Kar, B.; Bose, A.; Dwivedi, G.R.; Pandey, M.M. Current Treatment Strategies Against Multidrug-Resistant Bacteria: A Review. Curr. Microbiol. 2022, 79, 388. [Google Scholar] [CrossRef]
- Tufa, T.B.; Regassa, F.; Amenu, K.; Stegeman, J.A.; Hogeveen, H. Livestock Producers’ Knowledge, Attitude, and Behavior (KAB) Regarding Antimicrobial Use in Ethiopia. Front. Vet. Sci. 2023, 10, 1167847. [Google Scholar] [CrossRef]
- Philoppes, J.N.; Lamie, P.F. Design and Synthesis of New Benzoxazole/Benzothiazole-Phthalimide Hybrids as Antitumor-Apoptotic Agents. Bioorg. Chem. 2019, 89, 102978. [Google Scholar] [CrossRef]
- Guo, L.; Wan, Y.; Liu, M.; Zheng, F.; Shi, Y.; Wang, K.; Cao, X.; Bao, L.; Ke, S. Discovery of Structural Diversity Guided N-S Heterocyclic Derivatives Based on Natural Benzothiazole Alkaloids as Potential Cytotoxic Agents. J. Heterocycl. Chem. 2024, 61, 1740–1751. [Google Scholar] [CrossRef]
- Racané, L.; Kralj, M.; Šuman, L.; Stojković, R.; Tralić-Kulenović, V.; Karminski-Zamola, G. Novel Amidino Substituted 2-Phenylbenzothiazoles: Synthesis, Antitumor Evaluation in Vitro and Acute Toxicity Testing in Vivo. Bioorg. Med. Chem. 2010, 18, 1038–1044. [Google Scholar] [CrossRef]
- Patel, R.V.; Park, S.W. Catalytic N-Formylation for Synthesis of 6-Substituted-2-Benzothiazolylimino-5-Piperazinyl-4-Thiazolidinone Antimicrobial Agents. Res. Chem. Intermed. 2015, 41, 5599–5609. [Google Scholar] [CrossRef]
- Bhoi, M.N.; Borad, M.A.; Solanki, A.P.; Patel, H.D. Novel 4H-Pyrimido[2,1-b]Benzothiazoles Derivatives: Camphorsulphonic Acid Catalyzed Enantioselective Synthesis, Optimization, and Biological Study. Phosphorus Sulfur Silicon Relat. Elem. 2023, 198, 822–835. [Google Scholar] [CrossRef]
- Racané, L.; Ptiček, L.; Kostrun, S.; Raić-Malić, S.; Taylor, M.C.; Delves, M.; Alsford, S.; Olmo, F.; Francisco, A.F.; Kelly, J.M. Bis-6-Amidino-Benzothiazole Derivative That Cures Experimental Stage 1 African Trypanosomiasis with a Single Dose. J. Med. Chem. 2023, 66, 13043–13057. [Google Scholar] [CrossRef] [PubMed]
- Oyeneyin, O.E.; Moodley, R.; Mashaba, C.; Garnie, L.F.; Omoboyowa, D.A.; Rakodi, G.H.; Maphoru, M.V.; Balogun, M.O.; Hoppe, H.C.; Egan, T.J.; et al. In Vitro Antiplasmodium and Antitrypanosomal Activities, β-Haematin Formation Inhibition, Molecular Docking and DFT Computational Studies of Quinoline-Urea-Benzothiazole Hybrids. Heliyon 2024, 10, e38434. [Google Scholar] [CrossRef] [PubMed]
- Ghonim, A.E.; Ligresti, A.; Rabbito, A.; Mahmoud, A.M.; Di Marzo, V.; Osman, N.A.; Abadi, A.H. Structure-Activity Relationships of Thiazole and Benzothiazole Derivatives as Selective Cannabinoid CB2 Agonists with in Vivo Anti-Inflammatory Properties. Eur. J. Med. Chem. 2019, 180, 154–170. [Google Scholar] [CrossRef]
- Nagaraj; Chaluvaraju, K.C.; Niranjan, M.S.; Kiran, S. 1, 3, 4 Oxadiazole: A potent drug candidate with various pharmacological activities. Int. J. Pharm. Pharm. Sci. 2011, 3, 9–16. [Google Scholar]
- Li, K.; Frankowski, K.J.; Belon, C.A.; Neuenswander, B.; Ndjomou, J.; Hanson, A.M.; Shanahan, M.A.; Schoenen, F.J.; Blagg, B.S.J.; Aubé, J.; et al. Optimization of Potent Hepatitis C Virus NS3 Helicase Inhibitors Isolated from the Yellow Dyes Thioflavine S and Primuline. J. Med. Chem. 2012, 55, 3319–3330. [Google Scholar] [CrossRef]
- Khokra, S.L.; Arora, K.; Khan, S.A.; Kaushik, P.; Saini, R.; Husain, A. Synthesis, Computational Studies and Anticonvulsant Activity of Novel Benzothiazole Coupled Sulfonamide Derivatives. Iran. J. Pharm. Res. 2019, 18, 1. [Google Scholar]
- Singh, R.; Sindhu, J.; Devi, M.; Kumar, A.; Kumar, R.; Hussain, K.; Kumar, P. Solid-Supported Materials-Based Synthesis of 2-Substituted Benzothiazoles: Recent Developments and Sanguine Future. ChemistrySelect 2021, 6, 6388–6449. [Google Scholar] [CrossRef]
- Weekes, A.; Westwell, A. 2-Arylbenzothiazole as a Privileged Scaffold in Drug Discovery. Curr. Med. Chem. 2009, 16, 2430–2440. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.F.; Bradshaw, T.D.; Wrigley, S.; McCall, C.J.; Lelieveld, P.; Fichtner, I.; Stevens, M.F.G. Antitumor Benzothiazoles. 3. Synthesis of 2-(4-Aminophenyl)Benzothiazoles and Evaluation of Their Activities against Breast Cancer Cell Lines in Vitro and in Vivo. J. Med. Chem. 1996, 39, 3375–3384. [Google Scholar] [CrossRef] [PubMed]
- Kurbanova, M.S.; Ashfaq, M.; Sadigova, A.; Feizi-Dehnayebi, M.; Maharramov, A.M.; Tahir, M.N. A Hydrazone Derivative: Synthesis, Crystal Structure, Supramolecular Assembly Exploration by Hirshfeld Surface Analysis and Computational Study. J. Struct. Chem. 2024, 65, 92–106. [Google Scholar] [CrossRef]
- El-Etrawy, A.; Sherbiny, F. Design, Synthesis, Biological Evaluation and Molecular Modeling Investigation of New N’-(2-Thiouracil-5-Oyl) Hydrazone Derivatives as Potential Anti-Breast Cancer and Anti-Bacterial Agents. J. Mol. Struct. 2021, 1232, 129993. [Google Scholar] [CrossRef]
- Srovnalová, A.; Kaplánek, R.; Kejík, Z.; Hajduch, J.; Gurská, S.; Martásek, P.; Hajdúch, M.; Džubák, P.; Jakubek, M. Synthesis and Evaluation of Cyclobut-3-Ene-1,2-Dione-3-Hydrazones with Benzothiazole Moiety as Novel Anticancer Agents Inducing Nonapoptotic Oncosis-like Cell Death. Biomed. Pharmacother. 2025, 190, 118404. [Google Scholar] [CrossRef]
- Aljuhani, A.; Nafie, M.S.; Albujuq, N.R.; Alsehli, M.; Bardaweel, S.K.; Darwish, K.M.; Alraqa, S.Y.; Aouad, M.R.; Rezki, N. Discovery of New Benzothiazole-1,2,3-Triazole Hybrid-Based Hydrazone/Thiosemicarbazone Derivatives as Potent EGFR Inhibitors with Cytotoxicity against Cancer. RSC Adv. 2025, 15, 3570–3591. [Google Scholar] [CrossRef]
- Telvekar, V.N.; Bairwa, V.K.; Satardekar, K.; Bellubi, A. Novel 2-(2-(4-Aryloxybenzylidene) Hydrazinyl)Benzothiazole Derivatives as Anti-Tubercular Agents. Bioorg. Med. Chem. Lett. 2012, 22, 649–652. [Google Scholar] [CrossRef]
- Hitesh, B.K.; Bhavin, S.B.; Jitendra, S. Synthesis of Nitrogen Mustards of Fluoro-Benzothiazoles of Pharmacological Interest. Res. J. Pharm. Biol. Chem. Sci. 2010, 1, 124–129. [Google Scholar]
- Sun, T.M.; Wang, Y.C.; Wang, F.; Du, J.Z.; Mao, C.Q.; Sun, C.Y.; Tang, R.Z.; Liu, Y.; Zhu, J.; Zhu, Y.H.; et al. Cancer Stem Cell Therapy Using Doxorubicin Conjugated to Gold Nanoparticles via Hydrazone Bonds. Biomaterials 2014, 35, 836–845. [Google Scholar] [CrossRef]
- Sonawane, S.J.; Kalhapure, R.S.; Govender, T. Hydrazone Linkages in PH Responsive Drug Delivery Systems. Eur. J. Pharm. Sci. 2017, 99, 45–65. [Google Scholar] [CrossRef]
- Ye, W.L.; Zhao, Y.P.; Li, H.Q.; Na, R.; Li, F.; Mei, Q.B.; Zhao, M.G.; Zhou, S.Y. Doxorubicin-Poly (Ethylene Glycol)-Alendronate Self-Assembled Micelles for Targeted Therapy of Bone Metastatic Cancer. Sci. Rep. 2015, 5, 14614. [Google Scholar] [CrossRef]
- Zandi, R.; Larsen, A.B.; Andersen, P.; Stockhausen, M.T.; Poulsen, H.S. Mechanisms for Oncogenic Activation of the Epidermal Growth Factor Receptor. Cell. Signal. 2007, 19, 2013–2023. [Google Scholar] [CrossRef]
- Sharma, B.; Singh, V.J.; Chawla, P.A. Epidermal Growth Factor Receptor Inhibitors as Potential Anticancer Agents: An Update of Recent Progress. Bioorg. Chem. 2021, 116, 105393. [Google Scholar] [CrossRef]
- Gabr, M.T.; El-Gohary, N.S.; El-Bendary, E.R.; El-Kerdawy, M.M.; Ni, N. Synthesis, in Vitro Antitumor Activity and Molecular Modeling Studies of a New Series of Benzothiazole Schiff Bases. Chin. Chem. Lett. 2016, 27, 380–386. [Google Scholar] [CrossRef]
- Lindgren, E.B.; De Brito, M.A.; Vasconcelos, T.R.A.; De Moraes, M.O.; Montenegro, R.C.; Yoneda, J.D.; Leal, K.Z. Synthesis and Anticancer Activity of (E)-2-Benzothiazole Hydrazones. Eur. J. Med. Chem. 2014, 86, 12–16. [Google Scholar] [CrossRef]
- Sultana, F.; Saifi, M.A.; Syed, R.; Mani, G.S.; Shaik, S.P.; Osas, E.G.S.; Godugu, C.; Shahjahan, S.; Kamal, A. Synthesis of 2-Anilinopyridyl Linked Benzothiazole Hydrazones as Apoptosis Inducing Cytotoxic Agents. New J. Chem. 2019, 43, 7150–7161. [Google Scholar] [CrossRef]
- Liu, K.; Ding, Y.; Kang, C. Synthesis and Antiproliferative Activity of New N-Acylhydrazone Derivatives Containing Benzothiazole and Indole Based Moiety. Pharm. Chem. J. 2020, 54, 345–352. [Google Scholar] [CrossRef]
- Osmaniye, D.; Levent, S.; Karaduman, A.B.; Ilgın, S.; Zkay, Y.; Kaplancikli, Z.A. Synthesis of New Benzothiazole Acylhydrazones as Anticancer Agents. Molecules 2018, 23, 1054. [Google Scholar] [CrossRef]
- Mokhtar, A.M.; El-Messery, S.M.; Ghaly, M.A.; Hassan, G.S. Targeting EGFR Tyrosine Kinase: Synthesis, in Vitro Antitumor Evaluation, and Molecular Modeling Studies of Benzothiazole-Based Derivatives. Bioorg. Chem. 2020, 104, 104259. [Google Scholar] [CrossRef]
- Yurttaş, L.; Ertaş, M.; Çiftçi, G.A.; Temel, H.E.; Demirayak, Ş. Novel Benzothiazole Based Imidazole Derivatives as New Cytotoxic Agents against Glioma (C6) and Liver (HepG2) Cancer Cell Lines. Acta Pharm. Sci. 2017, 55, 39–47. [Google Scholar] [CrossRef]
- Xie, X.; Yan, Y.; Zhu, N.; Liu, G. Benzothiazoles Exhibit Broad-Spectrum Antitumor Activity: Their Potency, Structure-Activity and Structure-Metabolism Relationships. Eur. J. Med. Chem. 2014, 76, 67–78. [Google Scholar] [CrossRef]
- Reddy, V.G.; Reddy, T.S.; Jadala, C.; Reddy, M.S.; Sultana, F.; Akunuri, R.; Bhargava, S.K.; Wlodkowic, D.; Srihari, P.; Kamal, A. Pyrazolo-Benzothiazole Hybrids: Synthesis, Anticancer Properties and Evaluation of Antiangiogenic Activity Using in Vitro VEGFR-2 Kinase and in Vivo Transgenic Zebrafish Model. Eur. J. Med. Chem. 2019, 182, 111609. [Google Scholar] [CrossRef]
- Subba Rao, A.V.; Swapna, K.; Shaik, S.P.; Lakshma Nayak, V.; Srinivasa Reddy, T.; Sunkari, S.; Shaik, T.B.; Bagul, C.; Kamal, A. Synthesis and Biological Evaluation of Cis-Restricted Triazole/Tetrazole Mimics of Combretastatin-Benzothiazole Hybrids as Tubulin Polymerization Inhibitors and Apoptosis Inducers. Bioorg. Med. Chem. 2017, 25, 977–999. [Google Scholar] [CrossRef]
- Palkar, M.; Noolvi, M.; Sankangoud, R.; Maddi, V.; Gadad, A.; Nargund, L.V.G. Synthesis and Antibacterial Activity of a Novel Series of 2,3-Diaryl-Substituted-Imidazo(2,1-b)-Benzothiazole Derivatives. Arch. Pharm. 2010, 343, 353–359. [Google Scholar] [CrossRef]
- Zha, G.F.; Leng, J.; Darshini, N.; Shubhavathi, T.; Vivek, H.K.; Asiri, A.M.; Marwani, H.M.; Rakesh, K.P.; Mallesha, N.; Qin, H.L. Synthesis, SAR and Molecular Docking Studies of Benzo[d]Thiazole-Hydrazones as Potential Antibacterial and Antifungal Agents. Bioorg. Med. Chem. Lett. 2017, 27, 3148–3155. [Google Scholar] [CrossRef]
- Ghannam, I.A.Y.; Abd El-Meguid, E.A.; Ali, I.H.; Sheir, D.H.; El Kerdawy, A.M. Novel 2-Arylbenzothiazole DNA Gyrase Inhibitors: Synthesis, Antimicrobial Evaluation, QSAR and Molecular Docking Studies. Bioorg. Chem. 2019, 93, 103373. [Google Scholar] [CrossRef]
- Prajapati, N.P.; Vekariya, R.H.; Borad, M.A.; Patel, H.D. Recent Advances in the Synthesis of 2-Substituted Benzothiazoles: A Review. RSC Adv. 2014, 4, 60176–60208. [Google Scholar] [CrossRef]
- Bala, S.; Uppal, G.; Kajal, A.; Kamboj, S.; Sharma, V. Hydrazones as Promising Lead with Diversity in Bioactivity-Therapeutic Potential in Present Scenario. Int. J. Pharm. Sci. Rev. Res. 2013, 18, 65–74. [Google Scholar]
- Shi, S.M.; Li, Q.; Hu, S.L. A New Hydrazone-Based Colorimetric Chemosensor for Naked-Eye Detection of Copper Ion in Aqueous Medium. J. Chem. Res. 2019, 43, 426–430. [Google Scholar] [CrossRef]
- Sharma, N.K.; Bhadauria, R.S. Synthesis and Biological Evaluation of 1-(6-Bromobenzo [d]Thiazol-2-Yl)-2-(Disubstituted Methylene) Hydrazine Derivatives. J. Drug Deliv. Ther. 2019, 9, 577–586. [Google Scholar] [CrossRef]
- Pise, A.S.; Ingale, A.P.; Dalvi, N.R. Ultrasound-Assisted Efficient and Green Synthesis of 2-Substituted Benzothiazoles under Solvent-Free Condition Using Recyclable Sulfated Tungstate. Synth. Commun. 2021, 51, 3629–3641. [Google Scholar] [CrossRef]
- Rakas, A.; Persoons, L.; Daelemans, D.; Grgić, D.K.; Kraljević, T.G. A Sustainable Synthesis of Novel 2-(3,4-Disubstituted phenyl)benzoxazole Derivatives and Their Antiproliferative and Antibacterial Evaluation. Molecules 2025, 30, 1767. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T.; Mottillo, C.; Titi, H.M. Mechanochemistry for Synthesis. Angew. Chem. 2020, 132, 1030–1041. [Google Scholar] [CrossRef]
- Fantozzi, N.; Volle, J.N.; Porcheddu, A.; Virieux, D.; García, F.; Colacino, E. Green Metrics in Mechanochemistry. Chem. Soc. Rev. 2023, 52, 6680–6714. [Google Scholar] [CrossRef]
- Cabeza, J.A.; García, F.; García-Álvarez, P.; García-Soriano, R.; Cabeza, A.; García, F.; García-Álvarez, P.; García-Soriano, R. Fast and scalable Solvent-Free Access to Lappert’s Heavier Tetrylenes E{N(SiMe3)2}2(E = Ge, Sn, Pb). Chem. Sci. 2023, 14, 12477–12483. [Google Scholar] [CrossRef]
- Sharma, H.; Singh, N.; Jang, D.O. A Ball-Milling Strategy for the Synthesis of Benzothiazole, Benzimidazole and Benzoxazole Derivatives under Solvent-Free Conditions. Green Chem. 2014, 16, 4922–4930. [Google Scholar] [CrossRef]
- Bhosle, A.A.; Banerjee, M.; Hiremath, S.D.; Bhasikuttan, A.C.; Chatterjee, A. A New Series of D1-A-D2-Type ESIPT-TICT-AIE Active Orange-to-Red Emissive Unsymmetrical Azines: Their All-throughout Mechanochemical Synthesis and Exploration of Photophysical Properties. Chem. An. Asian J. 2023, 18, e202300048. [Google Scholar] [CrossRef]
- Colacino, E.; Porcheddu, A.; Halasz, I.; Charnay, C.; Delogu, F.; Guerra, R.; Fullenwarth, J. Mechanochemistry for “No Solvent, No Base” Preparation of Hydantoin-Based Active Pharmaceutical Ingredients: Nitrofurantoin and Dantrolene. Green Chem. 2018, 20, 2973–2977. [Google Scholar] [CrossRef]
- Do, J.L.; Friščić, T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef]
- Yadav, K.P.; Rahman, M.A.; Nishad, S.; Maurya, S.K.; Anas, M.; Mujahid, M. Synthesis and biological activities of benzothiazole derivatives: A review. Intell. Pharm. 2023, 1, 122–132. [Google Scholar] [CrossRef]
- Gilani, S.J.; Khan, S.A. Synthesis and Pharmacological Evaluation of N-(6-Chlorobenzo[d]Thiazol-2- Yl)Hydrazine Carboxamide Derivatives of Benzothiazole. Med. Chem. Res. 2013, 22, 3316–3328. [Google Scholar] [CrossRef]
- Munirajasekhar, D.; Himaja, M.; Sunil, M. Synthesis and Anthelmintic Activity of 2-Amino-6-Substituted Benzothiazoles. Int. Res. J. Pharm. 2011, 2, 114–117. [Google Scholar]
- Himaja, M.; Munirajasekhar, D.; Karigar, A.; Ramana, M.V.; Sikarwar, M. Synthesis, Anthelmintic, Insecticidal Activity of (E)-1-((1-(6-Chlorobenzo[d]Thiazol-2-Yl)-3- Phenyl-1H-Pyrazol-4-Yl)Methylene)-2-(6-Substituted Benzo[d]Thiazol-2-Yl)Hydrazine Derivatives. Asian J. Chem. 2012, 24, 2789–2792. [Google Scholar]
- Perin, N.; Hok, L.; Beč, A.; Persoons, L.; Vanstreels, E.; Daelemans, D.; Vianello, R.; Hranjec, M. N-Substituted Benzimidazole Acrylonitriles as in Vitro Tubulin Polymerization Inhibitors: Synthesis, Biological Activity and Computational Analysis. Eur. J. Med. Chem. 2021, 211, 113003. [Google Scholar] [CrossRef]
- Beč, A.; Hok, L.; Persoons, L.; Vanstreels, E.; Daelemans, D.; Vianello, R.; Hranjec, M. Synthesis, Computational Analysis, and Antiproliferative Activity of Novel Benzimidazole Acrylonitriles as Tubulin Polymerization Inhibitors: Part 2. Pharmaceuticals 2021, 14, 1052. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grows Aerobically, 9th ed.; CLSI Document MO7-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Matsui, M.; Marui, Y.; Kushida, M.; Funabiki, K.; Muramatsu, H.; Shibata, K.; Hirota, K.; Hosoda, M.; Tai, K. Second-Order Optical Nonlinearity of 6-(Perfluoroalkyl) Benzothiazolylazo Dyes. Dyes Pigments 1998, 38, 57. [Google Scholar] [CrossRef]
- Sokol, I.; Rakas, A.; Kučić Grgić, D.; Persoons, L.; Daelemans, D.; Gazivoda Kraljević, T. Biological Assessments of Novel Ultrasound-Synthesized 2-Arylbenzimidazole Derivatives: Antiproliferative and Antibacterial Effects. RSC Med. Chem. 2025, 16, 3197–3212. [Google Scholar] [CrossRef]
- Deep, A.; Jain, S.; Sharma, P.C.; Verma, P.; Kumar, M.; Dora, C.P. Design and Biological Evaluation of Biphenyl-4-Carboxylic Acid Hydrazide-Hydrazone for Antimicrobial Activity. Acta Pol. Pharm. Drug Res. 2010, 67, 255–259. [Google Scholar]

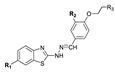 | |||||||||||
| IC50/µM | |||||||||||
| CMPD | R1 | R2 | R3 | CAPAN-1 | HCT-116 | LN-229 | NCI-H460 | DND-41 | HL-60 | K-562 | Z-138 |
| 19 | H | H |  | 2.0 ± 0.3 | 2.5 ± 0.5 | 1.9 ± 0.1 | 1.4 ± 0.5 | 6.5 ± 3.8 | 20.0 ± 0.4 | 5.3 ± 3.7 | 3.6 ± 0.3 |
| 20 | H | H |  | 54.8 ± 6.1 | >100 | >100 | 62.9 ± 4.5 | ≥90.4 | ≥81.6 | >100 | ≥77.5 |
| 21 | H | OMe | 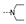 | 1.9 ± 0.3 | 2.2 ± 0.3 | 1.7 ± 0.1 | 1.7 ± 0.1 | 2.0 ± 0.0 | 2.3 ± 0.0 | 2.0 ± 0.1 | 2.2 ± 0.0 |
| 22 | H | OMe | 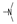 | 2.0 ± 0.6 | 6.0 ± 3.6 | 1.9 ± 0.1 | 2.7 ± 0.3 | 2.1 ± 0.1 | 2.4 ± 0.0 | 2.0 ± 0.1 | 5.2 ± 4.0 |
| 23 | H | OMe | 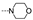 | >100 | >100 | >100 | 29.7 ± 9.8 | 81.1 ± 9.1 | 66.6 ± 2.9 | >100 | 64.1 ± 4.7 |
| 24 | H | OMe |  | 54.9 ± 7.2 | >100 | >100 | >100 | 65.4 ± 8.6 | >100 | >100 | ≥90.7 |
| 25 | H | F | 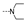 | 1.4 ± 0.9 | 8.4 ± 0.8 | 2.0 ± 0.3 | 4.2 ± 0.5 | 1.8 ± 0.6 | 5.6 ± 4.3 | 1.8 ± 0.2 | 5.0 ± 0.8 |
| 26 | H | F | 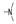 | 2.1 ± 0.6 | 1.2 ± 0.3 | 1.8 ± 0.3 | 1.0 ± 0.0 | 2.7 0.2 | 1.9 0.1 | 2.1 ± 0.3 | 2.4 ± 0.0 |
| 27 | H | F | 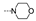 | 2.9 ± 0.3 | >100 | >100 | 3.0 ± 0.4 | ≥90.5 | 45.1 ± 9.6 | 62.7 ± 3.2 | 32.3 ± 5.9 |
| 28 | H | F | 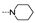 | 1.0 ± 0.3 | 6.7 ± 1.7 | 5.1 ± 4.3 | 5.8 ± 0.2 | 3.8 2.4 | 9.9 ± 2.9 | 4.3 ± 3.7 | 6.6 ± 4.2 |
| 29 | Cl | H | 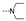 | 1.9 ± 0.6 | 9.5 ± 0.8 | 3.2 ± 1.5 | 7.7 ± 2.4 | 2.2 ± 0.6 | 3.9 ± 1.2 | 1.9 ± 0.3 | 2.4 ± 0.5 |
| 30 | Cl | H | 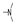 | 1.2 ± 0.6 | 5.3 ± 2.6 | 3.0 ± 1.7 | 7.1 ± 1.4 | 2.1 ± 0.4 | 5.0 ± 3.7 | 2.1 ± 1.3 | 6.0 ± 1.5 |
| 31 | Cl | H | 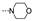 | 66.1 ± 11.2 | >100 | 68.3 ± 7.3 | ≥71.8 | 58.1 ± 6.3 | >100 | >100 | ≥86.0 |
| 32 | Cl | H | 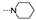 | 10.6 ± 0.7 | 9.1 ± 4.0 | 10.6 ± 1.8 | 12.4 ± 3.7 | 12.2 ± 0.2 | 10.3 ± 0.3 | 9.4 ± 2.0 | 8.8 ± 3.3 |
| 33 | Cl | OMe | 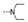 | 1.7 ± 0.4 | 2.4 ± 0.1 | 1.9 ± 0.2 | 4.1 ± 0.6 | 2.2 ± 0.0 | 6.1 ± 0.3 | 2.1 ± 0.1 | 3.0 ± 1.0 |
| 34 | Cl | OMe | 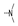 | 2.5 ± 1.1 | 10.4 ± 2.3 | 9.9 ± 0.8 | 8.1 ± 2.5 | 5.6 ± 1.2 | 10.7 ± 0.6 | 8.4 ± 2.2 | 4.9 ± 3.0 |
| 35 | Cl | OMe | 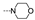 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 36 | Cl | OMe | 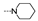 | 5.6 ± 2.4 | 21.7 ± 3.7 | 8.6 ± 1.9 | 14.8 ± 3.5 | 2.7 ± 1.0 | 16.7 ± 1.7 | 5.0 ± 2.8 | 9.1 ± 4.0 |
| 37 | Cl | F |  | 1.0 ± 0.3 | 1.9 ± 0.6 | 2.0 ± 0.2 | 1.4 ± 0.1 | 1.9 ± 0.3 | 1.9 ± 0.0 | 1.9 ± 0.0 | 1.3 ± 0.5 |
| 38 | Cl | F | 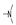 | 0.6 ± 0.2 | 1.4 ± 0.0 | 1.6 ± 0.0 | 0.9 ± 0.1 | 2.5 ± 0.2 | 2.1 ± 0.1 | 1.7 ± 0.2 | 2.2 ± 0.0 |
| 39 | Cl | F | 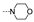 | 36.0 ± 4.6 | >100 | >100 | 39.9 ± 3.5 | 16.0 ± 0.5 | 15.7 ± 0.5 | >100 | ≥80.5 |
| 40 | Cl | F | 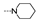 | 1.9 ± 0.2 | 1.8 ± 0.1 | 1.3 ± 0.3 | 1.8 ± 0.8 | 2.2 ± 0.1 | 5.2 ± 0.3 | 1.6 ± 0.4 | 1.9 ± 0.7 |
| 41 | OMe | H | 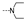 | 1.8 ± 0.5 | 1.8 ± 0.2 | 1.6 ± 0.3 | 2.7 ± 0.1 | 2.1 ± 0.1 | 3.5 ± 2.0 | 3.2 ± 1.8 | 3.4 ± 0.2 |
| 42 | OMe | H | 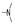 | 1.9 ± 0.1 | 2.6 ± 0.8 | 1.9 ± 0.2 | 6.7 ± 0.2 | 2.1 ± 0.1 | 4.2 ± 2.4 | 1.9 ± 0.1 | 2.0 ± 0.2 |
| 43 | OMe | H | 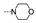 | 64.6 ± 12.9 | >100 | ≥87.4 | 54.7 ± 7.7 | 67.5 ± 12.4 | 65.2 ± 1.9 | ≥70.8 | 66.1 ± 2.2 |
| 44 | OMe | H | 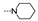 | 57.9 ± 3.3 | >100 | >100 | >100 | 46.6 ± 2.0 | >100 | 21.8 ± 1.6 | ≥80.6 |
| 45 | OMe | OMe | 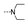 | 1.9 ± 0.5 | 1.8 ± 0.2 | 1.3 ± 0.0 | 1.8 ± 0.1 | 11.3 ± 1.5 | 1.7 ± 0.5 | 10.4 ± 0.4 | 1.7 ± 0.7 |
| 46 | OMe | OMe | 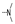 | 1.9 ± 0.4 | 2.3 ± 0.3 | 1.8 ± 0.4 | 1.7 ± 0.1 | 1.7 ± 0.2 | 1.6 ± 0.3 | 1.9 ± 0.0 | 1.7 ± 0.2 |
| 47 | OMe | OMe | 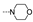 | 1.8 ± 0.4 | 4.9 ± 1.9 | 2.6 ± 0.1 | 1.7 ± 0.1 | 7.3 ± 0.0 | 1.8 ± 0.0 | 2.8 ± 1.2 | 5.1 ± 4.0 |
| 48 | OMe | OMe | 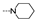 | 1.8 ± 0.2 | 1.9 ± 0.6 | 2.1 ± 0.4 | 6.4 ± 0.4 | 3.6 ± 0.5 | 12.8 ± 1.7 | 2.7 ± 0.5 | 5.2 ± 0.5 |
| 49 | OMe | F |  | 1.8 ± 0.3 | 1.8 ± 0.3 | 1.8 ± 0.1 | 1.4 ± 0.2 | 1.5 ± 0.3 | 1.3 ± 0.2 | 1.8 ± 0.1 | 3.8 ± 1.8 |
| 50 | OMe | F |  | 2.0 ± 0.5 | 2.5 ± 0.6 | 1.8 ± 0.1 | 1.7 ± 0.1 | 2.1 ± 0.3 | 1.8 ± 0.2 | 1.8 ± 0.3 | 3.8 ± 0.2 |
| 51 | OMe | F | 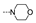 | 6.0 ± 4.8 | 2.8 ± 0.1 | 2.3 ± 0.8 | 1.6 ± 0.4 | 3.7 ± 2.1 | 1.9 ± 0.5 | 2.9 ± 1.0 | 1.6 ± 0.2 |
| 52 | OMe | F | 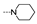 | 1.8 ± 0.4 | 2.0 ± 0.4 | 1.8 ± 0.1 | 1.4 ± 0.2 | 1.9 ± 0.1 | 3.0 ± 1.1 | 3.0 ± 1.3 | 2.1 ± 0.6 |
| etoposide | 0.03 ± 0.01 | 3.4 ± 0.1 | 3.7 ± 0.1 | 6.1 ± 0.4 | 1.0 ± 0.1 | 0.8 ± 0.1 | 4.0 ± 0.6 | 0.7 ± 0.1 | |||
| nocodazole | 0.02 ± 0.01 | 0.04 ± 0.01 | 0.4 ± 0.3 | 0.5 ± 0.1 | 0.7 ± 0.1 | 0.04 ± 0.00 | 0.04 ± 0.01 | 0.04 ± 0.01 | |||
| IC50/µM | S.I. | ||||||||
| CMPD | PBMC | CAPAN-1 | HCT-116 | LN-229 | NCI-H460 | DND-41 | HL-60 | K-562 | Z-138 |
| 19 | 17.7 ± 1.2 | 8.8 | 7.1 | 9.3 | 12.6 | 2.7 | 0.9 | 3.3 | 4.9 |
| 20 | ≥70.6 | 1.3 | 1.1 | 0.8 | 0.9 | 0.9 | |||
| 21 | 4.2 ± 4.3 | 2.2 | 1.9 | 2.4 | 2.4 | 2.1 | 1.8 | 2.1 | 1.9 |
| 22 | 7.5 ± 0.9 | 3.8 | 1.3 | 4.0 | 2.8 | 3.6 | 3.1 | 3.8 | 1.4 |
| 23 | 12.6 ± 10.2 | 0.4 | 0.2 | 0.2 | 0.2 | ||||
| 24 | 67.9 ± 10.9 | 1.2 | 1.0 | 0.7 | |||||
| 25 | 11.2 ± 1.7 | 8.0 | 1.3 | 5.6 | 2.7 | 6.2 | 2.0 | 6.2 | 2.2 |
| 26 | 12.1 ± 2.0 | 5.8 | 10.1 | 6.7 | 12.1 | 4.5 | 6.4 | 5.8 | 5.1 |
| 27 | 55.8 ± 0.4 | 19.2 | 18.6 | 0.6 | 1.2 | 0.9 | 1.7 | ||
| 28 | 8.9 ± 1.6 | 8.9 | 1.3 | 1.7 | 1.5 | 2.3 | 0.9 | 2.1 | 1.3 |
| 29 | 15.7 ± 2.8 | 8.3 | 1.7 | 4.9 | 2.0 | 7.2 | 4.0 | 8.3 | 6.6 |
| 30 | 13.5 ± 2.0 | 11.2 | 2.5 | 4.5 | 1.9 | 6.4 | 2.7 | 6.4 | 2.2 |
| 31 | 91.2 ± 1.4 | 1.4 | 1.3 | 1.3 | 1.6 | 1.1 | |||
| 32 | 44.6 ± 42.8 | 4.2 | 4.9 | 4.2 | 3.6 | 3.7 | 4.3 | 4.7 | 5.1 |
| 33 | 2.3 ± 1.2 | 1.3 | 1.0 | 1.2 | 0.6 | 1.0 | 0.4 | 1.1 | 0.8 |
| 34 | 7.5 ± 1.5 | 3.0 | 0.7 | 0.8 | 0.9 | 1.3 | 0.7 | 0.9 | 1.5 |
| 35 | >100 | ||||||||
| 36 | 13.2 ± 1.1 | 2.4 | 0.6 | 1.5 | 0.9 | 4.9 | 0.8 | 2.6 | 1.5 |
| 37 | 12.5 ± 2.1 | 12.5 | 6.6 | 6.3 | 8.9 | 6.6 | 6.6 | 6.6 | 9.6 |
| 38 | 2.9 ± 1.2 | 4.8 | 2.1 | 1.8 | 3.2 | 1.2 | 1.4 | 1.7 | 1.3 |
| 39 | 50.4 ± 0.2 | 1.4 | 1.3 | 3.1 | 3.2 | 0.6 | |||
| 40 | 6.1 ± 0.4 | 3.2 | 3.4 | 4.7 | 3.4 | 2.8 | 1.2 | 3.8 | 3.2 |
| 41 | 5.3 ± 2.3 | 2.9 | 2.9 | 3.3 | 2.0 | 2.5 | 1.5 | 1.7 | 1.6 |
| 42 | 24.6 ± 1.6 | 12.9 | 9.5 | 12.9 | 3.7 | 11.7 | 5.9 | 12.9 | 12.3 |
| 43 | 50.5 ± 10.4 | 0.8 | 0.6 | 0.9 | 0.7 | 0.8 | 0.7 | 0.8 | |
| 44 | 62.6 ± 31.1 | 1.1 | 1.3 | 2.9 | 0.8 | ||||
| 45 | 4.1 ± 0.1 | 2.2 | 2.3 | 3.2 | 2.3 | 0.4 | 2.4 | 0.4 | 2.4 |
| 46 | 3.7 ± 0.1 | 1.9 | 1.6 | 2.1 | 2.2 | 2.2 | 2.3 | 1.9 | 2.2 |
| 47 | 5.7 ± 6.2 | 3.1 | 1.2 | 2.2 | 3.3 | 0.8 | 3.1 | 2.0 | 1.1 |
| 48 | 17.3 ± 12.8 | 9.6 | 9.1 | 8.2 | 2.7 | 4.8 | 1.3 | 6.4 | 3.3 |
| 49 | 17.3 ± 17.3 | 9.6 | 9.6 | 9.6 | 12.3 | 11.5 | 13.3 | 9.6 | 4.5 |
| 50 | 7.2 ± 2.5 | 3.6 | 2.9 | 4.0 | 4.2 | 3.4 | 4.0 | 4.0 | 1.9 |
| 51 | ≥40.7 | 6.8 | 14.5 | 17.7 | 25.4 | 11.0 | 21.4 | 14.0 | 25.4 |
| 52 | 12.3 ± 10.3 | 6.8 | 6.1 | 6.8 | 8.8 | 6.4 | 4.1 | 4.1 | 5.8 |
| etoposide | >10 | ||||||||
| nocodazole | 1.4 ± 0.1 | 70.0 | 35.0 | 3.5 | 2.8 | 2.0 | 35.0 | 35.0 | 35.0 |
 | ||||||||
| MIC/µg/mL | ||||||||
| CMPD | R1 | R2 | R3 | E. coli | P. aeruginosa | K. pneumoniae | S. aureus | E. faecalis |
| 19 | H | H |  | >256 | >256 | >256 | 26.67 ± 7.54 | 32 ± 0.00 |
| 20 | H | H | 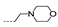 | >256 | >256 | >256 | >256 | >256 |
| 23 | H | OCH3 |  | >256 | >256 | >256 | 170.67 ± 60.33 | 8 ± 0.00 |
| 24 | H | OCH3 |  | >256 | >256 | >256 | >256 | >256 |
| 25 | H | F | 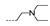 | >256 | 32 ± 0.00 | 16 ± 0.00 | 16 ± 0.00 | >256 |
| 26 | H | F | 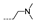 | >256 | >256 | >256 | >256 | 128 ± 0.00 |
| 27 | H | F | 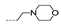 | >256 | >256 | >256 | >256 | >256 |
| 28 | H | F | 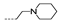 | >256 | >256 | 32 ± 0.00 | >256 | >256 |
| 29 | Cl | H | 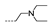 | >256 | >256 | >256 | 42.67 ± 15.08 | 32 ± 0.00 |
| 30 | Cl | H | 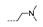 | >256 | >256 | >256 | 128 ± 0.00 | >256 |
| 31 | Cl | H | 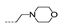 | >256 | >256 | >256 | >256 | >256 |
| 32 | Cl | H | 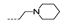 | >256 | >256 | 53.33 ± 15.07 | >256 | >256 |
| 33 | Cl | OCH3 |  | >256 | 16 ± 0.00 | >256 | >256 | >256 |
| 34 | Cl | OCH3 | 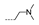 | >256 | >256 | >256 | 32 ± 0.00 | 32 ± 0.00 |
| 36 | Cl | OCH3 | 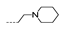 | >256 | >256 | >256 | >256 | >256 |
| 37 | Cl | F | 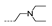 | >256 | 4 ± 0.00 | >256 | >256 | >256 |
| 38 | Cl | F | 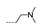 | >256 | >256 | >256 | >256 | >256 |
| 39 | Cl | F | 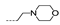 | >256 | >256 | >256 | >256 | >256 |
| 41 | OCH3 | H | 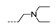 | >256 | >256 | >256 | >256 | >256 |
| 43 | OCH3 | H | 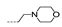 | >256 | >256 | >256 | >256 | >256 |
| 44 | OCH3 | H | 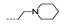 | >256 | >256 | >256 | >256 | >256 |
| 45 | OCH3 | OCH3 | 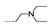 | >256 | >256 | >256 | >256 | >256 |
| CAZ | 0.5 ± 0.00 | 1.67 ± 0.47 | >256 | 53.33 ± 15.07 | >256 | |||
| CIP | <0.215 | 0.67 ± 0.24 | >256 | 0.5 ± 0.0 | 0.83 ± 0.24 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokol, I.; Mlinar, H.; Grgić, D.K.; Persoons, L.; Daelemans, D.; Mihovilović, M.; Kraljević, T.G. Mechanochemical Solvent-Free Synthesis and Biological Profiling of Novel 2-Hydrazone-Bridged Benzothiazoles as Potent Anticancer Agents. Sustain. Chem. 2025, 6, 41. https://doi.org/10.3390/suschem6040041
Sokol I, Mlinar H, Grgić DK, Persoons L, Daelemans D, Mihovilović M, Kraljević TG. Mechanochemical Solvent-Free Synthesis and Biological Profiling of Novel 2-Hydrazone-Bridged Benzothiazoles as Potent Anticancer Agents. Sustainable Chemistry. 2025; 6(4):41. https://doi.org/10.3390/suschem6040041
Chicago/Turabian StyleSokol, Ivana, Hanja Mlinar, Dajana Kučić Grgić, Leentje Persoons, Dirk Daelemans, Moris Mihovilović, and Tatjana Gazivoda Kraljević. 2025. "Mechanochemical Solvent-Free Synthesis and Biological Profiling of Novel 2-Hydrazone-Bridged Benzothiazoles as Potent Anticancer Agents" Sustainable Chemistry 6, no. 4: 41. https://doi.org/10.3390/suschem6040041
APA StyleSokol, I., Mlinar, H., Grgić, D. K., Persoons, L., Daelemans, D., Mihovilović, M., & Kraljević, T. G. (2025). Mechanochemical Solvent-Free Synthesis and Biological Profiling of Novel 2-Hydrazone-Bridged Benzothiazoles as Potent Anticancer Agents. Sustainable Chemistry, 6(4), 41. https://doi.org/10.3390/suschem6040041








